Abstract
Background:
Cognitive decline is a frequently cited concern among patients receiving hematopoietic cell transplantation (HCT), and patients often experience neurocognitive deficits (i.e., stable or worsening neurocognitive performance) throughout the transplant course. Deficits can be most severe during the acute transplant period (i.e., 90 days after transplantation), when patients also typically experience elevated systemic levels of inflammation. Previous studies have identified inflammation as a likely mechanism underlying neurocognitive deficits, primarily in women with breast cancer; however, longitudinal studies have been limited. In this study, our aim was to evaluate the relationship between changes in systemic inflammation and changes in cognition from pre-to post-transplant in patients receiving allogeneic HCT.
Methods:
Patients scheduled for allogeneic HCT (n=85) were assessed prior to HCT and 90 days after HCT. Biomarkers of inflammation included IL-6, sTNF-RII, CRP, and IL-1ra, which have been previously associated with neurocognitive deficits in cancer patients. Patients completed neuropsychological testing and self-report questionnaires.
Results:
Mixed models demonstrated that from pre-to post-HCT, increases in IL-6 and sTNF-RII were associated with neurocognitive deficits, and decreases in CRP were associated with better neurocognitive performance. There were no significant associations between changes in inflammation and self-reported cognitive performance.
Conclusions:
Our findings are the first to our knowledge to report a robust relationship between increasing inflammation and neurocognitive deficits from pre-to post-HCT. Additional studies are needed to confirm these findings in a larger sample.
1. Introduction
Patients receiving hematopoietic cell transplant (HCT) often experience neurocognitive deficits compared to non-transplant patients with hematologic cancers,1 non-cancer controls,2 and population norms.3–5 Neurocognitive deficits can take the form of worsening or stable performance (i.e., lack of expected improvements in performance due to practice effects).6 Deficits are often observed prior to transplantation3,5,7 and are most severe during the acute transplant period.4,7–9 By one year after transplantation, neurocognition in many patients recovers to pre-HCT levels,7,10 but deficits are evident in up to 40% of patients, and up to 60% self-report cognitive problems.11 Further, certain subgroups of patients (e.g., older adults, patients receiving myeloablative allogeneic HCT) are particularly susceptible to worse cognitive outcomes after HCT.2,12 Cognitive deficits can have profound detrimental consequences on quality of life and are a commonly-cited concern of patients treated with HCT.13,14
Experimental studies suggest that systemic inflammation may contribute to neurocognitive function,15 whereby higher levels of biomarkers of inflammation such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor (TNF) impair neurocognitive processes. For example, research with animal models has shown that administration of IL-1 impairs spatial learning,16 long-term memory,17 and working memory.18 Further, age-related memory loss co-occurs with increases in IL-6,19 and is worse among older mice who over-express TNF.20 Research has also shown that prolonged elevations in inflammation are associated with impaired cognition.21
Relationships between inflammation and neurocognitive function have not been investigated in HCT recipients. This state of affairs is surprising considering that elevated inflammation is a hallmark of allogeneic HCT.22 Nevertheless, a significant body of research has reported associations between elevated inflammation and neurocognitive deficits in cancer patients who have not received transplantation. In cancer patients, subjective and neurocognitive performance have been consistently associated with higher concurrent levels of TNF23–28 and IL-6,27–30 with less consistent evidence for higher levels of concurrent IL-128 and C-reactive protein (CRP).31–33 Longitudinal studies evaluating these associations have found that increasing inflammation (i.e., sTNF-RII, IL-6) is significantly associated with worsening neurocognition23,28 and subjective cognition34 over time in patients with breast cancer. Additional research is needed in patients with other cancer types.
The goal of the current study was to investigate the relationship between changes in inflammation and changes in subjective and neurocognitive performance in adult allogeneic HCT recipients during the acute transplant period (i.e., 90 days). We hypothesized that: 1) inflammation would increase from pre-to post-HCT, 2) subjective and objective neurocognitive performance would worsen or remain stable from pre-to post-HCT, and 3) increasing inflammation would be associated with worsening subjective and objective neurocognitive performance over time.
2. Methods
2.1. Participants
Eligible participants: were at least 18 years of age; were diagnosed with a hematologic cancer; were scheduled to receive allogeneic HCT with peripheral blood stem cells; had not previously been treated with HCT; had no history of cerebrovascular accident, head trauma with loss of consciousness within the past five years, or brain damage/injury; had completed at least six years of formal education; were willing and able to provide written informed consent; and were able to read and speak English. Participants were part of a larger IRB-approved study evaluating quality of life in patients receiving allogeneic HCT. Participants were paid $20 at each evaluation. The study was approved by the University of South Florida Institutional Review Board.
