Abstract
Objective:
To characterize the use of multiparametric magnetic resonance imaging (mpMRI) in male Medicare beneficiaries electing active surveillance for prostate cancer. Multi-parametric resonance imaging (mpMRI) has emerged as a tool that may improve risk-stratification and decrease repeated biopsies in men electing active surveillance. However, the extent to which mpMRI has been implemented in active surveillance has not been established.
Methods:
Using Surveillance, Epidemiology, and End Results (SEER) registry data linked to Medicare claims data, we identified men with localized prostate cancer diagnosed between 2008–2013 and managed with active surveillance. We classified men into two treatment groups: active surveillance without mpMRI and active surveillance with mpMRI. We then fit a multivariable logistic regression models to examine changing mpMRI utilization over time, and factors associated with the receipt of mpMRI.
Results:
We identified 9,467 men on active surveillance. Of these, 8,178 (86%) did not receive mpMRI and 1,289 (14%) received mpMRI. The likelihood of receiving mpMRI over the entire study period increased by 3.7% (p=0.004). On multivariable logistic regression, patients who were younger, white, had lower comorbidity burden, lived in the northeast and west, had higher incomes and lived in more urban areas had greater odds of receiving mpMRI (all p<0.05).
Conclusion:
From 2008–2013, use of mpMRI in active surveillance increased gradually but significantly. Receipt of mpMRI among men on surveillance for prostate cancer varied significantly across demographic, geographic and socioeconomic strata. Going forward, studies should investigate causes for this variation and define ideal strategies for equitable, cost-effective dissemination of mpMRI technology.
Keywords: SEER Program, Medicare, Prostatic Neoplasms, Magnetic Resonance Imaging, Health Services Research, Surveillance
Introduction
Active surveillance is the gold standard for men with lower risk prostate cancer. Traditional active surveillance protocols rely on transrectal ultrasound-guided biopsies to accurately risk-stratify patients. More recently, new imaging modalities1 and genomic classifiers2 have supplemented traditional protocols to attempt to improve this risk stratification.
Multi-parametric resonance imaging (mpMRI) is one of these tools, but its use in active surveillance remains uncharacterized. The potential for mpMRI to improve the monitoring of patients on active surveillance by detecting clinically significant cancers3 and limiting the number of repeat biopsies,4, 5 may drive widespread uptake. Alternatively, concerns that mpMRI may add unnecessary expense, inconvenience, and anxiety may limit acceptance and use of mpMRI6, 7. While most urologists believe mpMRI is beneficial in the management of prostate cancer, many feel that limited access and prohibitive cost are significant barriers to incorporating mpMRI into standard practice.8 Where there is uncertainty, there is often variation in practice. As with other e4–6xpensive technologies, mpMRI use may be more prevalent among higher socioeconomic groups, or within certain regions of the country9, 10.
For these reasons, we sought to characterize the use of mpMRI among men on active surveillance using population-level data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare data. Understanding factors associated with the adoption of mpMRI represents an initial step in developing strategies to optimize cost-effective and equitable dissemination.
Methods
Study Population
The SEER-Medicare data files were used to identify men with non-managed care Medicare coverage age 66 years or older, and localized prostate (International Classification of Diseases 9th Edition [ICD-9] code 185) between 2008 and 2013. Methods for cohort identification closely adhered to prior work using SEER-Medicare data to study prostate cancer active surveillance.11 We included patients 66 years or older who were continuously enrolled in Medicare Parts A and B during the 12 months prior and after diagnosis (henceforth “entitlement criteria”). Patients who did not undergo any definitive treatment within the first year of diagnosis (i.e., no surgery, radiation, cryotherapy, or hormone therapy) and who had at least one prostate-specific antigen (PSA) level and/or one prostate biopsy in the two years after diagnosis were assigned to active surveillance, based on previously described work11 as well as recent data supporting the high sensitivity of this definition for men on prostate cancer active surveillance12. We excluded patients who had Gleason score of 4+3=7 or greater pathology as surveillance is not a recommended management strategy for this group.13
Outcomes
The primary outcome of this analysis is receipt of mpMRI as part of active surveillance. To define this outcome, we identified whether men received a pelvic MRI during active surveillance (Healthcare Common Procedure Coding System [HCPCS] codes 72195, 72196, 72197, and 72198 with or without MRI guidance for needle placement [HCPCS 77021]) after their first biopsy (henceforth “index biopsy”).
