Abstract
Background:
Knee osteoarthritis (OA) is a pervasive musculoskeletal condition, often exacerbated by movement-evoked pain (MEP). Despite established research demonstrating significant racial differences in OA pain, few studies have investigated ethnic/racial group differences in MEP and lower extremity function and their association with psychosocial factors, such as perceived stress. Therefore, the primary aims were: (1) to identify ethnic/racial group differences in persons with or at risk for knee OA pain based on MEP, physical performance, and perceived stress measures, and (2) to determine if perceived stress explains the relationship between MEP and function in non-Hispanic Blacks (NHBs) and non-Hispanic Whites (NHWs).
Methods:
A total of 162 NHB and NHW community-dwelling older adults (50–78 years of age) were included in this analysis from the Understanding Pain and Limitations in Osteoarthritic Disease (UPLOAD) cross-sectional cohort study. Demographic, anthropometric, pain and functional parameters were assessed using a battery of validated instruments. Descriptive statistics, parametric, and multivariate analyses were conducted to determine ethnic/racial differences in perceived stress, MEP, and function.
Results:
Our results support the hypothesis that among persons with knee OA pain, NHBs have significantly greater MEP and lower functional level, despite similar levels of perceived stress. However, perceived stress was more strongly related to MEP in NHB compared to NHWs. Differences in function were limited to walking speed, where NHWs demonstrated faster gait speed.
Conclusions:
Our cross-sectional study demonstrated important ethnic/racial differences in MEP and function. Also, perceived stress had a stronger effect on MEP in NHBs, suggesting that perceived stress may more strongly influence pain with physical movement among NHB adults. MEP may be a clinically important pain outcome to measure in persons with OA, and these data warrant future research on the impact of stress on pain and functional outcomes in older adults, particularly in NHBs.
Keywords: pain, osteoarthritis, movement, function, stress, ethnicity/race
1. Introduction
Chronic pain is a global public health problem. Indeed, twelve of the most-disabling chronic conditions are associated with chronic pain (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017). Among these, osteoarthritis (OA) causes musculoskeletal pain that is often initiated or exacerbated by movement, which leads to significant limitations in physical function, participation in daily activities, and mobility (Cruz-Almeida, Rosso, et al., 2017; Cruz-Almeida, Cardoso, et al., 2017; March et al., 2014). While pain-related function is a strong predictor of current and future disability in older adults, especially older non-Hispanic Blacks (NHBs) (Walker et al., 2016a; Smith et al., 2016; Zakoscielna & Parmelee, 2013), movement-evoked pain (MEP) may be a stronger predictor of musculoskeletal disability than pain at rest or general pain (Mankovsky-Arnold et al., 2014).
MEP refers to pain that is triggered or exacerbated by active or passive movement and that is clearly differentiated by the person from their background ongoing spontaneous pain (Corbett et al., 2019). MEP is a unique construct (vs. pain-at-rest) to study in individuals with hip and knee OA, including those who subsequently undergo joint replacement, given its relationship to functional status and recovery outcomes (Sayers et al., 2016). Despite several examples of direct measurement of pain brought on by movement, typical approaches to pain assessment fail to specifically measure MEP and do not distinguish between chronic pain experienced at rest and with movement (He, Grant, Holden, & Gilron, 2017; Corbett et al., 2019; Butera, Fox, & George, 2016). This is problematic because increasing research suggests that MEP can be not only mechanistically distinct from rest pain (i.e., spontaneous pain) (He et al., 2017; Mankovsky-Arnold, Wideman, Larivière, & Sullivan, 2014), but is also likely one of the primary drivers of impaired mobility, particularly in older populations.
While MEP has been understudied in general, the extent to which MEP differs across ethnic/racially diverse older adults has not been investigated. Standard pain measures have revealed greater pain and disability among NHB adults with OA and other pain conditions compared to their non-Hispanic white (NHW) counterparts (Vina et al., 2018; Janevic et al., 2017). We recently found that higher MEP was significantly associated with poorer lower extremity functional performance (e.g., gait speed) in both NHBs and NHWs with or at risk for knee OA, with more NHBs having severe MEP (Cruz-Almeida, Cardoso et al., 2017). Taylor and colleagues (2018) did not find racial differences in the relationship between slow gait speed and pain in older adults and concluded that the underlying factors for this finding are more intrinsic to disparate environmental conditions rather than a function of ethnic/race itself. While numerous factors, such as pain catastrophizing, negative/positive affect, social demographics and perceived control, are recognized as contributing to ethnic/racial differences in OA-related pain (Cruz-Almeida, Cardoso, et al., 2017; Bartley et al., 2019; Cardoso et al., 2018), emerging evidence suggest that psychosocial stress (e.g., perceived stress) represents another important risk factor contributing to increased pain and disability in older adults with or at risk for knee OA (Sibille et al., 2018; Vaughn, Terry, Bartley, Schaefer, & Fillingim, 2018).
Living with a chronic pain condition like OA is stressful and can place further physiological and psychological burden on the body ultimately leading to accelerated aging and disability, especially in vulnerable older adult populations such as NHBs (Anton et al., 2015; Sibille et al., 2012). Stress has been identified as one of the top 10 determinants of health disparities and has been linked to coronary vascular disease, obesity, diabetes, autoimmune disorders, and chronic pain (Djuric et al., 2010; Abdallah & Geha, 2017). Stressors more frequently experienced by ethnic/racial minority groups, including socioeconomic disadvantage and racial discrimination, have been associated with health status and health behaviors (Ahmed, Mohammed, & Williams, 2007; Jackson, Knight, & Rafferty, 2010) including Black American women with OA (Walker Taylor et al., 2018). Of particular interest is perceived stress, defined as an individual’s perception or feelings about the degree to which one’s life is appraised as stressful over a given time period (Cohen et al., 1983). Chronic psychosocial and perceived stress may contribute to greater musculoskeletal pain (Tsuboi et al., 2017), experimental pain sensitivity (Mechlin et al., 2005; Gordon, Johnson Nau, Mechlin, & Girdler, 2017), and disability in chronic rheumatological conditions (Sumner et al., 2019). High perceived stress has been associated with heightened pain intensity and interference with normal household and work activities in older adults (White et al., 2014), and stress may contribute to previously documented racial/ethnic groups differences in OA pain (Vaughn et al., 2018). Indeed, recent research suggests that not only do NHBs with or at risk for OA have higher levels of clinical and experimentally-induced pain, functional limitations, and perceived stress than do NHWs, but also that high perceived stress predicts higher MEP and lower function in older NHBs (Sibille et al., 2018; Booker et al., 2018).
