Abstract
Rationale & Objective:
Vascular dysfunction, characterized by impaired vascular endothelial function and increased large-elastic artery stiffness, is evident early in autosomal dominant polycystic kidney disease (ADPKD) and is an important predictor of cardiovascular events and mortality. Aldosterone excess has been implicated in the development of endothelial dysfunction and arterial stiffness, in part by causing increased oxidative stress and inflammation. We hypothesized that aldosterone antagonism would reduce vascular dysfunction in patients with early-stage ADPKD.
Study Design:
Prospective, randomized, controlled, double-blind clinical trial
Setting & Participants:
61 adults 20–55 years of age with ADPKD, estimated glomerular filtration rate ≥60 mL/min/1.73 m2, and receiving a renin angiotensin aldosterone system inhibitor
Intervention:
Spironolactone (maximum dose of 50 mg/d) or placebo for 24 weeks
Outcomes:
Change in brachial artery flow-mediated dilation [FMDBA] was the primary endpoint and changes in carotid-femoral pulse-wave velocity [CFPWV]) was the secondary endpoint.
Results:
Sixty participants completed the trial. Participants had a mean age of 34±10 (s.d.) years, 54% were female, and 84% non-Hispanic White. Spironolactone did not change brachial artery flow-mediated dilation (8.0% ± 5.5% and 7.8% ± 4.3% at baseline and 24 weeks, respectively, versus corresponding values in placebo group of 8.4% ± 6.2% and 8.0% ± 4.6%; p=0.9 for comparison of change between groups) or carotid-femoral pulse-wave velocity (640 ± 127 cm/s and 603 ± 101 cm/s at baseline and 24 weeks, respectively, versus corresponding values in the placebo group of 659 ± 138 cm/s and 658 ± 131 cm/s; p=0.1). Brachial systolic blood pressure was reduced with spironolactone (median change of −6 [IQR, −15, 1] mm Hg, versus −2 [−7, 10] mmHg in the placebo group; p=0.04). Spironolactone did not change the majority of circulating and/or endothelial cell markers of oxidative stress/inflammation, nor did it change vascular oxidative stress.
Limitations:
Low level of baseline vascular dysfunction; lack of aldosterone measurements
Conclusions:
Twenty-four weeks of aldosterone antagonism reduced systolic blood pressure without changing vascular function in patients with early-stage ADPKD.
Keywords: autosomal dominant polycystic kidney disease (ADPKD), vascular dysfunction, cardiovascular disease, aldosterone antagonist, vascular endothelial function, large-elastic artery stiffness, spironolactone, clinical trial, endothelium, oxidative stress, polycystic kidney disease, pulse wave velocity (PWV), mineralocorticoid, randomized controlled trial (RCT)
Autosomal dominant polycystic kidney disease (ADPKD) is the most common potentially lethal monogenic kidney disorder, occurring worldwide and affecting all races.1 While ADPKD is characterized by the development and continued growth of multiple kidney cysts that result in end-stage renal disease in the majority of afflicted patients,2 the leading causes of death among patients with ADPKD are cardiovascular complications and disorders.3 Hypertension occurs early in the natural history of ADPKD4 and is known to be partly responsible for the excess risk of cardiovascular disease in ADPKD.1
Impaired vascular endothelial function, commonly assessed as reduced endothelium-dependent dilation, as well as increased large-elastic artery stiffness, occur early in the course of ADPKD, even in the presence of preserved glomerular filtration rate (GFR).5, 6 Notably, impaired endothelium-dependent dilation and increased large-elastic artery stiffness are both independent predictors of cardiovascular events and mortality.7, 8 Both oxidative stress and inflammation are also increased in ADPKD9, 10 and may be involved in the pathogenesis of vascular dysfunction and cardiovascular disease in this population. Thus, establishing strategies to delay, minimize and/or prevent arterial dysfunction, and thus mitigate cardiovascular risk, are a high priority in ADPKD.
In ADPKD, renin-angiotensin aldosterone system activation results from expansion of renal cysts and compression of the renal vasculature.11, 12 Angiotensin converting enzyme inhibitors (ACEi) are widely used for controlling hypertension in ADPKD, but aldosterone breakthrough may occur,13 which can promote vascular oxidative stress, vasoconstriction, and hypertension via vascular mineralocorticoid receptor activation.14 Aldosterone levels are high in patients with ADPKD and remain elevated after administration of ACEi when compared to patients with essential hypertension.12 Studies have implicated aldosterone excess in the development of endothelial dysfunction, decreased arterial compliance, and cardiovascular disease in patients with primary hyperaldosteronism, hypertension, heart failure, and even in the general population.15–18 Additionally, mineralocorticoid receptor antagonists have been shown to reduce vascular dysfunction in numerous chronic disease populations receiving ACEi, including heart failure,19, 20 resistant hypertension,21, 22 and chronic kidney disease.23
Accordingly, the aim of this randomized, placebo-controlled trial was to determine the efficacy of an aldosterone antagonist for improving vascular endothelial function (primary endpoint), as well as reducing large-elastic artery stiffness (secondary endpoint) in patients with early-stage ADPKD receiving the maximal tolerable dose of an ACEi or angiotensin receptor blocker (ARB). Additionally, we sought to obtain insight into the mechanisms by which aldosterone antagonism may reduce vascular dysfunction in ADPKD. We hypothesized that aldosterone antagonism would improve brachial artery flow-mediated dilation (FMDBA), a measure of endothelium-dependent dilation, and reduce carotid-femoral pulse-wave velocity (CFPWV), an index of large-elastic artery stiffness, and that this would be associated with reductions in circulating and endothelial cell markers of oxidative stress and inflammation, including changes in circulating bioactive lipid mediators.
METHODS
Additional details of the Methods are provided in Item S1.
Study Participants.
Eligible participants were enrolled at the University of Colorado Denver Anschutz Medical Campus between July 2014 and June 2016 (the trial concluded according to enrollment determined by power calculations described below). Participants eligible for inclusion were men and women 20–55 years of age with a diagnosis of ADPKD based on the Ravine criteria (in participants ≥30 years)24 with a PKD1 genotype. Total kidney volume was between 500–2,500 ml, estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation25 was ≥60 ml/min/1.73 m2, and all participants had a history of hypertension (SBP > 130 mm Hg and/or diastolic BP > 80 mmHg based on 3 separate measurements within the past year) treated with a stable dose of an ACEi or ARB (standard recommendation at the time the trial was designed) and currently controlled (to <160/90 mmHg).
