Abstract
Cytomegalovirus (CMV) and psychological stress are implicated as drivers of immunological aging. It is unknown, however, whether associations among CMV titers, stress, and immune aging are more stable or dynamic over time. The present investigation tested the between-person (stable differences) and within-person (dynamic fluctuations) associations of CMV titers and perceived stress on late-differentiated T and natural killer (NK) peripheral blood cells in a longitudinal study of older adults aged 64–92 years (N=149). Participants reported stress levels and provided blood biannually for 2.5 years (up to 5 waves per person) to assess CMV IgG titers and composites of late-differentiated CD8 T cells (CD28- and CD57+ subsets) and CD56dim NK cells (CD57+, NKG2C+, and FcεRIγ- subsets). In multilevel models that controlled for demographic variables, higher CMV titers were associated with higher proportions and counts of aged T and NK cells between people and lower counts of aged T cells within people. Perceived stress was associated with higher counts of aged T cells between people, but was not associated with aged NK cells. A significant interaction between stress and CMV titers on T cells between people indicated that older adults with lower stress levels and lower CMV titers had the lowest proportions of late-differentiated T cells, whereas those with higher stress levels had high proportions, regardless of CMV control. Our results provide evidence for longer-term, between-person associations among CMV titers, stress, and immunological aging, rather than dynamic within-person associations. We propose that targeting factors that promote low, stable perceived stress in older adults may retard T cell differentiation and ultimately support healthy aging.
Keywords: Cytomegalovirus, aging, longitudinal, psychological stress, immunosenescence
1. Introduction
Innate and adaptive immunity decline with normal aging and these changes are associated with increased morbidity and mortality in older adults (Franceschi et al., 2018; Pera et al., 2015; Pawelec 2017). Markers associated with immune aging include the progressive accumulation of late-differentiated CD8 T cells that lose co-stimulatory molecule CD28 and acquire maturation marker CD57 (Appay et al., 2008; Effros et al., 2005; Vallejo, 2005). Late-differentiated peripheral blood T cells are considered to be senescent or near senescent cells that are functional and cytotoxic, but generally have reduced proliferative capacity (except under certain stimulation conditions) (Griffin, Michel, Vallejo, 2018; Strioga, Pasukoniene, & Characiejus, 2011). Additionally, the proportion of CD56dim natural killer (NK) cells increases with age (Lutz et al., 2011) and acquire CD57, marking reduced proliferative capacity but high cytotoxicity, and a late stage of NK cell maturation (Björkström et al., 2010; Lopez-Vergès et al., 2010).
Cytomegalovirus (CMV) is associated with the accumulation of markers indicative of immune aging, including late-differentiated T cells (CD8+CD28− and CD8+CD57+) (Derhovanessian et al. 2011; Weritheimer et al., 2014) and “memory-like” NK cells that express CD57, activating receptor NKG2C, or lack the adaptor protein FcεRIγ (Lee et al., 2015; Muntasell et al., 2016; Solana et al., 2014; Zhang et al., 2013). Furthermore, CMV may have divergent effects on the functionality of these late-differentiated T cells and NK cells (e.g., Bigley et al., 2016). CMV is a highly prevalent betaherpes virus that infects 30–90% of the population – an effect that increases linearly with age (Staras et al., 2006). Once infected with CMV, a person is unable to eliminate the virus and establishes a life-long latency. Therefore, in an older adult sample in which the majority are likely CMV-positive, an important consideration is not only CMV serostatus but also viral control.
In immunocompetent individuals, persistent CMV infection typically remains subclinical and well-controlled. However, psychological stress can reactivate latent herpes viruses, resulting in higher virus-specific IgG antibody titers (Glaser et al., 1991; Rector et al., 2014). Therefore, stress may act synergistically with CMV titers to affect late-differentiated immune cell populations. In addition, stress and related psychological factors may act directly on number and or function of late-differentiated CD8+ T cells (CD57+ and CD28−) and CD56dim NK cells (CD57+) through various pathways including, for example, neuroendocrine factors (Amati et al., 2010; Bauer et al., 2015; Bosch, Fischer, & Fischer, 2009; Duggal et al., 2014; Segerstrom, Al-Attar, & Lutz, 2012).
It is unknown how stable or dynamic associations are among CMV titers, psychological stress, and late-differentiated immune cells. The aim of the current study was to examine the main and interactive effects of between-person differences and within-person fluctuations in CMV titers and perceived stress on late-differentiated T and NK cells in a community sample of older adults, assessed biannually over 2.5 years (i.e., up to 5 time points, or waves, per person). Focusing on older adults is relevant because control of persistent pathogens like CMV may weaken with age, resulting in reactivation and disease (Stowe et al., 2007; Thomasini et al., 2017), and because stress in older adulthood may exacerbate an already weakened immune system due to aging (Butcher & Lord, 2004; Graham, Christian, Kiecolt-Glaser, 2006). We hypothesized that CMV titers and perceived stress would be directly associated with higher levels of aged T and NK cells between and within people. We also hypothesized an interactive effect such that CMV titers would be positively associated with late-differentiated T and NK cells between and within people, and this effect would be particularly pronounced for older adults with high levels of stress. Models were adjusted for laboratory and demographic variables. In addition to concurrent models, we also explored time-lagged analyses of significant within-person effects to examine whether CMV titers or stress six months prior are associated with current levels of aged T and NK cells.
2. Materials and methods
2.1. Participants
Participants were 149 community-dwelling older adults. The sample was on average 77 years old at the first wave (range = 64–92 years). The majority of the sample was female (58%), white (94%), and the remainder was African American (4%) and Asian American/Pacific Islander (2%). The gender distribution of our sample is consistent with the older adult population in the area (56.1% female aged 65 years and older), but our sample has a slightly higher percentage of white and lower percentage of African American participants than the area (87.3% white, 8% African American) (American Fact Finder, 2019). Median household income was US$60,000 (range: $9,000 - $400,000) and median education was 16 years (range: 9–22).
2.2. Procedures
Participants were recruited from a volunteer subject pool maintained by the University of Kentucky Sanders-Brown Center on Aging. All procedures were approved by the University of Kentucky Institutional Review Board. Prospective participants were contacted and screened by telephone. Those who were interested and eligible were enrolled. Exclusion criteria included diseases or disorders affecting the immune system including autoimmune diseases (e.g., Grave’s disease, rheumatoid arthritis, type I diabetes), cancers (e.g., melanoma, lymphoma, breast cancer), immunosuppressive disorders (e.g., HIV, immunoglobulin deficiency), or chronic, severe infections (e.g., hepatitis); chemotherapy or radiation treatment in the 5 years prior to enrollment; unwillingness to undergo venipuncture; immunomodulatory medications including opioids and steroids; or more than two of the following classes of medications: psychotropics, anti-hypertensives, hormone replacement, or thyroid supplements. These criteria excluded major influences on immunity and allowed reasonably healthy adults to participate in this study.