2.2. Procedure
Participants were recruited during an outpatient appointment at Moffitt Cancer Center. Written informed consent was obtained prior to initiation of study procedures. Participants completed baseline self-report questionnaires and neurocognitive testing prior to hospital admission for transplantation. Neurocognitive testing was administered by a trained research coordinator or a doctoral student in clinical psychology at the University of South Florida. All neurocognitive testing was supervised by a clinical neuropsychologist with extensive experience working with individuals receiving HCT. Participants were asked to return at 90 days after transplant (i.e., a time when acute graft-versus-host disease (GVHD) had largely resolved, and chronic GVHD had yet to emerge), to complete the same assessments. A blood sample was drawn from participants at each assessment to measure circulating biomarkers of inflammation. Data were collected between September 2010 and April 2014. Neurocognitive findings for the full sample have been reported previously.12 Participants were included in the current analyses if they had biomarker data at baseline and 90 days after HCT.
2.3. Measures
2.3.1. Demographic and Clinical Information
Prior to transplantation, participants completed a sociodemographic questionnaire assessing age, sex, ethnicity, race, marital status, education, and annual household income. Comorbidities were assessed via medical chart review using the Hematopoietic Cell Transplantation Comorbidities Index (HCT-CI).35 Additional clinical information (i.e., disease type, full vs. reduced intensity conditioning, pre-HCT disease status, length of hospital stay, donor status, body mass index or BMI, history of total body irradiation, history of prophylactic cranial irradiation) was obtained via the Moffitt Department of Blood and Marrow Transplantation database and medical chart review.
2.3.2. Neurocognitive Performance
Neurocognitive tests were selected based on published recommendations from the International Cognition and Cancer Task Force.36 Premorbid intellectual ability was evaluated using the Wechsler Test of Adult Reading.37 Neurocognitive domains included verbal memory (Hopkins Verbal Learning Test-Revised38 Immediate and Delayed Recall), verbal fluency (Controlled Oral Word Association Test39), visuospatial memory (Brief Visuospatial Memory Test-Revised40 Immediate and Delayed Recall), attention (Wechsler Adult Intelligence Scale Digit Span41 and Color Trails Test Part 142), and executive functioning (Color Trails Test Part 242 and Stroop Neuropsychological Screening Test43). Participants’ scores on each test were standardized as t-scores based on age-adjusted population norms and averaged to create a domain score. Scores across each of the neurocognitive domains were averaged to derive a total neuropsychological performance (TNP) score. For each domain and TNP, higher scores indicate better neurocognitive performance. These measures are not intended to be pure tests of each domain; instead they provide a useful heuristic for characterizing neurocognitive functioning in individuals treated with HCT.
2.3.3. Self-Reported Cognitive Performance
Subjective cognition was evaluated using the Everyday Cognition (ECog) scale.44 The ECog yields a global cognition score and subscale scores for divided attention, language, memory, planning, organization, and visuospatial abilities. In this study, the global score (i.e., average of the six subscales) was used in analyses. Higher scores indicate worse cognitive performance.
2.3.4. Biomarkers of Inflammation
Blood was processed for serum, which was stored at −80 degrees C and shipped frozen to the UCLA Cousins Center for Psychoneuroimmunology Inflammatory Biology Laboratory for immunoassays. Consistent with previous studies examining inflammation and cognition in cancer patients, biomarkers included IL-6, interleukin-1 receptor antagonist (IL-1ra), soluble tumor necrosis factor receptor 2 (sTNF-RII), and CRP.23,24 High sensitivity (IL-6) and regular sensitivity (IL-1ra, sTNF-RII, CRP) enzyme-linked immunosorbent assays (R&D Systems Human Quantikine ELISA; Minneapolis, MN) were performed according to the manufacturer’s instructions with the following modifications. CRP assays were performed with a sample dilution of 1:500 and an extended standard curve, to yield a lower limit of detection of 0.2 mg/L. sTNF-RII assays were performed with a sample dilution of 1:30, yielding a lower limit of detection of 234 pg/mL. The lower limits of detection of the IL-6 and IL-1ra assays were 0.2 and 31.2 pg/mL, respectively. All samples were assayed in duplicate, with both samples from each participant tested on the same immunoassay plate. An internal control sample was included on every plate to monitor inter-assay (<10%) and mean intra-assay (<5%) variability.