Despite abundant evidence indicating that pain impacts physical function, there remains considerable knowledge gaps regarding MEP in ethnically/racially diverse older adults with or at risk for OA, and the contributions of stress thereto. To our knowledge, no studies to date have directly examined potential ethnic/racial differences in the association between perceived life stress and experimental measures of MEP and performance-based physical function among older NHBs and NHWs with knee OA. Therefore, the primary objectives of the present study were: (1) to identify ethnic/race group differences in persons with knee OA pain specific to MEP, physical performance, and perceived stress measures, and (2) to determine if perceived stress explains the relationship between MEP and function in NHBs and NHWs. We hypothesized that: 1): NHBs will demonstrate significantly higher MEP, lower physical function performance, and higher perceived stress in comparison to NHWs; and 2) Perceived stress will be a significant psychosocial factor that explains the relationship between higher MEP and lower function in NHBs, but not NHWs.
2. Materials and Methods
Reporting in this article follows the recommendations of the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines (Vandenbrouke et al., 2014).
2.1. Design.
This is a secondary data analysis of a prospective observational cohort study including older adults with knee pain who participated in the community-based study Understanding Pain and Limitations in Osteoarthritic Disease (UPLOAD) at the University of Florida (UF) and the University of Alabama at Birmingham (UAB). The parent study’s purpose is to identify the biopsychosocial factors that underlie ethnic differences in pain and functional limitations in adults with or at risk for symptomatic knee OA. Institutional Review Boards at the UF and UAB approved the study.
2.2. Participants.
The UPLOAD study enrolled individuals between 45 and 85 years of age, who identified themselves as either non-Hispanic Black/African American or non-Hispanic White/Caucasian or European. Participants reported unilateral or bilateral knee pain and screened positive for clinical knee OA, which has a high sensitivity and specificity for radiographically confirmed symptomatic knee OA (Altman et al., 1986; Roux et al., 2008). Given widespread variability in definitions of OA (Kraus et al., 2015), we adopted this approach to be as inclusive as possible in recruitment and enroll a cohort with a broad range of OA characteristics, from very early signs to more advanced disease. The goal of the study was to recruit individuals with or at risk of knee OA and follow prospectively to understand factors associated with disease progression, clinical pain, and functional limitations.
Additional details of the screening and inclusion/exclusion criteria are reported in several published articles (Cardoso et al., 2016; Cruz-Almeida, Cardoso et al., 2017). Briefly, exclusion criteria were applied to reduce the presence of medical conditions that could confound symptomatic knee OA-related outcomes or preclude successful completion of the protocol including: systemic rheumatic disease/condition, surgery to the index knee, uncontrolled hypertension (>150/95), loss of peripheral sensation, neurological disorders, cardiovascular or peripheral arterial disease, serious psychiatric disorder resulting in recent hospitalization (within the past 12 months), diminished cognitive function, and pregnancy due to unknown risk to the fetus. As shown in Figure 1, a total of 126 NHBs and 156 NHWs were enrolled. For the analysis reported in this paper, we selected individuals who were 50 years of age and older, resulting in a final sample size of 83 NHBs and 79 NHWs. We selected individuals 50 and older to (1) capture individuals entering “later life” to better understand the impact of aging on MEP and function, and (2) include individuals meeting one of the clinical criteria for knee OA (i.e. age 50 and older, Altman et al., 1986).
Fig. 1.
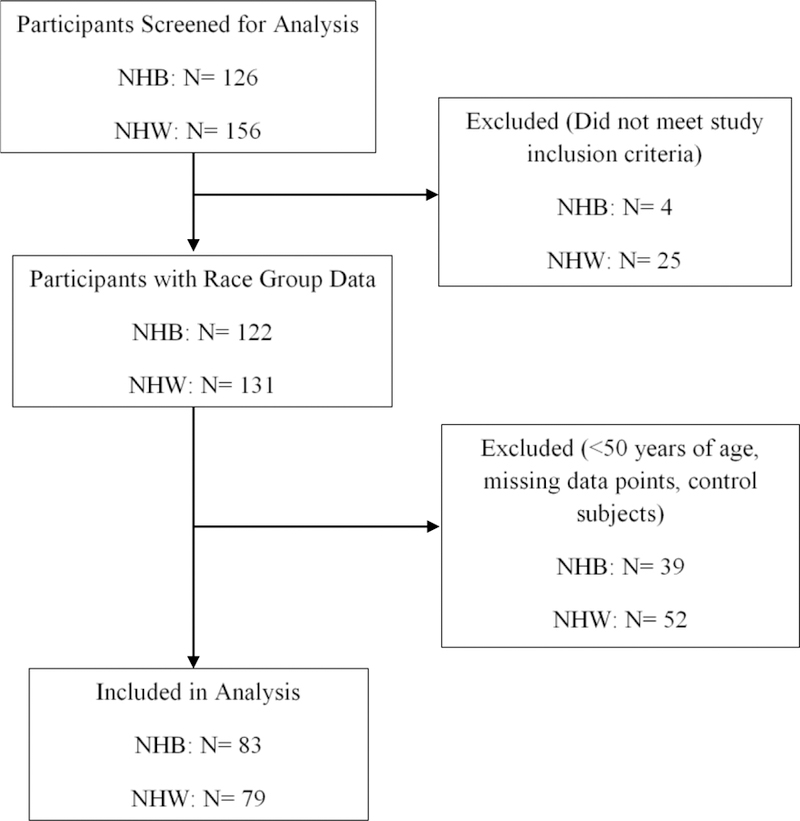
Flow Chart of Sample Enrollment
2.3. Procedures.
Upon arriving at the clinical laboratory, participants provided informed consent and completed a series of baseline questionnaires that assessed demographics, health and pain history, and perceived stress. Next, a physical exam of the knees and hands assessed for current pain, bony enlargement, and crepitus. The knee reported by the participant as most painful was designated as the index knee. In addition, posterior-anterior and lateral radiographs of the index knee were obtained, and study rheumatologists read the radiographs to provide a Kellgren-Lawrence (KL) score, which ranges from 0 (no joint changes) to 4 (severe joint changes) (Kellgren & Lawrence, 1957). The visit concluded by conducting physical performance (gait and balance tests) and lower extremity strength tests.
2.4. Measures
2.4.1. Perceived Stress.
The Perceived Stress Scale is a 10-item global measure of an individual’s perception of psychosocial stress during the past month (Cohen, Kamarck, & Mermelstein, 1983). Each item is rated on a 5-point scale ranging from never (0) to very often (4). Positively worded items are reverse scored, and the ratings are summed, with higher scores indicating more perceived stress. Total scores range from 0–40 and for our analysis, perceived stress scores were modeled as a continuous variable.
2.4.2. Physical Function.
The Short Physical Performance Battery (SPPB) consists of three measures of lower-extremity mobility function: three increasingly difficult standing balance tasks (side-by-side, semi-tandem, and tandem stance), 4-meter normal walking speed, and timed repeated chair stand (i.e., ability and time to rise from a chair safely 5 times) (Guralnik et al., 1995). The SPPB has been validated and used widely in older adults, including middle-aged adults ages 50–64 (Miller, Wolinksy, Andresen, Malmstrom, & Miller, 2008; Deshpande, Metter, Guralnik, Bandinelli, & Ferrucci, 2013), with various chronic conditions including chronic pain (Eggermont et al., 2014; Fowler-Brown et al., 2013). Participant performance on each of these three movements is scored from 0 (worst performance) to 4 (best performance), and a total score is calculated for a possible maximum score of 12; thus, 0= worst performance to 12= best performance. Lower scores indicate greater functional limitation and scores are analyzed as a continuous variable.