Participants were interviewed in their homes every 6 months for up to 2.5 years (up to 5 waves) between July 2011 and December 2016. Scales were verbally administered with the assistance of response cards. After each interview, study nurses drew blood by venipuncture in the morning hours to control for potential circadian variation. Participants received US$50 for their participation at each wave.
2.3. Measures
2.3.1. Perceived psychological stress.
Participants completed the Perceived Stress Scale (PSS; Cohen et al., 1983) to assess levels of psychological stress. This 10-item measure is widely used for assessing global stress perceptions over the past month. An example item includes: “In the past month, how often have you felt that you were unable to control the important things in your life?”; participants rate items on a 0 (never) to 4 (very often) Likert scale. Higher values indicate higher levels of perceived stress. PROC VARCOMP in SAS was used to estimate the variances associated with items, waves, people, and their interactions to calculate variance components to estimate reliability (equations #4 and #5 from Cranford et al., 2006). The measure was reliable both over people (α= .98) and over time (i.e., within people, α= .75).
2.3.2. CMV IgG titers.
CMV antibody titers were determined by ELISA (DRG International, Inc., Springfield, NJ) according to manufacturer specifications. The dynamic range of the kit is 1.2–18 IU/mL; initial runs were diluted 40-fold. Samples that were above 18 IU/mL were further diluted (80- and 160-fold) and re-run (n=11 samples). Additionally, n=161 person-wave CMV observations were extrapolated using a power curve; extrapolation was accounted for in analyses involving CMV titers using a categorical variable (0=not extrapolated, 1= extrapolated). The sensitivity, specificity, and accuracy of the test were 100%, 98%, and 99%, respectively. The intra-assay and inter-assay coefficients of variability were 5.1% and 9.9%. CMV IgG Index values of 1.0 or greater were considered seropositive (n = 106/149, 71% of the sample).
2.3.3. T and NK cell subsets.
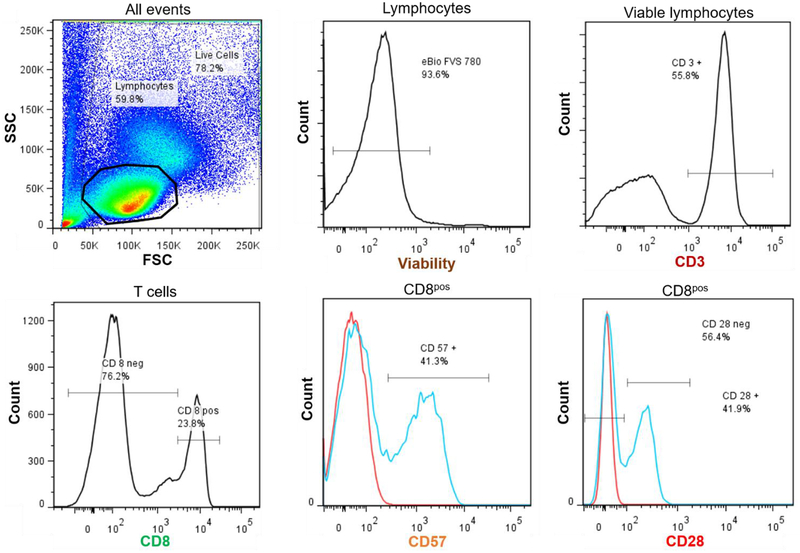
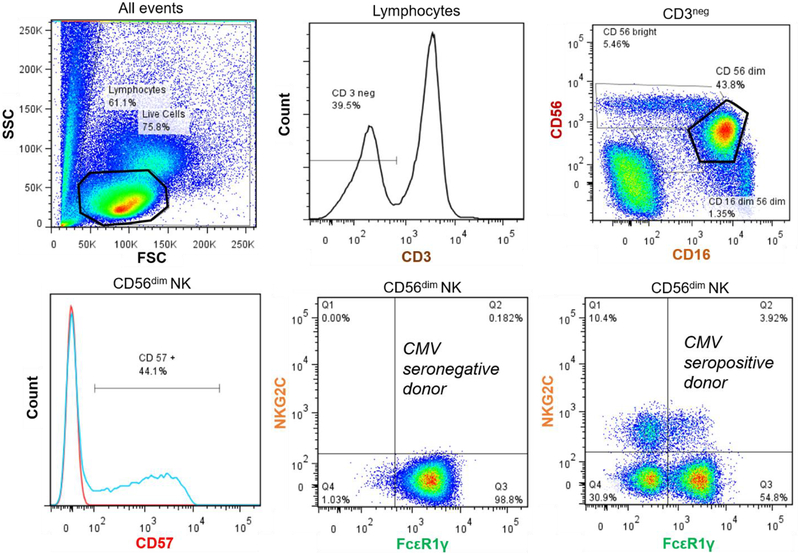
Selected panels of immunological markers of late-differentiated T and NK cells were evaluated by flow cytometry as previously described (Reed et al., 2018). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved. Thawed PBMCs were washed, counted, and treated with fixable viability stain for 30 minutes at room temperature. Cells were then washed with PBS + 10% FBS and incubated with human IgG for 15 min on ice to block surface Fc receptors. Next, samples were stained on ice for 30 min with combinations of fluorescently labelled monoclonal antibodies specific for surface proteins (see Supplemental Table 1 for clones and suppliers), washed, and then fixed. For some samples, intracellular staining was performed by first permeabilizing the cells with 1x permeabilization buffer (eBioscience, San Diego, CA) and then staining with antibodies for 20 min at room temperature. Percentages of lymphocytes of PBMCs were determined; T cells were defined as CD3+ cells and NK cells were defined as CD3-CD56+CD16+ within the lymphocyte population. Surface proteins on T cells were identified with CD8, CD28, and CD57. Surface and intracellular proteins on NK cells were identified with CD57, NKG2C, and FcεRIγ (intracellular). Other antibodies that are not the focus of this investigation but were included in the T and NK cell staining tubes were CD4, KIR, and CD14 (see Supplemental Table 1). Cells were analyzed on a LSR-II flow cytometer (BD, Franklin Lakes, NJ). Spectral overlap was electronically compensated for using OneComp ebeads (eBioscience) stained with individual fluorochorme-conjugated antibodies. Data were analyzed using FlowJo v7.6 software. Illustrative plots of the analyzed T and NK cell subsets are shown in Figures 1 and 2.
Figure 1.
Illustrative plots of analyzed T cell populations. Lymphocytes were identified on the forward scatter/side scatter plot and calculated as a percentage of live cells. Viable lymphocytes (eBio viability stain) were identified. CD3+ cells were selected for and further identified as CD8+ cells. Proportions of CD8+ T cells expressing CD57+ and CD28− were calculated.
Figure 2.