Participants were excluded if they had an average serum potassium >5.5 mEq/l or any single value >6.0 mEq/l in the past 6 months, received an aldosterone antagonist in the past 6 months, were using a potassium-sparing diuretic or other medication that could contribute to hyperkalemia (e.g. non-steroidal anti-inflammatory agents), body mass index ≥40 kg/m2 (for accuracy of vascular measurements), were currently smoking or had a history of smoking in the past 12 months, had a history of severe congestive heart failure (ejection fraction <35%), were hospitalized in the last 3 months, had a history of liver disease, received immunosuppressive therapy within the last year, used warfarin with an INR > 2.5, had an active infection or antibiotic use, had alcohol dependence or abuse, or were pregnant, nursing, or planning to become pregnant. If study participants were using antioxidants and/or omega-3 fatty acids, they were discontinued at least 4 weeks prior to study participants, and if participants used cannabis use was discontinued 2 weeks prior to vascular measurements.
Study Design.
A 24-week (±2 weeks) prospective, randomized, placebo-controlled (1:1 allocation), parallel group, double-blind study with the mineralocorticoid antagonist spironolactone was conducted at the University of Colorado Anschutz Medical Campus Division of Renal Diseases and Hypertension Clinical Vascular Physiology Laboratory. Randomization (without blocking) occurred using a computer-generated procedure run and kept by a statistician. After initial screening, subjects meeting inclusion criteria had baseline vascular measurements performed, as described below. Measurements were made in the supine position following standard recommendations.26 Participants were then randomized by the study coordinator according to a computer-generated random allocation sequence and received either the mineralocorticoid antagonist spironolactone or matching placebo. Participants received a dose of 25 mg/d for 4 weeks, with dosing escalated to 50 mg/d for the remainder of the study if tolerated by an individual participant.
All investigators, coordinators, analysts, and participants were blinded to group assignment, with only the nursing staff not affiliated with the study and the statistician aware of the randomization. Measurements were repeated after 24 weeks of the intervention. The primary outcome was the change in FMDBA in the spironolactone compared to placebo group.
Procedures.
Vascular Measurements.
FMDBA was determined using duplex ultrasonography (Xario 200, Toshiba, Tustin, CA) with ECG-gated end-diastolic ultrasound images analyzed by a single blinded analyst using a commercially available software package (Vascular Analysis Tools 5.8.1, Medical Imaging Applications, Coralville, IA), as described in detail previously.27–29 Doppler flow of the brachial artery was also measured and peak shear rate was calculated as a potential covariate.4–7 Following a minimum of 15 minutes of rest in the supine position, 30 seconds of B-mode ECG-gated end-diastolic ultrasound images were recorded to determine baseline brachial artery diameter. A forearm blood pressure cuff placed just distal to the olecranon process was inflated to 250 mmHg for 5 minutes. Thirty seconds prior to cuff release, brachial artery dimeter during occlusion was recorded for calculation of shear rate. Peak shear rate was calculated during the 15 seconds following cuff release, and peak diameter was determined as the largest six consecutive R-wave triggered frames during the two minutes following cuff release for calculation of percent FMDBA. Endothelium-independent dilation (brachial artery dilation to 0.4 mg of sublingual nitroglycerin) was assessed as a standard index of smooth muscle cell sensitivity to exogenous nitric oxide.27, 29, 30
PWV was measured as described in detail previously using a transcutaneous custom tonometer.27–29 Supine blood pressure was also measured using the auscultatory method. Additionally, as secondary indices of arterial stiffness, carotid artery compliance, carotid artery β-stiffness index, carotid intimal medial thickness, carotid augmentation index, and carotid SBP were measured.27–29
The influence of oxidative stress on FMDBA was assessed by infusing a supraphysiological dose of ascorbic acid and measuring FMDBA during the “drip infusion” when peak plasma concentrations occur, as described previously.27, 30, 31
Cellular Markers of Oxidative Stress and Inflammation.
Vascular endothelial cells were obtained from the intima of an antecubital vein and were later stained for positive identification of endothelial cells, assessment of nuclear factor κB (NFκB), interleukin 6 (IL-6), NAD(P)H oxidase (p47phox), and phosphorylated endothelial nitric oxide synthase (PeNOS) using immunofluorescence, as described previously.27, 29, 32
Circulating Markers of Oxidative Stress, Inflammation, and Bioactive Lipid Mediators.
Serum high-sensitivity C-reactive protein (hsCRP) and IL-6 levels were measured by ELISA. Targeted liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of markers of oxidative stress (prostaglandins [PG], including 8-isoprostane, as well as PGF2α, PGD2, and PGE2), and bioactive lipid mediators (hydroxyoctadecadienoic acids [9-HODE and 13-HODE], hydroxyeicosatetraenoic acids [5-HETE, 8-HETE, 9-HETE, 11-HETE, 12-HETE], epoxyeicosatrienoic acids [8,9-EET, 11,12-EET and 14–15-EET), and hydroxyeicosapentanoic acids [5-HEPE, 12-HEPE] was also performed on serum samples using validated assays, as described in detail previously.10, 33, 34
Outcome Measures.
The primary endpoint was change in FMDBA in the spironolactone compared to placebo group. Secondary outcomes were change in CFPWV, blood pressure, the change in FMDBA following acute infusion of ascorbic acid, and circulating and vascular endothelial cell protein expression of markers of oxidative stress, inflammation, and associated pathways. Pre-specified exploratory outcomes included change in carotid artery compliance, carotid artery β-stiffness index, carotid SBP, and carotid intimal medial thickness.
Statistical Analysis.
All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC). Differences in baseline variables between groups were assessed using independent samples t-tests, Chi-square tests, or Fishers exact test. The comparison of changes in percent FMDBA in response to treatment between groups were analyzed using an independent samples t-test. This same statistical approach was performed for other secondary and exploratory endpoints. Non-normally distributed variables were log-transformed prior to analysis. For variables that could not be log-transformed (change in SBP and DBP), a non-paramteric (Wilcoxon rank sums) test was used. In addition to comparison of change in percent FMDBA with ascorbic acid (compared to isovolumetric saline) between groups (independent samples t-test), a paired t-test was used for within group comparisons of the change in percent FMDBA with an acute ascorbic acid infusion (compared to saline infusion). Alpha was set at 0.05 (two-sided). All data are reported as means ± S.D (S.E. in figures), medians (interquartile range), or n (%).