2.4.3. Movement-evoked pain.
For the purposes of this study, we used two approaches to measure MEP. First, we examined the intensity of pain during weight-bearing lower extremity movements that rely heavily on the flexion of the knee as measured by the SPPB. Participants were asked for a numeric pain rating after each movement using a numerical rating scale from 0 (no pain) to 100 (the most intense pain imaginable).
Second, MEP was measured by having participants rate the intensity of pain during maximal isometric strength testing of the knee extensor muscles. This measure of bilateral (index knee and non-index knee) lower extremity strength was assessed by having the participant extend the leg with maximum force while a handheld dynamometer (Lafayette Hand-Held Dynamometer: Model 01165, Lafayette Instruments, Inc., Lafayette, IN) was placed just above the ankle to resist the participant’s movement. The tests were performed three times on each leg while the participant was in a sitting position with knee extended 75° from the horizontal position. The three pain ratings were averaged for each knee.
2.4.4. Pain and Function.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a reliable and well-validated self-report measure of knee and hip OA symptoms and function within the immediate past 48 hours (Bellamy et al., 1988; Collins et al., 2011). The WOMAC has three subscales: pain during passive and active activities, stiffness, and impairments in physical function. Scores for the five pain items are summed for a possible score of 0–20 and higher scores indicate worse pain. The WOMAC pain subscale was included in the models to control for the effects of “self-reported” OA pain distinct from measuring movement-evoked pain during the performance of the tasks.
2.4.5. Sample Characteristics.
Ethnicity and race were based on responses to the following questions: (1) are you Hispanic or Latino? (Yes or No), and (2) what is your race or origin: Asian or Asian American; Black or African American; White, Caucasian, or European; American Indian or Alaska Native; Native Hawaiian or Other Pacific Islander; and Some Other Race or Origin? Additional demographic and baseline anthropometric data, including age, gender, education level, past and current medical history, height, weight, and body mass index were assessed and reviewed for accuracy by a study-designated advanced practice nurse.
2.5. Analysis.
Ethnic/race differences in demographic characteristics and study variables were examined using chi-square (x2) for dichotomous variables and ANOVA for continuous variables. A composite proxy of SES was computed by averaging the z-scores of our measures of education and income, where higher education and higher income categories reflect higher SES status. Mean intensity ratings for MEP were generated for NHBs and NHWs. ANCOVAs determined differences in measures of perceived stress, MEP, and function. In order to examine the influence of ethnic/race group, perceived stress and their interaction on MEP and function, two sets of analyses are presented. First, crude unadjusted models were performed, followed by fully adjusted models, which included covariates of SES index, age, KL-score on the index knee, BMI, study site (UF & UAB), and WOMAC scores to adjust for the chronicity of ongoing OA pain. Statistical significance was considered at p <.05.
3. Results
3.1. Participant Characteristics.
A total of 162 participants (83 NHBs, 79 NHWs) were included in the analyses (Table 1). Our study sample was mostly female (61.1%) and recruited from the UF location (63.6%). There were significant ethnic/race group differences in age and SES. NHBs were younger, more economically and educationally disadvantaged, and less likely to be married compared to NHWs.
Table 1.
Demographic Characteristics of Study Sample
| Characteristic | NHBs N= 83 | NHWs N= 79 | x2 or F-value | p |
|---|---|---|---|---|
| Male (N, %) | 36 (43.4) | 27 (34.2) | 1.44 | 0.23 |
| Female (N, %) | 47 (56.6) | 52 (65.8) | ||
| UF Site (N, %) | 50 (60.24) | 53 (67.1) | 0.82 | 0.37 |
| UAB Site (N, %) | 33 (39.8) | 26 (32.9) | ||
| Age (M±SD) | 58.04 ± 5.63 | 61.47 ± 7.63 | 10.69 | 0.001 |
| SES Index | −0.26 | 0.28 | 16.69 | 0.001 |
| KL Score (Index | 7.98 | 0.09 | ||
| Knee) | ||||
| 0 | 25 (30.1) | 19 (24.1) | ||
| 1 | 7 (8.4) | 19 (24.1) | ||
| 2 | 17 (20.5) | 13 (16.5) | ||
| 3 | 15 (18.1) | 14 (17.78) | ||
| 4 | 18 (21.7) | 12 (15.2) | ||
| BMI (kg/m2) | 32.02 ± 6.92 | 31.00 ± 7.48 | 0.81 | 0.37 |
| WOMAC | 8.8 ± 3.89 | 6.29 ± 4.16 | 14.65 | 0.0002 |
3.2. Ethnic/Racial Differences in MEP, Function, and Perceived Stress.
There were significant racial/ethnic differences in all measures of MEP in both the crude unadjusted and fully adjusted models (Table 2), such that NHBs reported greater MEP than NHWs. In addition, NHBs were more likely to report the maximum intensity of pain (pain intensity = 100) for MEP, while more NHWs were likely to report no MEP (pain intensity = 0). In general, both groups demonstrated moderate functional performance on the SPPB, but NHBs showed significantly lower overall function (p= 0.01) and walking speed (p <0.01). Balance and chair standing performance were similar across ethnic/race groups. Perceived stress did not differ across groups.
Table 2.
Average MEP, Function, and Perceived Stress in NHBs and NHWs
| NHB (N= 83) | NHW (N= 79) | |||||||
|---|---|---|---|---|---|---|---|---|
| MEP | Mean ± SD | Range | N (%) Reporting No MEP | Mean ± SD | Range | N (%) Reporting No MEP | Unadjusted p | Adjusted p |
| Balance pain | 25.44 ± 27.43 | 100 | 23 (28.40) | 11.62 ± 16.81 | 73 | 42 (53.16) | <0.01 | <0.01 |
| Walking pain | 29.05 ± 30.34 | 92 | 28 (34.15) | 12.68 ± 19.83 | 80 | 40 (50.63) | <0.01 | 0.03 |
| Chair stand pain | 33.62 ± 29.71 | 100 | 14 (18.18) | 17.57 ± 21.31 | 80 | 24 (31.17) | <0.01 | 0.03 |
| Index knee strength pain | 27.65 ± 27.58 | 100 | 7 (8.43) | 12.73 ± 17.68 | 64 | 28 (35.44) | <0.01 | 0.02 |
| Non-index knee strength pain | 18.47 ± 23.32 | 100 | 21 (25.30) | 9.64 ± 15.38 | 77 | 36 (45.57) | <0.01 | 0.22 |
| Function | Mean ± SD | Range | Mean ± SD | Range | Unadjusted p | Adjusted p | ||
| Total Function Score | 9.11 ± 1.82 | 33 | 9.70 ± 1.66 | 8 | <0.01 | 0.08 | ||
| Balance | 3.69 ± 0.66 | 2 | 3.72 ± 0.70 | 3 | 0.69 | 0.90 | ||
| Walking speed | 3.55 ± 0.69 | 2 | 3.80 ± 0.54 | 3 | <0.01 | 0.05 | ||
| Chair stand | 1.94 ± 1.34 | 4 | 2.18 ± 1.13 | 4 | 0.12 | 0.34 | ||
| Perceived Stress | 14.08 ± 6.65 | 33 | 13.31 ± 6.71 | 28 | 0.49 | N/A | ||
3.3. Relationship between Perceived Stress and MEP and Function
Separate general linear models were conducted to examine the association of perceived stress and ethnicity/race with MEP (Table 3) and function (Table 4). Overall, there was not a significant main effect for the association of perceived stress with any measure of MEP; although, walking pain approached statistical significance (p= 0.06). However, significant interactions between perceived stress and ethnicity/race emerged for most measures of MEP, even after controlling for demographics and WOMAC. This significant interaction was observed for balance pain [F(1, 143) = 87.6, p= 0.006)], walking pain (F(1,144) = 7.71, p= 0.006)], and marginally for chair stand pain [F(1, 137)= 7.40, p= 0.007)] and index knee strength pain [F(1, 145) = 3.10, p= 0.08]. As shown in Figure 2, the steeper slope is evidence that perceived stress is more strongly related to MEP in NHBs than NHWs.