Illustrative plots of analyzed NK cell populations. Lymphocytes were identified on the forward scatter/side scatter plot and calculated as a percentage of live cells. Viable CD3- cells were selected for and further identified as CD56dim NK cells (low to moderate CD56 expression and high CD16 expression). Percentages of CD56dim cells expressing CD57+ were calculated. In addition, the CD56dim cells were divided into quadrants according to their NKG2C and FcεR1γ expression (quadrants Q1–4). Cells (%) expressing NKG2C+ were calculated by summing quadrants Q1 (FcεR1γ− NKG2C+) and Q2 (FcεR1γ+ NKG2C+). Cells (%) expressing FcεR1γ− were calculated by summing quadrants Q1 (NKG2C+ FcεR1γ−) and Q4 (and NKG2C- FcεR1γ-). Expression of NKG2C and FcεR1γ is displayed for a representative CMV seronegative donor and CMV seropositive donor.
Composites of aged T and NK cells (expressed in both proportions and counts) were identified by correlational analyses previously described (Reed et al., 2018). Immune cell composites were used instead of individual subsets for several reasons: (1) the individual T and NK cell subsets were significantly correlated within cell compartments (see below for details) and so likely index similar effects; (2) composite variables can provide more robust descriptive power than single variables alone (Song et al., 2013); and (3) using two reliable composites (T and NK cell composites) instead of the five individual subsets decreases the number of statistical tests run and the probability of committing Type I error (Song et al., 2013). The T cell composite included the mean proportions or cell counts of CD3+CD8+CD28− and CD3+CD8+CD57+ cells (individual subsets correlated r=.94 – .98, p values < .0001 for proportion and counts; composite α = .96 for proportions, α = .98 for counts, across all participants and observations). The T cell composite proportion is expressed as a percentage of total CD8+ cells. The NK cell composite included the mean proportions or cell counts of CD57+, NKG2C+, and FcεRIγ- markers on CD3-CD16+CD56dim cells (individual subsets correlated r=.29- .58, p values < .0006 for proportions, and r=.54- .72, p values < .0001 for counts; α= .67 for proportions, α = .69 for counts, across all participants and observations). The NK cell composite proportion is expressed as a percentage of total CD56dim cells. Cell counts (per μL) were obtained by multiplying total leukocyte count via hemocytometer by the percentages of gated lymphocytes. A natural log transformation was applied to the T cell counts composite and the NK cell proportions and counts composites to improve normality (see Table 1 for descriptive statistics). Two laboratory scientists completed the standardized staining and flow cytometry protocol and a categorical variable was included in all models to control for any inter-individual differences (1= scientist #1; 2 = scientist #2).
Table 1.
Descriptive information for study variables
| Entire Sample (N=149) | CMV+ sub-sample (n=106) | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | ICC | Mean (SD) | Range | ICC | |
| Age at first wave | 77.8 (5.4) | 64.7 – 92.8 | -- | 78.0 (5.2) | 64.7 – 92.8 | -- |
| Gender (reference = males) | 42% | 1 – 2 | -- | 40% | 1 – 2 | -- |
| Education (years) | 16.5 (2.5) | 9 – 22 | -- | 16.3 (2.6) | 9 – 22 | -- |
| Income (USD) | 80,040 (60,140) | 9,000 – 400,000 | -- | 76,229 (53,877) | 9,000 – 350,000 | -- |
| Perceived Stress | ||||||
| Overall mean | 10.6 (5.4) | 1.6 – 30.2 | 0.66 | 11.2 (5.7) | 1.6 – 30.2 | 0.72 |
| Between-person version | 0 (5.4) | −9.0 – 19.6 | 0 (5.7) | −9.6 – 19.0 | ||
| Within-person version | 0 (3.2) | −9.8 – 21.8 | 0 (3.0) | −9 .8 – 13.8 | ||
| CMV titers (IU/mL) | ||||||
| Overall mean | 19.2 (21.9) | 0.12 – 111.4 | 0.66 | 26.5 (21.8) | 1.5 – 111.4 | 0.56 |
| Between person version | 0 (21.9) | −19.1 – 92.2 | -- | 0 (21.8) | −25.0 – 84.9 | -- |
| Within-person version | 0 (13.3) | −44.7 – 117.9 | -- | 0 (15.6) | −44.7 – 117.9 | -- |
| Immune Composite (%) | ||||||
| T cell composite | 52.5 (21.5) | 2.2 – 91.3 | 0.93 | 59.4 (17.9) | 11.4 – 91.3 | 0.91 |
| NK cell composite | 8.9 (11.9) | 0.3 – 78.0 | -- | 11.5 (12.8) | 0.6 – 78.0 | -- |
| ln (NK cell composite) | 1.4 (1.3) | −1.2 – 4.4 | 0.95 | 1.8 (1.2) | −0.5 – 4.4 | 0.97 |
| Immune Composite (cells/μL) | ||||||
| T cell composite | 107.0 (110.1) | 1.3 – 698.4 | -- | 135.0 (115.2) | 3.3 – 698.4 | -- |
| ln (T cell composite) | 4.1 (1.2) | 0.2 – 6.6 | 0.87 | 4.5 (1.0) | 1.2 – 6.6 | 0.81 |
| NK cell composite | 14.2 (27.0) | 0.2 – 331.2 | -- | 18.7 (30.2) | 0.2 – 331.2 | -- |
| ln (NK cell composite) | 1.5 (1.6) | −1.9 – 5.8 | 0.72 | 2.0 (1.5) | −1.9 – 5.8 | 0.72 |
Note. Means, standard deviations (SD), observed ranges (min-max), and intraclass correlation coefficients (ICCs) presented for all study variables. Natural log transformed variables used in analyses indicated by ln ( ).
2.3.4. Covariates.
Three covariates (in addition to CMV extrapolation and laboratory scientist) were selected that could account for extraneous variance in immunological aging without overcontrolling or compromising degrees of freedom (Segerstrom, 2009): time (entered as wave number, centered at the first wave), age at first wave (centered around the grand mean, 77 years), and gender (reference is males). Age was calculated as the difference in years between date of birth and the first interview date. Gender was self-reported at study entry. Including time and age in the models adjusted for within-person changes over time and between-person age differences in T and NK cell subsets (Apoil et al., 2017; Campos et al., 2014; Wertheimer et al., 2014). Including gender in the models adjusted for differences in immune subsets between males and females (Al-Attar et al., 2016; Wikby et al., 2008). An additional set of models further adjusted for education and income due to known relationships among socioeconomic status, CMV, and late-differentiated immune cells (Aiello et al., 2016; Dowd & Aiello, 2009). Missing income data (n=17, 11%) were imputed using multiple imputation and the expectation-maximization algorithm in R software using the Amelia function.
2.4. Data Analysis
Multilevel models with repeated immune assessments (Level 1) within person (Level 2) were used to accommodate missing data and utilize all available data without the need for either list-wise deletion or data imputation (Singer & Willett, 2003). Data were analyzed using the lme (linear mixed-effects) function from the nlme library (version 3.1.118) in R (version 3.0.3). Models were estimated using maximum likelihood estimation and included a random intercept to account for individual differences in proportions and counts of T and NK cell composites.
