Abstract
Plethysmograph measurement of respiratory phenotypes provides a highly-sensitive means to study nicotine response in experimental model animals. We measured average respiratory frequency, tidal volume, minute volume and inspiratory time in C3H/HeJ and C57BL/6J mice subcutaneously administered 0.35 and 0.70 mg/kg nicotine. Both mouse strains showed significantly altered respiratory and locomotion phenotypes relative to saline injected controls when administered the higher dose, but only C57BL/6J responded to the lower nicotine dose. Respiratory and locomotion phenotypes rarely differed significantly by sex. To investigate whether the strain-specific differences in nicotine sensitivity were related to differences in clearance, we followed up by measuring nicotine clearance in C3H/HeJ and C57BL/6J mice (0.35 mg/kg subcutaneous) and found sex differences in both strains, but no difference between strains.
Keywords: respiration, ventilation, nicotine, mouse, strain, plethysmography
INTRODUCTION
Exposure to nicotine, the main psychoactive agent in tobacco, induces a variety of physiological responses in humans and model organisms, including changes in heart rate, vascular resistance, blood pressure (Omvik, 1996; Tonstad and Andrew Johnston, 2006), respiration or ventilation, arousal (Henningfield, 1984), glucose homeostasis and insulin sensitivity (Vu et al., 2014), locomotion (Umezu, 2012), altered cognition (Campos et al., 2016), and attention (Hahn, 2015), with different studies reporting both stimulatory and sedative effects, depending on dose, method of administration, organism, or genetic background. Nicotine both increases and reduces respiratory rate in human smokers, depending on subject, correlated with its effects on heart rate (Jones, 1987), and in mice, depending on strain (Collins et al., 1988; Marks et al., 1989). Altered ventilation is also a symptom of anxiety associated with nicotine withdrawal (Malin and Goyarzu, 2009; Van Duinen et al., 2010), but respiration has not yet been measured in model organism studies of nicotine withdrawal.
An extensive literature describes the effects of prenatal nicotine exposure upon respiration in rat and mouse pups (Bamford and Carroll, 1999; Robinson et al., 2002; Huang et al., 2004; Eugenin et al., 2008; Avraam et al., 2015). A few prior studies also measured the effects of acute nicotine upon respiration in restrained adult mice (Collins et al., 1988; Marks et al., 1989). More recent technological advances that better reduce atmospheric background now provide for the precise measurement of respiratory parameters, including tidal volume, by whole-body plethysmography on conscious, unrestrained experimental animals over long periods and with repeated measures in the same animals (Lim et al., 2014), allowing for investigations under conditions more relevant to tobacco use and withdrawal. We also hypothesized that this technology would demonstrate differences in nicotine sensitivity extending over the half-life of nicotine, and that some of those differences would correlate with differences in nicotine metabolism related to strain and sex. Here we utilize high-precision whole-body plethysmography to demonstrate congenital differences between two commonly studied inbred mouse strains regarding sensitivity to acute nicotine but find those differences are not driven by variation in nicotine metabolism. These investigations lay valuable groundwork for further studies using highly sensitive respiratory phenotypes to dissect the genetically-determined mechanisms underlying variation in physiological responses to nicotine.
MATERIALS AND METHODS
Respiration measurements
All protocols were approved by the Washington University Institutional Animal Care and Use Committee and comply with the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals. 8-week-old C57BL/6J and C3H/HeJ mice were acquired from Jackson Laboratory (Bar Harbor, ME, USA) and used in experiments within two weeks. Respiratory parameters were measured in a Buxco Small Animal Whole Body Plethysmograph (Data Sciences International, St. Paul, MN, USA). Eight mice of each sex and strain were used for each experiment. Mice were acclimated to the plethysmograph chamber for one hour prior to each experiment. They were then removed from the chambers, injected subcutaneously with sterile 0.9% saline or saline containing 0.35 or 0.70 mg/kg nicotine (nicotine hydrogen tartate, Sigma-Aldrich, St. Louis, MO, USA), returned to the chambers and parameters measured for thirty minutes. Each mouse was used in three separate experiments performed at 24 hour intervals. Half of the mice in each strain/sex group were injected sequentially with 0.35, 0.00, and 0.70 mg/kg nicotine, while the other half were injected with 0.00, 0.70 and 0.35 mg/kg nicotine.
Time to first rear
To measure the sedative effects of nicotine, mice were observed in the plethysmograph chamber following injection. Mice that appeared sedated all lay flat on the bottom of the chamber within one minute following injection. The time each mouse first rose with both front paws off the bottom of the chamber was recorded as the time of first rearing, with the time of injection as time zero. Time of first rearing was recorded as zero for mice that did not lay flat in the chamber within one minute following injection.