2.4. Data Analyses
Means and standard deviations (continuous variables) and frequencies and percentages (categorical variables) were used to describe sociodemographic and clinical characteristics of the sample. Differences in sociodemographic and clinical characteristics between participants included in analyses and participants in the larger study were assessed using independent samples t-tests, chi-square tests, and fisher’s tests. Associations among biomarkers of inflammation and neurocognitive domains at each time point were assessed using Spearman’s correlations. Associations between participant characteristics (i.e., age, gender, premorbid IQ, comorbidities) and TNP at baseline were assessed using Spearman’s correlations, and variables significant at p<.10 were included as covariates in longitudinal analyses. Inflammatory biomarker levels were halved when below the limit of detection (e.g., if the lower limit of detection was <.2 mg/L, the datum was entered as .1 mg/L), and estimated when above the upper limit of detection (e.g., 25 mg/L). Biomarkers of inflammation were natural log-transformed to normalize their distributions. Changes in biomarkers of inflammation, neurocognitive performance, and subjective cognition over time were evaluated using Wilcoxon sign rank tests. Relationships between changes in biomarkers of inflammation and changes in global cognition and TNP over time were examined using mixed models, which included all available data at each time point.45,46 Biomarkers of inflammation were mean-centered and included as time-varying covariates. The associations between biomarkers of inflammation and cognitive performance were examined by including the time x biomarkers of inflammation interaction while controlling for main effects and covariate x time interactions. To reduce the potential for Type 1 error, further probing of subjective and objective cognitive domains was conducted if the omnibus tests of global cognition and TNP were significant, respectively. Changes in biomarkers of inflammation are depicted as binary variables (above median change versus below median change) in figures for illustrative purposes, but were analyzed as continuous variables. All inferential statistical analyses were conducted with an alpha of 0.05. SAS version 9.4 (Cary, NC) was used for all statistical analyses.
3. Results
Of the 225 participants who provided informed consent, 89 provided both neurocognitive data and blood samples at both time points and were initially included in the current analyses (see Supplemental Figure 1 for a participant flow diagram). Compared to participants who did not provide blood samples in the larger study, participants included in the current analyses were more likely to be married (p=.03). There were no other significant differences between these groups on sociodemographic or clinical variables (i.e., age, premorbid IQ, comorbidities, sex, ethnicity, race, marital status, education, annual household income, disease type, conditioning regimen, pre-HCT disease status, length of hospital stay, donor type, BMI, total body irradiation, or prophylactic cranial irradiation) (see Supplemental Table 1) (ps>.05). Of the 89 participants initially included, three participants were removed for having two or more biomarkers of inflammation considered to be an outlier (i.e., ≥3 SD above the sample mean), and one for having both a documented infection and one biomarker of inflammation outlier. Patients taking steroids (n=4) or without documented infection who had one biomarker of inflammation outlier (n=9) were included in analyses. Thus, our final sample was comprised of 85 participants.
Demographic and clinical characteristics are displayed in Table 1. Participants were mostly male (58%), Caucasian (94%), married (75%), and reported a household income over $40,000/year (69%). Most had acute myeloid leukemia (31%), followed by myelodysplastic syndrome (18%), non-Hodgkin lymphoma (15%), and acute lymphocytic leukemia (12%). The majority were in complete or partial remission (71%) at the time of HCT. Gender and IQ were associated with TNP at baseline (ps<.01) and were included as covariates in multivariate analyses. Additionally, consistent with previous literature, BMI47 and age12 were included as covariates.
Table 1.
Participant characteristics, N=85
| Variable | Means (SD) / N (%) |
|---|---|
| Age | 52.0 (12.9) |
| PreMorbid IQ | 104.8 (10.5) |
| Comorbidities | 3.0 (1.8) |
| Sex (female) | 36 (42%) |
| Ethnicity (non-Hispanic) | 72 (86%) |
| Race (Caucasian) | 80 (94%) |
| Marital Status (married) | 64 (75%) |
| Education | |
| High school or less | 20 (24%) |
| Post-high school or college graduate | 55 (65%) |
| Post-college education | 10 (12%) |
| Annual household income (% ge $40k) | 44 (69%) |
| Disease type | |
| AML | 26 (31%) |
| MDS | 15 (18%) |
| NHL, B-Cell | 13 (15 %) |
| ALL | 10 (12%) |
| Other | 21 (25%) |
| Conditioning regimen | |
| Full intensity | 65 (73%) |
| Reduced intensity | 24 (27%) |
| Pre-HCT disease status | |
| Complete remission | 44 (53%) |
| Partial remission | 15 (18%) |
| Stable disease or no response | 17 (20%) |
| Progressive disease | 7 (8%) |
| Length of stay in hospital | 23.7 (5.8) |
| Donor | |
| Related | 27 (32%) |
| Matched unrelated | 42 (49%) |
| Mismatched unrelated | 16 (19%) |
| Body mass index (BMI) | 28.4 (5.5) |
| Total Body Irradiation (TBI) | 10 (13%) |
| Prophylactic Cranial Irradiation (PCI) | 1 (1%) |
Note: AML=acute myeloid leukemia, MDS=myelodysplastic syndrome, NHL=non-Hodgkin lymphoma, ALL=acute lymphocytic leukemia.
