Abstract
Objectives:
To describe the distribution of post-void residual (PVR) volumes across patients with and without lower urinary tract symptoms (LUTS) and examine relationships between self-reported voiding symptoms, storage symptoms, and PVR.
Methods:
PVR and demographic data were obtained from the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) observational cohort study. Selfreported symptoms were collected using the American Urological Association Symptom Index (AUA-SI) and the LUTS Tool. PVR values were obtained from two other cohorts:living kidney donors with unknown LUTS from the Renal and Lung Living Donors Evaluation Study (RELIVE), and continent women in the Establishing the Prevalence of Incontinence (EPI) study, a population-based study of racial differences in urinary incontinence prevalence.
Results:
Across the three studies, median PVRs were similar: 26mL in LURN (n=880, range 0–932mL), 20mL in EPI (n=166, range 0–400mL), and 14mL in RELIVE (n=191, range 0–352mL). In LURN, males had 3.6 times higher odds of having PVR>200mL (95% CI=1.72–7.48). In RELIVE, median PVR was significantly higher for males (20mL vs. 0mL, p=0.004). Among women, only the intermittency severity rating was associated with a probability of an elevated PVR. Among men, incomplete emptying and burning severity rating were associated with a higher odds of elevated PVR, but urgency severity ratings were associated with lower odds of elevated PVR.
Conclusions:
Care-seeking patients have PVRs similar to those in people with unknown history of LUTS (RELIVE) and without self-reported LUTS (EPI). Although PVR was correlated with voiding symptoms, the mean differences only explain ~2% of the variance.
Keywords: voiding, lower urinary tract symptoms, emptying, urgency, incontinence
INTRODUCTION
The initial evaluation of lower urinary tract symptoms (LUTS) includes a history, physical exam, urinalysis, and post-void residual (PVR), measured by bladder scan or straight catheterization.1,2 Conventional wisdom states that elevated PVR may correlate with clinically-relevant issues, such as risk of urinary tract infection (UTI) and the severity of LUTS, and may be an indication for clinical intervention. However, there is no standard for what constitutes an abnormal PVR,3,4 or at what volume residual urine may cause symptoms. In the available literature, the definition of urinary retention has been pragmatically set as a PVR volume ranging from 100mL to 500mL.5 While this is still debated, current expert opinion defines urinary retention as an elevated PVR of >300mL, without differentiation among males and females.5–10 There is no guideline or evidence stronger than expert panel consensus to establish the use of PVR as a clinical tool.
Given this knowledge gap, we assessed PVR and patient-reported urinary symptoms in the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) and compared them with PVR distributions in other large cohorts with and without LUTS. We hypothesized that: 1) there would be wide variation in retained urine volume, with a substantial number having what is currently considered to be a clinically-significant elevated PVR; and 2) absolute PVR volume would not correlate with self-reported storage and voiding symptoms.
MATERIALS and METHODS
Study design and population
Data were obtained from the LURN Observational Cohort Study, which has been described previously. Briefly, men and women presenting to a physician at one of six LURN tertiary care centers were recruited between June 2015 and January 2017. These persons were free of major neurologic diseases or injuries known to directly affect bladder function, as outlined in the LURN protocol for inclusion and exclusion.11 Patients were not excluded if they had already sought care from another provider, such as primary care practitioners, urologists, or gynecologists for LUTS. At the baseline visit, participants completed a medical history and clinical exam as well as questionnaires related to LUTS, bowel function, sexual function, and psychological health.
Additional data for patients without LUTS were obtained from two prior published studies: the Renal and Lung Living Donors Evaluation (RELIVE) study and the Establishing the Prevalence of Incontinence (EPI) study. The RELIVE study explored long-term outcomes, including measured iothalamate glomerular filtration rates (iGFR), for living kidney donors who donated between 1963 and 2007 at three large US transplant centers.12 Calculation of the iGFRs required several complete urine voids, with a PVR measured after each void. The first PVR measurement was used for the current analysis. The RELIVE study had no information about urinary symptoms. LUTS The EPI study’s primary purpose was to estimate the prevalence of incontinence in black and white women in southeast Michigan using population-based samples. Women aged 35–64 years old were recruited between 2002 and 2004 and completed telephone surveys. A subset was invited to the clinic for urodynamic and pelvic floor testing where the PVR was obtained.13
Procedures and measures
At the LURN baseline clinical visit, comorbidities were assessed using the Functional Comorbidity Index.14 UTIs were self-reported by asking women if they had more than two UTIs in the past year, and men if they had ever had a UTI in their lifetime. PVR was measured either by ultrasound (n=676) or catheterization (n=181). In the RELIVE study (n=413), PVR was measured using ultrasound; in the EPI study (n=166), PVR was measured by catheterization.