Plasma nicotine measurements
8-week-old C57BL/6J and C3H/HeJ mice (Jackson Laboratory, Bar Harbor, ME, USA) were injected with 0.35 mg/kg nicotine (nicotine hydrogen tartate, Sigma-Aldrich, St. Louis, MO, USA), anesthetized with isoflurane and blood was drawn by terminal cardiac puncture after 5, 10, 20 or 30 minutes. Five males and five females of each strain were used for each time-point. Blood samples were immediately placed on ice, and centrifuged to separate plasma. Analytes were extracted with methanol including deuterated (d3)-nicotine-N-oxide (Toronto Research Chemicals, Toronto Canada) as an internal standard, evaporated to dryness in a Savant Speedvac (Thermo Fisher Scientific, Waltham, MA, USA), and resuspended in 100 mM Tricine buffer. LC-MS\MS analyses were performed on an API 4000 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems Sciex, Foster City, CA) equipped with an electrospray source. The HPLC system consisted of two LC 20AC pumps with a CTO-20A column over, SIL-20A autosampler, DGU-20A3 degasser, FCF-11AL valve, and a CBM 20A controller (Shimadzu, Columbia, MD, USA). The chromatographic separation was performed on an xBridge column (150 × 2.1, 3.5μm, Waters, Milford, MA, USA) with a pre-column inline 0.2 μm filter. The injection volume was 5 μl and the oven temperature was 40 °C. Mobile phase (0.3 ml/min) was (A) 4.5 mM ammonium acetate pH 4.0 and (B) 4.5 mM ammonium acetate in acetonitrile using the following program: 1% B for 4 minutes, linear gradient to 25% B between 4 and 5 minutes, held at 25% B until 6.0 minutes, and then re-equilibrated to initial conditions between 6.5 and 8.5 minutes. The instrument was operated in positive-ion mode at 450 °C with an ion spray voltage of 5500 V, entrance potential of 10 V and exit potential of 22 V. The curtain gas was set at 20, ion source gas 1 at 30, and ion source gas 2 at 40. Transitions monitored for nicotine were m/z 163→130 for nicotine and m/z 182→132 for (d3)-nicotine-N-oxide.
Data analysis
Respiratory parameters were measured and analyzed using FinePointe software (Data Sciences International, St. Paul, MN, USA) for thirty minutes following injection.. The four parameters measured were: 1) frequency, number of breaths per minute, 2) minute volume, total volume inhaled per minute, 3) tidal volume, volume per breath, and 4) inspiratory time, seconds per breath. Averages for a baseline period spanning the ten minutes prior to injection, and for three ten minute periods post-injection (0-10, 10-20 and 20-30 minutes) were calculated. Additional statistical tests were performed using the statistical software R (R Core Team).