Means for biomarkers of inflammation (absolute and natural log-transformed) and cognitive performance at both time points are shown in Table 2. Correlations among biomarkers of inflammation and among neurocognitive domains at each time point are in Supplemental Tables 2 and 3, respectively. Over time, there were significant increases in circulating levels of IL-6 and sTNF-RII and a significant decrease in CRP. There was no significant change in IL-1ra. For neurocognitive performance, TNP remained stable over time, in spite of the potential for repeat practice effects. Further probing of TNP revealed that all domains worsened (verbal fluency) or remained stable (verbal memory, visual memory, attention, and executive functioning). There was no significant change in self-reported (subjective) global cognition.
Table 2.
Biomarkers of inflammation and measures of cognitive performance, Means (SD) (N=85)
| Variables | Pre-HCT | 90 Days After HCT | p-value |
|---|---|---|---|
| Serum Biomarker Concentrationsa | |||
| IL-6 (pg/mL) | |||
| Absolute | 5.9 (12.7) | 6.4 (6.1) | |
| Log-transformed | 13 (.8) | 15 (.9) | 0.03 |
| sTNF-RII (pg/mL) | |||
| Absolute | 4510 (3401) | 5253 (2389) | |
| Log-transformed | 8.3 (.5) | 8.5 (.4) | <.0001 |
| CRP (mg/L) | |||
| Absolute | 7.9 (10.8) | 7.0 (12.6) | |
| Log-transformed | 1.3 (1.3) | 9 (1.5) | <.01 |
| IL-1ra (pg/mL) | |||
| Absolute | 632 (744) | 439 (204) | |
| Log-transformed | 6.1 (.7) | 6.0 (.5) | 0.35 |
| Cognitive Performance | |||
| Global cognition | 9.3 (3.1) | 9.5 (4.0) | 0.52 |
| TNPb | 47.4 (7.1) | 47.9 (7.7) | 0.79 |
| Verbal memory | 41.0 (11.3) | 42.2 (10.8) | 0.26 |
| Verbal fluency | 45.6 (11.4) | 43.8 (11.7) | 0.05 |
| Visual memory | 47.6 (11.0) | 49.3 (11.5) | 0.07 |
| Attention | 52.1 (7.4) | 51.7 (8.1) | 0.60 |
| Executive functioning | 51.0 (8.3) | 50.2 (9.4) | 0.40 |
Note: IL-1ra=Interleukin-1 receptor antagonist, IL-6=interleukin-6, sTNF-RII=soluble tumor necrosis factor receptor 2, CRP=C-reactive protein, TNP=total neuropsychological performance.
Serum concentrations of circulating biomarkers were natural log-transformed. Mean concentrations are shown in absolute values for ease of interpretation.
Objective cognitive performance domain scores are t-scores, which were averaged to generate the TNP.
