Abstract
Background:
Inflammation has been hypothesized to contribute to reward dysfunction across psychiatric conditions, but little is known about this relationship in youth. Therefore, the present study investigated the associations between general and specific markers of inflammation and neural activation during reward processing, including anticipation and attainment, in youth with diverse psychiatric symptoms.
Methods:
Forty-six psychotropic medication-free youth with diverse psychiatric symptoms underwent a blood draw to measure 41 cytokines, as well as structural and functional magnetic resonance imaging. The Reward Flanker Task examined neural activation during reward anticipation and attainment. Relationships between inflammation and neural activation were assessed using data reduction techniques across the whole-brain, as well as in specific reward regions of interest (basal ganglia, anterior and mid-cingulate cortex [ACC/MCC]).
Results:
Whole-brain principal component analyses showed that factor 3 (12 cytokines: FGF-2, Flt3-L, frac-talkine, GM-CSF, IFN-α2, IFN-γ, IL-3, IL-4, IL-7, IL-17A, MDC, and VEGF) was negatively correlated with pre-cuneus/posterior cingulate cortex activity during anticipation. Factor 2 (11 cytokines: eotaxin, IL-1α, IL-1Rα, IL-2, IL-5, IL-9, IL-12P40, IL-13, IL-15, MCP-3, and TNF-β) was negatively correlated with angular gyrus activity during attainment. ROI analyses additionally showed that multiple cytokines were related to activity in the basal ganglia (EGF, FGF-2, Flt-3L, IL-2, IL-13, IL-15, IL-1Rα, MCP-3) and ACC/MCC (Flt-3L) during attainment. C-reactive protein (CRP) was not associated with neural activation.
Conclusions:
Investigation of specific markers of immune function showed associations between inflammatory processes and activation of posterior default mode network, prefrontal cortex, and basal ganglia regions during multiple phases of reward processing.
Keywords: Reward processing, Functional magnetic resonance imaging, Reward anticipation, Reward attainment, Peripheral inflammation, Youth
1. Introduction
There has been extensive evidence in both adults (Felger, 2017; Felger et al., 2016; Felger and Lotrich, 2013; Felger and Treadway, 2017; Miller et al., 2013) and adolescents (Gabbay et al., 2009b; Gabbay et al., 2009c; Miklowitz et al., 2016; Mitchell and Goldstein, 2014) that inflammation has a role in the development of major depressive disorder (MDD). However, patterns of increased inflammation have also been noted across other psychiatric disorders and age groups (Gabbay et al., 2009a; Najjar et al., 2013). Together, these findings suggest that inflammation is not specific to one diagnostic category, but rather related to some overlap of symptoms between disorders and shared etiology.
One theory to explain this phenomenon is that inflammation induces alterations within the reward circuitry, a salient feature in many psychiatric conditions (Der-Avakian and Markou, 2012; Felger and Treadway, 2017; Swardfager et al., 2016). There is support for the link between immune system functioning and reward dysfunction in both preclinical and clinical research. For example, in animal models, immunological challenges, such as exposure to endotoxins (e.g., lipopolysaccharide; LPS), often produce sickness behaviors characterized by sleep disturbances, reduced consumption of food, as well as decreased locomotion, social interaction, and sexual behavior (Dantzer, 2009; De La Garza, 2005; Hart, 1988; Konsman et al., 2002; Yirmiya et al., 2001). All of these behaviors share phenomenological similarity to clinical symptoms that reflect reward dysfunction, such as anhedonia, fatigue, malaise, anorexia, and decreased sexual desire (Maes et al., 2012). Sickness behaviors such as these may be an adaptive response that allows the conservation of energy to facilitate healing and recovery from illness.
Clinical studies of healthy adults have also shown that administration of immunological challenges such as endotoxins alters activation within reward circuits (Eisenberger et al., 2010; Inagaki et al., 2015; Muscatell et al., 2016). Eisenberger et al. (2010) found that in healthy adults, exposure to endotoxin increased depressed mood and reduced activation in a key reward-related region, the ventral striatum (VS), in response to monetary reward anticipation. Inagaki et al. (2015) alternately found that healthy adults exposed to endotoxin had increased motivation to be near social support figures, and this was associated with greater VS activation. Similarly, endotoxin administered to healthy adults also induced increased activation in reward regions, namely the VS and ventromedial prefrontal cortex (vmPFC), in response to social reward feedback (Muscatell et al., 2016). From these studies, it seems that the type of reward given (i.e., monetary or social) influences the direction of VS activation in response to an immunological challenge; monetary rewards elicit reduced VS activation, while social rewards elicit increased VS activation. Another study by Slavich et al. (2010) similarly documented that psychological stress was associated with increased interleukin-6 and a soluble receptor for tumor necrosis factor-a, the latter of which was subsequently correlated with increased neural activation in the anterior cingulate cortex (ACC) and insula during social rejection. Taken together, these data support the possible role of inflammation in neural function during different types of monetary and social reward tasks.
Despite the above evidence linking inflammation to alterations in activation of the reward circuitry in adults, there has been scarce neuroimmunological research in relation to reward processing in adolescence. It is important for investigations to target adolescence, as this is a period of time during which many psychiatric disorders first emerge (Galvan, 2017; Jaworska and MacQueen, 2015; Kessler et al., 2005). It has been suggested that maturational alterations within the rapidly changing reward circuitry during adolescence may trigger the emergence of psychiatric symptoms (Fairchild, 2011). Moreover, investigations of youth also allow us to better examine neurobiological mechanisms of dysfunction prior to the cumulative effects of aging, treatment, and disease chronicity.