Participants in LURN completed the LUTS Tool (Version 1.0. Copyright 2007 by Pfizer, Inc. Used with permission.)15 and the American Urological Association Symptom Index (AUA-SI). The LUTS Tool includes 22 symptom severity questions rated on a scale from “never” to “almost always”, except for questions about daytime and nighttime frequency, which were rated on a scale of 1–3, 4–7, 8–10, 11–13, and 14 or more times per day and never, 1, 2, 3, or 4 or more times per night, respectively.
Statistical analysis
Demographic and clinical characteristics of LURN participants with measured PVR are presented by sex using frequencies and percentages for categorical variables, and means and standard deviations (SD) or median and interquartile ranges (IQR) for continuous variables. Differences between sexes and between participants with and without PVR were assessed using chi-square and Wilcoxon two-sample tests for categorical and continuous variables, respectively.
Potential associations between measured PVR and self-reported responses from the LUTS Tool and the AUA-SI were assessed descriptively by sex using box plots of PVR by self-reported symptom rating and Pearson correlation. The distribution of PVR by presence and absence of LUTS was explored using medians and IQRs of PVR and Wilcoxon two-sample tests. Multivariable Weibull and logistic regression was used to test for adjusted associations between multiple LUTS and PVR as a continuous variable and probability of elevated PVR (defined a priori as PVR>200mL) by sex. Weibull regression is an accelerated failure time model commonly used for survival analysis. It specifies that the covariates act multiplicatively on the outcome; in this case, measured PVR model selection was guided by the method of best subsets. P-values were adjusted for multiple comparisons by controlling the false discovery rate using the method developed by Benjamini and Hochberg.16 All analyses were completed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 1064 participants in the LURN Observational Cohort Study, 880 had PVR measured at enrollment. Participants with measured PVR were more likely to be female (53% vs. 44%, p=0.03), but were similar on all other demographic and clinical characteristics. In the cohort with PVR data recorded, women were younger than men (mean age [SD] 56.6 [14.5] vs. 61.0 [13.7], p<0.001, Table 1). Anti-cholinergic or anti-constipation medication use was similar between men and women, while alpha-blocker use was higher in men (40% vs. 2%), and 15% of men reported using 5-alpha reductase inhibitors. Forty-eight percent of women reported more than two UTIs in the past year, and 22% of men reported ever having at least one UTI within their lifetime.
Table 1:
Demographics and medical history of LURN participants with measured PVR by sex
| Male (n=416) | Female (n=464) | p-value* | |
|---|---|---|---|
| Age mean (SD) | 61.0 (13.7) | 56.6 (14.5) | <.001 |
| Race n (%) | 0.073 | ||
| African-American | 39 (9%) | 59 (13%) | |
| Other | 43 (10%) | 32 (7%) | |
| White | 333 (80%) | 373 (80%) | |
| Ethnicity n (%) | 0.968 | ||
| Hispanic/Latino | 17 (4%) | 18 (4%) | |
| Non-Hispanic/Non-Latino | 390 (94%) | 435 (94%) | |
| Ethnicity unknown | 9 (2%) | 11 (2%) | |
| BMI median (IQR) | 28.6 (25.6–32.7) | 29.3 (24.9–35.0) | 0.343 |
| Diabetes n (%) | 80 (19%) | 68 (15%) | 0.070 |
| Anticholinergic medication use n (%) | 13 (3%) | 10 (2%) | 0.368 |
| Anti-constipation medication use n (%) | 36 (9%) | 28 (6%) | 0.135 |
| Alpha blocker use n (%) | 168 (40%) | 9 (2%) | <.001 |
| 5-alpha reductase inhibitor use n (%) | 64 (15%) | - | |
| Functional comorbidity index median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | 0.475 |
| History of UTI** n (%) | 90 (22%) | 219 (48%) | <.001 |
p-value from chi-square or Wilcoxon 2-sample test;
History of UTI assessed as more than two UTIs in the past year for women and any previous UTIs for men
Abbreviations: BMI, body mass index; IQR, interquartile range; LURN, Symptoms of Lower Urinary Tract Dysfunction Research Network; PVR, postvoid residual volumes; SD, standard deviation; UTI, urinary tract infection
In LURN, PVR measurement was done predominately by ultrasound for men (99%). Among women, 61% of measured PVR was obtained by ultrasound, and median PVR was 10mL lower for those measured with ultrasound (median [IQR]=20 [0–52] for ultrasound, 30 [10–70] for catheter, p=0.001). Other demographics and LUTS severity did not differ by method of PVR measurement. The median PVR in the LURN cohort was 26mL (IQR 6–67mL, range 0–932mL, Supplemental Figure 1). The distribution of PVR was similar by sex (median PVR=27mL [IQR=0–78.5mL] in men, 25mL [IQR=10–60mL] in women), but the maximum PVR observed was higher in men (932mL vs. 420mL).