Patient demographic and pathological information were obtained using the SEER Patient Entitlement and Diagnosis Summary File (PEDSF). Local census tract information (i.e., ZIP code-level educational attainment, population of the county of residence, and ZIP code-level median household income) were obtained. Geographic region (northeast, south, central, west) was categorized based on SEER region at the time of the prostate cancer diagnosis. Pathologic information included tumor grade and stage classification based on the 2005 International Society of Urological Pathology Consensus.14 Patient comorbidity was determined using the Charlson-Klabunde method in the 12 months leading up to diagnosis.15
Statistical Analysis
We summarized demographic, socioeconomic, and pathologic characteristics of men in the cohort, stratified by receipt of mpMRI, using frequencies and percentages. Tests for associations were conducted using Chi-square or Fisher exact tests for categorical variables, and Students T-tests or Wilcoxon Ran-Sum tests for continuous variables. Strength and direction of associations between covariates and receipt of mpMRI were assessed using odds ratios (OR) and 95% confidence intervals (CI). Unadjusted ORs were calculated based on unadjusted logistic regression models. We then fit a multivariable logistic regression model to examine patient factors that were independently associated with receipt of mpMRI. We included variables from the unadjusted analysis with a p <0.05 and also enhanced the model by adding additional factors into the multivariable model whose effects were deemed clinically important (i.e., age, sex, race, comorbidity, marital status, stage, and grade). We then used this model to calculate the adjusted yearly probability of receiving mpMRI. To test the hypothesis that MRI uptake may be greater in men with Gleason 3+4 disease, we assessed for an interaction between Gleason score and MRI use/year. We also performed a sensitivity analysis calculating the adjusted yearly probability of receiving MRI stratified by Gleason 3+3 and 3+4.
All analysis were performed using SAS v9.4 (Cary, NC) and R (version 3.4.1) using the packages dpylr16 for data management, compareGroups17 for descriptive tables, and ggplot218 for graphics. Statistical significance was set at 0.05. The study protocol was deemed exempt by the University of Pittsburgh Institutional Review Board.
Results
We identified 9,467 male Medicare beneficiaries diagnosed with prostate cancer and managed with active surveillance between 2008 and 2013. Of these, 1,289 (14%) received mpMRI. The demographic, socioeconomic, and clinical information for the men in the prostate cancer surveillance cohort is summarized in Table 1. Overall, most of the men in the cohort were under 75 years, white, married, had low co-morbidity burden, Gleason score 3+3, lived in higher educational attainment areas, higher income areas, densely populated areas, and there was greater representation in SEER from the northeast and west (Table 1). Median time on surveillance was 44 months overall (interquartile range [IQR] 29–63 months). MRI took place at a median of 14.1 months from the time of diagnosis (IQR 3.8–27 months). Most (62%) MRI were performed after the initial biopsy establishing diagnosis, but before the first biopsy on surveillance (sometimes referred to as “confirmatory biopsy”)19. However, 494 (38%) MRI were performed after the confirmatory biopsy. Within the cohort, follow-up ceased because of mortality (all-cause) in 8%, treatment in 24% (and end of entitlement criteria 68%. Among the men having MRI, 394 were treated after receiving MRI and 194 of these treatment events (49%) occurred within 90 days post-MRI. Among the 1,289 men receiving mpMRI, the median number of mpMRI performed was 0.38 per year (IQR 0.25–0.61) presenting approximately one mpMRI every 3 years. This corresponds to 950 (74%) men receiving 1 MRI during surveillance, 256 (20%) men receiving 2 MRI and 83 (6%) receiving ≥3 MRI.
