Introduction
Eosinophilic esophagitis (EoE) is an increasingly prevalent, food and aero- allergen mediated, chronic disorder of the esophagus affecting over 37 per 100,000 children in the United States [1]. It is characterized by symptoms of esophageal dysfunction and an eosinophil-predominant inflammation in the esophagus in absence of other causes of esophageal eosinophilia [2].
Infants and toddlers with EoE frequently present with feeding difficulties and feeding aversion, and a careful history in school aged-children and adolescents with this condition may reveal that they have learned to compensate by eating slowly, chewing excessively, taking small bites, drinking excessively with meals, or avoiding specific food consistencies such as meats [3,4]. Such alterations in the feeding or eating behavior in children with chronic conditions including EoE, can result in stressful interactions with their caregivers, especially surrounding meal time and may lead to social isolation [5,6]. Taken together, EoE is an emerging esophageal condition which can result in altered eating behavior in children and this in turn may affect their caregiver’s quality of life (cQoL).
The clinical evaluation of eating behavior in children involves gathering in-depth history, conducting a thorough evaluation of oropharyngeal anatomy, behavioral observations, relational aspects of feeding, and assessing the feeding environment. Although not routinely performed, some centers may also assess the esophageal motility and the epithelial integrity. As a result, this can be time-consuming and financially burdensome to the caregivers, and requires careful consideration [7,8].
Caregivers’ observe the feeding or eating behavior of their child longitudinally across a number of mealtimes and situations. As such, they can provide a comprehensive perspective on a child’s typical feeding behaviors compared to that of an observation made by a healthcare personnel during a single mealtime in an unfamiliar clinical environment [9]. There are multiple reports demonstrating that the caregiver questionnaires can serve as an efficient way to assess child’s feeding behavior. For example, caregiver report has been shown to correspond to direct observation of some eating behaviors, including meal length and parent coaxing in children [10,11]. However, to date, caregivers’ perspective on the eating behaviors of children with EoE and its impact on their cQoL has not been examined. Taking caregivers’ perspective into account could allow identification of certain eating styles in children with EoE and may lead to interventions to diminish their problematic impact on the child as well as their caregiver.
In this study, we compared caregivers’ perception of eating behaviors in school-aged children with EoE (cEoE) to that of non-EoE controls (cControl), and investigated the impact of EoE-related feeding/swallowing problems on cQoL. Based on published literature and our own clinical observations, we hypothesized that caregivers would report maladaptive eating behaviors in their school-aged child diagnosed with EoE, and that the maladaptive eating behavior would negatively impact their quality of life.
Methods
Study design and Participants
We prospectively enrolled 80 caregivers of children between 6 – 18 years of age presenting to our multidisciplinary Eosinophilic Gastrointestinal Disorders clinic and the Gastroenterology clinic between January 2016 and December 2017. Forty-two (53%) were caregivers to children with EoE which was defined per the 2011 consensus guidelines wherein children needed to have ≥15 eosinophils per high power field (eos/hpf) and at least one typical symptom of esophageal dysfunction (i.e., vomiting, feeding intolerance, dysphagia) with other causes of esophageal eosinophilia excluded, and without a response to adequate acid suppression. The non-EoE control group comprised of 38 (47%) children presenting for upper gastrointestinal complaints and did not meet the criteria for EoE. Of the 42 children with EoE, 33 (79%) had active EoE, and 9 (21%) had inactive EoE (defined as < 15 eos/hpf in their esophageal biopsies at the time of most recent esophagogastroduodenoscopy) [2].
The study protocol was developed and the data collection was completed before the updated international consensus diagnostic criteria for eosinophilic esophagitis was published wherein the proton-pump inhibitors (PPI) were classified as a treatment for esophageal eosinophilia that may be due to EoE than as a diagnostic criteria [12].
Caregivers of children receiving tube feedings (e.g., feeding via nasgogastric tube or gastrostomy tube) or parenteral feeds, or with neurodevelopmental, genetic, metabolic and behavioral disorders, inflammatory bowel disease, celiac disease, and those refusing to provide informed consent were excluded.
Demographic and clinical data
Demographic information (including age, gender, ethnicity) and clinical and histologic data (such as symptoms, duration of complaints, allergic co-morbidities, peak eosinophil count from their most recent esophageal biopsies, and ongoing treatment) was gathered from medical records.