To specify the stable and dynamic effects of CMV and perceived stress on immune outcomes, CMV titers and stress were each partitioned into two orthogonal terms (Hoffman & Stawaski, 2009): an average score per person across all waves (centered around the grand mean), which captured between-person differences, and a deviation from each person’s average score at each wave, which captured within-person variance. For example, the between-person version of perceived stress was computed by averaging each person’s stress levels across all of their waves and then subtracting the sample mean from each person’s mean (i.e., , where is each person j’s mean stress level across all of their waves, and is the sample mean stress level); the within-person version was computed by subtracting each person’s average stress level (calculated across all of their waves) from their stress level at each wave (i.e., where xij is person j’s stress level at time i and is person j’s stress level across all of their waves). The same calculations were performed to create between- and within-person versions of CMV titers.
Separate models tested main and interacting fixed effects of between- and within-person CMV titers and perceived stress on the proportions and counts of T and NK cell composites. The within-person versions of CMV and perceived stress were also tested as random effects and included in the model when they significantly improved model fit by log likelihood comparisons. All models controlled for lab scientist and CMV extrapolation, and further adjusted models included time, age at first wave, gender, income, and education as fixed effects. In adjusted models, a random effect of time was also tested and included when it significantly improved model fit by log likelihood comparisons. Following methods outlined by Aiken and West (1991), significant interactions were probed at low (−1 SD) and high (+1 SD) levels of CMV titers and perceived stress. Fixed effects are reported as unstandardized γ weights, which can be interpreted in the same manner as unstandardized B coefficients in regression. To provide a sense of relative magnitude of effects, we also report standardized betas for the simple slopes involved in significant interactions. Although we focused on immune composites, results from individual immune subsets may be of particular interest to readers and are presented in Supplemental Table 10 and Supplemental Figure 1A–F. Analyses were conducted in the entire sample (N=149) as well as in the subset of CMV-positive adults (n=106) to enable a discussion regarding viral control. Additional post-hoc analyses were conducted in the CMV-negative adults (n=43) to test the main effect of stress.
Last, the primary analyses tested concurrent associations among CMV, stress, and late-differentiated immune cells. Additional exploratory analyses examined time-lagged effects of significant within-person associations. These analyses addressed whether within-person deviations in CMV titers or stress from six months prior relate to current levels of T and NK cell subsets, controlling for concurrent within-person deviations.
3. Results
Table 1 presents descriptive information for all study variables. Full multilevel model results are presented in supplemental material.
3.1. Available Immune Data
Of the 149 participants, 139 had cryopreserved cells stored for one or more waves; 69 participants had valid data at wave 1, 66 at wave 2, 107 at wave 3, 117 at wave 4, and 109 at wave 5. Of the possible 149 (people)*5 (waves) = 745 observations, missing data were present because 36 people missed one or more blood draw appointments or had problems with blood withdrawal (47 person-waves missing), 23 people dropped out (57 person-waves missing), 3 people died (6 person-waves missing), and 3 people did not allow their blood to be drawn (9 person-waves missing). Additionally, cells for flow cytometry analyses were collected and cryopreserved beginning in November 2012; therefore 76 participants did not have cells available from earlier waves for analysis (158 person-waves missing). The lower number of observations at waves 1 and 2 are attributable to this mechanism; these missing data are missing completely at random and thus do not bias the parameter estimates (Singer & Willet, 2003). Overall, 468 person-waves were available for analysis. Of the 139 participants with cryopreserved cells, participants had on average 3.4 available immune data points (SD = 1.26, median= 3, range= 1–5). The number of available immune data points did not correlate with mean CMV titers (r=−.05, p =.51), mean levels of perceived stress (r=−.10, p=.24), age at the first wave (r=.09, p=.26), income (r=−.10, p =.24), education (r=.00, p =.97), nor did it differ across gender (t(143)=.36, p=.72).
3.2. Results in the entire sample
3.2.1. T cell composite.
There were main effects of CMV titers and perceived stress1 on the T cell composite, which is the mean expression of CD8+CD28− and CD8+CD57+ (calculated for both proportions and counts, as described in Materials and Methods). Between people, higher CMV titers across all waves were associated with a higher proportion (γ= 0.32, SE= 0.077, t(136)= 4.13, p=.0001; see Supplemental Figure 2A) and counts (γ= 0.021, SE= 0.004, t(136)= 4.96, p <.0001; see Supplemental Figure 2B) of the late-differentiated T cell composite. Covariate adjustments did not alter these effects (p values <.0001). Additionally, within people, at waves when a person had higher than average CMV titers, he or she also had lower counts of the late-differentiated T cell composite (γ= −0.003, SE=0.001, t(307)=−2.42, p=.016; with covariates p=.031; see Supplemental Figure 2C). An exploratory model examined the lagged effect of this within-person association, which was not significant (γ= .00001, SE=0.001, t(226)=0.006, p=.99; with covariates p=.56); thus, a person’s deviation in their CMV titers at a previous wave did not predict lower T cell composite counts at the following wave. The within-person effect was only significant concurrently in time. Between people, higher perceived stress was associated with higher T cell composite counts (γ= 0.037, SE= 0.017, t(137)= 2.14, p=.034; with covariates p=.034; see Supplemental Figure 2D) and was nearly statistically significant for the T cell composite proportion (γ= 0.57, SE= 0.32, t(137)= 1.82, p=.071; with covariates p=.051; see Supplemental Figure 2E).
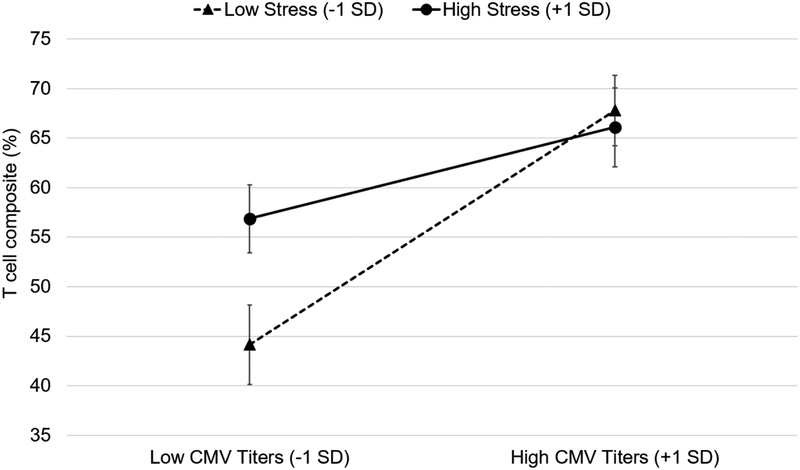
There was a significant, between-person interaction of CMV titers and stress on the T cell composite proportion (γ= −0.028, SE= 0.014, t(134)= −2.03, p= .045; with covariates p= .025). As shown in Figure 3, older adults with low stress and low CMV titers had the lowest proportions of late-differentiated T cells (estimate: 44%), significantly lower than older adults with low stress and high CMV titers (estimate: 68%, simple main effect: γ =0.54, Beta =0.55, SE=.12, p<.0001) and older adults with high stress and low CMV titers (estimate: 57%, γ =1.18, SE=0.45, p=.010). Older adults with high stress and high CMV titers had significantly higher proportions of late-differentiated T cells (estimate: 66%) than older adults with high stress and low CMV titers (simple main effect: γ =0.21, Beta =0.21, SE=.095, p=.029).