A sample size of 24 subjects per group was calculated based on 97% power and a two-side type I error rate of 0.05 in order to detect a difference in mean change in FMD of 3.8 percent (baseline: 5.5%, end-of-study: 9.3%), assuming standard deviations of 2.1 and 3.4, respectively, based on previously published data (open-label) assessing the effect of an aldosterone antagonist of FMDBA.20 To account for potential dropout and experimental failure of about 20%, approximately 30 participants were randomized to each group.
Study Approval
All procedures were approved by the Institutional Review Board of the University of Colorado Anschutz Medical Campus (13–1440) and adhere to the Declaration of Helsinki. The nature, benefits and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation. The trial was registered at ClinicalTrials.gov (NCT01853553).
Results
Enrollment and Baseline Clinical Characteristics.
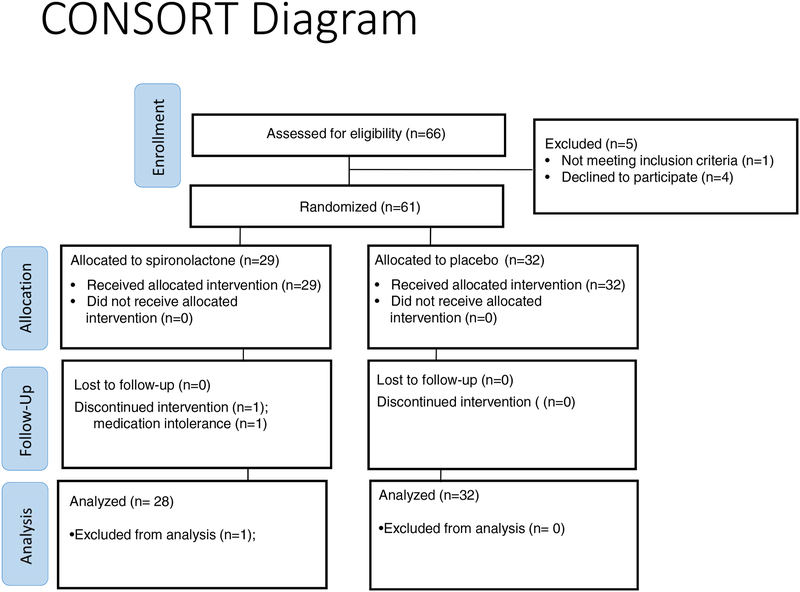
Of the 66 participants who were screened for participation, 61 were randomized to receive either the aldosterone antagonist spironolactone or placebo (Figure 1). One participant in the spironolactone group (group 1) discontinued the intervention prior to the final study visit at 24 weeks due to inability to tolerate the medication (frequent urination interfering with daily living). Participants had a mean age of 34±10 (s.d.) years; 54% were female and 84%, non-Hispanic White. Groups did not differ significantly in terms of baseline characteristics, including gender, race/ethnicity, body mass index, blood pressure, lipids, eGFR, and medications (Table 1).
Figure 1:
Flow diagram of patient enrollment, randomization, and completion.
Table 1.
Baseline Characteristics of Study Participants
| Variable | Spironolactone (n=29) | Placebo (n=32) | P-Value |
|---|---|---|---|
| Age, y | 34±10 | 34±9 | 0.9 |
| Male sex | 55% | 38% | 0.2 |
| Non-Hispanic White | 79% | 88% | 0.4 |
| BMI, kg/m2 | 27.2±4.7 | 27.1±5.3 | 0.9 |
| SBP, mmHg | 122±13 | 120±12 | 0.5 |
| DBP, mmHg | 79±9 | 76±11 | 0.2 |
| eGFR, ml/min/1.73m2 | 96±23 | 93±19 | 0.6 |
| LDL, mg/dL | 95±30 | 95±26 | 0.9 |
| HDL, mg/dL | 47±11 | 51±14 | 0.2 |
| Total Cholesterol, mg/dL | 162±36 | 167±29 | 0.5 |
| ACEi/ARB, % | 100% | 100% | 0.9 |
| Diuretic, % | 17% | 19% | 0.9 |
| CCB, % | 3% | 6% | 0.6 |
| Statin, % | 31% | 16% | 0.2 |
Continuous data expressed as mean±S.D. BMI, body-mass index; SBP, systolic blood pressure; SBP, systolic blood pressure, eGFR; estimated glomerular filtration rate; LDL, low density lipoprotein, HDL, high density lipoprotein; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker. P-values are a comparison of the spironolactone and placebo groups using an independent samples t-test, chi-square test, or Fisher’s exact test.
Effect of Aldosterone Antagonism on Vascular Function.
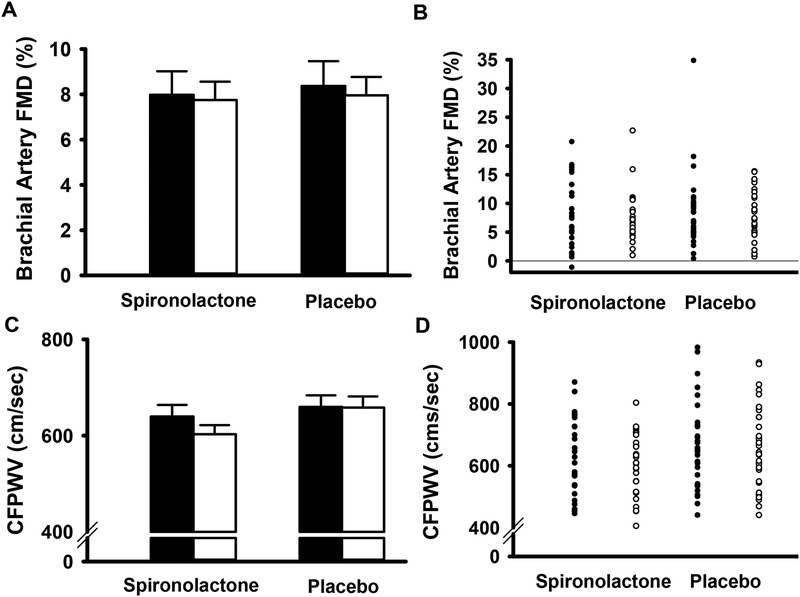
The primary endpoint, change in FMDBA (expressed as percent change), did not differ after 24 weeks in the spironolactone group as compared to the placebo group (Figure 2A and 2B; Table 2). Results were similar when FMD was expressed as an absolute change (Table 2). Baseline diameter and shear rate did not differ across the study, thus modeling was not performed to include these variables as covariates (Table 2). Endothelium-independent dilation to sublingual nitroglycerin, a measure of smooth muscle cell responsiveness to nitric oxide, was also unaffected by aldosterone antagonism (Table 2).