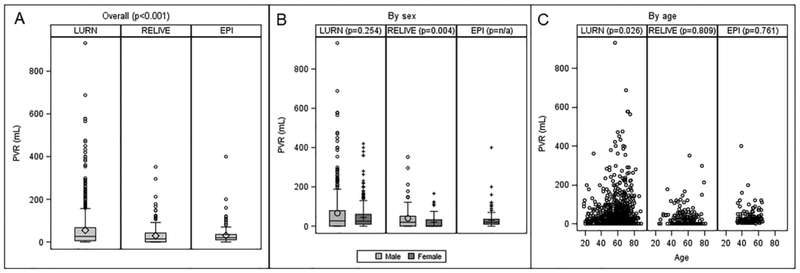
Observed PVR in RELIVE (n=413) and EPI (n=166), although statistically significantly lower, was clinically similar to LURN, with medians of 14mL (IQR=0–42mL) and 20mL (IQR=10–35mL), respectively (Figure 1A). Elevated PVR (defined as PVR>200mL) was more prevalent in LURN (5.2%) compared with RELIVE (1.6%) and EPI (0.6%, p=0.004). In RELIVE, PVR was statistically lower in women (median PVR 0mL IQR=0–32mL compared with 20mL IQR=0–51mL in men, Figure 1B). No association between PVR and age was detected in any of the studies (Figure 1C).
Figure 1:
Comparison of PVR across three studies. Boxplots show the distribution of PVR in LURN, RELIVE, and EPI overall and by sex. Relationships between age and PVR in each study are shown using scatter plots. P-values from non-parametric analysis of variance (ANOVA; Kruskal-Wallis tests) are shown testing for differences in distributions across the studies (panel A) and differences in distributions by sex within each study (panel B). P-values (panel C) are from Pearson correlations between age and PVR within each study.
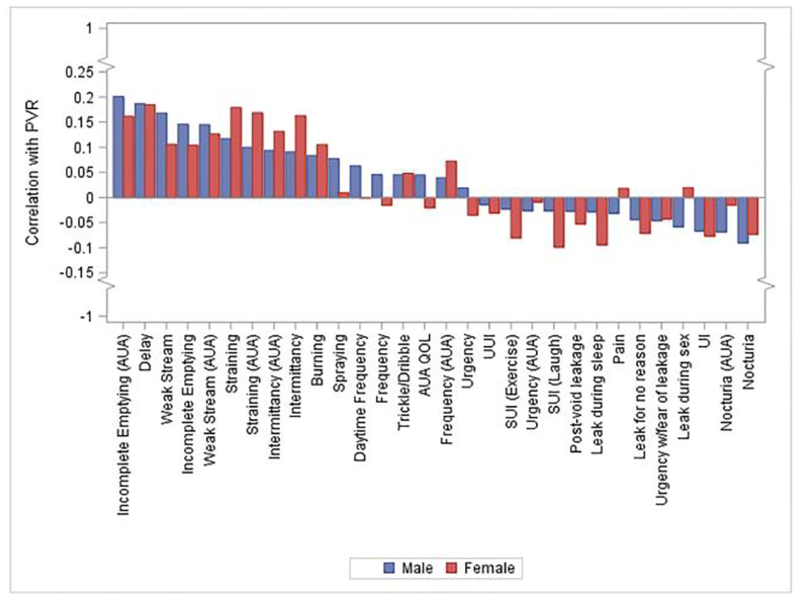
Correlations between PVR and symptom severity ratings on the LUTS Tool and AUA-SI ranged from −0.1 to 0.2, demonstrating weak correlation (Figure 2). In men, voiding and post-micturition symptoms of incomplete emptying, delay, and weak stream showed very weak positive correlation with PVR; nocturia demonstrated weak negative correlation. In women, delay, straining, and intermittency were weakly positively correlated with PVR; symptoms of stress urinary incontinence were weakly negatively correlated. When men taking outlet medications were excluded, results were unchanged (Supplemental Figure 2). When comparing the distribution of PVR by symptom presence or absence, only straining and hesitancy in women and intermittency, weak stream, and delay in men demonstrated statistically significant differences in PVR, compared with those without symptoms; however, the maximum difference in PVR between symptom presence and absence was only 20mL (Supplemental Table 1). A comparison of symptom ratings between participants with and without elevated PVR (>200mL) showed higher median scores for incomplete emptying (AUA-SI), weak stream (AUA-SI and LUTS Tool), and delay (LUTS Tool) reported for male participants with elevated PVR (Supplemental Table 2). Among women, higher incomplete emptying (AUA-SI), straining (AUA-SI and LUTS Tool), and intermittency (AUA-SI and LUTS Tool) ratings were observed for those with elevated PVR (Supplemental Table 3).
Figure 2:
Correlation between PVR and LUTS symptom severity measured by the LUTS Tool, by sex, in LURN.