Table 1:
Characteristics of the Medicare cohort on active surveillance stratified by receipt of multiparametric MRI (mpMRI)
| No mpMRI (N=8178) |
mpMRI (N=1289) |
P value1 | |
|---|---|---|---|
| Age at diagnosis (%) | <0.001 | ||
| 66–69 | 1664 (20) | 422 (33) | |
| 70–74 | 2661 (33) | 497 (39) | |
| 75–79 | 2155 (26) | 267 (21) | |
| 80 and older | 1698 (21) | 103 (7) | |
| Race (%) | <0.001 | ||
| White | 6861 (84) | 1119 (87) | |
| Black | 788 (10) | 76 (6) | |
| Other | 529 (6) | 94 (7) | |
| Marital Status (%) | <0.001 | ||
| Married | 4196 (51) | 771 (60) | |
| Not married | 1191 (15) | 171 (13) | |
| Unknown | 2791 (34) | 347 (27) | |
| Comorbidity (%) | <0.001 | ||
| 0 | 4721 (58) | 892 (69) | |
| 1 | 1941 (24) | 246 (19) | |
| 2 or more | 1516 (18) | 151 (12) | |
| Gleason score (%) | 0.002 | ||
| 3+3 or less | 6184 (76) | 1027 (80) | |
| 3+4 | 1994 (24) | 262 (20) | |
| Education level in the ZIP code of residence (%) | <0.001 | ||
| Low (<75% with high school education) | 882 (11) | 92 (7) | |
| High(>75% with high school education) | 7296 (89) | 1197 (93) | |
| County of residence population (%) | <0.001 | ||
| 1,000,000 or more | 4420 (54) | 861 (66) | |
| 250,000– 999,999 | 1378 (17) | 214 (17) | |
| Less than 250,000 | 2380 (29) | 214 (17) | |
| Median household income in ZIP code of residence, $ (%) | <0.001 | ||
| $60,000 or less | 4221 (52) | 422 (33) | |
| $60,001 or more | 3957 (48) | 867 (67) | |
| U.S. geographic region (%) | <0.001 | ||
| Northeast | 1322 (16) | 366 (28) | |
| South | 2060 (25) | 170 (14) | |
| Central | 1608 (20) | 120 (9) | |
| West | 3188 (39) | 633 (49) | |
| Year of Diagnosis (%)1 | <0.001 | ||
| 2008 | 1346 (17) | 165 (13) | |
| 2009 | 1382 (17) | 205 (16) | |
| 2010 | 1457 (18) | 199 (15) | |
| 2011 | 1536 (19) | 243 (19) | |
| 2012 | 1212 (14) | 233 (18) | |
| 2013 | 1245 (15) | 244 (19) |
Due to the requirement of 2 years claims data follow up, rates of AS in the later study years should not be taken as a true denominator.
The results of the unadjusted and multivariable analyses are shown in Table 2. Men who were younger, white, healthier, lived in more populated communities, higher income communities, lived in the northeast or west (compared to the south and midwest), and those diagnosed more recently had greater odds of receiving an mpMRI (Table 2). Gleason score and education level were not significantly different for those who did or did not receive an mpMRI (Table 2). There was no interaction in the model between Gleason score and MRI use over time. Similarly, when the yearly probability of receiving MRI was re-calculated by Gleason score, there was no difference in direction of effect or effect size.
Table 2.
Unadjusted and adjusted logistic regression models assessing odds of receiving mpMRI among Medicare beneficiaries on active surveillance for localized prostate cancer
| Covariate | Unadjusted analysis | Multivariable analysis* | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) |
P value | Adjusted Odds Ratio (95% CI) |
P value | |
| Age at Diagnosis | <0.001 | <0.0001 | ||
| 66–69 | reference | reference | ||
| 70–74 | 0.74 (0.64–0.85) | 0.77 (0.66–0.89) | ||
| 75–79 | 0.49 (0.41–0.58) | 0.54 (0.45–0.63) | ||
| 80 or more | 0.24 (0.19–0.30) | 0.26 (0.21–0.33) | ||
| Race (self-reported) | <0.001 | 0.04 | ||
| White | reference | reference | ||
| Black | 0.59 (0.46–0.75) | 0.72 (0.55–0.93) | ||
| Other | 1.09 (0.86–1.36) | 0.99 (0.78–1.26) | ||
| Marital status | <0.001 | 0.006 | ||