To test for significant differences in respiratory parameters between sexes within each strain/treatment group, two-tailed t-tests were performed for each parameter at each time point. Differences between the three treatment groups (saline, 0.35, and 0.70 mg/kg nicotine) were subsequently tested by analysis of variance (ANOVA) combining both sexes within each strain for each parameter at each time point. To correct for multiple testing, individual ANOVA tests were considered statistically significant at a threshold of p<0.002. All reported p-values are for post-hoc two-tailed t-tests.
To test for significant differences in plasma nicotine concentrations between sexes within each strain, and between strains (combining both sexes), two-tailed t-tests were performed for each time point (3 tests per time point). To correct for multiple testing, individual tests were considered statistically significant at a threshold of p<0.003.
RESULTS
Effects of Acute Nicotine on Mouse Respiration
Male and female mice of two inbred strains, C57BL/6J and C3H/HeJ, were injected subcutaneously with two doses of nicotine, 0.35 or 0.70 mg/kg, and compared to controls injected with saline alone. 0.35 mg/kg was chosen and initially tested because it was previously shown to be reinforcing in C57BL/6J mice (Grieder et al., 2017). The C57BL/6J and C3H/HeJ strains were chosen because of their high prevalence in the scientific literature and distant relationship (Petkov et al., 2004). Four respiratory parameters were measured for thirty minutes by full-body plethysmography: 1) frequency, 2) minute volume, 3) tidal volume, and 4) inspiratory time. For analysis, each measurement was treated as the average over three ten minute intervals following injection. Baseline measurements were also collected for ten minutes prior to injection. Baseline respiratory parameters did not significantly differ between saline, 0.35, and 0.70 mg/kg nicotine-injected mice (data not shown).
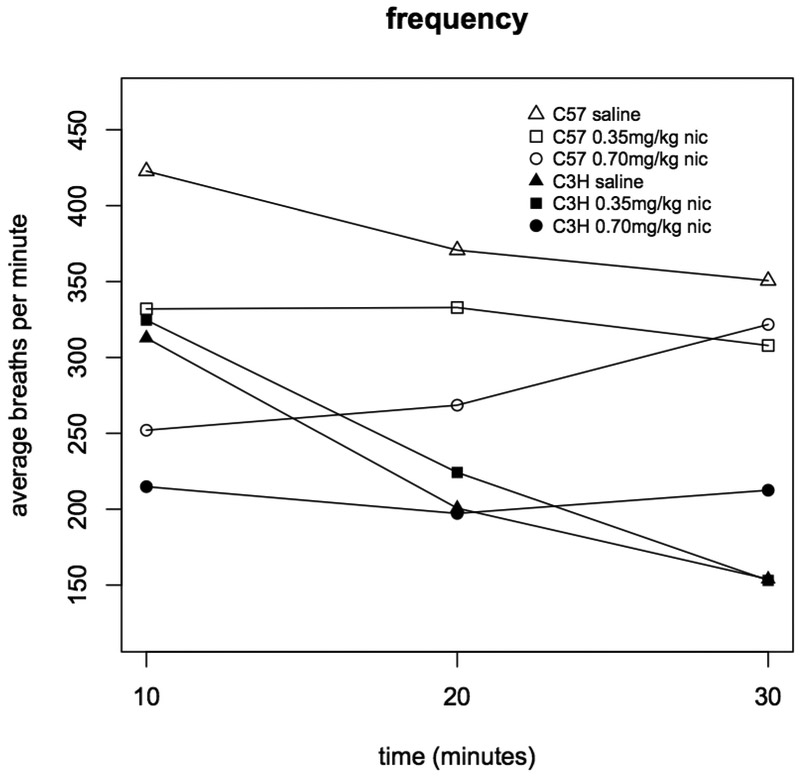
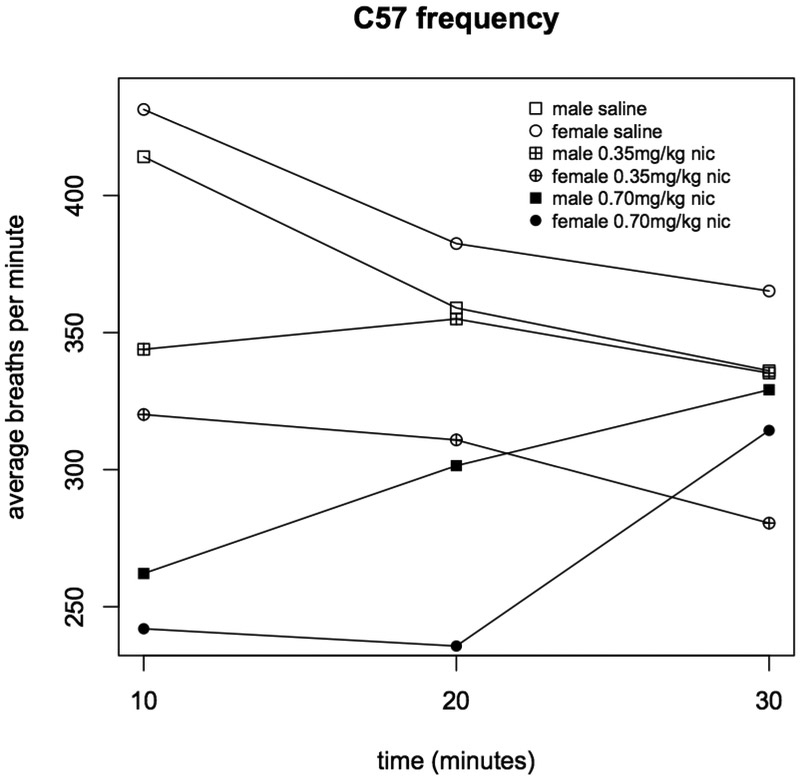
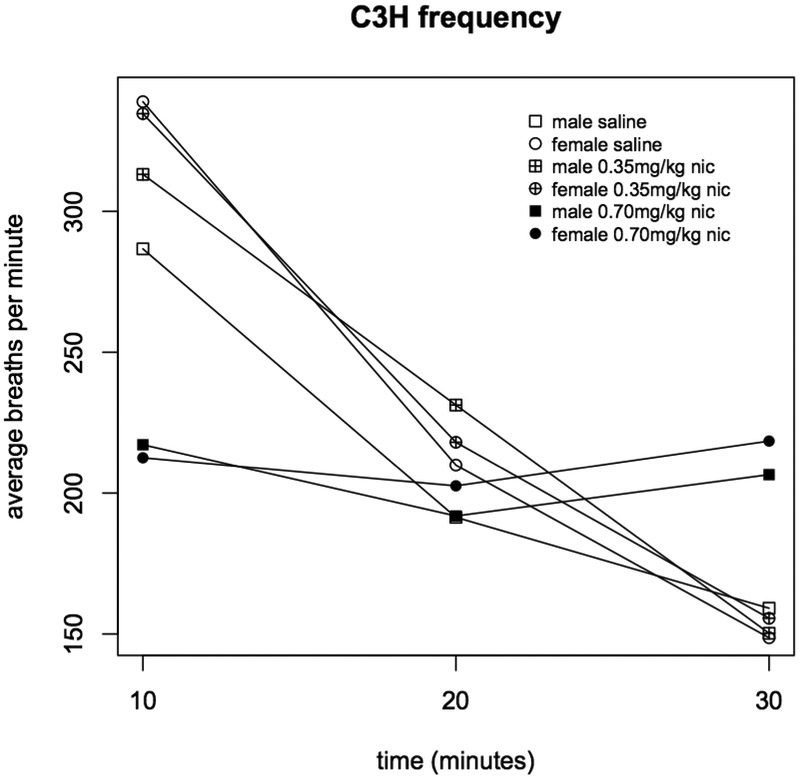
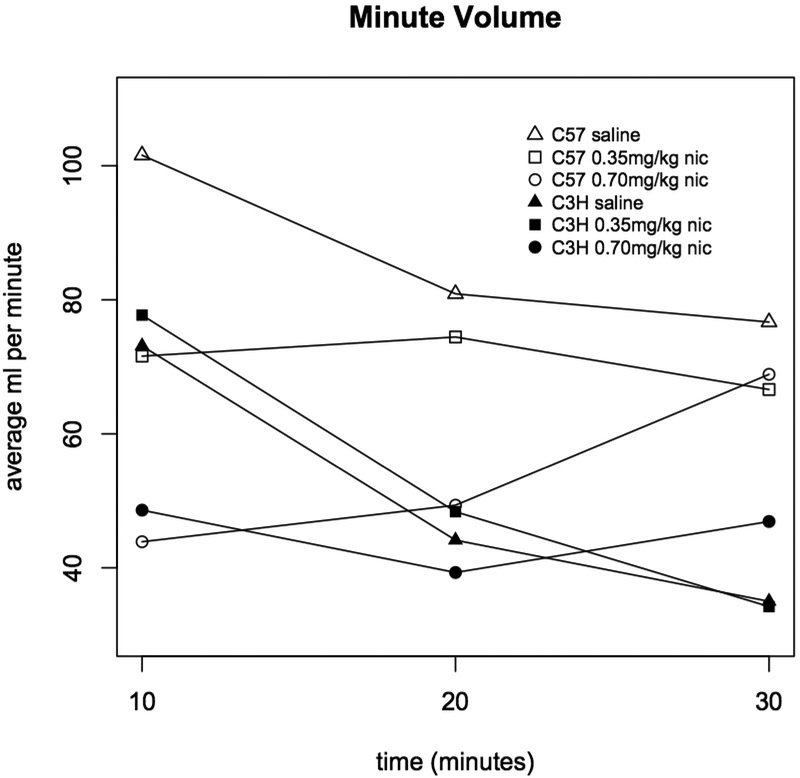
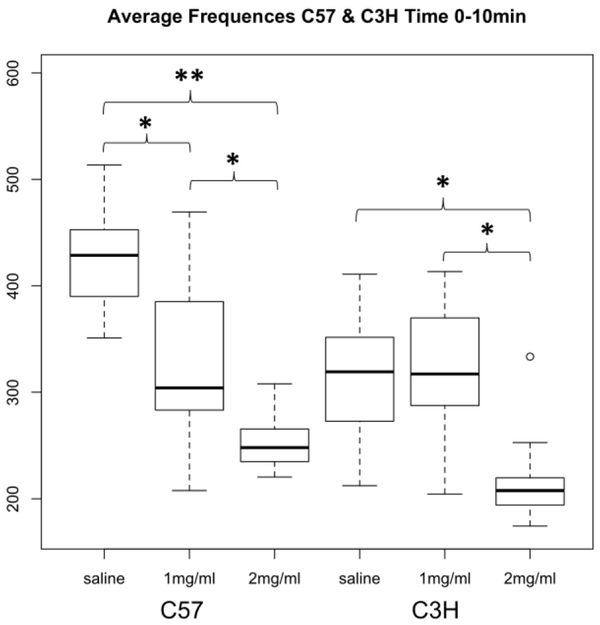