Administration of survey and Survey Instruments
On the day of the clinic visit, caregivers were informed about the study and were invited to participate. Upon obtaining an informed consent, caregivers were requested to complete a Child Eating Behavior Questionnaire (CEBQ) [13] and a Feeding/Swallowing Impact on Children’s Caregivers questionnaire (FS-IS) [14] one after the other.
Child Eating Behavior Questionnaire (CEBQ):
The CEBQ is a 35-item caregiver-rated questionnaire frequently used to investigate eating behavior in children over 2 years of age. It is broadly comprised of “Eating approach” and “Eating avoidance” traits. The “Eating approach” trait identifies positive inclinations for eating and is comprised of 4 subscales: Food Responsiveness (FR, 5 items), Desire to Drink (DD, 3 items), Emotional Over-Eating (EOE, 4 items) and Enjoyment of Food (EF, 4 items). The “Eating avoidance” trait identifies negative inclinations to food intake and is also comprised of 4 subscales: Satiety Responsiveness (SR, 5 items), Food Fussiness (FF, 6 items), Slowness in Eating (SE, 4 items), and Emotional Under-Eating (EUE, 4 items). The responses are scored on a five-point Likert frequency scale from 1 (never) to 5 (always) with higher scores indicating a stronger display of “Eating approach” or “Eating avoidance” behavior (Supplementary Material 1).
Feeding/Swallowing Impact on Children’s Caregivers questionnaire (FS-IS):
The FS-IS is an 18-item survey instrument commonly used to measure the impact of feeding/swallowing problems in children with chronic conditions on the quality of life of their caregivers within the past one month. The items on FS-IS are grouped into 3 major categories including the caregiver’s perceptions of: (1) time demands on daily activities, (2) worry about the child’s well-being, and (3) challenges related to the delivery of care specific to feeding/swallowing needs. Responses are assessed on a 5-point Likert scale ranging from a score of 1 (never) to a score of 5 (almost always) with higher scores signifying higher impact of child’s feeding/swallowing problem on the cQoL (Supplementary material 2).
Statistical analysis
Statistical analysis was performed using Stata version 14.0 (Stata Corp, College Station, TX). Bivariate analysis for continuous variables was performed using t tests, and chi-square test for categorical variables. We dichotomized CEBQ responses into “Not often” (Never + Rarely + Sometimes) and Often (Often + Always), and FS-IS responses into “Less than or equal to half the time” (Never +Almost never + Half the time) and “More than half the time” (Very Often + Almost always) to gain a deeper understanding of the differences between study groups. To compare the responses between cEoE and the cControl, unadjusted logistic regression was used to estimate the odds ratio (OR) with 95% confidence intervals (95% CI) for each question. Multivariate logistic regression was performed to adjust for potential independent predictors such as age, gender, ethnicity, allergic co-morbidities, EoE activity status, duration of complaints or EoE and ongoing treatment. To determine statistical significance, we used the conventional cut-off of p ≤ 0.05 as threshold for cohort characteristics and logistic regression models. We used a more stringent cut-off of p ≤ 0.001 (calculated as 0.05/35 questions) for CEBQ, and p ≤ 0.002 (calculated as 0.05/18 questions) for FS-IS to account for multiple comparisons and determine statistical significance.
Ethical Considerations
This study was approved by the Institutional Review Board at Vanderbilt University (protocol number 151846). All participants provided written informed consent before enrolling.
Results
Demographic and clinical characteristics
The average age was 11 ± 4 years (Mean ± SD), and it was similar for both children in both groups. Our cohort predominantly comprised of Caucasians (85%), and this is reflective of our general patient population. A significantly higher proportion of children in the EoE group were male compared to children in the non-EoE control group (85% vs. 26%; p < 0.001). When compared to the children in the non-EoE group, majority of children in the EoE group complained of reflux (8% vs. 31%; p = 0.01) and dysphagia (0% vs 45%; p < 0.001). Whereas, in the non-EoE control group, majority of children complained of abdominal pain (76% vs. 50%; p = 0.01) and constipation (47% vs. 24%; p = 0.03) when compared to children with EoE.