| Married | reference | reference | ||
| Not married | 0.78 (0.65–0.93) | 0.88 (0.73–1.06) | ||
| Unknown | 0.68 (0.59–0.77) | 0.80 (0.69–0.92) | ||
| Charlson-Klabunde Comorbidity Index | <0.001 | <0.0001 | ||
| 0 | reference | reference | ||
| 1 | 0.67 (0.58–0.78) | 0.73 (0.63–0.86) | ||
| 2 or more | 0.53 (0.44–0.63) | 0.66 (0.54– 0.79) | ||
| Gleason score | 0.002 | 0.593 | ||
| 3+3 or less | reference | reference | ||
| 3+4 | 0.79 (0.68–0.91) | 1.04 (0.90–1.21) | ||
| Education level in the ZIP code of residence | <0.001 | 0.864 | ||
| Low (<75% with high school education) | reference | reference | ||
| High (>75% with high school education) | 1.57 (1.26–1.98) | 0.98 (0.76–1.26) | ||
| County of residence population | <0.001 | 0.0008 | ||
| 1,000,000 or more | reference | reference | ||
| 250,000–999,999 | 0.80 (0.68–0.94) | 0.86 (0.72–1.01) | ||
| Less than 250,000 | 0.46 (0.39–0.54) | 0.71 (0.59–0.86) | ||
| Median household income in ZIP code of residence | <0.001 | 0.0001 | ||
| $60,000 or less | reference | reference | ||
| $60,001 or more | 2.19 (1.94–2.48) | 1.36 (1.16– 1.59) | ||
| U.S. geographic region | <0.001 | <0.0001 | ||
| Northeast | reference | reference | ||
| South | 0.30 (0.25–0.36) | 0.40 (0.32–0.49) | ||
| Central | 0.27 (0.22–0.33) | 0.35 (0.28–0.45) | ||
| West | 0.72 (0.62–0.83) | 0.75 (0.65–0.88) | ||
| Year of Diagnosis | <0.001 | 0.004 | ||
| 2008 | Reference | Reference | ||
| 2009 | 1.21 (0.97–1.51) | 1.19 (0.95–1.49) | ||
| 2010 | 1.11 (0.89–1.39) | 1.02 (0.81–1.28) | ||
| 2011 | 1.29 (1.05–1.60) | 1.16 (0.93–1.44) | ||
| 2012 | 1.57 (1.27–1.94) | 1.39 (1.11–1.73) | ||
| 2013 | 1.60 (1.29–1.98) | 1.39 (1.12–1.74) | ||
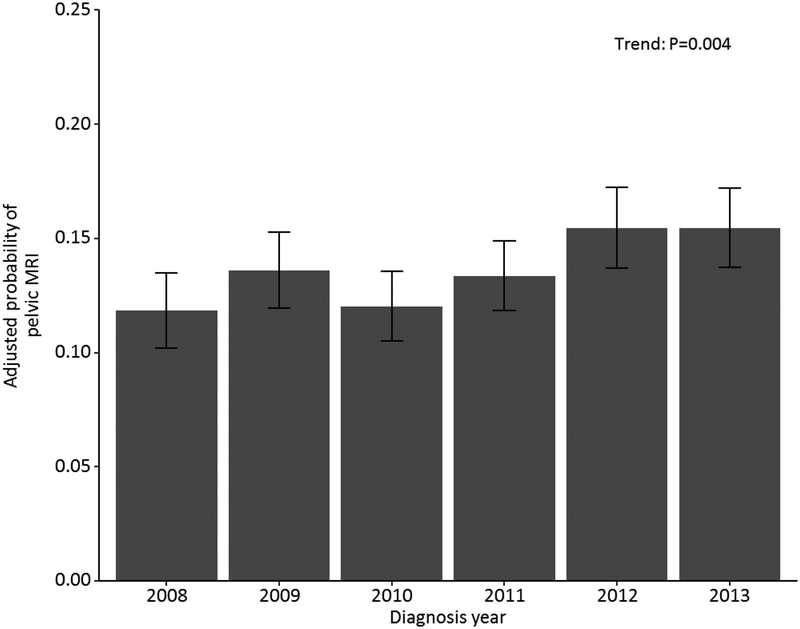
The adjusted probability of receiving an mpMRI during the study period increased from 11.8% to 15.5% from 2008 to 2013, representing an overall increase of 3.7% for the entire study period (p=0.004; test for trend, Figure 1).
Figure 1:
*Adjusted for age, race, marital status, Charlson-Klabunde comorbidity index, Gleason score, ZIP code-level educational attainment, county population, ZIP code-level median household income and geographic region.
Discussion
In this population-based study we characterize patterns of mpMRI utilization for men on surveillance for localized prostate cancer between 2008 and 2013. There are several key implications from this analysis. First, mpMRI was uncommon, with only 14% of men on surveillance receiving mpMRI. Second, the uptake of mpMRI in active surveillance was relatively slow with an increase of only 3.7% over the 5 years analyzed. Finally, use of mpMRI varied significantly across geographic regions and among socioeconomic strata.
There are several factors that may explain the slow uptake of mMRI from 2008 to 2013. During this time-period, there was considerable variation in the way prostate MRI sequences were reported.20 This variation likely contributed to the slow adoption of this technology due to difficulty of interpretation by both radiologists and urologists. The prostate imaging reporting and data system (PI-RADS) classification for structured reporting of the probability of cancer risk was introduced in 2012.21 PI-RADS version 2 further refined the classification system and allowed for more confident and uniform reporting of MRI-detected prostate lesions.22 With better uniformity of reporting and interpretation, we suspect that provider confidence and application of this technology will increase and will be reflected in future population-based studies.