Figure 2: Changes in brachial artery flow-mediated dilation and aortic pulse-wave velocity with spironolactone and placebo.
Brachial artery flow-mediated dilation (FMD) mean±s.e group values (Panel A) and individual data points (Panel B) at baseline (black bars; closed circles) and following 24 weeks of treatment (white bars; open circles) with spironolactone or placebo. Carotid-femoral pulse-wave velocity (CFPWV mean±s.e group values (Panel C) and individual data points (Panel D) at baseline (black bars; closed circles) and following 24 weeks of treatment (white bars; open circles) with spironolactone or placebo.
Table 2.
Vascular Parameters
| Spironolactone n=28 | Placebo n=32 | ||||||
|---|---|---|---|---|---|---|---|
| BL | 24 wk | Δ | BL | 24 wk | Δ | P-Value | |
| Brachial artery FMD | |||||||
| percent change | 8.0±5.5 | 7.8±4.3 | −0.2±3.2 | 8.4±6.2 | 8.0±4.6 | −0.4±4.7 | 0.9 |
| absolute change; mm | 0.26±0.16 | 0.26±0.12 | −0.00±0.11 | 0.26±0.14 | 0.25±0.13 | −0.01±0.13 | 0.8 |
| Baseline brachial artery diameter (mm) | 3.6±0.7 | 3.6±0.6 | −0.1±0.2 | 3.4±0.6 | 3.3±0.6 | −0.0±0.2 | 0.5 |
| Peak shear rate (s−1) | 1009±424 | 1100±336 | 91±329 | 1121±489 | 1174±426 | 54±410 | 0.7 |
| Brachial artery dilation to nitroglycerin | |||||||
| percent change * | 27.9±9.1 | 28.6±6.8 | 0.7±4.4 | 28.0±6.1 | 28.9±7.3 | 0.9±7.4 | 0.9 |
| absolute change; mm * | 0.94±0.22 | 0.97±0.19 | 0.03±0.12 | 0.95±0.21 | 0.94±0.17 | −0.01±0.19 | 0.5 |
| Brachial SBP (mmHg) | 124±13 | 117±11 | −6 (−15, 1) | 124±10 | 125±13 | −2 (−7, 10) | 0.04 |
| Brachial DBP (mmHg) | 74±10 | 70±9 | −4 (−10, 3) | 75±9 | 75±10 | −1 (−7, 9) | 0.2 |
| Carotid-femoral PWV (cm/s) | 640±125 | 603±101 | −37±78 | 659±138 | 658±131 | −1±89 | 0.1 |
| Carotid-radial PWV (cm/s) | 924±126 | 903±147 | −21±106 | 920±185 | 937±187 | 18±188 | 0.3 |
| Carotid intimal medial thickness (mm) | 0.48±0.09 | 0.48±0.09 | −0.01±0.05 | 0.47±0.07 | 0.47±0.06 | −0.00±0.07 | 0.7 |
| Carotid augmentation index (%) | 9.3±15.0 | 2.5±16.5 | −6.8±14.6 | 8.3±15.4 | 13.4±14.1 | 5.2±13.3 | 0.002 |
| Carotid artery compliance (mm/mm Hg * 10−1) | 0.12±0.04 | 0.12±0.04 | 0.01±0.02 | 0.12±0.04 | 0.12±0.03 | 0.00±0.03 | 0.5 |
| Carotid β-stiffness index (A.U.) | 6.1±2.0 | 5.8±1.7 | −0.3±1.3 | 6.0±2.1 | 5.8±2.1 | −0.2±1.9 | 0.7 |
| Carotid SBP (mmHg) | 118±15 | 110±12 | −8±15 | 120±15 | 120±16 | 0±17 | 0.06 |
Data are mean±S.D or median [IQR]. P-value is a comparison of the change from baseline to 24 weeks (Δ) between the 2 groups using a t-test for normally distributed variables and a non-parametric (Wilcoxon rank sums) test for non-normally distributed variables. Blood pressures were taken in the supine position. BL, baseline; FMD, flow-mediated dilation; NTG, nitroglycerin; SBP, systolic blood pressure; DBP, diastolic blood pressure; PWV, pulse-wave velocity.
N=16 for spironolactone and n=15 for placebo for NTG variables.
The secondary endpoint, CFPWV, also did not change after 24 weeks in the spironolactone group as compared to the placebo group Figure 2C and 2D; Table 2). Carotid-radial PWV, a measure of peripheral arterial stiffness, did not change in either group (Table 2). Change in resting brachial SBP was −6 [IQR, −15, 1] mmHg in the spironolactone group, compared with a change of 12 [IQR, −7, 10] mmHg in the placebo group (Table 2; p=0.04). Carotid augmentation index, a measure of wave reflection, was reduced with spironolactone (Table 2; p=0.002), and carotid SBP, an index of central blood pressure, tended to be reduced with spironolactone (Table 2; p=0.06). Additional secondary endpoints (carotid artery intimal medial thickness, carotid artery compliance, and carotid β-stiffness index) were unchanged (Table 2).
Vascular Oxidative Stress, Inflammation, and Bioactive Lipid Mediators.
At baseline, infusion of ascorbic acid significantly raised plasma ascorbic acid levels (pre: 34.0±5.1 μmol/L; post: 1093.8±330.1 μmol/L; p=0.002), as reported previously.29 This infusion significantly improved FMDBA as compared to isovolumetric saline, as also reported previously.29 At 24 weeks, ascorbic acid infusion also improved FMDBA in both groups (saline vs ascorbic acid: 7.7±4.4% vs 9.9±5.4% [p=0.001] for spironolactone; 7.1±4.1% vs 9.0±5.7% [p=0.008] for placebo); however, the comparison of change in percent FMDBA with ascorbic acid between groups revealed no reduction in vascular oxidative stress with aldosterone antagonism (p=0.8).