Expectedly, majority of children in the EoE group reported concomitant atopic comorbidities [such as food allergies (86%), asthma (15%), and allergic rhinitis (67%)]. The duration of upper gastrointestinal complaints or the time since EoE diagnosis was largely comparable between two groups except for a higher proportion of children in the EoE group had the condition for 19 – 24 months when compared to duration of gastrointestinal complaints in the children in non-EoE control group (10% vs. 3%; p = 0.02) (Table 1).
Table 1:
Demographic and clinical characteristics of children enrolled in the study
| Non-Eosinophilic esophagitis controls (N=38) | Eosinophilic esophagitis (N=42) | p value | |
|---|---|---|---|
| Age (years) [Mean ± SD] | 11 ± 4 | 11 ± 4 | 1.00 |
| Males, [Number (%)] | 10 (26) | 34 (81) | < 0.001 |
| Ethnicity, [Number (%)] | |||
| Caucasian | 36 (95) | 31 (74) | 0.01 |
| African American | 1 (3) | 11 (26) | 0.004 |
| Hispanic | 1 (3) | -- | 0.51 |
| Presenting symptoms, [Number (%)] | |||
| Reflux | 3 (8) | 13 (13) | 0.01 |
| Nausea | 7 (18) | 5 (12) | 0.06 |
| Dysphagia | -- | 19 (45) | 0.004 |
| Abdominal pain | 29 (76) | 21 (50) | 0.01 |
| Vomiting | 11 (29) | 20 (48) | 0.08 |
| Constipation | 18 (47) | 10 (24) | 0.03 |
| Diarrhea | 11 (4) | 10 (4) | 0.88 |
| Known eosinophilic esophagitis | -- | 39 (93) | < 0.001 |
| Duration of gastrointestinal complaints or eosinophilic esophagitis, [Number (%)] | |||
| ≤ 6 months | 10 (26) | 9 (22) | 0.67 |
| 7 – 12 months | 6 (16) | 7 (17) | 0.90 |
| 13 – 18 months | 3 (8) | 4 (10) | 0.75 |
| 19 – 24 months | 1 (3) | 4 (10) | 0.02 |
| > 24 months | 18 (47) | 17 (42) | 0.65 |
| Allergic comorbidities, [Number (%)] | |||
| Food allergies | 6 (16) | 36 (86) | < 0.001 |
| Asthma | -- | 15 (38) | 0.01 |
| Eczema | 5 (2) | 19 (8) | 0.05 |
| Allergic rhinitis | 8 (3) | 67 (28) | < 0.001 |
| Median (IQR) peak eosinophils per high power field (eos/hpf) | |||
| -- | 59 (0–105) | ||
| Eosinophilic esophagitis activity status, [Number (%)] | |||
| Active (≥ 15 eos/hpf) | 33 (79) | ||
| Inactive (< 15 eos/hpf) | 9 (21) | ||
| Ongoing Treatment, [Number (%)] | |||
| Proton pump | -- | 37 (88) | < 0.001 |
| inhibitors | |||
| Topical steroids | -- | 31 (74) | < 0.001 |
| Dietary elimination | -- | 30 (71) | < 0.001 |
SD: Standard deviation; IQR: Interquartile range
Caregivers’ perception of eating behavior as assessed by CEBQ
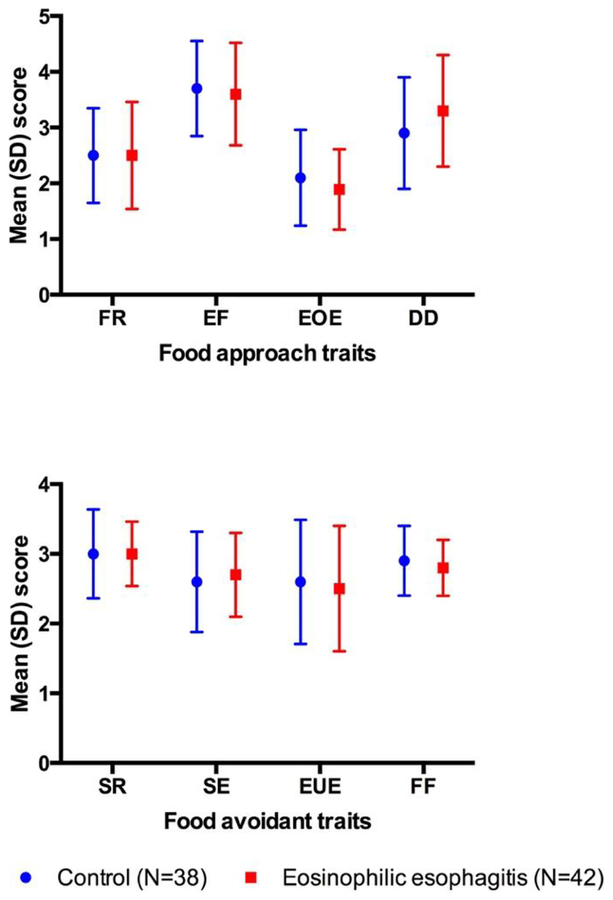