In women, intermittency severity on the LUTS Tool and sensation of incomplete emptying on the AUA-SI were associated with elevated PVR (26% increase in PVR per unit increase in intermittency severity rating, 95% confidence interval [CI] 13%−42%, p<0.001, 9% increase in PVR per unit increase in incomplete emptying severity rating, 95% CI=2%−18%, p=0.01, Table 2a). Higher nocturia severity, leakage just after voiding, and spraying severity ratings were associated with lower measured PVR (range 8%−11% decrease in PVR).
Table 2a:
Multivariable associations with PVR among female LURN participants
| Covariate | % change or OR | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|---|
| Weibull model of continuous PVR | Intermittency (LUTS tool) | +26% | +13% | +42% | <.001 |
| Nocturia (LUTS tool) | −8% | −16% | +0% | 0.045 | |
| Spraying (LUTS tool) | −11% | −19% | −3% | 0.013 | |
| Incomplete emptying (AUA-SI) | +9% | +2% | +18% | 0.014 | |
| Post-void leakage | −9% | −17% | −1% | 0.027 | |
| Logistic model of probability of PVR>200 | Intermittency (LUTS tool) | 1.49 | 1.06 | 2.10 | 0.023 |
Among men, self-reported incomplete emptying on the AUA-SI and weak stream severity ratings on the LUTS Tool were associated with higher PVR (21% increase in PVR per unit increase in incomplete emptying severity rating, 95% CI=11%−31%, p<0.001, 19% increase in PVR per unit increase in weak stream severity rating, 95% CI=8%−32%, p<0.001, Table 2b). Higher urgency severity rating on the AUA-SI and bladder pain rating on the LUTS Tool were associated with lower PVR.
Table 2b:
Multivariable associations with PVR among male LURN participants
| Covariate | % change or OR | Lower 95% CI | Upper 95% CI | P-value | |
|---|---|---|---|---|---|
| Weibull model of continuous PVR | Incomplete emptying (AUA-SI) | +21% | +11% | +31% | <.001 |
| Urgency (AUA-SI) | −11% | −18% | −4% | 0.002 | |
| Pain (LUTS tool) | −15% | −26% | −4% | 0.011 | |
| Weak stream (LUTS tool) | +19% | +8% | +32% | <.001 | |
| Logistic model of probability of PVR>200 | Incomplete emptying (AUA-SI) | 1.50 | 1.17 | 1.92 | 0.001 |
| Urgency (AUA-SI) | 0.76 | 0.60 | 0.97 | 0.027 | |
| Burning (LUTS tool) | 1.40 | 0.98 | 2.01 | 0.066 |
Abbreviations: AUA-SI, American Urological Association-Symptom Index; CI, confidence interval; IQR, interquartile range; LURN, Symptoms of Lower Urinary Tract Dysfunction Research Network; LUTS, lower urinary tract symptoms; OR, odds ratio; PVR, post-void residual volume
Among women, only intermittency rating on the LUTS Tool was associated with probability of elevated PVR (odds ratio [OR] = 1.49, 95% CI=1.06–2.10, p=0.02). Among men, each unit increase in incomplete emptying severity rating on the AUA-SI was associated with a 50% increase in odds of elevated PVR (OR=1.50, 95% CI=1.17–1.92, p=0.001). In addition, higher severity ratings of burning during urination were associated with higher odds of elevated PVR (OR=1.40, 95% CI=0.98–2.01), while higher urgency severity ratings on the AUA-SI were associated with 24% lower odds of elevated PVR (OR=0.76, 95% CI=0.60–0.97, p=0.03).