In addition to codification of mpMRI reporting, process-based improvements may yield greater uptake of mpMRI in prostate cancer surveillance in subsequent years relative to this cohort. For example, the 3 Tesla magnet improved imaging quality and eliminated the requirement for an uncomfortable endorectal coil, both of which were potential barriers to use of mpMRI. Patient adherence to prostate cancer surveillance, which relies on serial testing, places a high premium on any improvement in patient comfort23. Moreover, the widespread use of MRI-ultrasound fusion-guided biopsies did not occur until after 2013.24, 25 Other MRI-guided biopsies are challenged by lack of accuracy (e.g. cognitive biopsy) or patient discomfort (e.g. in-bore biopsy) as compared to fusion-guided biopsies.26 As the use of fusion technology increases, given its advantages over other biopsy techniques19, we hypothesize that mpMRI use in active surveillance will continue to accelerate and will be reflected in future population-based studies.
Finally, there is still a significant ideological barrier to adoption of mpMRI. While most urologists feel it is beneficial in the management of prostate cancer, many believe that access and cost are significant barriers to incorporating this technology into routine practice.8 The manner in which mpMRI access improves, and costs diminish, may also determine the extent of use in the future.
Our multivariable analysis suggests that mpMRI use varies significantly across racial, socioeconomic and geographic groups. This observation is concordant with investigations of other costly technologies (i.e. stereotactic body radiation for prostate cancer), which demonstrate greater use among white, higher socioeconomic groups and within certain regions of the country9, 10, 27. In this analysis, increasing income was associated with higher odds of receiving mpMRI. The finding that black men had lower odds of receiving mpMRI is concerning given the large body of evidence supporting worse prostate cancer outcomes overall, and surveillance outcomes specifically, among this group28. Finally, mpMRI use was more likely in the US northeast and west regions compared to the midwest and south. The northeast and west regions contain markets with high hospital and physician capacity in close geographic proximity23 which results in significant market competition. This competition may drive increased adoption of new technologies in order to gain a competitive advantage and increase market share.29 Thus, the market dynamic of these regions may explain greater use of mpMRI compared to other regions.
The findings of this study should be considered in the context of several limitations. Using claims data, it is difficult to distinguish active surveillance from watchful waiting as prostate cancer management strategies. However, we took several measures to limit misclassification. Our definition of active surveillance has prior precedent11, and required evidence of surveillance with billing claims for PSA or prostate biopsy, which would be less commonly performed in men on watchful waiting. Recent work validates the ability of this definition to detect surveillance in administrative claims data12. In addition, we limited our cohort to patients with Gleason score ≤3+4=7 to eliminate patients with higher risk disease, who would be more likely to receive watchful waiting in lieu of active surveillance. An additional limitation is that there is no specific billing code for prostate MRI. MRI of the pelvis can be performed for other indications beyond prostate cancer surveillance. However, we restricted our cohort to men with billing codes for pelvic MRI and prostate cancer as their only cancer diagnosis, thus minimizing misclassification of MRIs ordered for other malignancies. While pelvic MRI could theoretically be ordered for non-malignant indications, this is likely rare in this patient population. Lastly, our findings are based on retrospective administrative and registry data, which is limited by coding errors, uncaptured claims and unmeasured confounding that may not be fully adjusted for in our multivariable models. Furthermore, we were not able to account for the impact of hospital and provider characteristics in this analysis (e.g. academic center, provider volume). However, we adjusted for several measurable clinical and nonclinical factors to minimize confounding, such as age, comorbidity, and Gleason score.
Conclusion
From 2008 to 2013, the use of mpMRI in prostate cancer active surveillance in the Medicare population increased incrementally, but significantly. The overall rate of mpMRI use was 14% and increased by a mere 3.7% over the 5 years analyzed. Receipt of mpMRI among men on surveillance for prostate cancer varied significantly across demographic and socioeconomic strata. This may represent provider uncertainty and access disparities and is a potential target for future work determining ideal implementation of mpMRI technology in prostate cancer active surveillance.
Funding/Disclosures
Mina M. Fam is supported in part by the Shadyside Hospital Foundation.
Liam C. Macleod is supported in part by the Shadyside Hospital Foundation and in part by the Conquer Cancer Foundation.