Table 3.
Coefficients for ANCOVA with MEP as Outcome (Adjusted Models)
| Balance Pain | Walking Pain | Chair Stand Pain | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | SS | F | p | SS | F | p | SS | F | p |
| Perceived stress | 494.55 | 1.37 | 0.24 | 1521.38 | 3.59 | 0.06 | 740.99 | 1.69 | 0.20 |
| Race | 551.98 | 1.53 | 0.22 | 909.06 | 2.15 | 0.15 | 862.2 | 1.97 | 0.16 |
| Perceived stress*race | 2747.19 | 7.60 | 0.006 | 3264.68 | 7.71 | 0.006 | 3236.27 | 7.40 | 0.007 |
| C1 SES index | 725.46 | 2.01 | 0.158 | 36.94 | 0.09 | 0.77 | 57.57 | 0.13 | 0.71 |
| C2 Age | 173.89 | 0.48 | 0.49 | 228.52 | 0.54 | 0.46 | 442.66 | 1.01 | 0.32 |
| C3 KL score | 451.35 | 1.25 | 0.27 | 1707.77 | 4.03 | 0.05 | 3922.67 | 8.97 | 0.003 |
| C4 BMI | 70.06 | 0.19 | 0.66 | 432.84 | 1.02 | 0.31 | 1131.92 | 2.59 | 0.11 |
| C5 Study site | 360.04 | 1 | 0.32 | 1306.72 | 3.09 | 0.08 | 13.95 | 0.03 | 0.86 |
| C6 WOMAC | 13888.37 | 38.42 | <.0001 | 17837 | 42.13 | <.0001 | 18922.74 | 43.27 | <.0001 |
| Index Knee Strength Pain | Non-Index Knee Strength Pain | |||||
|---|---|---|---|---|---|---|
| Predictor | SS | F | p | SS | F | p |
| Perceived stress | 9.71 | 0.02 | 0.88 | 1137.53 | 3.52 | 0.06 |
| Race | 139.84 | 0.32 | 0.58 | 48.42 | 0.15 | 0.7 |
| Perceived stress*race | 1374.23 | 3.1 | 0.08 | 353.64 | 1.09 | 0.3 |
| C1 SES index | 441.87 | 1 | 0.32 | 26.21 | 0.08 | 0.78 |
| C2 Age | 59.4 | 0.13 | 0.71 | 514.38 | 1.59 | 0.21 |
| C3 KL score | 47.25 | 0.11 | 0.74 | 258.59 | 0.8 | 0.37 |
| C4 BMI | 664.48 | 1.5 | 0.22 | 211.21 | 0.65 | 0.42 |
| C5 Study site | 1996.7 | 4.51 | 0.04 | 379.41 | 1.17 | 0.28 |
| C6 WOMAC | 10239.43 | 23.12 | <.01 | 8095.38 | 25.02 | <.01 |
SES= socioeconomic status, BMI= body mass index, WOMAC= Western Ontario and McMaster Universities Osteoarthritis Index
Table 4.
Coefficients for ANCOVA with Function as Outcome (Fully Adjusted Models)
| Total Function | Balance | Walking Speed | Chair Stand | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | SS | F | p | SS | F | p | SS | F | p | SS | F | p |
| Perceived stress | 18.42 | 7.94 | 0.006 | 0.76 | 1.87 | 0.17 | 1.51 | 4.54 | 0.03 | 4.12 | 3.17 | 0.08 |
| Race | 1.62 | 0.7 | 0.41 | 0.005 | 0.01 | 0.91 | 0.03 | 0.1 | 0.75 | 0.92 | 0.7 | 0.4 |
| Perceived stress*race | 0.01 | 0 | 0.94 | 0.002 | 0 | 0.95 | 0.13 | 0.39 | 0.53 | 0.27 | 0.21 | 0.65 |
| C1 SES index | 10.16 | 4.38 | 0.04 | 0.605 | 1.5 | 0.22 | 1.59 | 4.77 | 0.03 | 1.35 | 1.04 | 0.31 |
| C2 Age | 14.65 | 6.31 | 0.01 | 2.734 | 6.77 | 0.01 | 1.07 | 3.21 | 0.07 | 1.25 | 0.96 | 0.33 |
| C3 KL score | 1.61 | 0.69 | 0.41 | 0.046 | 0.11 | 0.74 | 0.39 | 1.17 | 0.28 | 0.003 | 0 | 0.96 |
| C4 BMI | 17.51 | 7.54 | 0.007 | 3.36 | 8.31 | 0.005 | 0.03 | 0.09 | 0.77 | 6.76 | 5.19 | 0.02 |
| C5 Study site | 20.54 | 8.85 | 0.003 | 2.45 | 6.05 | 0.02 | 2.01 | 6.04 | 0.02 | 18.36 | 14.09 | <0.01 |
| C6 WOMAC | 8.16 | 3.51 | 0.06 | 0.76 | 1.88 | 0.17 | 0.08 | 0.27 | 0.61 | 2.81 | 2.15 | 0.14 |
Fig. 2.