We further performed a subgroup analysis of all patients in LURN taking medications that may affect PVR, including alpha blockers; 5-alpha reductase inhibitors; and bladder relaxants, such as anticholinergic medications and beta-3 agonists. Among men taking alpha-blockers or 5-alpha reductase inhibitors, the median PVR was 41mL (IQR 15–119) for those on an alpha blocker alone (n=127), 27mL (IQR 0–88) for those on 5-alpha reductase inhibitors alone (n=23), and 28mL (IQR 13–92) for men on both medications (n=41), compared with a median PVR of 21mL (0–53) for men not taking either medication (p <0.001). The proportion of men with elevated PVR (>200mL) did not differ between the four groups (p=0.27). In men and women, there was no statistically significant difference in PVR between those on any form of bladder relaxant medications (19 men, 11 women) and those not taking these medications (median PVR in men=14.5mL on bladder relaxant vs. 27mL not on bladder relaxant, p=0.19, median PVR in women=26mL on bladder relaxant vs. 25mL not on bladder relaxant, p=0.74).
COMMENT
In our treatment-seeking population from LURN, median PVR was clinically similar to the other community populations (RELIVE and EPI), while the range was wider and slightly higher in the LURN cohort. We identified statistically significant but very weak correlations between symptom severity and PVR in both sexes that endorsed a sensation of incomplete emptying.
The proportions of people seeking care for LUTS with elevated PVR, as defined by >300cc in prior studies,3 were lower than anticipated at less than 2%. Using lower thresholds of PVR (i.e., 250mL, 200mL, and 150mL) found only 3%, 5%, and 9% of care-seeking patients in these groups respectively (Figure 1A). Most importantly, the subgroup analysis of those patients with PVR greater than 200mL did not show any clinically-relevant association between symptoms when compared with the overall cohort.
Other longitudinal community-based studies associating PVR with LUTS have yielded weak associations as well. In a study of 329 men, baseline PVRs were measured, and patients were followed for 5 years.17 In those with PVR of <400cc, the rates of proceeding to prostate surgery and/or the need for indwelling or intermittent catheterization ranged from 4% (<200cc) to 18% (200–400cc). However, when the baseline PVR was over 400, it was found that over 75% of these men ultimately underwent interventions, suggesting that a PVR < 400cc may only need intervention in less than a quarter of patients. Our cohort had very few men with PVR >400, and we did not follow men longitudinally, so cannot comment on the natural history for elevated PVR.
In a separate study of 1688 asymptomatic community-dwelling men, PVR, along with six other factors, including family history of an enlarged prostate or LUTS, were associated with the future development of symptomatic LUTS.18 However, in a different study of randomly-selected men, PVR alone was not associated with present or future symptoms, similar to our findings. Finally, in a longitudinal study of community-dwelling men that measured PVR every 2 years for up to 12 years, the average yearly increase in PVR was only 2.2%.19 However, there was significant variability in PVR trajectory where a rapid rise, not just the overall one-time PVR, was associated with higher baseline AUA-SI. These studies all agree with our findings that symptoms alone may not be able to predict the presence of an elevated PVR.