Bruce L. Jacobs is supported in part by the University of Pittsburgh Physicians Academic Foundation, P30CA047904 from the National Cancer Institute and the Henry L. Hillman Foundation.
The other authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Futterer JJ, Briganti A, De Visschere P, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. European urology. 2015;68:1045–1053. [DOI] [PubMed] [Google Scholar]
- 2.Cucchiara V, Cooperberg MR, Dall’Era M, et al. Genomic Markers in Prostate Cancer Decision Making. European urology. 2018;73:572–582. [DOI] [PubMed] [Google Scholar]
- 3.Bryant RJ, Yang B, Philippou Y, et al. Does the introduction of prostate multiparametric magnetic resonance imaging into the active surveillance protocol for localized prostate cancer improve patient re-classification? BJU Int. 2018. [DOI] [PubMed] [Google Scholar]
- 4.Perlis N, Al-Kasab T, Ahmad A, et al. Defining a Cohort that May Not Require Repeat Prostate Biopsy Based on PCA3 Score and Magnetic Resonance Imaging: The Dual Negative Effect. J Urol. 2018;199:1182–1187. [DOI] [PubMed] [Google Scholar]
- 5.Curci NE, Lane BR, Shankar PR, et al. Integration and Diagnostic Accuracy of 3T Nonendorectal coil Prostate Magnetic Resonance Imaging in the Context of Active Surveillance. Urology. 2018;116:137–143. [DOI] [PubMed] [Google Scholar]
- 6.Sherrer RL, Lai WS, Thomas JV, Nix JW, Rais-Bahrami S. Incidental findings on multiparametric MRI performed for evaluation of prostate cancer. Abdom Radiol (NY). 2018;43:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston E, Pye H, Bonet-Carne E, et al. INNOVATE: A prospective cohort study combining serum and urinary biomarkers with novel diffusion-weighted magnetic resonance imaging for the prediction and characterization of prostate cancer. BMC Cancer. 2016;16:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthigi A, Sidana A, George AK, et al. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol. 2017;35:32 e31–32 e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wennberg J, Gittelsohn. Small area variations in health care delivery. Science. 1973;182:1102–1108. [DOI] [PubMed] [Google Scholar]
- 10.Cary KC, Punnen S, Odisho AY, et al. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the Urologic Diseases in America project. Prostate Cancer Prostatic Dis. 2015;18:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filson CP, Schroeck FR, Ye Z, Wei JT, Hollenbeck BK, Miller DC. Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol. 2014;192:75–80. [DOI] [PubMed] [Google Scholar]
- 12.Modi PK, Kaufman SR, Qi J, et al. National Trends in Active Surveillance for Prostate Cancer: Validation of Medicare Claims-based Algorithms. Urology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll PH, Mohler JL. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16:620–623. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JI, Allsbrook WC Jr., Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. The American journal of surgical pathology. 2005;29:1228–1242. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- 16.Wickham H, Francois R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. R package version 0.7.4 https://CRAN.R-project.org/package=dplyr2017. [Google Scholar]
- 17.Subirana I, Sanz H., Vila J. Building Bivariate Tables: The compareGroups Package for R. Journal of Statistical Software, 57(12), 1–16. http://www.jstatsoft.org/v57/i12/.2014.25400517 [Google Scholar]
- 18.Hadley W Ggplot2. New York, NY: Springer Science+Business Media, LLC; 2016. [Google Scholar]
- 19.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson L, Ahmed HU, Allen C, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37:48–58. [DOI] [PubMed] [Google Scholar]
- 21.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. European radiology. 2012;22:746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas HA, Hotker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. European radiology. 2016;26:1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J Clin. 2016;66:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arsov C, Rabenalt R, Quentin M, et al. Comparison of patient comfort between MR-guided in-bore and MRI/ultrasound fusion-guided prostate biopsies within a prospective randomized trial. World J Urol. 2016;34:215–220. [DOI] [PubMed] [Google Scholar]
- 27.Halpern JA, Sedrakyan A, Hsu WC, et al. Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer. 2016;122:2496–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gokce MI, Sundi D, Schaeffer E, Pettaway C. Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis. 2017;20:127–136. [DOI] [PubMed] [Google Scholar]
- 29.Adler JT, Chang DC. Implications of Market Competition, Technology Adoption, and Cost for Surgical Patients. JAMA Surg. 2016;151:621. [DOI] [PubMed] [Google Scholar]