Figure 3.
Simple slopes depicting the between-person differences in cytomegalovirus (CMV) titers and perceived stress on T cell composite (%) in the entire sample. Low CMV titers and low stress are 1 standard deviation (SD) units below the grand means; high CMV titers and high stress are 1 SD units above. Error bars represent standard errors of the estimates. The model was adjusted for lab scientist, CMV extrapolation, time, age at first wave, gender, income, and education.
3.2.2. NK cell composite.
The NK cell composite is the mean expression of CD57+, NKG2C+, and FcεRIγ- markers on CD3-CD16+CD56dim cells (calculated for both proportions and cell counts, as described in Materials and Methods). Between people, higher CMV titers across all waves were associated with a higher proportion of late-differentiated NK cells (γ= 0.003, SE= 0.001, t(132)= 2.13, p =.035; with covariates p=.012; see Supplemental Figure 2F). Perceived stress was not significantly associated with the NK cell composite either within or between people. Additionally, there were no significant interactions between CMV titers and perceived stress on the NK cell composite.
3.3. Results in the subset of CMV-positive older adults
3.3.1. T cell composite.
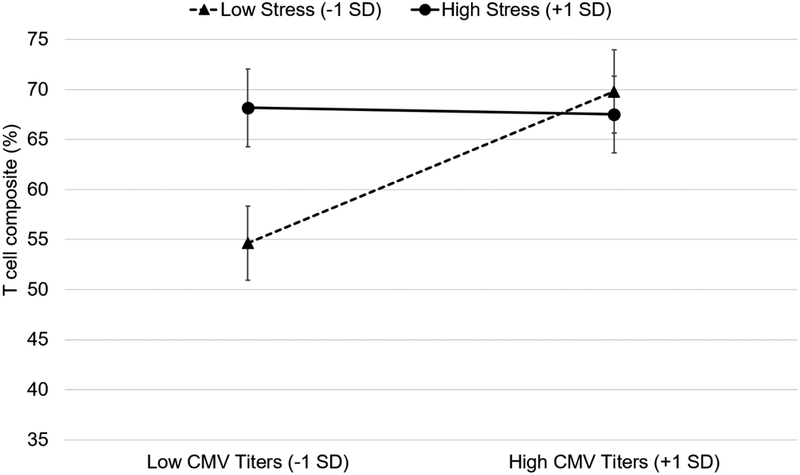
Between people, poorer CMV control (i.e., higher CMV titers among CMV-positive older adults) across all waves was associated with higher counts of the late-differentiated T cell composite (γ= 0.010, SE=0.005, t(93)=2.10, p=.038 including covariates; unadjusted model p=.055) (see Supplemental Figure 2G). In addition, higher levels of perceived stress tended to be associated with higher counts of the late-differentiated T cell composite between people (γ= 0.027, SE=0.016, t(97)=1.71, p=.090; with covariates p=.063), but this effect was not significant. There was, however, a significant between-person interaction of CMV titers and stress on the T cell composite proportion (γ= −0.032, SE= 0.014, t(91)= −2.34, p=.022 including covariates; unadjusted model p= .071). As shown in Figure 4, older adults with low stress and low CMV titers had the lowest proportions of late-differentiated T cells (estimate: 55%), significantly lower than older adults with low stress and high CMV titers (estimate: 70%, simple main effect: γ = 0.35, Beta = 0.42, SE=.12, p=.005) and older adults with low CMV titers and high stress (estimate: 68%, γ =1.18, SE=0.41, p=.005). Older adults with high stress had similarly high proportions of aged T cells (67–68%) at both low and high CMV titers (simple main effect: γ =−0.015, Beta = −0.018, SE=.10, p=.88).
Figure 4.
Simple slopes depicting the between-person differences in cytomegalovirus (CMV) titers and perceived stress on T cell composite (%) in the subset of CMV-positive older adults. Low CMV titers and low stress are 1 standard deviation (SD) units below the grand means; high CMV titers and high stress are 1 SD units above. Error bars represent standard errors of the estimates. The model was adjusted for lab scientist, CMV extrapolation, time, age at first wave, gender, income, and education.
3.3.2. NK cell composite.
Among CMV-positive older adults, there were no significant main or interacting effects of CMV titers or perceived stress on NK cell composite proportions or counts within or between people.
3.4. Results in the subset of CMV-negative older adults
Among the CMV-negative older adults, there were no significant main effects of perceived stress on T or NK cell composite proportions or counts within or between people.
4. Discussion
The present study tested stable between-person differences and dynamic within-person fluctuations in CMV titers and perceived stress on late-differentiated T and NK cell composites in a longitudinal sample of older adults. Overall, stable between-person differences but not dynamic within-person fluctuations tended to govern the associations between CMV titers and perceived stress on makers indicative of immunological aging.
Between people, older adults with higher CMV titers across all waves had consistently higher proportions and cell counts of the late-differentiated T and NK cell composites across months to years. These findings align with previous cross-sectional reports of associations between CMV seropositivity or titers and T and NK cells (Derhovanessian et al. 2011; Muntasell et al., 2016; Solana et al., 2014). One important consideration regarding terminology: although the NK cell subsets (CD57+, NKG2C+, and FcεRIγ- on CD56dim NK cells) that together form the NK cell composite are considered later differentiated cells (Björkström et al., 2010; Luetke-Eversloh, Killig, & Romagnani, 2013; Muntasell et al., 2016), CMV is a main driver of this differentiation and therefore they may also be appropriately termed “CMV-exposed” NK cells. Impaired CMV control (i.e., higher titers in CMV-positive older adults) was associated with higher counts of late-differentiated T cells between people, suggesting there may be gradations in the accumulation of late-differentiated T cells that could be missed if only CMV serostatus is assessed. Importantly, although longitudinal, the reported associations are not experimental and reverse causality remains a possibility. We expected that within-person increases in CMV titers would be associated with increases in late-differentiated cells (e.g., as evidenced in transplant patients when CMV reactivation induces transient quantitative and qualitative changes in the T cell compartment; Cantisán et al. 2017). However, contrary to our hypothesis, at waves when a person’s CMV titers increased, they had lower proportions of late-differentiated T cells at the same wave (but not the future wave). One possibility that would require further investigation is that CMV reactivation may cause late-differentiated T cells to migrate out of the blood and travel to tissues where CMV may be reactivating.