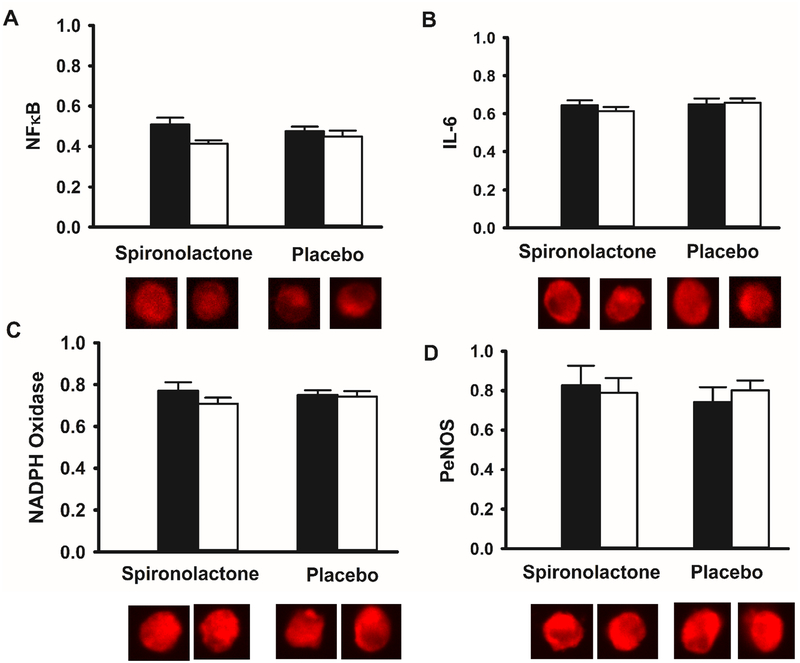
Serum hsCRP levels were increased at 24 weeks in the spironolactone compared to placebo group (Table 3; p=0.04) without a change in IL-6 levels (Table 3; p =0.2). In contrast to circulating inflammatory markers, vascular endothelial cell protein expression of the pro-inflammatory transcription factor NFκB and IL-6 were unchanged with spironolactone compared to placebo (Figure 3A and 3B; p=0.2 and 0.2, respectively). Protein expression of the oxidant enzyme NADPH oxidase (Figure 3C; p=0.3) and PeNOS (Figure 3D; p=0.3) were also unchanged with spironolactone compared to placebo group. Circulating levels of the oxidative stress marker 8-isoprostane were nominally decreased in the spironolactone compared to placebo group, but this finding was not statistically significant (Table 3, p=0.05). Other bioactive lipid mediators assessed did not differ in change between groups (Table 3). We have previously reported a comparison of these biomarkers at baseline to levels in normal healthy controls.29
Table 3.
Circulating Markers
| Spironolactone n=28 | Placebo n=31 | ||||||
|---|---|---|---|---|---|---|---|
| BL | 24 wk | Δ | BL | 24 wk | Δ | P-Value | |
| hsCRP, mg/L | 0.97 (0.32, 1.85) | 2.39 (0.62, 6.12) | 0.28 (−0.21, 2.18) | 1.19 (0.52, 4.36) | 1.74 (0.62, 4.89) | 0.19 (−0.29, 1.1) | 0.04 |
| IL-6, pg/mL | 0.56 (0.39, 0.80) | 0.76 (0.52, 1.22) | 0.14 (0.00, 0.44) | 0.56 (0.32, 1.24) | 0.60 (0.40, 1.01) | 0.02 (−6.1, 3.9) | 0.2 |
| 8-isoprostane, pg/mL | 5.0 (3.8, 6.6) | 4.7 (4.1, 6.5) | −0.50 (−1.6, 1.3) | 4.7 (3.8, 5.8) | 5.2 (4.4, 6.7) | 0.90 (−0.80, 1.5) | 0.05 |
| PGF2a. pg/mL | 31.7 (11.6, 80.7) | 38.0 (13.7, 74.0) | −0.35 (−32.1, 13.6) | 21.3 (13.7, 53.8) | 34.0 (27.2, 51.2) | 3.8 (−6.8, 19.6) | 0.5 |
| PGD2, pg/mL | 85.7 (46.5, 163.7) | 94.5 (45.8, 141.7) | −7.3 (−51.5, 33.0) | 77.9 (55.3, 120.3) | 88.0 (59.4, 115.7) | −3.5 (−45.3, 50.1) | 0.9 |
| PGE2, pg/mL | 195.8 (49.0, 475.4) | 203.3 (110.5, 335.1) | 7.1 (−175.7, 73.7) | 133.0 (63.0, 300.8) | 158.9 (103.0, 229.0) | 7.2 (−134.4, 118.0) | 0.9 |
| 9-HODE, ng/mL | 13.3 (8.4, 21.0) | 10.2 (5.1, 14.6) | −5.1 (−10.0, 0.20) | 11.4 (7.1, 20.2) | 10.2 (6.9, 17.1) | −1.6 (−6.9, 3.1) | 0.3 |
| 13-HODE, ng/mL | 10.9 (7.6, 19.7) | 10.0 (8.1, 13.6) | −1.3 (−9.3, 3.7) | 9.6 (6.3, 16.8) | 9.9 (7.6, 14.8) | −0.2 (−6.1, 3.9) | 0.6 |
| 5-HETE, ng/mL | 2.1 (0.76, 2.9) | 2.2 (1.6, 2.6) | 0.33 (−0.82, 1.4) | 2.0 (1.1, 3.0) | 2.1 (1.1, 3.4) | 0.15 (−0.29, 1.3) | 0.7 |
| 8-HETE, ng/mL | 0.79 (0.45, 2.0) | 0.87 (0.65, 1.8) | 0.00 (−0.32, 0.41) | 0.58 (0.39, 0.87) | 0.84 (0.60, 1.2) | 0.21 (−0.21, 0.50) | 0.7 |
| 9-HETE, ng/mL | 4.1 (1.8, 6.5) | 2.9 (1.5, 5.2) | −0.67 (−3.5, 0.57) | 1.4 (1.0, 2.6) | 1.8 (1.3, 3.1) | 0.21 (−0.21, 0.50) | 0.2 |
| 11-HETE, ng/mL | 1.4 (0.58, 3.3) | 1.5 (0.80, 2.6) | 0.10 (−1.3, 0.67) | 1.1 (0.62, 2.2) | 1.3 (1.1, 2.0) | 0.41 (−0.96, 0.91) | 0.9 |
| 12-HETE, pg/mL | 63.8 (24.9, 138.8) | 72.9 (32.3, 139.1) | 0.65 (−48.7, 38.7) | 39.9 (18.3, 64.3) | 49.5 (27.5, 75.5) | 13.3 (−26.4, 33.2) | 0.5 |
| 8,9-EET, ng/mL | 4.8 (2.5, 13.6) | 5.6 (3.5, 12.6) | −0.40 (−4.4, 3.2) | 3.0 (1.8, 5.7) | 4.5 (2.7, 6.8) | 0.30 (−1.5, 2.1) | 0.9 |
| 11,12-EET, ng/mL | 1.4 (0.45, 4.4) | 1.5 (0.79, 2.3) | 0.13 (−2.0, 0.71) | 1.2 (0.48, 2.7) | 1.5 (0.98, 2.1) | 0.16 (−1.2, 0.95) | 0.7 |
| 14,15-EET, ng/mL | 1.3 (0.69, 2.2) | 1.0 (0.82, 1.6) | −0.08, (−1.1, 0.49) | 0.94 (0.62, 1.3) | 1.1 (0.87, 1.3) | 0.13 (−0.37, 0.53) | 0.2 |
| 5-HEPE, ng/mL | 0.84 (0.56, 1.1) | 0.68 (0.49, 1.2) | −0.22 (−0.57, 0.38) | 0.63 (0.41, 0.78) | 0.71 (0.60, 0.91) | 0.24 (−0.04, 0.46) | 0.08 |
| 12-HEPE, pg/mL | 38.3 (13.4, 80.4) | 51.0 (21.3, 84.6) | −11.60 (−40.2, 34.2) | 19.5 (10.8, 52.9) | 30.2 (19.9, 48.4) | 4.8 (−16.0, 28.4) | 0.5 |
Data are median [interquartile range]. P-value is a comparison of the change from baseline to 24 weeks (Δ) between the 2 groups using log-transformed values. Non-normally distributed variables were log-transformed prior to analysis.