Injection with both 0.35 and 0.70 mg/kg nicotine significantly reduced respiratory frequency in C57BL mice (during the 0-10 min interval, saline vs. 0.35 mg/kg p=9.2×10−5, and saline vs. 0.70 mg/kg p=2.2×10−15, Figure 1). But, among C3H mice, only 0.70 mg/kg nicotine significantly affected respiratory frequency (0-10 min interval, saline vs. 0.70 mg/kg p=1.2×10−6, 0.35 vs. 0.70 mg/kg p=7.8×10−7, Figure 1). Frequency was not significantly associated with sex (Figures 2 & 3)nor was any other respiratory parameter (data not shown) for either strain under any condition. Both 0.35 and 0.70 mg/kg nicotine significantly affected minute volume, tidal volume, and inspiratory time among C57 mice, while only 0.70 mg/kg affected these parameters among C3H mice, mirroring nicotine effects on frequency (Figures 4, 5 & 6). For C57 mice, the higher dose (0.70 mg/kg) of nicotine had larger effects on all respiratory parameters than the lower dose (0.35 mg/kg).
Figure 1A. Respiratory frequency in C3H and C57 mice administered nicotine.
Average respiratory frequency (breaths per minute) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine. Each data point represents eight male and eight female mice. Statistically significant differences: C57 0-10 min saline vs. 0.35 mg/kg (p=9.2×10−5), saline vs. 0.70 mg/kg (p=2.2×10−15), and 0.35 vs. 0.70 mg/kg (p=3.9×10−4); C57 10-20 min saline vs. 0.70 mg/kg (p=6.3×10−6); C3H 0-10 min saline vs. 0.70 mg/kg (p=1.2×10−6) and 0.35 vs. 0.70 mg/kg (p=7.8×10−7); C3H 20-30 min saline vs. 0.70 mg/kg (p=1.1×10−5) and 0.35 vs. 0.70 mg/kg (p=1.8×10−5).
Figure 2. Respiratory frequency in C57 male and female mice administered nicotine.
Average respiratory frequency (breaths per minute) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine (n=8).
Figure 3. Respiratory frequency in C3H male and female mice administered nicotine.
Average respiratory frequency (breaths per minute) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine (n=8).
Figure 4. Minute volume in C3H and C57 mice administered nicotine.
Average minute volume (ml inhaled per minute) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine. Each data point represents eight male and eight female mice. Statistically significant differences: C57 0-10 min saline vs. 0.35 mg/kg (p=2.0×10−4), saline vs. 0.70 mg/kg (p=2.2×10−16), and 0.35 vs. 0.70 mg/kg (p=0.001); C57 10-20 min saline vs. 0.70 mg/kg (p=1.0×10−6), and 0.35 vs. 0.70 mg/kg (p=8.4×10−4); C3H 0-10 min saline vs. 0.70 mg/kg (p=1.6×10−6) and 0.35 vs. 0.70 mg/kg (p=6.0×10−6).
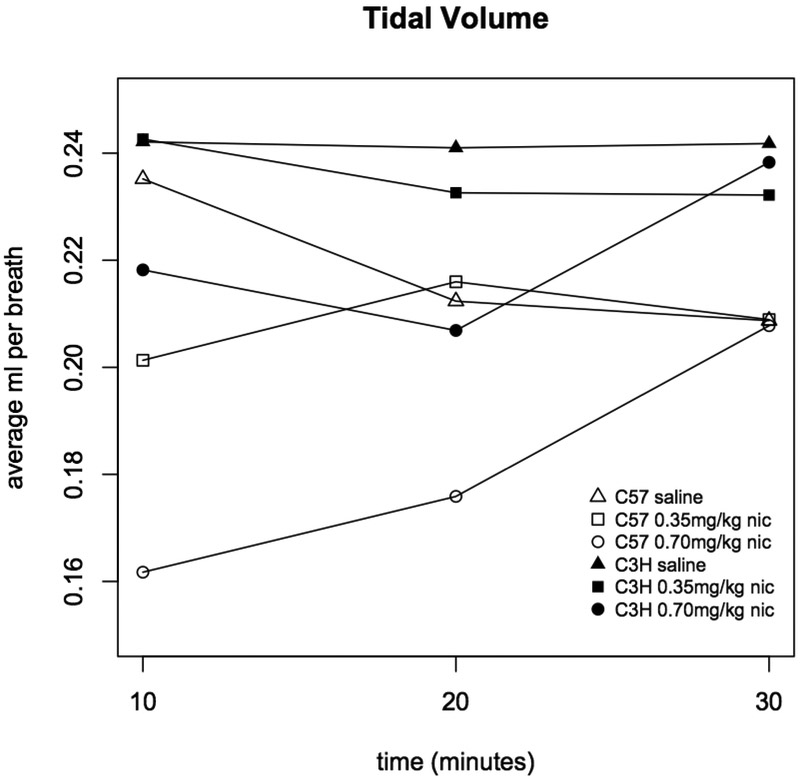
Figure 5. Tidal volume in C3H and C57 mice administered nicotine.