The caregivers’ perception for each of the eating behavior subscales as well as the “Food approach” trait and the “Food avoidant” trait scores were similar between children with EoE and the non-EoE controls (Figure 1). The scores within the EoE group did not differ by the disease activity status. In the “Food approach” trait, relatively higher proportion of cEoE indicated that their children ‘often’ demonstrated ‘Desire to Drink’ when compared to cControl but this observation did not achieve statistical significance (43% vs 34%; p = 0.41). In the “Food avoidant” traits, when compared to cControls relatively small proportion of cEoE indicated that their child ‘Often’ ate slowly (24% vs. 19%; p = 0.58) or exhibited food fussiness (34% vs. 31%; p = 0.84) (Supplementary Table 1).
Figure 1: Caregivers perception of eating behavior in children with EoE and non-EoE controls as assessed by CEBQ.
FR: Food Responsiveness; EF: Enjoyment of Food; EOE: Emotional Over-Eating; DD: Desire to Drink; SR: Satiety Responsiveness; SE: Slowness in Eating; EUE: Emotional Under-Eating; FF: Food Fussiness
Impact of feeding/swallowing problems on their caregivers’ quality of life
The cEoE reported that their child’s feeding or swallowing problems adversely impacted the quality of their life in all three categories. Compared to the cControl, the cEoE found it challenging to make plans to go out to eat (1.72 ± 0.21 vs. 2.78 ± 0.26; p < 0.001). cEoE also frequently found it challenging to get help from others as they were scared to feed or take care of the child (1.36 ± 0.12 vs. 2.19 ± 0.24; p < 0.001). Along the same lines, a significantly higher proportion of cEoE found it difficult to get help from others more than half the time because they were scared to feed or take care of the child when compared to that of cControls (31% vs. 11%; p = 0.02).
cEoE were more worried about the way their child would breathe while feeding and were worried if their child will choke when compared to cControl (2.28 ± 0.16 vs.1.25 ± 0.13; p < 0.001). cEoE were also more worried about their child’s general health when compared to the cControl (3.50 ± 0.19 vs. 2.67 ± 0.23; p < 0.001). A significantly higher proportion of cEoE were concerned that their child will never eat or drink like other children when compared to the cControls (31% vs. 11%; p = 0.02).
In the problems with feeding their child category, cEoE indicated that it was hard to feed their child as it took a long time to prepare liquids and foods the “right” way, and because their family members or professionals had different opinions about taking care of their child’s feeding/swallowing problems compared to cControl [(2.1 ± 0.20 vs. 1.17 ± 0.09; p < 0.001), and (2.14 ± 0.22 vs. 1.25 ± 0.13; p < 0.001), respectively]. A higher proportion of cEoE when compared to cControl tended to indicate that they experienced difficulties in feeding their children more than half of the time as they did not get enough information on how to get their child to eat and drink like other children (12% vs. 8%; p = 0.55) (Table 2, and Supplementary Table 2).