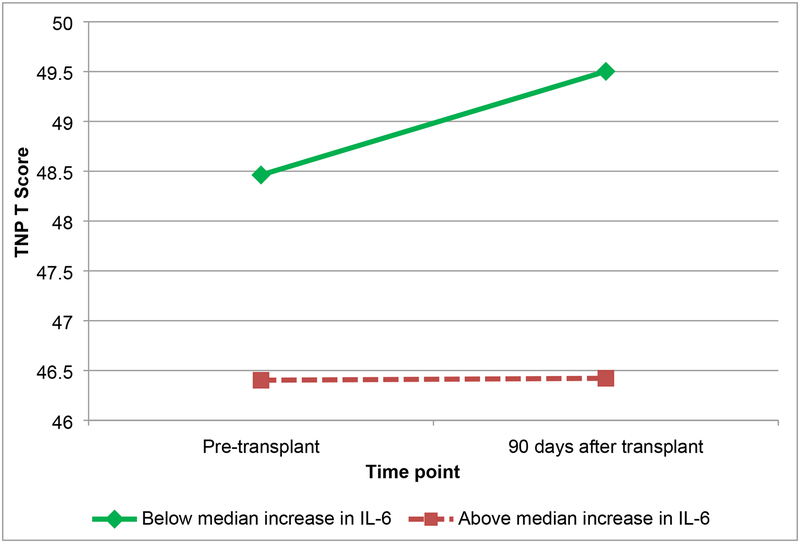
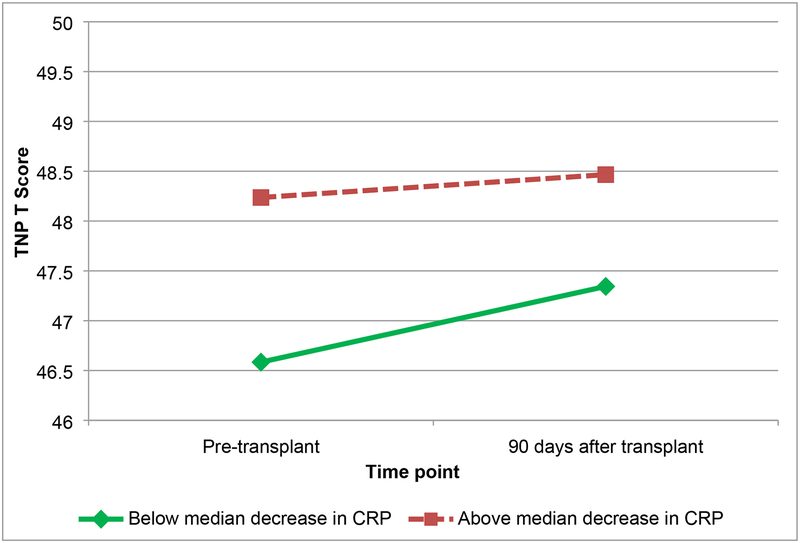
Results of the linear mixed models are shown in Table 3. Regarding TNP, while IL-6 increased on average for the entire sample, patients with greater increases in IL-6 demonstrated worse TNP that did not improve over time (Figure 1). Patients with greater increases in sTNF-RII demonstrated worsening TNP over time compared to patients with less increase in sTNF-RII (Figure 2). Patients with greater decreases in CRP demonstrated improvements in TNP relative to patients with smaller decreases in CRP (Figure 3). There were no significant associations between change in IL-1ra and change in TNP over time (p>.05), nor between any of the biomarkers of inflammation and subjective cognition over time (ps>.05).
Table 3.
Linear mixed model unstandardized parameter estimates for cognitive performance
| Biomarker of inflammation | Effect | Global cognition | TNP | Verbal memory | Verbal fluency | Visual memory | Attention | Executive functioning |
|---|---|---|---|---|---|---|---|---|
| IL-6 | Intercept | 8.76** | 48.52*** | 36.14*** | 41.50*** | 63.06*** | 56.74*** | 43.64*** |
| Time | 0.01 | 8.67* | 6.86 | 8.58 | 14.93* | 7.40^ | 2.80 | |
| Time*IL-6 | −0.15 | −1.73^ | −4.40** | −2.30 | −0.95 | −2.06^ | −1.92^ | |
| sTNF-RII | Intercept | 8.82** | 47.51*** | 35.71*** | 41 14*** | 62.01*** | 56.39*** | 42 73*** |
| Time | 0.37 | 8.05* | 8.12 | 10.51 | 14.30* | 6.84 | 2.47 | |
| Time*sTNF-RII | 0.88 | −3.85* | −5.12^ | −0.42 | −4.13 | −5.23** | −2.91 | |
| CRP | Intercept | 9.02 | 47.40*** | 34.37*** | 41.30*** | 61.12*** | 56.08*** | 42.92*** |
| Time | 0.34 | 7.29^ | 5.61 | 8.84 | 12.31^ | 7.23 | 2.00 | |
| Time*CRP | 0.08 | −1.10* | −2.00* | −1.34 | −1.91* | −0.63 | −0.68 | |
| IL-1ra | Intercept | 8.43** | 48.91*** | - | - | - | - | - |
| Time | 0.08 | 8.36* | - | - | - | - | - | |
| Time*IL-1ra | −0.01 | −0.53 | - | - | - | - | - |
Note: IL-6=interleukin-6, sTNF-RII=soluble tumor necrosis factor receptor 2, CRP=C-reactive protein, IL-1ra=Interleukin-1 receptor antagonist, TNP=total neuropsychological performance. Models adjusted for gender, mean–centered IQ, BMI, age, and each covariate*time interaction. Biomarkers of inflammation were log-transformed and mean–centered. The intercept represents the value of the outcome at 90 days after HCT. Longitudinal changes are modeled as time effects, and the association between each biomarker and time is examined using the time x biomarker interaction.