Average tidal volume (ml inhaled per breath) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine. Each data point represents eight male and eight female mice. Statistically significant differences: C57 0-10 min saline vs. 0.35 mg/kg (p=0.002), saline vs. 0.70 mg/kg (p=2.0×10−13), and 0.35 vs. 0.70 mg/kg (p=0.002); C57 10-20 min saline vs. 0.70 mg/kg (p=4.8×10−5), and 0.35 vs. 0.70 mg/kg (p=6.2×10−4); C3H 10-20 min saline vs. 0.70 mg/kg (p=0.001).
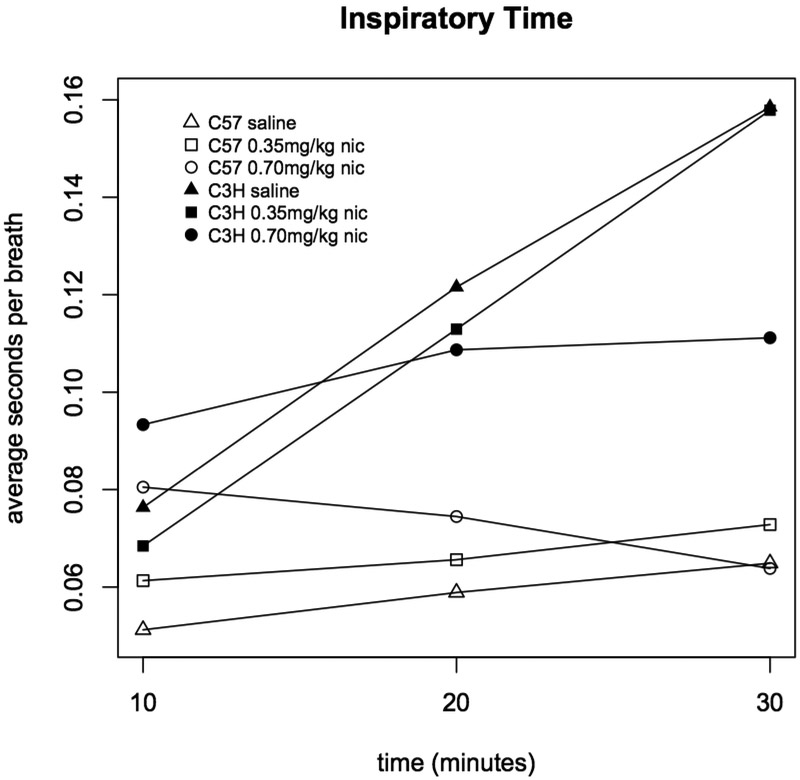
Figure 6. Inspiratory time in C3H and C57 mice administered nicotine.
Average inspiratory time (seconds per breath) was measured during three ten minute intervals following administration of saline, 0.35 or 0.70 mg/kg nicotine. Each data point represents eight male and eight female mice. Statistically significant differences: C57 0-10 min saline vs. 0.35 mg/kg (p=9.6×10−4), saline vs. 0.70 mg/kg (p=5.8×10−11), and 0.35 vs. 0.70 mg/kg (p=1.2×10−4); C57 10-20 min saline vs. 0.70 mg/kg (p=2.4×10−4); C3H 0-10 min 0.35 vs. 0.70 mg/kg (p=6.3×10−6).
For both mouse strains, frequency was highly correlated with minute volume and inspiratory time (R2>0.90 for minute volume and >0.77 for inspiratory time across the three intervals), and minute volume and inspiratory time were highly correlated (R2>0.64). The exceptional parameter was tidal volume; among the C57BL/6J mice, across the three intervals, tidal volume was moderately correlated with the other three parameters (R2>0.47), but, interestingly, among the C3H/HeJ mice, tidal volume was poorly correlated with minute volume (R2=0.11) and not correlated with frequency (R2=0.005) or inspiratory time (R2=0.006).
Sedative Effects of Acute Nicotine
During plethysmograph experiments, mouse rearing was monitored. Many of the mice injected with nicotine appeared sedated within one minute following injection, i.e. they flattened their bodies against the bottom of the chamber and reduced their movements for several minutes. Parallel to nicotine effects on respiration, this was true of all C57 mice and fifteen of sixteen C3H mice injected with 0.70 mg/kg, 56% of C57 mice injected with 0.35 mg/kg and only one of sixteen C3H mice injected with 0.35 mg/kg nicotine. None of the mice injected with saline displayed sedation.