Table 2:
Comparison of the caregivers’ responses to FS-IS questionnaire
| Non-Eosinophilic esophagitis controls (N=38) | Eosinophilic esophagitis (N=42) | |
|---|---|---|
| In the past ONE month, as a result of your child’s feeding/swallowing problems, how often have you had problems carrying out your daily activities? [Mean ± SD] | ||
| It is hard for me to do my job, go to school, or work around the house | 1.47 ± 0.11 | 1.40 ± 0.09 |
| It is hard for me to get help from others because they are scared to feed or take care of my child. | 1.36 ± 0.12 | 2.19 ± 0.24 |
| It is hard for me to leave my child because I am scared to have other people feed or take care of my child. | 1.51 ± 0.16 | 2.17 ± 0.23 |
| It is hard for my family to make plans or go out to eat. | 1.72 ± 0.21 | 2.78 ± 0.26 |
| I am too tired to do the things I want or need to do. | 1.55 ± 0.17 | 2.17 ± 0.20 |
| In the past ONE month, as a result of your child’s feeding/swallowing problems, how often have you had problems with worrying? [Mean ± SD] | ||
| I worry about my child’s general health. | 2.67 ± 0.23 | 3.50 ± 0.19 |
| I worry that my child does not get enough to eat or drink. | 2.01 ± 0.19 | 2.81 ± 0.20 |
| I worry about how others will react to my child’s feeding/swallowing problems. | 1.51 ± 0.18 | 2.76 ± 0.20* |
| I worry about how my child breathes when feeding and whether my child will choke. | 1.25 ± 0.13 | 2.28 ± 0.16* |
| I worry that my child will never eat and drink like other children. | 1.34 ± 0.15 | 2.57 ± 0.22* |
| I worry about whether I am doing enough to help with my child’s feeding/swallowing problems. | 1.74 ± 0.22 | 3.04 ± 0.19* |
| I worry about how my child’s feeding/swallowing problems affect others in my family. | 1.34 ± 0.16 | 2.30 ± 0.20* |
| In the past ONE month, as a result of your child’s feeding/swallowing problems, how often have you had problems feeding your child? [Mean ± SD] | ||
| It is hard to feed my child because it takes a long time to prepare liquids and foods the “right” way. | 1.17 ± 0.09 | 2.12 ± 0.20* |
| It is hard to feed my child because I don’t know how to prepare liquids and foods. | 1.08 ± 0.07 | 1.67 ± 0.18 |
| It is hard to feed my child because others give my child liquids or foods that are not allowed. | 1.05 ± 0.07 | 1.92 ± 0.18* |
| It is hard to feed my child because I don’t know how long these problems will last. | 1.51 ± 0.17 | 2.11 ± 0.22 |
| It is hard to feed my child because family members or professionals have different opinions about taking care of my child’s feeding/swallowing problems. | 1.25 ± 0.13 | 2.14 ± 0.22* |
| It is hard to feed my child because I do not get enough information about how to get my child to eat and drink like other children. | 1.17 ± 0.10 | 1.75 ± 0.17 |
t-Test p value ≤ 0.001
Bivariate logistic regression revealed multiple differences between cEoE and cControl, but only one of these differences in persisted after the multivariate logistic analysis. The cEoE expressed greater concern for breathing and choking while feeding [aOR (95% CI): 3.24 (1.17 – 8.95); p = 0.02], with the odds for male being 44.16 (4.44 – 439.20) times greater than that for a female and presence of food allergies increasing the odds by 49.21 (4.74 – 509.50) times.
Discussion
In a questionnaire-based study we investigated caregiver’s perception of the impact of EoE on eating behavior of their children and examined how this affected caregiver’s quality of life. Our results were unexpected and mixed. While the caregivers’ perceptions of the eating behaviors between school-aged children with EoE and non-EoE controls did not differ significantly, the EoE-related feeding or swallowing problems in their child adversely impacted cQoL in the past month.
We theorized that the caregivers of children with EoE would report atypical eating behaviors, and in particular they would reveal their concerns which would be grouped in the ‘Food Avoidant’ traits defined in the CEBQ. Specifically, we were expecting the caregivers of children with EoE to indicate that frequently their child ate slow and/or often demonstrated food fussiness. However, our data from the 8 eating behavior sub-scales included in the CEBQ revealed that the eating behaviors were similar between children with EoE and age and ethnicity matched non-EoE controls, and this relationship was irrespective of the EoE activity status or the duration of disease or presenting complaints. Interestingly, only a relatively small proportion of caregivers of children with EoE report slowness in eating or food fussiness.