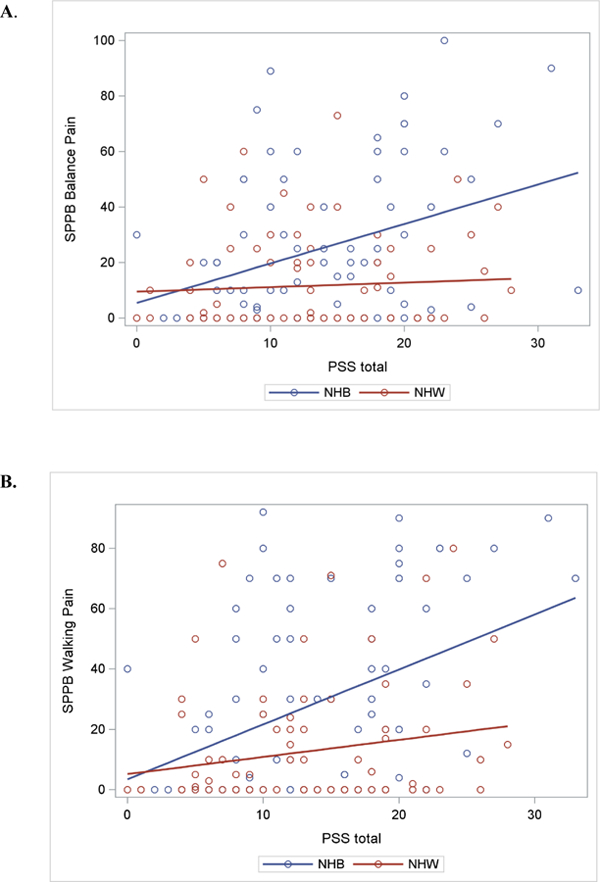
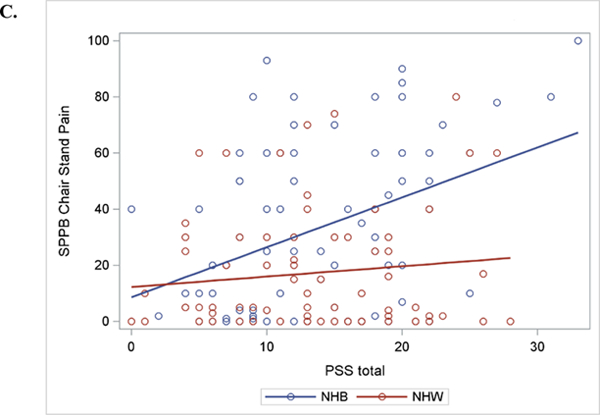
Linear Regression Slopes for Movement-evoked Pain for Perceived Stress*Race Interaction . A. Balance Pain; B. Walking Pain; C. Chair Stand Pain
† Perceived Stress Score (PSS)
There was a significant main effect of perceived stress for total functional performance [F(1, 145) = 7.94, p= 0.006] and walking speed (p= 0.03). There was a trend toward significance for chair stand function (p=0.08). In contrast to MEP, there were no interaction effects for perceived stress and race for any of the physical function performance measures.
4. Discussion
4.1. Significance of Results
This is the first study to explore how perceived stress differentially impacts non-Hispanic Blacks and non-Hispanic Whites in the context of MEP and function. Our findings are novel in demonstrating that (1) NHBs experience greater MEP, and perceived stress was more strongly related to MEP in NHBs compared to NHWs, and (2) perceived stress was significantly associated with physical function performance across both racial/ethnic groups.
Although the average intensity of MEP was relatively low for both groups, NHBs reported significantly greater intensity of MEP (i.e., twice as intense pain ratings) compared to NHWs. Furthermore, not only do NHBs report greater MEP but fewer NHBs report zero pain intensity as compared to NHW, suggesting that MEP is experienced by a greater proportion of NHBs. Notably, these differences in MEP remained significant even after controlling for background pain as measured by the WOMAC pain scores. These findings support our first hypothesis that NHBs would demonstrate greater MEP. The question becomes “why do NHBs have more MEP?” Research has consistently found that NHBs report increased sensitivity to laboratory pain stimuli (Rahim-Williams et al., 2012; Kim et al., 2017; Bell et al., 2018), and similar findings have emerged among individuals with or at risk for knee OA (Cruz-Almeida, Cardoso, et al., 2017). Like laboratory-induced pain, MEP represents a form of evoked pain due to internal mechanical stimuli induced by movement. Hence, if NHBs exhibit a pain modulatory profile characterized by increased pain facilitation and decreased inhibition, this could explain their greater pain in response to movement-evoked stimuli. More severe joint damage could also contribute to greater MEP among NHBs, as some previous work has reported more severe radiographic OA among NHBs (Braga et al., 2009; Nelson et al., 2011), in which case movement may actually generate a more intense nociceptive stimulus in the joint, thereby inducing greater pain. However, KL scores did not differ significantly between ethnic/race groups in this study, and MEP was greater in the NHB group even after controlling for KL scores.
There were no group differences in levels of perceived stress, but the association of perceived stress with MEP was moderated by race/ethnicity. Specifically, higher perceived stress was more strongly associated with greater MEP during balance, walking, and chair stand tasks among NHBs but not among NHWs. The reasons for this pattern of results are not completely clear; however, it is conceivable that stress may be associated with biological and/or psychosocial responses that differentially influence MEP among NHBs versus NHWs. For example, Gordon and colleagues (2017) demonstrated similar chronic stress exposure and acute stress reactivity among NHBs and NHWs; however, stress reactivity was protective against pain in NHWs but not NHBs. Similarly, Herbert and colleagues (2017) found that pain intensity was negatively associated with a biological measure of stress (cortisol) in NHWs, but not in NHBs. Some have even suggested that epigenetic alterations in the stress response receptor gene (NR3C1) and immune cytokine genes, which are associated with chronic pain and chronic stress, may notably contribute to the differential modulation of chronic pain by ethnicity/race, leaving Black Americans more vulnerable to severe and disabling pain (Aroke et al., 2019).
Our findings of similar magnitude of stress are consistent with previous literature showing no significant ethnic/racial differences (Cohen & Williamson, 1988; Kim et al., 2009), while other studies have shown significantly greater perceived stress in NHWs (Carson et al., 2018; O’Neal et al., 2015). There are several potential explanations for the lack of ethnic/race group differences in perceived stress. One, models were adjusted for socioeconomic and demographic factors that could in fact mediate the relationship between perceived stress and ethnicity/race. Second, differences in stress appraisal and coping factors may obscure or balance out any potential differences in perceived stress. Third, the presence of chronic knee pain may represent a persistent stressor that equalizes perceived stress in both ethnic groups. While the magnitude of perceived stress did not differ across racial/ethnic groups, the nature of the underlying exposures to stress (e.g. nature of exposure, life interference, duration, timing) likely does differ, which may explain differential associations between stress and MEP. For example, NHBs experience far greater levels of discrimination, and this type of stress has been associated with increased pain in prior work (Goodin et al., 2013). Hence, it may be the nature or the duration of stressors rather than their perceived magnitude that contributes to greater MEP.
In terms of function, our observed differences in performance were driven primarily by slower walking speed in NHBs versus NHWs. This is consistent with epidemiological studies that demonstrate that older NHBs and Hispanics experience significantly greater arthritis-attributable physical limitations and disability as compared to NHWs and other ethnic/racial groups (Barbour, Helmick, Boring, & Brady, 2017; Vaughn et al., 2018). Perceived stress was significantly associated with overall functional performance, walking speed, and chair stand completion time; however, this relationship was not race-dependent in our study.