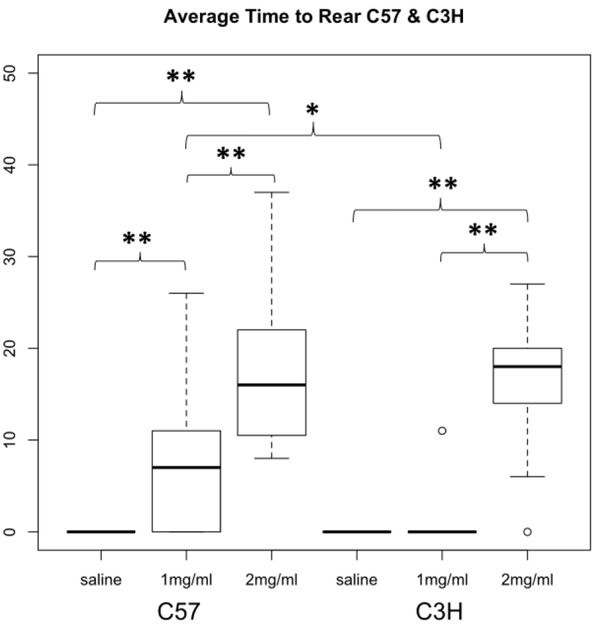
In order to quantify these affects, we measured the time at which mice first rose on their hind legs (time until first rear) following the sedated period after injection, sometimes exceeding thirty minutes. Mice that did not appear sedated were measured as zero minutes. Interestingly, time until first rear did not significantly differ between C57 and C3H mice injected with 0.70 mg/kg (17.4 vs. 16.8 minutes respectively, Figure 7).
Figure 7. Time to first rear following nicotine administration in C3H and C57 mice.
Average time to first rear (minutes). Each data point represents eight male and eight female mice. The boxplots provide summaries of the data distributions for each group consisting of eight male and eight female mice. A box represents the interquartile range, which includes 50% of values. The line across the box indicates the median. The whisker lines extend to the highest and lowest values that are within 1.5x the interquartile range. Further outliers are marked with circles. * p=0.01, ** p<0.004.
Nicotine Plasma Concentrations
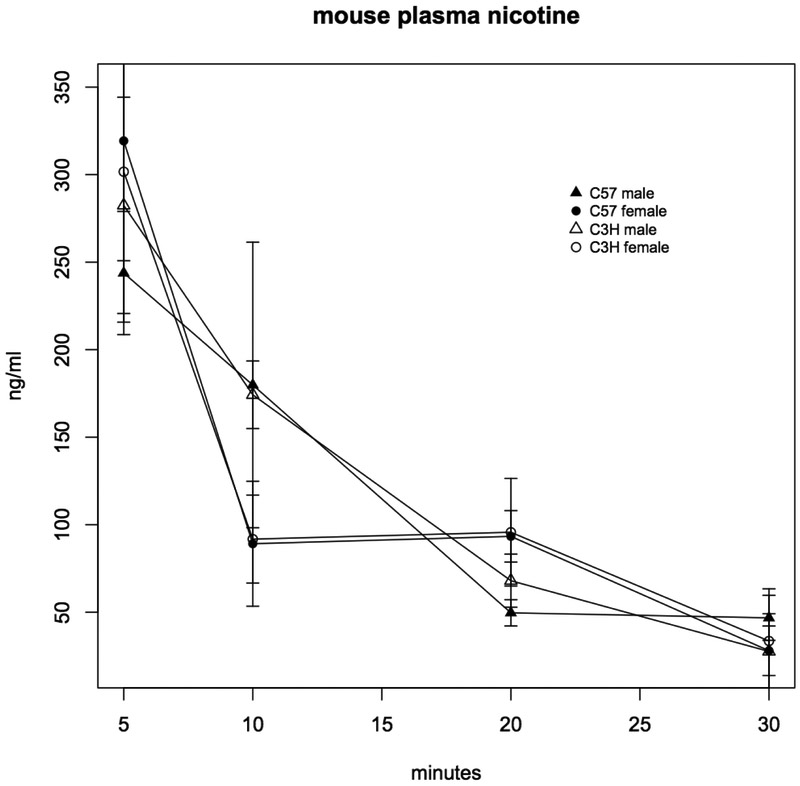
To determine whether strain differences in nicotine effects on mouse respiration and activity were related to differences in nicotine plasma concentrations, C57BL/6J and C3H/HeJ mice were injected subcutaneously with 0.35 mg/ml nicotine, and plasma nicotine measurements were made 5, 10, 20 and 30 minutes following injection. Although plasma nicotine concentrations differed by sex, with nicotine concentrations lower after 20 minutes in both strains (C3H p=0.001, C57 p=0.052), there was no difference in concentrations between strains (Figure 8).
Figure 8. Plasma nicotine concentrations in C3H and C57 mice.
Nicotine concentrations were measured in plasma 5, 10, 20 and 30 minutes following subcutaneous injection with 0.35 mg/kg nicotine. Each data point is the mean ± SD (n=5). Only the difference between C3H males and females at 10 minutes is statistically significant (p=0.001). C57 males vs. females p=0.052.
DISCUSSION
Both acute nicotine and nicotine withdrawal alter respiratory parameters in human subjects and experimental animals, but nicotine’s effects on respiration are little studied in whole animals due to the challenges of measuring respiratory metrics compared to phenotypes such as heart-rate, blood-pressure or locomotion. Unrestrained whole-body plethysmography offers a precise, non-invasive method to measure respiratory phenotypes in experimental animals, avoiding the stresses or anesthesia common to traditional plethysmography techniques (Gargiulo et al., 2012; Lim et al., 2014).