=p<.001
=p<.01
=p<.05
=p<.10
Figure 1.
Total neuropsychological (TNP) scores by change in interleukin-6 (IL-6) (below median increase, and above median increase) from pre-transplant to 90 days after transplant
Figure 2.
Total neuropsychological (TNP) scores by change in soluble tumor necrosis factor receptor 2 (sTNF-RII) (below median increase, and above median increase) from pre-transplant to 90 days after transplant
Figure 3.
Total neuropsychological (TNP) scores by change in c-reactive protein (CRP) (below median decrease, and above median decrease) from pre-transplant to 90 days after transplant
Based on findings of significant and trending relationships of changes in IL-6, sTNF-RII, and CRP with change in TNP, post hoc analyses were conducted to explore relationships of these biomarkers with the objective cognitive domains that comprise TNP. Results indicated that patients with greater increases in IL-6 demonstrated worse verbal memory and a trend toward worsening attention and executive functioning over time compared to patients with less increase in IL-6. Patients with greater increases in sTNF-RII demonstrated worse attention and a trend toward worsening verbal memory over time compared to patients with less increase in sTNF-RII. Patients with greater decreases in CRP demonstrated improvements in verbal memory and visual memory compared to patients with smaller decreases in CRP.
4. Discussion
This is the first study to our knowledge to examine relationships among changes in inflammation and changes in subjective and objective cognition during the acute transplant period in patients receiving allogeneic HCT. As hypothesized, increases in IL-6 and sTNF-RII from pre-HCT to 90 days after HCT were associated with declines in TNP, verbal memory, attention, and executive functioning. Similarly, decreases in CRP over time were associated with improvements in TNP, verbal memory, and visual memory. There were no associations between systemic inflammation and subjective cognition.
Existing literature indicates that patients receiving HCT often have higher levels of inflammation22,48–50 which increase from pre- to post-HCT.51 Elevated levels of inflammation in this population could be due to factors such as previous treatment, disease, transplant related factors (e.g., donor match), and post-HCT progression. Consistent with research examining pre-to post-treatment changes in other cancer samples not receiving HCT, there were increases in circulating levels of IL-628,34,52 and sTNF-RII23 while IL-1ra remained unchanged23,28,34 from pre-to post-transplantation. Conversely, CRP levels, which were very elevated, decreased post-HCT. We speculate that the reduction in CRP levels over time may have been due to a negative impact of HCT on liver function as levels of IL-6 and sTNF-RII (more direct measures of systemic inflammation) increased from pre- to post-HCT. With respect to neurocognitive deficits, worsening and/or stable performance on all neurocognitive domains was consistent with a 2013 meta-analysis6 of patients receiving HCT indicating a lack of expected improvements over time on neurocognitive tests due to repeated testing (i.e., practice effects).
Few studies have examined changes in both inflammation and cognition from pre-to post-treatment, but in this study there were robust patterns of association between increasing inflammation (IL-6 and sTNF-RII) and worsening neurocognitive performance. There were significant associations between IL-6, sTNF-RII, and/or CRP within every neurocognitive domain, with the most robust relationships observed for TNP and verbal memory. Existing literature suggests that patients receiving allogeneic HCT may be particularly vulnerable to deficits in verbal memory,2,7 and our data show a clear and consistent association between higher levels of systemic inflammation and verbal memory deficits.