Older adults with higher levels of perceived stress across all waves had higher cell counts and tended to have higher proportions of late-differentiated T cells. Our findings in healthy older adults support previous evidence of links between more psychological distress (including job stress) and higher counts and proportions of late-differentiated T cells in young and middle-aged adults (Amati et al., 2010; Bosch, Fischer, & Fischer, 2009) and older hip fracture patients (Duggal et al., 2014). Perceived stress has also been associated with shorter telomeres from CD8+CD28− cells in younger adults (Murdock et al., 2018). The lack of significant within-person associations suggests that these markers indicative of an aging immune system may be resilient to fluctuations in stress (at least in the present measurement context of months to years), such that deviations in a person’s perceived stress are not reliably associated with changes in late-differentiated T or NK cells. However, the between-person associations suggest that those particularly at immunological risk are older adults with consistently higher levels of perceived stress.
Older adults who reported lower stress and had lower CMV titers across all waves had the lowest proportions of late-differentiated T cells. Additionally, among the CMV-positive subset, older adults with low stress and good CMV control (i.e., low titers) also had the lowest proportions of late-differentiated T cells. Previous reports of psychological stress and aged T cells have not considered the effects of CMV (Amati et al. 2010; Bosch et al., 2009; Duggal et al., 2014), despite the association between this latent virus and expansion of late-differentiated immune cells. Interestingly, among the CMV-positive subset, older adults who reported high stress across all waves displayed high proportions of late-differentiated T cells regardless of their level of CMV control. In one recent study of young to middle-age women, high-stress mothers of children with Autism Spectrum Disorder had higher proportions of effector memory (CD45RA-CD62L-) T cells compared to low-stress mothers of neurotypical children, independent of CMV serostatus (Prather et al., 2018). Our results add to the growing literature in this area and suggest that stress continues to affect T cell aging in older adulthood. Late-differentiated effector memory T cells may accumulate in those older adults with either good or impaired CMV control who experience consistently high levels of stress. Therefore, targeting CMV control may be necessary but not sufficient to promote healthy immune aging; identifying ways to also reduce psychological stress may yield the most robust health effects for older adults.
Potential health implications of the current observations are worth noting. Increases in late-differentiated CD8+ T cells predict 4-year mortality (Wikby et al., 2005), frailty (Lu et al., 2016), and impaired vaccination responses in older adults (Effros, 2007; Trzonkowski et al., 2003). It has also been proposed that late-differentiated T cells exhibit a senescence-associated secretory phenotype (SASP) (Callender et al., 2018) and may be a source of chronic low-grade inflammation associated with the development of age-related morbidity (Tchkonia et al., 2013). Therefore, consistently high stress levels and high CMV titers may be detrimental to healthy aging insofar as they increase the accumulation of late-differentiated cells and an immune risk phenotype that has clinical health implications.
Notably, the majority of results dealt with late-differentiated T cell subsets and not NK cells. One possibility is that CMV-associated T cells are more stress-responsive than NK cells. Evidence for this theory has been examined in the context of a physical stressor – exercise. In both CMV-positive and CMV-negative adults, exercise induces similar increases in catecholamines, which interact with beta-adrenergic receptors (β-AR) on lymphocytes. However, CMV-positive adults show decreased β-AR expression and sensitivity on late-differentiated NK cells (NKG2C+ / CD57+) but not on late-differentiated T cells (Bigley et al., 2015; Simpson, et al., 2016; Spielmann et al., 2014). A similar mechanism of impaired adrenergic sensitivity may explain why late-differentiated NK cells may be less responsive to chronic stress than late-differentiated T cells. In the context of CMV infection, late-differentiated T cells may be especially adept at “hearing” stress hormones, whereas this might not be the case for NK cells.
The results should be considered in the context that the immunological aging composites had low levels of within-person variability over time. The intraclass correlation coefficients for T and NK cell composites ranged from .72 to .95 in the entire sample (see Table 1), suggesting that the majority of variability in these markers exists at the between-person level (at least across months to years). The lack of within-person variability could be explained in part by the temporal distance between measurement occasions (i.e., every 6 months as opposed to, for example, every few years), or other individual factors (e.g., genetics) that may more strongly influence the stability of late-differentiated immune cells beyond within-person fluctuations in CMV and stress levels. Perhaps as a result of there being more between-person than within-person variability, the results were dominated by between-person effects. Future work that incorporates more dynamic measures of immunological aging over time may find significant within-person associations. In general, however, longitudinal studies that provide estimates of variability and stability in markers of immunological aging are lacking, and future work in this area is warranted (Reed et al., 2018).
Strengths of the present study include its longitudinal design and up to five repeated measurements within person, which allowed us to evaluate between- versus within-person effects. Additionally, as recommended, we present findings both in terms of immune cell proportions and counts (Nikolich-Žugich & van Lier, 2017). Given the well-known redistributive effects of the sympathetic nervous system on lymphocytes in peripheral blood (e.g., Atanackovic et al. 2006), and the fact that proportions can be affected by changes in either the numerator (e.g., CD8+CD28−) or the denominator (e.g., total CD8 T cells), presenting immunological aging findings in terms of proportions only may provide a biased view (particularly in the context of stress). Furthermore, much of the existing CMV and immune aging literature is devoted to the T cell compartment, whereas the present study investigated both late-differentiated T cell and NK cell composites.
The study was limited by a sample that included relatively healthy older adults, which may result in a restriction of range in terms of late-differentiated immune cells, but also this minimized the confounding role of concurrent health status. Future studies should include diverse older adult samples, as well as additional conceptualizations and measures of stress (Epel et al., 2018). It is unknown when CMV-positive older adults in the current sample contracted CMV, and timing of the infection may be an important indicator of how well CMV is controlled as well as downstream health effects (Aiello, Chiu, Frasca, 2017). Nevertheless, CMV IgG titers provided an indication of latent viral activity, however, other methods such as CMV DNA measures may be more sensitive (Leng et al., 2011). Although other persistent sources of antigen (e.g., EBV, HSV) may have cumulative effects on immunological aging, CMV likely exerts the most robust effect on markers of immune aging and on health outcomes such as frailty (Béziat et al., 2013; Derhovanessian et al., 2011; Thomasini et al., 2017). An additional consideration is that quantitative changes in T and NK cell subsets are not directly related to functional changes, and so future studies should investigate both phenotypic markers and functional assays to better describe the role of perceived stress and CMV control on immune function in aging.
In conclusion, these findings report novel insights into the longitudinal associations among CMV titers, perceived stress, and aspects of immunological aging. There were stable associations across all waves between CMV titers and aged T and NK cells and between perceived stress and aged T cells. Older adults with low stress levels and low CMV titers (i.e., better CMV control) displayed the lowest proportions of late-differentiated T cells. Conversely, older adults with good CMV control but high levels of stress displayed similarly high proportions of late-differentiated T cells as older adults with poor CMV control. Therefore, targeting not only CMV control but also factors that promote low, stable stress in older adults (e.g., via emotion regulation and self-regulation skills or health behaviors such as physical activity, etc.) may further retard T cell differentiation and ultimately support healthy aging.
Supplementary Material
Highlights.
Higher CMV titers across waves associated with higher levels of aged T and NK cells.