This observational cohort gives notable information on a large group of care-seeking patients with respect to measured PVR values. We acknowledge certain limitations in our research. Notably, the control groups (those from RELIVE) were not prospectively enrolled in the study at the same time as the cases (LURN and EPI), and we drew their data from other studies. In the LURN cohort, we do not have longitudinal PVR data to track changes over time, or with respect to various treatments, age and time, bladder volume, medications, and infections. Because of this, multiple sets of measurements with analysis of median values would be valuable. The RELIVE cohort was not confirmed to be free of LUTS, just presumed to be asymptomatic because they were not care-seeking individuals. The EPI patients were only women who may have had LUTS other than incontinence. There may be a difference between the ultrasound measurements and the volumes obtained by catheterization; ultrasound measurements are more operator-dependent, and the catheterization result is more difficult to be confounded by other physical issues, such as pelvic masses, ovarian cysts, etc.20 A portion of the observational cohort (184 participants, or 17%) did not have PVR measurements and were not included in this analysis. The absence of this group may have biased the analysis; however, these patients were demographically and clinically similar to included LURN participants. Finally, we did not exclude patients who had already obtained specialty care elsewhere with either primary care practitioners, gynecologists, or urologists. This contributed a small cohort that were already on medicines, such as alpha blockers and 5-alpha reductase inhibitors. Inclusion of this group could have skewed the PVR downward due to prior treatment or upward due to recalcitrant symptoms that prompted additional care-seeking. However, exclusion of these participants did not change the results of the whole cohort.
CONCLUSIONS
This study demonstrates that the proportions of men and women seeking care for LUTS with an elevated PVR were lower than we anticipated, making up less than 2% of the cohort. Also in this cohort, self-reported LUTS are only weakly associated with PVR. Most importantly, the subgroup analysis of those patients with PVR greater than 200mL did not show any clinically-relevant association between symptoms when compared with the overall cohort. Thus, clinicians cannot estimate the likelihood that a given patient has a normal or elevated PVR, based on PROs. Because of this, we feel the measurement of a PVR during the initial evaluation of all patients with LUTS is advisable.
Supplementary Material
Supplemental Figure 1: Histograms of PVR in LURN, overall and by sex. The distribution of PVR in LURN for male participants (left panel), female participants (middle panel), and all participants (right panel).
Supplemental Figure 2: Correlation between measured PVR and LUTS, excluding men on alpha blockers or 5-alpha reductase inhibitors. The height of each bar represents the correlation between measured PVR and responses to the LUTS Tool and AUA symptom items for men not taking the noted medications (blue bars) and women (red bars).
ACKNOWLEDGEMENTS
Heather Van Doren, MFA, senior medical editor with Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
SUPPORT
This is publication number 11 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Siddiqui is supported by grant K23-DK110417 from the NIDDK.
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Eric Jelovsek, MD, Aaron Lentz, MD, Drew Peterson, MD, Nazema Siddiqui, MD, Alison Weidner, MD; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O’Donnell, MD, Vivian Sung, MD; Study Coordinator: Ahmad Alzubaidi
Northwestern University, Chicago, IL (DK097779): PIs: David Cella, Brian Helfand, MD, PhD; Co-Is: James W Griffith, PhD, Kimberly Kenton, MD, MS, Christina Lewicky-Gaupp, MD, Todd Parrish, PhD, Jennie Yufen Chen, PhD, Margaret Mueller, MD; Study Coordinators: Sarah Buono, Maria Corona, Beatriz Menendez, Alexis Siurek, Meera Tavathia, Veronica Venezuela, Azra Muftic, Pooja Talaty, Jasmine Nero. Dr. Helfand, Ms. Talaty, and Ms. Nero are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, John Wei, MD; Study Coordinators: Morgen Barroso, Linda Drnek, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Alice Liu, MPH, Brenda Vicars, RN Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald L. Andriole, MD, H. Henry Lai; Co-I: Joshua Shimony, MD, PhD; Study Coordinators: Susan Mueller, RN, BSN, Heather Wilson, LPN, Deborah Ksiazek, BS, Aleksandra Klim, RN, MHS, CCRC
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: Christopher Mullins, PhD; NIH Personnel: Tamara Bavendam, MD, Robert Star, MD
Arbor Research Collaborative for Health, Data Coordinating Center (DK097776 and DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Gang Liu, PhD, Abigail Smith, PhD; Project Manager: Melissa Fava, MPA, PMP; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Clinical Monitor: Timothy Buck, BS, CCRP; Research Analysts: Margaret Helmuth, MA, Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: The authors report “none”.