Jordan and colleagues’ (1998) asked, “are ethnic or cultural differences in the psychosocial determinants of… arthritis pain…worthy of study?” (p. 81). Our findings demonstrate the need to understand how psychosocial factors, such as perceived stress, contribute to MEP in NHBs specifically. This calls for a scientific paradigm shift for more within-group research to fully explicate the unique contextual relationship of perceived stress and pain. What is unclear is the causal relationship between stress and MEP: does greater MEP increase psychological and physical stress or vice versa? Direction of causation notwithstanding, perceived stress remains an important factor to measure, and attenuate, given its association with multiple pain-related outcomes such as function (Booker et al., 2018), pain interference (White et al., 2014), sleep disturbances (Eslami, Zimmerman, Grewal, Katz, & Lipton, 2016), and cellular aging (Sibille, et al., 2012). Individuals with more severe MEP and worse functional performance also demonstrate significantly greater depressive symptoms, higher use of active and passive coping strategies, and more catastrophizing, pain hypervigilance and negative affect (Cruz-Almeida, Cardoso, et al., 2017). Differences in MEP and perceived stress are clinically meaningful and should be further elucidated through clinical and research efforts.
4.2. Clinical Applications and Research Implications.
Measurement obscurity has greatly hampered our recognition and response to MEP. Nonetheless, our findings indicate the importance of measuring MEP in addition to spontaneous and rest pain in clinical and research settings in individuals with musculoskeletal conditions. Understanding MEP in the context of function provides new insight on the adverse effects of movement (Cruz-Almeida, Cardoso, et al., 2017; Corbett, et al, 2019). Particularly, studying how movement produces or exacerbates knee pain may lend greater interpretive value for common outcomes of OA, such as decreased physical activity and avoidance of activity. Further, given the relationship with function, MEP (i.e., objective measurement rather than self-report) may also represent an important patient-reported outcome measure (PROM) to be considered in initiatives such as IMMPACT (Dworkin et al., 2005), Patient-Reported Outcomes Measurement Information System (PROMIS®, Cook et al., 2016), and Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION; Fillingim et al., 2014), especially when studying chronic conditions of aging such as OA. In managing knee OA, MEP may be an important target to treat through behavioral modification to sustain optimal joint and muscle function. Future mechanism-based research is needed to accurately phenotype MEP in older individuals with OA.
Even with low perceived arthritis-related stress, NHBs with functional limitations still experience high life stressors compared to NHWs (McIlvane, Baker, & Mingo, 2008). Thus, to fully comprehend ethnic/race group differences in pain-related outcomes, research is needed to address psychosocial sources of perceived and actual stress, such as discrimination and socioeconomic/financial stress, given the differential effects across the ethnic/race groups (Baker, Buchanan, & Corson, 2008; Burgess et al., 2009; Dugan et al., 2018; Herbert et al., 2017; Walker Taylor et al., 2018). Providers must also be more attentive to social and environmental conditions as key aspects in application of the biopsychosocial model of pain and disability (Fillingim, 2017). More comprehensive measurement of stress may reveal specific environmental exposures or life events, and cognitive-affective responses thereto, that explain the stronger association of stress with MEP and function in NHBs. Meints and Edwards (2018) present an array of stress-related psychological factors to consider when interpreting chronic pain outcomes, especially since NHBs engage in more negative pain coping, such as catastrophizing, that are associated with poor pain outcomes (Meints, Miller, & Hirsch, 2017). This might inform positive coping interventions designed to reduce perceived stress and MEP in groups of people at high-risk. The current study contributes to the literature by investigating mechanisms that have previously been underappreciated and may highlight potentially important opportunities to intervene on psychosocial and environmental distress through stress management.
4.3. Strengths and Limitations
We acknowledge strengths and limitations in our study. One strength of this study involves multiple performance-based techniques to measure MEP, a more objective method to assess current pain rather than recalled pain. As previously noted, there is presently no standardized method to clinically (re)produce and measure MEP giving rise to potential measurement error. Subsequently, our primary measure of clinical MEP (i.e., SPPB) requires further validation and the lab-based measurement of MEP may not be representative of the most painful movement-based activities. More dynamic tests of MEP are needed to distinguish MEP during passive and active flexion/extension of the knee. Nonetheless, significant ethnic/race differences in MEP intensity were observed. Specifically, two parameters of MEP, walking pain and balance pain, show indication that the SPPB can accurately distinguish movement and mobility activities associated with more/less pain. While the SPPB is valid and reliable for use in older adults and in chronic pain populations, its reliability in younger adults (50–64 years) and across ethnic groups is limited which may introduce age-related physical performance bias; and is therefore a limitation in our study. Given the heterogeneity of our sample inclusion age, physical performance could differ by age group; however, we were unable to examine possible cohort differences due to unequal distribution across age groups and subsequent insufficient power for statistical analyses.
Data reported are cross-sectional preventing causal inferences. Lastly, our models had limited predictors, and there are likely other factors that may further explain these racial/ethnic differences. Specifically, factors that contribute disproportionately to increased stress among NHBs include lower socioeconomic status (greater unemployment, lower income, and lower education level, unsafe neighborhoods), racial discrimination, higher chronic disease burden, and reduced access to healthcare (Poleshuck & Green, 2008; Vaughn et al., 2018). Therefore, future studies should critically examine the effect of ‘race, place, and income base’ to disentangle the stress exposures that contribute to greater MEP. Despite these limitations, our study provides new insights into racial differences in MEP, function, and perceived stress.
5. Conclusions
Movement-evoked pain is a significant aspect of the chronic pain experience in aging adults with or at risk for knee OA. In particular, our findings show that NHBs experience more MEP compared to NHWs. Perceived stress is an interesting and meaningful psychological mechanism contributing to the relationship between ethnic/race and MEP during physical performance tasks. In conclusion, these results suggest that future research on the association between ethnicity/race, MEP, and function requires more careful attention to identifying precise factors that explain differences in symptomatic knee OA.
Highlights.
Older non-Hispanic Blacks (NHBs) report nearly twice as much MEP as compared to their older non-Hispanic White (NHWs) peers.
Perceived stress was not significantly different by race, yet the association between perceived stress and MEP was stronger in NHBs compared to NHWs.
Higher perceived stress is negatively associated with physical performance.
Additional intraracial and interracial group research is needed to fully explicate the unique contextual relationship of perceived stress, pain, and function.
Acknowledgments
Funding: This work was supported by the National Institutes of Health/National Institute on Aging (R37AG033906; T32AG049673; R01AG054370; P30 AG059297–01); the University of Florida Clinical and Translational Science Institute (UL1TR000064); the University of Alabama at Birmingham Center for Clinical and Translational Science Institute (UL1TR000165). Other funding for authors: NIH/NIA 4R00AG052642–03 (EJB) and NINDS K22NS102334 and UF McKnight Brain Institute Career Enhancement Award (ELT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
Research Data Availability Statement: These findings were generated from the baseline wave of a prospective cohort study, and collection of follow-up data is still ongoing. These data derive from an NIH-funded grant, and we have developed a data sharing plan that has been approved by NIH. This plan states that upon completion of data collection and analyses to test the primary study hypotheses, we will make the data publicly available. At that time, we will provide public access to a de-identified dataset.