In the FS-IS survey, caregivers of children with EoE indicated that the perceived feeding/swallowing problems in the child impacted their quality of life in all three components. Notably, they were most concerned about breathing and choking while feeding, and that it took a long time for them to prepare liquids and foods the “right” way. These observations are congruent with our clinical experience, previously published reports from EoE population [15–17], and other chronic disorders in children [18].
While our results from the CEBQ and FS-IS appear discordant, they raise several important questions. First, is it possible that we as healthcare providers directly extrapolate our impressions about altered eating behaviors in infants and toddlers with EoE to school aged and older children? If so, then we might be overestimating the impact of EoE on feeding behavior in this age group. Second, is there an examiners bias wherein we as healthcare providers during clinical history taking are seeking to elicit accounts suggestive of ‘food avoidant’ traits? Third, it is generally accepted that by 5 years of age children are presumed to have mastered most of their oral motor milestones, participate more fully in family mealtimes [19,20], and express relatively more autonomy to choose what they want to eat and how much. In this study we enrolled children between 6 and 18 years of age, so it is possible that the children enrolled in our study had adjusted their eating behaviors (e.g., self-limiting to the foods that do not result in discomfort while eating) and now were at their new baseline eating behavior. Finally, could our findings be due to reverse causation? Is it possible that a diagnosis of EoE could have led caregivers to change their perception of their child’s eating behavior, and/or that the caregivers were working extra hard to adjust to their child’s eating behavior which in turn was negatively impacting their quality of life as reflected in our FS-IS result.
This study has limitations. The main limitation of this study is a small and convenient sample. As a result, this study may have been underpowered to detect anything other than large effect sizes. This was particularly evident in the CEBQ analysis, and in the logistic regression models where the confidence intervals for the adjusted odds ratio for gender and food allergies were very wide. We used a cross-sectional study design which is not an ideal to assess alterations in the eating behaviors as it can evolve or be modified over a period of time. Our study did not have a healthy control group for comparisons and this may limit the generalizability of our findings. Finally, children in the non-EoE control group predominantly complained of abdominal pain, vomiting and constipation, and none of them complained of dysphagia. It is generally accepted that deficits in the esophageal physiology can manifest as physiologic impairments in the pharyngo-esophageal region. As a result, this might have modified CEBQ scores which largely assess the volume and desire to eat. Nonetheless, it will be of great interest to compare CEBQ scores across true controls to examine differences in all three groups.
Despite these limitations, this study has several strengths. In absence of a validated tool to evaluate caregiver’s perception of eating behaviors in children with EoE and its impact on their caregiver’s quality of life, we used one of the most commonly used survey tools to measure these outcomes. This is the first study to examine caregiver’s perception of eating behavior in children with EoE and to investigate the impact of feeding or swallowing issues in the child on their caregivers’ quality of life simultaneously. This is important as caregiver’s well-being has been shown to play an important role on the health outcomes in children with chronic conditions such as EoE. The response rate for both questionnaires were very high (100% for CEBQ and 99% for FS-IS) and we used rigorous statistical methods to analyze our data and used a stricter significance threshold to account for multiple comparisons. Finally, our data indicates that caregivers’ perspectives can perhaps supplement clinical (or objective) assessment of feeding behaviors in children with EoE and may provide healthcare providers with information on which to base management recommendations and ultimately improve the care provided to children with EoE.
In conclusion, our study raises important questions directly related to the clinical care of children with EoE and their caregivers’ quality of life. Using a sensitive and validated survey tool in a longitudinal study with adequate sample size and a healthy control group is warranted to unravel the trajectory of eating-behavior development in children, and its interactions with other risk factors (such as food allergies), and chronic esophageal inflammatory conditions such as EoE.
Supplementary Material
Acknowledgements
G.H. is supported by American College of Gastroenterology Junior Faculty Career Development Award, Vanderbilt University Turner Hazinski award, Vanderbilt University Katherine Dodd Faculty Scholar program, and the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR; U54 AI117804) training award. CEGIR is part of the Rare Disease Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Sciences, and is funded through collaboration between the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Center for Advancing Translational Sciences. CEGIR also is supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, Campaign Urging Research for Eosinophilic Diseases, and Eosinophilic Family Coalition.