REFERENCES
- 1.Kaplan SA. AUA Guidelines and Their Impact on the Management of BPH: An Update. Rev Urol. 2004;6 Suppl 9:S46–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572–1580. [DOI] [PubMed] [Google Scholar]
- 3.Stoffel JT, Peterson AC, Sandhu JS, Suskind AM, Wei JT, Lightner DJ. AUA White Paper on Nonneurogenic Chronic Urinary Retention: Consensus Definition, Treatment Algorithm, and Outcome End Points. J Urol. 2017;198(1):153–160. [DOI] [PubMed] [Google Scholar]
- 4.Brasure M, Fink HA, Risk M, et al. In: Chronic Urinary Retention: Comparative Effectiveness and Harms of Treatments. Rockville (MD)2014. [PubMed] [Google Scholar]
- 5.Kaplan SA, Wein AJ, Staskin DR, Roehrborn CG, Steers WD. Urinary retention and postvoid residual urine in men: separating truth from tradition. J Urol. 2008;180(1):47–54. [DOI] [PubMed] [Google Scholar]
- 6.Asimakopoulos AD, De Nunzio C, Kocjancic E, Tubaro A, Rosier PF, Finazzi-Agro E. Measurement of post-void residual urine. Neurourol Urodyn. 2016;35(1):55–57. [DOI] [PubMed] [Google Scholar]
- 7.Abrams PH, Dunn M, George N. Urodynamic findings in chronic retention of urine and their relevance to results of surgery. Br Med J. 1978;2(6147):1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal DE, Styles RA, Powell PH, Ramsden PD. Relationship between detrusor function and residual urine in men undergoing prostatectomy. Br J Urol. 1987;60(6):560–566. [DOI] [PubMed] [Google Scholar]
- 9.Di Pierdomenico AA, Radomski SB. Success rates of patients with poor emptying on clean intermittent catheterization. Can J Urol. 2014;21(2):7188–7193. [PubMed] [Google Scholar]
- 10.Ghalayini IF, Al-Ghazo MA, Pickard RS. A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int. 2005;96(1):93–97. [DOI] [PubMed] [Google Scholar]
- 11.Cameron AP, Lewicky-Gaupp C, Smith AR, et al. Baseline Lower Urinary Tract Symptoms in Patients Enrolled in LURN: A Prospective, Observational Cohort Study. J Urol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taler SJ, Messersmith EE, Leichtman AB, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant. 2013;13(2):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLancey JO, Fenner DE, Guire K, Patel DA, Howard D, Miller JM. Differences in continence system between community-dwelling black and white women with and without urinary incontinence in the EPI study. Am J Obstet Gynecol. 2010;202(6):584 e581–584 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. [DOI] [PubMed] [Google Scholar]
- 15.Coyne KS, Barsdorf AI, Thompson C, et al. Moving towards a comprehensive assessment of lower urinary tract symptoms (LUTS). Neurourology and urodynamics. 2012;31(4):448–454. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 17.Noguchi N, Chan L, Cumming RG, et al. Natural history of post-void residual urine volume over 5 years in community-dwelling older men: The Concord Health and Ageing in Men Project. Neurourol Urodyn. 2017. [DOI] [PubMed] [Google Scholar]
- 18.Kok ET, Schouten BW, Bohnen AM, Groeneveld FP, Thomas S, Bosch JL. Risk factors for lower urinary tract symptoms suggestive of benign prostatic hyperplasia in a community based population of healthy aging men: the Krimpen Study. J Urol. 2009;181(2):710–716. [DOI] [PubMed] [Google Scholar]
- 19.Rule AD, Jacobson DJ, McGree ME, Girman CJ, Lieber MM, Jacobsen SJ. Longitudinal changes in post-void residual and voided volume among community dwelling men. J Urol. 2005;174(4 Pt 1):1317–1321; discussion 1321–1312; author reply 1322. [DOI] [PubMed] [Google Scholar]
- 20.Simforoosh N, Dadkhah F, Hosseini SY, Asgari MA, Nasseri A, Safarinejad MR. Accuracy of residual urine measurement in men: comparison between real-time ultrasonography and catheterization. J Urol. 1997;158(1):59–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Histograms of PVR in LURN, overall and by sex. The distribution of PVR in LURN for male participants (left panel), female participants (middle panel), and all participants (right panel).
Supplemental Figure 2: Correlation between measured PVR and LUTS, excluding men on alpha blockers or 5-alpha reductase inhibitors. The height of each bar represents the correlation between measured PVR and responses to the LUTS Tool and AUA symptom items for men not taking the noted medications (blue bars) and women (red bars).