Nicotinic acetylcholine receptors expressed in the brainstem and spinal cord mediate central cholinergic regulation of respiration, including through their role in chemosensitivity, the ability of the nervous system to sense and respond to changing concentrations of CO2 in the blood. Acetylcholine and nicotine activate receptors in the preBötzinger complex, the site of normal respiratory rhythm generation in mammals, depolarizing inspiratory neurons and increasing respiratory rate (Shao and Feldman, 2009). Consistent with this, prior studies showed increased respiratory rate in most mouse strains tested one minute following intraperitoneal injection of nicotine at most concentrations (Marks et al., 1989). On the other hand, nicotine can also produce sedative effects in both humans and rodents (Acri, 1994; Umezu, 2012; Bernardi and Spanagel, 2014).
In the current study, we used the power of high-precision whole-body plethysmography to compare nicotine sensitivity between two well-studied inbred mouse strains, C57BL/6J and C3H/HeJ. We saw significant effects on both respiratory parameters and activity (as measured by time to first rear) at a high dose of nicotine in both strains, but a clear strain difference in sensitivity to nicotine at a lower nicotine dose. Interestingly, while the effects of nicotine on respiration were the most obvious immediately following nicotine administration, i.e. within ten minutes of subcutaneous injection, they persisted for thirty minutes, and in paradoxical ways. For example, there is a consistent pattern among all subsets of mice that displayed significantly decreased respiratory rate immediately following nicotine injection—i.e. male and female C57BL/6J mice that received 0.35 or 0.70 mg/kg, and male and female C3H/HeJ mice that received 0.70 mg/kg nicotine—relative to control mice, and C3H/HeJ mice that only received 0.35 mg/kg nicotine (Figures 1, 2 & 3). Saline-injected control mice, and C3H/HeJ mice injected with 0.35 mg/kg, displayed relatively rapid respiration initially, slowing as the mice remained in the plethysmograph chamber over the full thirty-minute duration of the experiment. By comparison, the groups of nicotine-injected mice that displayed relatively reduced respiratory rates initially, also displayed steady or increasing respiratory rates over time, sometimes rising above saline-injected controls by the final ten-minute period. Among the C57BL/6J mice, the trajectories of changing respiration rates even nicely parallel nicotine dose, with saline-injected mice displaying the highest initial rate, falling over time, 0.70 mg/kg injected mice displaying the lowest initial rate, rising over time, and 0.35 mg/kg injected mice displaying an intermediate initial rate that remained steady (Figure 1). These patterns also appeared consistent across the other three correlated phenotypes (Figures 4, 5 & 6). Our unintuitive results highlight the complexity of nicotine’s effects on arousal and respiration, and reinforce the value of whole body plethysmography performed over relatively long periods, to assay these sensitive phenotypes.
Strain-specific variation in nicotine metabolism, an obvious mechanism to explain differences in nicotine-sensitivity, has also been demonstrated (Poca et al., 2017). We therefore assayed nicotine clearance in both male and female C57BL/6J and C3H/HeJ mice subcutaneously injected with the nicotine dose (0.30 mg/kg) where we saw divergent sensitivity to nicotine. Sexual dimorphism has been demonstrated for various nicotine-related phenotypes in mice (Damaj, 2001), as well as for nicotine metabolism in humans (Bloom et al., 2011), and consistent with this, we found differences in clearance between male and female mice of both strains (Figure 8). However, we did not find significant differences between strains for either male or female mice, leading us to conclude that variation in nicotine metabolism is not driving the difference in nicotine sensitivity.
Activation of nicotinic acetylcholine receptors in the hypothalamic melanocortin system also alters blood glucose homeostasis in rodents (Takahashi et al., 1998; Mineur et al., 2011), with acute injections of nicotine inducing hypoglycemia and insulin resistance in C57BL/6J mice (Vu et al., 2014). In turn, alterations in metabolic rate directly influence ventilation parameters (Ishiguro et al., 2006). Thus, respiration phenotypes can reflect factors that mediate nicotine’s effects on metabolic rate, and in future studies it will be important to measure serum glucose and lipid levels in tandem with whole-body plethysmography in order to isolate this mechanism of nicotine’s action.
Genetic differences between mouse strains, especially natural variability effecting nicotinic receptor subunits, is associated with divergent responses to nicotine (Jackson et al., 2009; Wilking and Stitzel, 2015), including effects on respiratory rate (Marks et al., 1989). Our results extend that prior work by assaying further respiratory phenotypes over a relatively extended period. Importantly, although the phenotypes measured were largely correlated with one another, this was not always the case. In particular, nicotine’s differing effect on inspiratory time between the two studied mouse strains was not consistent with differences in other phenotypes i.e. respiratory frequency and volume. Additionally, the relationship between dose and its effects upon respiratory metrics reversed over time following acute administration and could not have been extrapolated from a briefer time course. Thus, precise measurement of respiratory phenotypes by whole body plethysmography in conscious, unrestrained animals, provides another powerful tool to tease apart the factors, including genetic variation, that underlie nicotine sensitivity.
Figure 1B. Distribution of respiratory frequency in C3H and C57 mice during the 10 minutes following nicotine administration.
Average respiratory frequency (breaths per minute) during the first ten minutes following administration of saline, 0.35 or 0.70 mg/kg nicotine. The boxplots provide summaries of the data distributions for each group consisting of eight male and eight female mice. A box represents the interquartile range, which includes 50% of values. The line across the box indicates the median. The whisker lines extend to the highest and lowest values that are within 1.5x the interquartile range. Further outliers are marked with circles. * p<4.0×10−4, ** p=2.2×10−15.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Drug Abuse, grant K01 DA034035, and The Longer Life Foundation, a Reinsurance Group of America/ Washington University Partnership. Thanks to Prof. Evan Kharasch at Duke University for his guidance, comments on this manuscript, and the use of his mass spectrometer. Thanks to Prof. Qin Liu at Washington University in St. Louis for assistance and the use of her plethysmograph.