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; PGF2α, prostaglandin F2α; PGD2, prostaglandin D2; PGE2, prostaglandin E2; HODE, hydroxyoctadecadienoic acid; HETE, hydroxyeicosatetraenoic acid; EET epoxyeicosatrienoic acid; HEPE, hydroxyeicosapentanoic acid.
Figure 3. Change in vascular endothelial cell protein expression with spironolactone and placebo.
Vascular endothelial cell protein expression of nuclear factor κ B (NFκB; n=20 spironolactone; n=14 placebo; Panel A), interleukin 6 (IL-6; n=20 spironolactone; n=13 placebo; Panel B), NADPH oxidase (n=20 spironolactone; n=13 placebo; Panel C), and phosphorylated endothelial nitric oxide synthase (PeNOS; n=12 spironolactone; n=10 placebo; Panel D) at baseline (black bars) and following 24 weeks of treatment (white bars) with spironolactone or placebo. Expression is relative to human umbilical vein endothelial cell (HUVEC) control. Representative images shown below. Date are mean±s.e.
Adherence and Adverse Events.
Total compliance, assessed by pill count over the year period, was 96% (95% in the spironolactone group and 96% in the placebo group). Overall, spironolactone was well tolerated. 14 participants experienced an adverse event related or possibly related to the drug (n=7 in the spironolactone group, n=7 in the placebo group, Table 4). There were no serious adverse events in either group. One participant in the spironolactone group dropped out of the study due to inability to tolerate frequent urination, despite dose reduction to 12.5 mg/d. No other participants discontinued the study. The vast majority of participants were able to tolerate the 50 mg/d dose, although two participants were reduced to 25 mg/d (one spironolactone/one placebo) and one participant was reduced to 12.5 mg/d (placebo) due to side-effects. There was no difference in the change in eGFR at 24 weeks between groups (−1.3±11 vs +0.7±17 ml/min/1.73 m2 for spironolactone and placebo, respectively; p=0.6).
Table 4.
Adverse Events
| Spironolactone (n=29) | Placebo (n=32) | |
|---|---|---|
| Dizziness/lightheadedness | 3 (10%) | 3 (9%) |
| Muscle cramping/soreness | 2 (7%) | 3 (9%) |
| Vision changes | 2 (7%) | 1 (3%) |
| Increased urination | 2 (7%) | 0 (0%) |
| Hyperkalemia | 0 (0%) | 1 (3%) |
| Fatigue | 1 (3%) | 1 (3%) |
| Increased thirst | 0 (0%) | 1 (3%) |
| Nausea | 0 (0%) | 1 (3%) |
| Mildly elevated AST/ALT | 0 (0%) | 1 (3%) |
Spironolactone groups includes n=1 participant who discontinued study participation due to inability to tolerate frequent urination.
ALT, alanine transaminase; aspartate aminotransferase, AST.
Discussion
In a randomized, controlled trial evaluating the efficacy of aldosterone antagonism for reducing vascular dysfunction in individuals with ADPKD, we found no change in FMDBA or CFPWV following 24 weeks of treatment with spironolactone. This was despite a significant reduction in SBP with spironolactone treatment, as would be clinically expected. Aldosterone antagonism also failed to change the majority of circulating, vascular, and endothelial cell markers of oxidative stress and inflammation, including bioactive lipid mediators.
Vascular dysfunction is present very early in the course of ADPKD5, 6, 28 and is an important and independent predictor of cardiovascular events and mortality.7, 8 This clinical trial was novel as it evaluated vascular dysfunction (FMDBA and CFPWV) as endpoints in response to an intervention in ADPKD. We found a modest 6% reduction in CFPWV with spironolactone treatment, although this change failed to meet statistical significance in comparison to change in the placebo group. Additionally, SBP was lowered by 7 mmHg with mineralocorticoid antagonism, which likely contributed to the nominally reduced CFPWV, given the dynamic interconnection between blood pressure and arterial stiffness.35 However, there was no improvement in endothelium-dependent dilation, as measured by FMDBA (primary outcome).