Higher CMV titers within-person associated with lower levels of aged T cells.
Higher perceived stress across waves associated with higher levels of aged T cells.
Stressed older adults had higher proportions of aged T cells regardless of CMV control.
Acknowledgements
The UK Flow Cytometry & Cell Sorting core facility is supported in part by the Office of the Vice President for Research, the Markey Cancer Center and an NCI Center Core Support Grant (P30 CA177558) to the University of Kentucky Markey Cancer Center.
Funding
This work was supported by the National Institute on Aging (K99-AG056635 [RGR], R01-AG026307 [SCS], K02-AG033629 [SCS], P30-AG028383).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Correlations between CMV titers and perceived stress ranged from r =.10-.28, p =.25-.004 at measurement waves 1–5. On average, across all waves, CMV titers and perceived stress correlated r =.16, p =.052.
References
- Aiello AE, Chiu YL, & Frasca D (2017). How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms?. Geroscience, 39, 261–271. doi: 10.1007/s11357-017-9983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Feinstein L, Dowd JB, Pawelec G, Derhovanessian E, Galea S, … & Simanek AM (2016). Income and markers of immunological cellular aging. Psychosomatic Medicine, 78, 657. doi: 10.1097/PSY.0000000000000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Sage, Newbury Park, CA. [Google Scholar]
- Al-Attar A, Presnell SR, Peterson CA, Thomas DT, & Lutz CT (2016). The effect of sex on immune cells in healthy aging: Elderly women have more robust natural killer lymphocytes than do elderly men. Mechanisms of Ageing and Development, 156, 25–33. doi: 10.1016/j.mad.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Amati M, Tomasetti M, Ciuccarelli M, Mariotti L, Tarquini LM, Bracci M, … & Mocchegiani E (2010). Relationship of job satisfaction, psychological distress and stress-related biological parameters among healthy nurses: a longitudinal study. Journal of Occupational Health, 52, 31–38. doi: 10.1539/joh.L9042 [DOI] [PubMed] [Google Scholar]
- Appay V, van Lier RA, Sallusto F, & Roederer M (2008). Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry Part A, 73, 975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, Peres M, Tkaczuk J, Roubinet F, & Blancher A (2017). Influence of age, sex and HCMV-serostatus on blood lymphocyte subpopulations in healthy adults. Cellular Immunology, 314, 42–53. doi: 10.1016/j.cellimm.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Atanackovic D, Schnee B, Schuch G, Faltz C, Schulze J, Weber CS, … & Deter HC (2006). Acute psychological stress alerts the adaptive immune response: stress-induced mobilization of effector T cells. Journal of Neuroimmunology, 176, 141–152. doi: 10.1016/j.jneuroim.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Bauer ME, Wieck A, Petersen LE, & Baptista TS (2015). Neuroendocrine and viral correlates of premature immunosenescence. Annals of the New York Academy of Sciences, 1351, 11–21. doi: 10.1111/nyas.12786 [DOI] [PubMed] [Google Scholar]
- Béziat V, Liu L, Malmberg JA, Ivarsson MA, Sohlberg E, Björklund AT, … & Schaffer M (2013). NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood, 121, 2678–88. doi: 10.1182/blood-2012-10-459545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley AB, Rezvani K, Pistillo M, Reed J, Agha N, Kunz H, … & Simpson RJ (2015). Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Part II: impact of latent cytomegalovirus infection and catecholamine sensitivity. Brain, Behavior, and Immunity, 49, 59–65. doi: 10.1016/j.bbi.2014.12.027 [DOI] [PubMed] [Google Scholar]
- Bigley AB, Spielmann G, Agha N, O’Connor DP, & Simpson RJ (2016). Dichotomous effects of latent CMV infection on the phenotype and functional properties of CD8+ T-cells and NK-cells. Cellular Immunology, 300, 26–32. doi: 10.1016/j.cellimm.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, … & Guzmán CA (2010). Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK cell differentiation uncoupled from NK cell education. Blood, 116, 3853–3864. doi: 10.1182/blood-2010-04-281675 [DOI] [PubMed] [Google Scholar]
- Bosch JA, Fischer JE, & Fischer JC (2009). Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain, Behavior, and Immunity, 23, 527–534. doi: 10.1016/j.bbi.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Butcher SK, & Lord JM (2004). Stress responses and innate immunity: aging as a contributory factor. Aging Cell, 3, 151–160. doi: 10.1111/j.1474-9728.2004.00103.x [DOI] [PubMed] [Google Scholar]
- Callender LA, Carroll EC, Beal RW, Chambers ES, Nourshargh S, Akbar AN, & Henson SM (2018). Human CD 8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell, 17, e12675. doi: 10.1111/acel.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, … & Solana R (2014). Effect of age and CMV on NK cell subpopulations. Experimental Gerontology, 54, 130–137. doi: 10.1016/j.exger.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Cantisán S, Páez-Vega A, Santos F, Rodríguez-Benot A, Aguado R, Rivero A, … & Spanish Network for Research in Infectious Diseases. (2017). Impact of age and cytomegalovirus on CD8+ T-cell compartment remodeling after solid organ transplantation: A one-year follow-up study. Experimental Gerontology, 95, 98–106. doi: 10.1016/j.exger.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceivedstress. Journal of Health and Social Behavior, 24, 385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, & Bolger N (2006). A procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Personality and Social Psychology Bulletin, 32, 917–929. 10.1177/0146167206287721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Hähnel K, Beck R, de Craen AJ, Slagboom EP, … & Pawelec G (2011). Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. Journal of General Virology, 92, 2746–2756. doi: 10.1099/vir.0.036004-0 [DOI] [PubMed] [Google Scholar]
- Dowd JB, & Aiello A (2009). Socioeconomic differentials in immune response in the US. Epidemiology, 20, 902–908. doi: 10.1097/EDE.0b013e3181bb5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NA, Upton J, Phillips AC, Hampson P, & Lord JM (2014). Depressive symptoms post hip fracture in older adults are associated with phenotypic and functional alterations in T cells. Immunity & Ageing, 11, 1–16. doi: 10.1186/s12979-014-0025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB (2007). Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine, 25, 599–604. doi: 10.1016/j.vaccine.2006.08.032 [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, & Man J (2005). The role of CD8+ T-cell replicative senescence in human aging. Immunological Reviews, 205, 147–157. 10.1111/j.0105-2896.2005.00259.x [DOI] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: a unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. doi: 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, … & Salvioli S (2018). The continuum of aging and age-related diseases: Common mechanisms but different rates. Frontiers in Medicine, 5(61), 1–23. doi: 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao H, & Kiecolt-Glaser JK (1991). Stress-related activation of Epstein-Barr virus. Brain, Behavior, and Immunity, 5, 219–232. [DOI] [PubMed] [Google Scholar]
- Graham JE, Christian LM, & Kiecolt-Glaser JK (2006). Stress, age, and immune function: toward a lifespan approach. Journal of Behavioral Medicine, 29, 389–400. doi: 10.1007/s10865-006-9057-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P Michel JJ, & Vallejo AN (2019). Diversity of CD28null T cells in the elderly: A glimpse in a biological adaptation of aging In Fulop T, Franceschi C, Hirokawa K, & Pawelec G (Eds.), Handbook of immunosenescence: Basic understanding and clinical implications (pp. 1–33, 2nd ed). Switzerland: Springer Nature. [Google Scholar]