Footnotes
DECLARATION OF INTEREST STATEMENT
The authors declare no interests.
REFERENCES
- Acri JB (1994) Nicotine modulates effects of stress on acoustic startle reflexes in rats: dependence on dose, stressor and initial reactivity. Psychopharmacology (Berl) 116:255–265. [DOI] [PubMed] [Google Scholar]
- Avraam J, Cohen G, Drago J, and Frappell PB (2015) Prenatal nicotine exposure increases hyperventilation in alpha4-knock-out mice during mild asphyxia. Respiratory physiology & neurobiology 208:29–36. [DOI] [PubMed] [Google Scholar]
- Bamford OS and Carroll JL (1999) Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol 117:29–40. [DOI] [PubMed] [Google Scholar]
- Bernardi RE and Spanagel R (2014) Basal activity level in mice predicts the initial and sensitized locomotor response to nicotine only in high responders. Behav Brain Res 264:143–150. [DOI] [PubMed] [Google Scholar]
- Bloom J, Hinrichs AL, Wang JC, von Weymarn LB, Kharasch ED, Bierut LJ, Goate A, and Murphy SE (2011) The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics 21:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MW, Serebrisky D, and Castaldelli-Maia JM (2016) Smoking and Cognition. Current drug abuse reviews 9:76–79. [DOI] [PubMed] [Google Scholar]
- Collins AC, Miner LL, and Marks MJ (1988) Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol Biochem Behav 30:269–278. [DOI] [PubMed] [Google Scholar]
- Damaj MI (2001) Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther 296:132–140. [PubMed] [Google Scholar]
- Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, and von Bernhardi R (2008) Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci 28:13907–13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, and Vesce G (2012) Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR journal 53:E55–69. [DOI] [PubMed] [Google Scholar]
- Grieder TE, George O, Yee M, Bergamini MA, Chwalek M, Maal-Bared G, Vargas-Perez H, and van der Kooy D (2017) Deletion of alpha5 nicotine receptor subunits abolishes nicotinic aversive motivational effects in a manner that phenocopies dopamine receptor antagonism. Eur J Neurosci 46:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B (2015) Nicotinic receptors and attention. Current topics in behavioral neurosciences 23:103–135. [DOI] [PubMed] [Google Scholar]
- Henningfield JE (1984) Pharmacologic basis and treatment of cigarette smoking. J Clin Psychiatry 45:24–34. [PubMed] [Google Scholar]
- Huang YH, Brown AR, Costy-Bennett S, Luo Z, and Fregosi RF (2004) Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respiratory physiology & neurobiology 143:1–8. [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Iwase M, Kanamaru M, Izumizaki M, Ohshima Y, Homma I (2006) Contribution of histamine type-1 receptor to metabolic and behavioral control of ventilation. J Physiol Sci 56:287–95. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Walters CL, Miles MF, Martin BR, and Damaj MI (2009) Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology 57:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA (1987) Cigarettes, respiratory rate, and the relaxation paradox. Int J Addict 22:803–809. [DOI] [PubMed] [Google Scholar]
- Lim R, Zavou MJ, Milton PL, Chan ST, Tan JL, Dickinson H, Murphy SV, Jenkin G, and Wallace EM (2014) Measuring respiratory function in mice using unrestrained whole-body plethysmography. Journal of visualized experiments : JoVE:e51755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH and Goyarzu P (2009) Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol:401–434. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, and Collins AC (1989) Genetic influences on nicotine responses. Pharmacol Biochem Behav 33:667–678. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR (2011) Nicotine decreases food intake through activation of POMC neurons. Science 332:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omvik P (1996) How smoking affects blood pressure. Blood Press 5:71–77. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, and Wiles MV (2004) An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 14:1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poca KS, Parente TE, Chagas LF, Leal BS, Leal HS, Paumgartten FJ, and De-Oliveira AC (2017) Interstrain differences in the expression and activity of Cyp2a5 in the mouse liver. BMC research notes 10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, and Funk GD (2002) Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol 538:957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM and Feldman JL (2009) Central cholinergic regulation of respiration: nicotinic receptors. Acta pharmacologica Sinica 30:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A1, Kishi E, Ishimaru H, Ikarashi Y, Maruyama Y (1998) Stimulation of rat hypothalamus by microdialysis with K+: increase of ACh release elevates plasma glucose. Am J Physiol 275 1647–53. [DOI] [PubMed] [Google Scholar]
- Tonstad S and Andrew Johnston J (2006) Cardiovascular risks associated with smoking: a review for clinicians. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology 13:507–514. [DOI] [PubMed] [Google Scholar]
- Umezu T (2012) Unusual effects of nicotine as a psychostimulant on ambulatory activity in mice. ISRN pharmacology 2012:170981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duinen MA, Niccolai V, and Griez EJ (2010) Challenging anxiety: a focus on the specificity of respiratory symptoms. Current topics in behavioral neurosciences 2:229–250. [DOI] [PubMed] [Google Scholar]
- Vu CU, Siddiqui JA, Wadensweiler P, Gayen JR, Avolio E, Bandyopadhyay GK, Biswas N, Chi NW, O’Connor DT, Mahata SK (2014) Nicotinic acetylcholine receptors in glucose homeostasis: the acute hyperglycemic and chronic insulin-sensitive effects of nicotine suggest dual opposing roles of the receptors in male mice. Endocrinology 155:3793–805. [DOI] [PubMed] [Google Scholar]
- Wilking JA and Stitzel JA (2015) Natural genetic variability of the neuronal nicotinic acetylcholine receptor subunit genes in mice: Consequences and confounds. Neuropharmacology 96:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]