Mineralocorticoid receptors are expressed extrarenally in the vasculature (endothelial and vascular smooth muscle cells), thus aldosterone has pleiotropic effects influencing vascular endothelial function and blood pressure beyond regulation of sodium and water balance.14 Aldosterone excess is associated with reduced large-elastic artery compliance15, 16 and impaired endothelium-dependent dilation.17, 18 Additionally, high levels of aldosterone and mineralocorticoid receptor activation stimulate a pro-inflammatory response via generation of reactive oxygen species.36
Trials of mineralocorticoid receptor antagonism have been efficacious for reducing vascular dysfunction in other chronic diseases with or without uncontrolled hypertension, typically also being treated with an ACEi. Aldosterone antagonism improves both resistance vessel19 and conduit artery endothelium-dependent dilation20 in individuals with heart failure. Additionally, treatment reduces mortality in heart failure patients.37 Mineralocorticoid receptor antagonism also improves FMDBA21 and reduced CFPWV22 in resistant hypertension. In these populations that have demonstrated improved FMDBA with aldosterone antagonism, baseline function was notably lower than in the present study, despite treatment with renin-angiotensin aldosterone system blockers. In individuals with hypertension, aldosterone antagonism attenuates a decline in FMDBA,38 reduces CFPWV,39 reduces arterial wall stiffness and media collagen to elastin ratio,40 and reduces left-ventricular mass index,41 without changing resistance vessel endothelium-dependent dilation.40 This is likely mediated by a large reduction in SBP.38–41 However, aldosterone antagonism does not improve endothelium-dependent dilation in type 2 diabetes mellitus42 or coronary artery disease without heart failure.43 Notably, in mild-to-moderate chronic kidney disease, spironolactone23 but not eplerenone44 reduces CFPWV, in addition to promoting a large reduction in SBP and improved left ventricular mass index and aortic distensibility.23
Both oxidative stress and inflammation are increased even early in the course of ADPKD,9, 10 which may be mediated in part by mineralocorticoid receptor activation.36 In various rodent models, aldosterone antagonism reduces aortic messenger RNA expression of NADPH oxidase p22phox,45 NADPH oxidase activity,46 plasma thiobarbituric acid-reactive substances,46 macrophage oxidation of LDL,47 superoxide production,47 and hepatic malonyl dialdehyde levels,45 in addition to increasing the glutathione-oxidized glutathione ratio.45 Less evidence is available in humans; however, 3 months of treatment with spironolactone reduces urinary 8-isoprostane levels in patients with diabetic nephropathy.48 We also found a suggestion of reduced circulating 8-isoprostane levels with spironolactone in early-stage ADPKD. In contrast, we found no reduction in endothelial cell markers of oxidative stress in response to aldosterone antagonism, as well as no reduction in vascular oxidative stress, as evidenced by continued improvement in FMDBA following an acute infusion of ascorbic acid that produces plasma concentrations that have been shown to inhibit superoxide production in vitro.49
Aldosterone antagonism also reduces circulating inflammatory markers in patients with hypertension40 and diabetic nephropathy,48 but not following 60 days of treatment in individuals with type 2 diabetes mellitus and moderately or severely increased albuminuria.50 While we found a surprising increase in hsCRP levels with spironolactone treatment, perhaps due to the variability of the test, there was no change in inflammation local to the vascular endothelium, as evidenced by a lack of change in endothelial cell protein expression of NFκB and IL-6. Aldosterone antagonism also increases eNOS protein and mRNA expression in rodents,45 and the improvement in endothelium-dependent dilation with spironolactone in heart failure patients treated with an ACEi is mediated by increased NO bioavailability.19 However, we found no change in endothelial cell protein expression of PeNOS with spironolactone treatment compared to the placebo group.
Notably, there was no difference in adverse events between the active and placebo groups. The major potential risk of spironolactone is hyperkalemia, particularly if receiving an ACEi/ARB. However, this was not a side-effect noted in the present trial. Overall, the study treatment was well-tolerated and drop out from the trial was extremely low.
The major strength of this study is that it was a randomized controlled trial to evaluate vascular endpoints in response to an intervention in individuals with ADPKD, which are both novel and clinically significant endpoints in this population. Additionally, we provided mechanistic insight through the collection of vascular endothelial cells from participants, acute infusion of ascorbic acid to measure vascular oxidative stress, and state-of-the art LC-MS/MS analyses to assess circulating markers. Participant retention in the study was excellent, with only one drop-out in a trial 24 weeks in duration. A potential limitation to the study is a relatively small sample size; however, we were appropriately powered to detect an improvement in the primary endpoint given expected vascular endothelial function at the time the trial was designed. An additional limitation is that it is difficult to separate the effects of blood pressure lowering from potential reductions in CFPWV, as regulation of blood pressure and arterial stiffness are closely related. Furthermore, measurements of plasma or urinary aldosterone were not available; however, accurate aldosterone measurements are challenging due to sensitivity to external factors including sodium intake, medications, position, and time of day, as well as assay limitations with low concentrations. These results may not apply to ADPKD patients who have more advanced chronic kidney disease, who may exhibit greater baseline vascular dysfunction, but would also be at higher risk of hyperkalemia with spironolactone. The overall level of vascular dysfunction in this cohort was lower than previously reported in early ADPKD,29 which may have limited the ability to detect an improvement in vascular function with aldosterone antagonism and altered the assumptions underlying the power calculations for the trial. Finally, there is a potential for inflated type-1 error for all significant differences reported, except for the primary endpoint, given multiple comparisons.
In conclusion, in individuals with early-stage ADPKD, mineralocorticoid antagonism does not improve FMDBA or reduce CFPWV, despite reducing blood pressure. This is consistent with the general lack of evidence for a reduction in oxidative stress and inflammation and associated pathways. Alternative interventions targeting an improvement in vascular endothelial function and reduced arterial stiffness in early ADPKD should be evaluated.
Supplementary Material
Supplementary File 1 (PDF) Item S1. Additional details of methods.
Funding:
NIH (grants R01DK097081, K01DK103678, UL1TR002535) and Zell Family Foundation.
Support: This trial was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), R01DK097081. Kristen Nowak is also supported by NIDDK, K01DK103678. Additional support was provided by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences CTSA grant number UL1 TR002535. Additional funding was provided by the Zell Family Foundation. The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Registered at ClinicalTrials.gov with study number NCT01853553.
Data Sharing: De-identified participant data, including data dictionaries, that underlie the results reported in this article will be made available, along with the study protocol. Data will be available beginning 6 months and ending 36 months following article publication to researchers who provide a methodologically sound proposal, in order to achieve the aims in the approved proposal. Proposals should be directed to Michel.Chonchol@ucdenver.edu. Requestors will need to sign a data access agreement and data will be sent through a third-party website.