- Hoffman L, & Stawski RS (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–120. doi: 10.1080/15427600902911189 [DOI] [Google Scholar]
- Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, … & Kim S (2015). Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity, 42, 431–442. doi: 10.1016/j.immuni.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Qu T, Semba RD, Li H, Yao X, Nilles T, … & Fried LP (2011). Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp65 495–503-specific CD8+ T cells in older adults. Age, 33, 607–614. doi: 10.1007/s11357-011-9205-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, … & Lanier LL (2010). CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK cell subset. Blood, 116, 3865–74. doi: 10.1182/blood-2010-04-282301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tan CTY, Nyunt MSZ, Mok EWH, Camous X, Kared H, … & Larbi A (2016). Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget, 7, 28783–95. doi: 10.18632/oncotarget.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke-Eversloh M, Killig M, & Romagnani C (2013). Signatures of human NK cell development and terminal differentiation. Frontiers in Immunology, 4, 499. doi: 10.3389/fimmu.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CT, Karapetyan A, Al-Attar A, Shelton BJ, Holt KJ, Tucker JH, & Presnell SR (2011). Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. The Journal of Immunology, 186, 4590–4598. doi: 10.4049/jimmunol.1002732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Pupuleku A, Cisneros E, Vera A, Moraru M, Vilches C, & López-Botet M (2016). Relationship of NKG2C copy number with the distribution of distinct cytomegalovirus-induced adaptive NK cell subsets. The Journal of Immunology, 196, 3818–27. doi: 10.4049/jimmunol.1502438 [DOI] [PubMed] [Google Scholar]
- Murdock KW, Zilioli S, Ziauddin K, Heijnen CJ, & Fagundes CP (2018). Attachment and telomere length: more evidence for psychobiological connections between close relationships, health, and aging. Journal of Behavioral Medicine, 41, 333–343. doi: 10.1007/s10865-017-9895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Žugich J, & van Lier RA (2017). Cytomegalovirus (CMV) research in immune senescence comes of age: Overview of the 6th International Workshop on CMV and Immunosenescence. GeroScience, 39, 245–249. doi: 10.1007/s11357-017-9984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. (2005). Human immunosenescence: is it infectious? Immunological Reviews, 205, 257–68. 10.1111/j.0105-2896.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, & Solana R (2015). Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas, 82, 50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Prather AA, Epel ES, Parra EP, Coccia M, Puterman E, Aiello AE, & Dhabhar FS (2018). Associations between chronic caregiving stress and T cell markers implicated in immunosenescence. Brain, Behavior, and Immunity. Epub ahead of print. doi: 10.1016/j.bbi.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, … & Bosch JA (2014). Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain, Behavior, and Immunity, 38, 133–141. doi: 10.1016/j.bbi.2014.01.012 [DOI] [PubMed] [Google Scholar]
- Reed RG, Al-Attar A, Presnell SR, Lutz CT, & Segerstrom SC A longitudinal study of the stability, variability, and interdependencies among late-differentiated T and NK cell subsets in older adults. Manuscript submitted for publication, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC (2009). Biobehavioral controls: threats to psychoneuroimmunology research? Brain, Behavior, and Immunity, 23, 885–886. doi: 10.1016/j.bbi.2009.05.053 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Al-Attar A, & Lutz CT (2012). Psychosocial resources, aging, and natural killer cell terminal maturity. Psychology and Aging, 27, 892–902. doi: 10.1037/a0029093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Bigley AB, Spielmann G, LaVoy EC, Kunz H, & Bollard CM (2016). Human cytomegalovirus infection and the immune response to exercise. Exercise Immunology Review, 22, 8–26. [PubMed] [Google Scholar]
- Singer JD, & Willett JB, 2003. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press, New York, NY. [Google Scholar]
- Solana R, Campos C, Pera A, & Tarazona R (2014). Shaping of NK cell subsets by aging. Current Opinion in Immunology, 29, 56–61. doi: 10.1016/j.coi.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Song MK, Lin FC, Ward SE, & Fine JP (2013). Composite variables: When and how. Nursing Research, 62, 45–49. doi:: 10.1097/NNR.0b013e3182741948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann G, Bollard CM, Bigley AB, Hanley PJ, Blaney JW, LaVoy EC, … & Simpson RJ (2014). The effects of age and latent cytomegalovirus infection on the redeployment of CD8+ T cell subsets in response to acute exercise in humans. Brain, Behavior, and Immunity, 39, 142–151. doi: 10.1016/j.bbi.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, & Cannon MJ (2006). Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clinical Infectious Diseases, 43, 1143–1151. 10.1086/508173 [DOI] [PubMed] [Google Scholar]
- Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, & Glaser R (2007). Chronic herpesvirus reactivation occurs in aging. Experimental Gerontology, 42, 563–570. doi: 10.1016/j.exger.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, & Characiejus D (2011). CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology, 134, 17–32. doi: 10.1111/j.1365-2567.2011.03470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, Van Deursen J, Campisi J, & Kirkland JL (2013). Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of Clinical Investigation, 123, 966–972. doi: 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasini RL, Pereira DS, Pereira FSM, Mateo EC, Mota TN, Guimarães GG, … & Junior ALT (2017). Aged-associated cytomegalovirus and Epstein-Barr virus reactivation and cytomegalovirus relationship with the frailty syndrome in older women. PloS One, 12, e0180841. doi: 10.1371/journal.pone.0180841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Myśliwska J, Szmit E, Wieckiewicz J, Łukaszuk K, Brydak LB, … & Myśliwski A (2003). Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine, 21, 3826–3836. [DOI] [PubMed] [Google Scholar]
- Vallejo AN (2005). CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunological Reviews, 205, 158–169. 10.1111/j.0105-2896.2005.00256.x [DOI] [PubMed] [Google Scholar]
- Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, … & Nikolich-Žugich J (2014). Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. The Journal of Immunology, 192, 2143–55. doi: 10.4049/jimmunol.1301721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, et al. (2005). An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. Journal of Gerontology: Series A Biological Sciences Medical Sciences, 60, 556–65. 10.1093/gerona/60.5.556 [DOI] [PubMed] [Google Scholar]
- Wikby A, Månsson IA, Johansson B, Strindhall J, & Nilsson SE (2008). The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology, 9, 299–308. doi: 10.1007/s10522-008-9138-6 [DOI] [PubMed] [Google Scholar]
- Zhang T, Scott JM, Hwang I, & Kim S (2013). Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. The Journal of Immunology, 190, 1402–6. doi: 10.4049/jimmunol.1203034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.