References
- 1.Abdallah CG, Geha P. Chronic Pain and chronic stress: Two sides of the same coin? Chronic Stress (Thousand Oaks) 2017;1. doi: 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed AT, Mohammed SA, Williams DR. Racial discrimination & health: pathways & evidence. Indian J Med Res 2007;126(4):318–27. [PubMed] [Google Scholar]
- 3.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis & Rheumatology 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 4.Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, …Pahor M Successful aging: Advancing the science of physical independence in older adults. Ageing Res Rev 2015;24:304–27. doi: 10.1016/j.arr.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroke EN, Joseph PV, Roy A, Overstreet DS, Tollefsbol TO, Vance DE, Goodin BR. Could epigenetics help explain racial disparities in chronic pain? J Pain Res 2019;12:701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker TA, Buchanan NT, Corson N. Factors influencing chronic pain intensity in older Black women: Examining depression, locus of control, and physical health. J Women’s Health 2008;17(5):869–78. doi: 10.1089/jwh.2007.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013–2015. MMWR Morb Mortal Wkly Rep 2017;66(9):246–53. doi: 10.15585/mmwr.mm6609e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell BA, Ruscheweyh R, Kelley BJ, Ness TJ, Vetter TR, Sellers AB. Ethnic differences identified by pain sensitivity questionnaire correlate with clinical pain responses. Reg Anesth Pain Med 2018;43(2):200–4. doi: 10.1097/AAP.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 10.Booker SQ, Cardoso JS, Terry EL, Sibille KT, Bradley LA, Goodin BR, Fillingim RB. Perceived Stress Predicts Function and Movement-Evoked Joint Pain in Aging African Americans with Osteoarthritis. Innovation in Aging 2018;2(Suppl. 1):485. [Google Scholar]
- 11.Braga L, Renner JB, Schwartz TA, Woodard J, Helmick CG, Hochberg MC, Jordan JM. Differences in radiographic features of knee osteoarthritis in African-Americans and Caucasians: The Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2009;17(12):1554–61. doi: 10.1016/j.joca.2009.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briggs AM, Cross MJ, Hoy DG, et al. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016;56(S2):S2443–S255. [DOI] [PubMed] [Google Scholar]
- 13.Burgess DJ, Grill J, Noorbaloochi S, Griffin JM, Ricards J, van Ryn M, & Partin MR. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med 2009;10(8):1341–52. doi: 10.1111/j.1526-4637.2009.00742.x [DOI] [PubMed] [Google Scholar]
- 14.Cardoso JS, Riley JL III, Glover T, Sibille KT, Bartley EJ, Goodin BR, Bulls HW, Herbert M, Addison AS, Staud R, Redden DT, Bradley LA, Fillingim RB, Cruz-Almeida Y. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain 2016;157:2104–14. 10.1097/j.pain.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc.Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 16.Cohen S, Williamson G. (1988). Perceived stress in a probability sample of the United States. In Spacapan S & Oskamp S (Eds.), The social psychology of health: Claremont Symposium on applied social psychology Newbury Park, CA: Sage. [Google Scholar]
- 17.Collins NJ, Misra D, Felson DT, et al. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res 2011;63(Suppl 11):S208–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, Dewalt DA, Fries JF, Pilkonis PA, Reeve BB, Stone AA, Weinfurt KP, Cella D. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett DB, Simon CB, Manini TM, George SZ, Riley JL 3rd, Fillingim RB. Movement-evoked pain: Transforming the way we understand and measure pain. Pain 2019;160(4):757–61. doi: 10.1097/j.pain.0000000000001431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Almeida Y, Cardoso J, Riley JL III, Goodin B, King CD, Petrov M, Bartley EJ, Sibille KT, Glover TL, Herbert MS, Bulls HW, Addison A, Staud R, Redden D, Bradley LA, Fillingim RB. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol 2017;98:186–91. 10.1016/j.exger.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz-Almeida Y, Rosso A, Marcum Z, Harris T, Newman AB, Nevitt M, Satterfield S, Yaffe K, Rosano C. for the Health ABC Study. Associations of musculoskeletal pain with mobility in older adults: potential cerebral mechanisms. J Gerontol A Biol Sci Med Sci 2017;72:1270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande N, Metter EJ, Guralnik J, Bandinelli S, Ferrucci L. Predicting 3-year incident mobility disability in middle-aged and older adults using physical performance tests. Arch Phys Med Rehabil 2013;94(5):994–7. doi: 10.1016/j.apmr.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djuric Z, Bird C, Furumoto-Dawson A, Rauscher G, Ruffin M, Stowe R, Tucker K, Masi C. Biomarkers of psychological stress in health disparities research. Open Biomark J 2008;1:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugan SA, Lewis TL, Everson-Rose SA, Jacobs EA, Harlow SB, Janssen I. Chronic discrimination and bodily pain in a multiethnic cohort of midlife women in the Study of Women’s Health Across the Nation. Pain 2018;158:1656–65. 10.1097/j.pain.0000000000000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1–2):9–19. [DOI] [PubMed] [Google Scholar]
- 26.Eggermont LHP, Leveille SG, Shi L, Kiely DK, Shmerling RH, Jones RN, Guralnik JM, Bean JF. J Am Geriatr Soc 2014;62:1007–16. doi: 10.1111/jgs.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eslami V, Zimmerman ME, Grewal T, Katz M, Lipton RB. Pain grade and sleep disturbance in older adults: Evaluation the role of pain, and stress for depressed and non-depressed individuals. Int J Geriatr Psychiatry 2016;31:450–7. doi: 10.1002/gps.4349 [DOI] [PubMed] [Google Scholar]
- 28.Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain 2017;158(Suppl 1):S11–S18. doi: 10.1097/j.pain.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, Widerstrom-Noga E, Arnold L, Bennett R, Edwards RR, Freeman R, Gewandter J, Hertz S, Hochberg M, Krane E, Mantyh PW, Markman J, Neogi T, Ohrbach R, Paice JA, Porreca F, Rappaport BA, Smith SM, Smith TJ, Sullivan MD, Verne GN, Wasan AD, Wesselmann U. The ACTTION-American Pain Society Pain Taxonomy (AAPT): An evidence-based and multidimensional approach to classifying chronic pain conditions. J Pain 2014;15(3):241–9. doi: 10.1016/j.jpain.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler-Brown A, Wee C, Marcantonio E, Ngo L, Leveille S. The mediating effect of chronic pain on the relationship between obesity and physical function and disability in older adults. J Am Geriatr Soc 2013;61: 2079–86. doi: 10.1111/jgs.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon JL, Johnson J, Nau S, Mechlin B, Girdler SS. The role of chronic psychosocial stress in explaining racial differences in stress reactivity and pain sensitivity. Psychosom Med 2017;79:201–12. doi: 10.1097/PSY.0000000000000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332(9):556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He D, Grant B, Holden RR, Gilron I. Methodology for self-report of rest pain (or spontaneous pain) vs evoked pain in chronic neuropathic conditions: A prospective observational pilot study. Pain Rep 2017;2(2):e587. doi: 10.1097/PR9.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbert MS, Goodin BR, Bulls HW, Sotolongo A, Petrov ME, Edberg JC, Bradley LA, Fillingim RB. Ethnicity, cortisol, and experimental pain responses among persons with symptomatic knee osteoarthritis. Clin J Pain 2017;33(9):820–6. doi: 10.1097/AJP.0000000000000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: Chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health 2010;100(5):933–9. doi: 10.2105/AJPH.2008.143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, Piette JD. Racial and socioeconomic disparities in disabling chronic pain: Findings from the Health and Retirement Study. J Pain 2017;18(12):1459–67. doi: 10.1016/j.jpain.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG. Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain 2017;158(2):194–211. doi: 10.1097/j.pain.0000000000000731 [DOI] [PubMed] [Google Scholar]