Peer Review: Received August 20, 2018. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form December 17, 2018.
References
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–1301. [DOI] [PubMed] [Google Scholar]
- 2.Gabow PA, Johnson AM, Kaehny WD, et al. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41(5):1311–1319. [DOI] [PubMed] [Google Scholar]
- 3.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5(12):2048–2056. [DOI] [PubMed] [Google Scholar]
- 4.Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocaman O, Oflaz H, Yekeler E, et al. Endothelial dysfunction and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2004;43(5):854–860. [DOI] [PubMed] [Google Scholar]
- 6.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69(2):350–357. [DOI] [PubMed] [Google Scholar]
- 7.Covic A, Gusbeth-Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis. 2005;45(6):965–977. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. [DOI] [PubMed] [Google Scholar]
- 9.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klawitter J, Reed-Gitomer BY, McFann K, et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol. 2014;307(11):F1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham PC, Lindop GB. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33(6):1084–1090. [DOI] [PubMed] [Google Scholar]
- 12.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1091–1096. [DOI] [PubMed] [Google Scholar]
- 13.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3(9):486–492. [DOI] [PubMed] [Google Scholar]
- 14.McCurley A, Pires PW, Bender SB, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18(9):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blacher J, Amah G, Girerd X, et al. Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens. 1997;10(12 Pt 1):1326–1334. [DOI] [PubMed] [Google Scholar]
- 16.Duprez DA, De Buyzere ML, Rietzschel ER, et al. Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur Heart J. 1998;19(9):1371–1376. [DOI] [PubMed] [Google Scholar]
- 17.Duffy SJ, Biegelsen ES, Eberhardt RT, Kahn DF, Kingwell BA, Vita JA. Low-renin hypertension with relative aldosterone excess is associated with impaired NO-mediated vasodilation. Hypertension. 2005;46(4):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannemann A, Wallaschofski H, Ludemann J, et al. Plasma aldosterone levels and aldosterone-to-renin ratios are associated with endothelial dysfunction in young to middle-aged subjects. Atherosclerosis. 2011;219(2):875–879. [DOI] [PubMed] [Google Scholar]
- 19.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101(6):594–597. [DOI] [PubMed] [Google Scholar]
- 20.Abiose AK, Mansoor GA, Barry M, Soucier R, Nair CK, Hager D. Effect of spironolactone on endothelial function in patients with congestive heart failure on conventional medical therapy. Am J Cardiol. 2004;93(12):1564–1566. [DOI] [PubMed] [Google Scholar]
- 21.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109(23):2857–2861. [DOI] [PubMed] [Google Scholar]
- 22.Kalizki T, Schmidt BMW, Raff U, et al. Low dose-eplerenone treatment decreases aortic stiffness in patients with resistant hypertension. J Clin Hypertens (Greenwich). 2017;19(7):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54(6):505–512. [DOI] [PubMed] [Google Scholar]
- 24.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343(8901):824–827. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak KL, Chonchol M, Ikizler TA, et al. IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol. 2017;28(3):971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak KL, Farmer H, Cadnapaphornchai MA, Gitomer B, Chonchol M. Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2017;32(2):342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak KL, Wang W, Farmer-Bailey H, et al. Vascular dysfunction, oxidative stress, and inflammation in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13(10):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonski KL, Racine ML, Geolfos CJ, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61(3):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(Pt 1):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. [DOI] [PubMed] [Google Scholar]
- 33.Klawitter J, Klawitter J, McFann K, et al. Bioactive lipid mediators in polycystic kidney disease. J Lipid Res. 2014;55(6):1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klawitter J, Haschke M, Shokati T, Klawitter J, Christians U. Quantification of 15-F2t-isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun Mass Spectrom. 2011;25(4):463–468. [DOI] [PubMed] [Google Scholar]
- 35.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension. 2018;71(3):389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51(2):161–167. [DOI] [PubMed] [Google Scholar]
- 37.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. [DOI] [PubMed] [Google Scholar]
- 38.Yamanari H, Nakamura K, Miura D, Yamanari S, Ohe T. Spironolactone and chlorthalidone in uncontrolled elderly hypertensive patients treated with calcium antagonists and angiotensin II receptor-blocker: effects on endothelial function, inflammation, and oxidative stress. Clin Exp Hypertens. 2009;31(7):585–594. [DOI] [PubMed] [Google Scholar]
- 39.White WB, Duprez D, St Hillaire R, et al. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41(5):1021–1026. [DOI] [PubMed] [Google Scholar]
- 40.Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51(2):432–439. [DOI] [PubMed] [Google Scholar]
- 41.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108(15):1831–1838. [DOI] [PubMed] [Google Scholar]
- 42.Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK. Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus. J Clin Endocrinol Metab. 2007;92(7):2552–2558. [DOI] [PubMed] [Google Scholar]
- 43.Shah NC, Pringle SD, Donnan PT, Struthers AD. Spironolactone has antiarrhythmic activity in ischaemic cardiac patients without cardiac failure. J Hypertens. 2007;25(11):2345–2351. [DOI] [PubMed] [Google Scholar]
- 44.Boesby L, Elung-Jensen T, Strandgaard S, Kamper AL. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3–4--a randomized controlled study. PLoS One. 2013;8(5):e64549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanz-Rosa D, Oubina MP, Cediel E, et al. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxid Redox Signal. 2005;7(9–10):1294–1301. [DOI] [PubMed] [Google Scholar]
- 46.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40(4):504–510. [DOI] [PubMed] [Google Scholar]
- 47.Keidar S, Hayek T, Kaplan M, et al. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2003;41(6):955–963. [DOI] [PubMed] [Google Scholar]
- 48.Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. J Clin Endocrinol Metab. 2006;91(6):2214–2217. [DOI] [PubMed] [Google Scholar]
- 49.Jackson TS, Xu A, Vita JA, Keaney JF, Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83(9):916–922. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen SE, Schjoedt KJ, Rossing K, et al. Levels of NT-proBNP, markers of low-grade inflammation, and endothelial dysfunction during spironolactone treatment in patients with diabetic kidney disease. J Renin Angiotensin Aldosterone Syst. 2013;14(2):161–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1 (PDF) Item S1. Additional details of methods.