J.H is supported by a Food Allergy Research and Education Clinical Network Grant and the Vanderbilt University Katherine Dodd Faculty Scholar program.
We acknowledge Ms. Cindy Womack-Ramirez and Ms. Melissa Beavers for their assistance with data collection and data management.
Footnotes
Compliance with Ethical Standards
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
All authors disclose that they have no conflict of interest.
References
- 1.Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol 2011. [DOI] [PubMed] [Google Scholar]
- 3.Haas AM, Maune NC. Clinical Presentation of Feeding Dysfunction in Children with Eosinophilic Gastrointestinal Disease. Immunol Allergy Clin North Am. 2009;29:65–75. [DOI] [PubMed] [Google Scholar]
- 4.Cannington EM, Dolen WK. Feeding Dysfunction in Children With Eosinophilic Gastrointestinal Diseases. Pediatrics [Internet]. 2011;128:S110.2–S111. Available from: http://pediatrics.aappublications.org/lookup/doi/10.1542/peds.2011-2107EE [Google Scholar]
- 5.Dodrill P, Gosa MM. Pediatric dysphagia: Physiology, assessment, and management. Ann Nutr Metab. 2015; [DOI] [PubMed] [Google Scholar]
- 6.Flood EM, Beusterien KM, Amonkar MM, Jurgensen CH, Dewit OE, Kahl LP, et al. Patient and caregiver perspective on pediatric eosinophilic esophagitis and newly developed symptom questionnaires. Curr Med Res Opin [Internet]. 2008;24:3369–81. Available from: http://www.tandfonline.com/doi/full/10.1185/03007990802536900 [DOI] [PubMed] [Google Scholar]
- 7.Borowitz KC, Borowitz SM. Feeding Problems in Infants and Children: Assessment and Etiology. Pediatr. Clin. North Am 2018. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard JJ, Malandraki GA. Pediatric dysphagia. Swallowing - Physiol Disord Diagnosis Ther. 2015. [Google Scholar]
- 9.Arvedson JC. Assessment of pediatric dysphagia and feeding disorders: Clinical and instrumental approaches. Dev. Disabil. Res. Rev 2008. [DOI] [PubMed] [Google Scholar]
- 10.Piazza-Waggoner C, Driscoll KA, Gilman DK, Powers SW. A comparison using parent report and direct observation of mealtime behaviors in young children with cystic fibrosis: Implications for practical and empirically based behavioral assessment in routine clinical care. Child Heal Care. 2008; [Google Scholar]
- 11.Whelan E, Cooper PJ. The association between childhood feeding problems and maternal eating disorder: a community study. Psychol Med. 2000; [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry [Internet]. 2001;42:963–70. Available from: http://doi.wiley.com/10.1111/1469-7610.00792 [DOI] [PubMed] [Google Scholar]
- 14.Lefton-Greif MA, Okelo SO, Wright JM, Collaco JM, McGrath-Morrow SA, Eakin MN. Impact of children’s feeding/swallowing problems: Validation of a new caregiver instrument. Dysphagia. 2014;29:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menard-Katcher C, Henry M, Furuta GT, Atkins D, Maune NC, Haas AM. Significance of feeding dysfunction in eosinophilic esophagitis. World J Gastroenterol. 2014;20:11019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta P, Furuta GT, Brennan T, Henry ML, Maune NC, Sundaram SS, et al. Nutritional State and Feeding Behaviors of Children with Eosinophilic Esophagitis and Gastroesophageal Reflux Disease. J Pediatr Gastroenterol Nutr. 2018;66:603–8. [DOI] [PubMed] [Google Scholar]
- 17.Taft TH, Ballou S, Keefer L. Preliminary evaluation of maternal caregiver stress in pediatric eosinophilic gastrointestinal disorders. J Pediatr Psychol. 2012;37:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoner JB, Bailey RL, Angell ME, Robbins J, Polewski K. Perspectives of parents/guardians of children with feeding/swallowing problems. J Dev Phys Disabil. 2006; [Google Scholar]
- 19.Rommel N, De Meyer A-M, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003; [DOI] [PubMed] [Google Scholar]
- 20.Illingworth RS, Lister J. The critical or sensitive period, with special reference to certain feeding problems in infants and children. J Pediatr. 1964; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.