- 40.Kim KH, Bursac Z, DiLillo V, White DB, West DS. Stress, race, and body weight. Health Psychol 2009;28(1):131–5. doi: 10.1037/a0012648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015;23:1233–41. doi: 10.1016/j.joca.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mankovsky-Arnold T, Wideman TH, Lariviere C, Sullivan MJ. Measures of spontaneous and movement-evoked pain are associated with disability in patients with whiplash injuries. J Pain 2014;15(9):967–75. doi: 10.1016/j.jpain.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 43.March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, Buchbinder R, Vos T, Woolf AD. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol 2014;28(3):353–66. [DOI] [PubMed] [Google Scholar]
- 44.McIlvane JM, Baker TA, Mingo CA. Racial differences in arthritis-related stress, chronic life stress, and depressive symptoms among women with arthritis: a contextual perspective. J Gerontol B Psychol Sci Soc Sci 2008. September;63(5):S320–7. [DOI] [PubMed] [Google Scholar]
- 45.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med 2005;67(6):948–56. [DOI] [PubMed] [Google Scholar]
- 46.Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 2018;87(Pt B):168–82. doi: 10.1016/j.pnpbp.2018.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meints SM, Stout M, Abplanalp S, Hirsh AT. Pain-Related rumination, but not magnification or helplessness, mediates race and sex differences in experimental pain. J Pain 2017;18(3):332–9. doi: 10.1016/j.jpain.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Miller DK, Wolinsky FD, Andresen EM, Malmstrom TK, Miller JP. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. J Gerontol A Biol Sci Med Sci 2008;63:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson AE, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM. Differences in multijoint radiographic osteoarthritis phenotypes among African Americans and Caucasians: the Johnston County Osteoarthritis project. Arthritis Rheum 2011;63(12):3843–52. doi: 10.1002/art.30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain 2008;136(3):235–8. doi: 10.1016/j.pain.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Neal WT, Qureshi W, Judd SE, Glasser SP, Ghazi L, Pulley L, Howard VJ, Howard G, Soliman EZ. Perceived stress and atrial fibrillation: The REasons for Geographic and Racial Differences in Stroke Study. Ann Behav Med 2015;49(6):802–8. doi: 10.1007/s12160-015-9715-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahim-Williams FB, Riley JL III, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain 2007;129:177–84. doi: 10.1016/j.pain.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roux CH, Saraux A, Mazieres B, Pouchot J, Morvan J, Fautrel B, et al. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis 2008;67:1406–11. [DOI] [PubMed] [Google Scholar]
- 54.Sayers A, Wylde V, Lenguerrand E, Beswick AD, Gooberman-Hill R, Pyke M, Dieppe P, Blom AW. Rest Pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res (Hoboken) 2016;68(2):237–45. doi: 10.1002/acr.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sibille KT, Langaee T, Burkley B, Gong Y, Glover TL, King C, Riley JL 3rd, Leeuwenburgh C, Staud R, Bradley LA, & Fillingim RB. Chronic pain, perceived stress, and cellular aging: An exploratory study. Mol Pain 2012;8, 12. doi: 10.1186/1744-8069-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sibille KT, King C, Garrett TJ, Glover TL, Zhang H, Chen H, Reddy D, Goodin BR, Sotolongo A, Petrov ME, Cruz-Almeida Y, Herbert M, Bartley EJ, Edberg JC, Staud R, Redden DT, Bradley LA, Fillingim RB. Omega-6: Omega-3 PUFA ratio, pain, functioning, and distress in adults with knee pain. Clin J Pain 2018;34(2):182–189. doi: 10.1097/AJP.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sumner LA, Olmstead R, Azizoddin DR, Ormseth SR, Draper TL, Ayeroff JR, Zamora-Racaza G, Weisman MH, Nicassio PM. The contributions of socioeconomic status, perceived stress, and depression to disability in adults with systemic lupus erythematosus. Disabil Rehabil 2019;18:1–6. [Epub ahead of print]. doi: 10.1080/09638288.2018.1522550 [DOI] [PubMed] [Google Scholar]
- 58.Taylor JL, Parker LJ, Szanton SL, Thorpe RJ Jr. The association of pain, race and slow gait speed in older adults. Geriatr Nurs 2018;39(5):580–3. doi: 10.1016/j.gerinurse.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsuboi Y, Ueda Y, Naruse F, Ono R. The association between perceived stress and low back pain among eldercare workers in Japan. J Occup Environ Med 2017;59(8):765–7. doi: 10.1097/JOM.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 60.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg 2014;12(12):1500–24. doi: 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 61.Vaughn IA, Terry EL, Bartley EJ, Schaefer N, Fillingim RB. Racial-ethnic differences in osteoarthritis pain and disability: A Meta-Analysis. J Pain 2018; [Epub ahead of print] doi: 10.1016/j.jpain.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vina ER, Ran D, Ashbeck EL, Kwoh CK. Natural history of pain and disability among African-Americans and Whites with or at risk for knee osteoarthritis: A longitudinal study. Osteoarthritis Cartilage 2018;26(4):471–9. doi: 10.1016/j.joca.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker JL, Thorpe RJ Jr., Harrison TC, Baker TA, Cary M, Allaire JC, Szanton SL, Whitfield KE. The relationship between pain, disability, and sex in African Americans. Pain Manage Nurs 2016;17(5):294–301. 10.1016/j.pmn.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker Taylor JL, Campbell CM, Thorpe RJ Jr., Whitfield KE, Nkimbeng M, Szanton SL. Pain, racial discrimination, and depressive symptoms among African American women. Pain Manage Nurs 2018;19(1):79–87. 10.1016/j.pmn.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White RS, Jiang J, Hall CB, Katz MJ, Zimmerman ME, Sliwinski M, Lipton RB. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults J Am Geriatr Soc 2014;62(12):2350–6 doi: 10.1111/jgs13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zakoscielna KM, Parmelee PA. Pain variability and its predictors in older adults: depression, cognition, functional status, health, and pain. J Aging Health 2013;25(8):1329–39. doi: 10.1177/0898264313504457 [DOI] [PubMed] [Google Scholar]