Subjective reports of cognitive deficits are common after receiving HCT,53 but patients receiving HCT may not perceive changes in cognition from pre-to post-HCT.54 In this study, patients receiving HCT did not perceive worsening cognition during the acute transplant period. Further, in contrast to previous studies with mostly breast cancer patients,23,30,31,34 changes in inflammation were not associated with changes in subjective cognition from pre- to post-HCT. Cognitive deficits may be less noticeable due to other factors such as morbidities throughout the acute transplant period. Moreover, a large body of research indicates that subjective cognition is not significantly correlated with objective cognition in cancer patients.55
This study had several strengths, including the focus on allogeneic HCT, the inclusion of both subjective and objective measures of cognitive performance, and a longitudinal study design. Limitations include a relatively homogenous sample in terms of race and ethnicity (predominantly white and non-Hispanic) and a high level of education that limits generalizability of findings to less educated patients. Thus, additional research is needed to confirm our results in more heterogeneous samples. Despite these limitations, these results extend our understanding of the role of systemic inflammation and neurocognitive impairment to HCT recipients. Future research on inflammation and cognition in this population should include a comparison group, larger samples, and interventions to preserve or improve neurocognitive performance in patients with higher inflammation during the transplant period. Existing nonpharmacological efforts including cognitive behavioral therapy delivered via videoconferencing56 and computerized training programs57,58 have demonstrated preliminary success in patients with breast cancer, but these interventions have not been implemented with patients being treated with HCT. In addition, regular exercise decreases inflammation59 and improves neurocognitive function60 in non-cancer populations and may be a useful intervention target for patients undergoing allogeneic HCT. In summary, inflammation may be a mechanism by which cognitive deficits occur in allogeneic transplant patients. Further research is needed to improve supportive care to reduce inflammation and improve cognition during the survivorship period as patients prepare to return to the cognitive demands of their daily lives.
Supplementary Material
Highlights.
In acute transplant period, inflammation and neurocognitive deficits increased
Over time, increasing inflammation and worsening neurocognition robustly associated
No association with systemic inflammation and subjective cognition
Acknowledgements:
The authors would like to thank Mr. Christian Perez for his technical support and performance of biomarker immunoassays.
Funding: This work was supported by the National Cancer Institute (K07-CA138499, R25-CA090314). This work was also supported by the UCLA Norman Cousins Center for Psychoneuroimmunology, and the Biostatistics Core and the Survey Methods Core at the H. Lee Moffitt Cancer Center & Research Institute, a National Cancer Institute-designated Comprehensive Cancer Center (NIH/NCI Grant Number: P30-CA076292).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: HJ: Consultant: RedHill Biopharma, Janssen Scientific Affairs
Disclaimer: The opinions expressed are the authors’ and do not represent those of the National Cancer Institute.
References
- 1.Harder H, Van Gool AR, Duivenvoorden HJ, et al. Case-referent comparison of cognitive functions in patients receiving haematopoietic stem-cell transplantation for haematological malignancies: two-year follow-up results. Eur J Cancer. 2007;43(14):2052–2059. [DOI] [PubMed] [Google Scholar]
- 2.Sharafeldin N, Bosworth A, Patel SK, et al. Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: Results from a prospective longitudinal study. J Clin Oncol. 2018;36(5):463–475. [DOI] [PubMed] [Google Scholar]
- 3.Scherwath A, Schirmer L, Kruse M, et al. Cognitive functioning in allogeneic hematopoietic stem cell transplantation recipients and its medical correlates: a prospective multicenter study. Psychooncology. 2013;22(7):1509–1516. [DOI] [PubMed] [Google Scholar]
- 4.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104(10):3386–3392. [DOI] [PubMed] [Google Scholar]
- 5.Schulz-Kindermann F, Mehnert A, Scherwath A, et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;39(12):789–799. [DOI] [PubMed] [Google Scholar]
- 6.Phillips KM, McGinty HL, Cessna J, et al. A systematic review and meta-analysis of changes in cognitive functioning in adults undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(10):1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones D, Vichaya EG, Wang XS, Sailors MH, Cleeland CS, Wefel JS. Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer. 2013;119(23):4188–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman MA, Fernandez M, Wefel JS, Myszka KA, Champlin RE, Meyers CA. Course of cognitive decline in hematopoietic stem cell transplantation: a within-subjects design. Arch Clin Neuropsychol. 2009;24(7):689–698. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SR, Small BJ, Booth-Jones M, Jacobsen PB, Fields KK. Changes in cognitive functioning in the year after hematopoietic stem cell transplantation. Cancer. 2007;110(7):1560–1567. [DOI] [PubMed] [Google Scholar]
- 11.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transplant. 2017;23(4):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoogland AI, Nelson AM, Small BJ, et al. The role of age in neurocognitive functioning among adult allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2017;23(11):1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchbinder D, Kelly DL, Duarte RF, et al. Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the Late Effects and Quality of Life Working Committee of the CIBMTR and Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transplant. 2018;53(5):535–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366. [DOI] [PubMed] [Google Scholar]
- 16.Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9(2):113–128. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez PV, Schioth HB, Lasaga M, Scimonelli TN. Memory impairment induced by IL-1beta is reversed by alpha-MSH through central melanocortin-4 receptors. Brain Behav Immun. 2009;23(6):817–822. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Yamaguchi T, Watanabe S, Yamamoto T. Involvement of arachidonic acid cascade in working memory impairment induced by interleukin–1 beta. Neuropharmacology. 2004;46(8):1195–1200. [DOI] [PubMed] [Google Scholar]
- 19.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94(4):1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore M, Angelucci F, Alleva E, Branchi I, Probert L, Aloe L. Learning performances, brain NGF distribution and NPY levels in transgenic mice expressing TNF–alpha. Behav Brain Res. 2000;112(1–2):165–175. [DOI] [PubMed] [Google Scholar]
- 21.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. [DOI] [PubMed] [Google Scholar]
- 22.Copelan EA. Hematopoietic stem–cell transplantation. N Engl J Med. 2006;354(17):1813–1826. [DOI] [PubMed] [Google Scholar]
- 23.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor–alpha (TNF–alpha) play a role in post–chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30 Suppl:S99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel SK, Wong AL, Wong FL, et al. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J Natl Cancer Inst. 2015;107(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amidi A, Agerbaek M, Wu LM, et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11(3):769–783. [DOI] [PubMed] [Google Scholar]
- 27.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin–6 and tumor necrosis factor-alpha levels in chemotherapy–treated breast cancer survivors. Brain Behav Immun. 2013;30 Suppl(0):S109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol. 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. [DOI] [PubMed] [Google Scholar]
- 30.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7(4):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist. 2013;18(9):1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starkweather A, Kelly DL, Thacker L, Wright ML, Jackson–Cook CK, Lyon DE. Relationships among psychoneurological symptoms and levels of C–reactive protein over 2 years in women with early–stage breast cancer. Support Care Cancer. 2017;25(1):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy–associated cognitive impairment in breast cancer patients: a multi–centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 37.The Psychological Corporation. Wechsler Test of Adult Reading. San Antonio TX: Harcourt Assessment; 2001. [Google Scholar]
- 38.Brandt J The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5(2):125–142. [Google Scholar]
- 39.Benton A, Hamsher Kd, Sivan A. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates. In: Inc; 1989. [Google Scholar]
- 40.Benedict RH. Brief visuospatial memory test--revised: professional manual. PAR; 1997. [Google Scholar]
- 41.Wechsler D WAIS–III: Administration and scoring manual: Wechsler adult intelligence scale. Psychological Corporation; 1997. [Google Scholar]
- 42.Maj M, D’Elia L, Satz P, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV–1 seropositive persons: A WHO study. Arch Clin Neuropsychol. 1993;8(2):123–135. [PubMed] [Google Scholar]
- 43.Trenerry MR, Crosson B, DeBoe J, Leber W. Stroop neuropsychological screening test In. Psychological Assessment Resources. Odessa, FL1989. [Google Scholar]
- 44.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23(4):323–355. [Google Scholar]
- 46.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- 47.O’Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C–reactive protein is a predictor for outcomes after reduced–intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(11):1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aki SZ, Suyani E, Bildaci Y, Cakar MK, Baysal NA, Sucak GT. Prognostic role of pre-transplantation serum C-reactive protein levels in patients with acute leukemia undergoing myeloablative allogeneic stem cell transplantation. Clin Transplant. 2012;26(5):E513–521. [DOI] [PubMed] [Google Scholar]
- 50.Tvedt TH, Lie SA, Reikvam H, et al. Pretransplant Levels of CRP and Interleukin-6 Family Cytokines; Effects on Outcome after Allogeneic Stem Cell Transplantation. Int J Mol Sci. 2016;17(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SW, Kitko CL, Braun T, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112(4):1539–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Booth-Jones M, Jacobsen PB, Ransom S, Soety E. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant. 2005;36(8):695–702. [DOI] [PubMed] [Google Scholar]
- 54.Ghazikhanian SE, Dorfman CS, Somers TJ, et al. Cognitive problems following hematopoietic stem cell transplant: relationships with sleep, depression and fatigue. Bone Marrow Transplant. 2017;52(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat Rev. 2012;38(7):926–934. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122(11):1782–1791. [DOI] [PubMed] [Google Scholar]
- 57.Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy–treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kesler SR, Wefel JS . Targeted treatment for cognitive impairment associated with cancer and cancer treatment In: Keefe RSE, Reichenberg A, Cummings J, eds. Cognitive Enhancement in CNS Disorders and Beyond. Oxford University Press; 2017. [Google Scholar]
- 59.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29(2):381–393. [DOI] [PubMed] [Google Scholar]
- 60.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.