In order to bridge the abovementioned gap in the literature, we aimed to assess the relationships between a wide 41-cytokine panel, as well as a more general marker of inflammation, C-reactive protein (CRP), and reward circuitry activation in youth with diverse psychiatric symptoms. We used a research domain criteria (RDoC) approach and did not sample one specific diagnostic category since reward deficits are salient across many psychiatric conditions (Freed et al., 2018; Hagele et al., 2015) and might therefore share the same biological underpinnings. Additionally, we investigated a wide panel of cytokines, including hematopoietic growth factors, chemokines, proinflammatory and anti-inflammatory cytokines, due to the complexity of the immune system. Since reward function reflects multiple reward processes, we used the Reward Flanker Task (RFT) during functional magnetic resonance imaging (fMRI) to study brain activation during two key reward processes, reward anticipation and attainment. In our prior pilot of the RFT, we examined neural activation patterns during distinct phases of reward processing in youth with diverse psychiatric symptoms. We documented a much larger network of regions was involved in reward processing beyond the traditionally identified PFC and VS, especially during reward attainment (Bradley et al., 2016). More specifically, we showed that while certain regions such as the PFC and VS were more activated during reward anticipation, other more posterior regions such as the cuneus, precuneus, posterior cingulate cortex (PCC), etc. were more activated during reward attainment (Bradley et al., 2016).
Here, as an extension of this pilot, we utilized a novel data-driven analytical approach that used principal component analysis to condense a 41-cytokine panel into ‘inflammatory factors’ that were then correlated with whole-brain activation during the RFT. Given the exploratory nature of the factor analysis that reduced the 41-cytokine panel into ‘inflammatory factors’, we additionally included an examination of the relationship between a more general and well-known marker of peripheral inflammation, CRP, and whole-brain neural activation during the RFT. Lastly, due to the preponderance of evidence specifically implicating the PFC and VS in reward dysfunction in depression (Bradley et al., 2016; Haber, 2011; Silverman et al., 2015; Urban et al., 2012; Zhang et al., 2013), we also included an analysis of the correlations between a priori selected reward-related regions of interest (ROIs; the bilateral basal ganglia and anterior cingulate cortex [ACC]/mid-cingulate cortex [MCC]) and all cytokines.
Based on prior research in adults, we hypothesized that reduced VS and PFC activity would be associated with increased cytokine plasma levels, especially during reward anticipation, based on findings in adults (Eisenberger et al., 2010). Furthermore, we also hypothesized that more posterior reward-related brain regions (e.g., precuneus, PCC, etc.) would show reduced activation in association with increased inflammation, especially during consummatory reward processes such as attainment of reward, given the findings from our original pilot of the RFT (Bradley et al., 2016).
2. Methods
2.1. Participants
The current sample consisted of 46 youth, all psychotropic medication-free, ages 12–20 years old, 22 of which were included in a prior published pilot of the Reward Flanker Task (Bradley et al., 2016). In order to examine of a full range of reward function and examine the relationship between inflammation and reward processing dimensionally according to the RDoC framework, both youth with and without psychiatric symptoms were sampled. Individuals with current psychiatric symptomatology were recruited, whether or not diagnostic criteria for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-Text Revision (DSM-IV-TR) disorders (APA, 2000) were met. Thirty-four participants displayed psychiatric symptoms, predominantly of mood and anxiety disorders, but also included obsessions, compulsions, attention, and impulse control difficulties (see Section 3.1 and Table 2 for full diagnostic details). Twelve participants did not display any current or past psychiatric illnesses. All participants were free of major neurological or medical conditions, including acute inflammatory conditions such as the flu or common cold.
Table 2.
Demographic and Clinical Characteristics of the Study Sample.
| Demographic Variables | Sample (n = 46) |
|---|---|
| Age [mean ± SD] (Range) | 16.43 ± 2.24 (12–20) |
| Sex [n female] (%) | 25 (54%) |
| Ethnicity [n] (%) | |
| Caucasian | 19 (41%) |
| African American | 17 (37%) |
| Asian | 1 (2%) |
| Other | 9 (20%) |
| Clinical Variables | |
| [mean ± SD] (Range) | |
| SHAPS | 22.49 ± 6.42 (14–38) |
| CDRS-R | 31.80 ± 15.69 (17–78) |
| BDI-II | 10.09 ± 11.16 (0–47) |
| BSSI | 1.98 ± 3.71 (0–15) |
| MASC Total | 37.25 ± 18.67 (2–78) |
| Illness History | |
| No history of psychiatric illness [n] (%) | 12 (26%) |
| Psychiatric symptoms [n] (%) | 34 (74%) |
| Depressive disorder | 19 (56%) |
| Bipolar disorder | 3 (9%) |
| Anxiety disorder | 19 (56%) |
| ADHD | 10 (29%) |
| Behavior disorder | 6 (18%) |
Abbreviations: ADHD = attention-deficit/hyperactivity disorder; BDI-II = Beck Depression Inventory, Second Edition; BSSI = Beck Scale of Suicidal Ideation; CDRS-R = Children’s Depression Rating Scale-Revised; MASC = Multidimensional Anxiety Scale for Children; Snaith-Hamilton Pleasure Scale.
General exclusionary criteria included an IQ below 80 on the Kaufmann Brief Intelligence Test [KBIT; (Kaufman and Kaufman, 1990)] and MRI contraindications. Additionally, a positive drug toxicology test on the day of the MRI scan and a positive pregnancy test in females were exclusionary. Immune-modulating medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), were not allowed in the two weeks prior to the blood draw and scan for all participants. Moreover, suicidal ideations requiring immediate medical attention were also exclusionary. As noted, participants with psychiatric symptoms were required to be psychotropic medication-free for at least one month prior to the scan (or 3 months for drugs with a longer half-life such as fluoxetine). Current psychosis and substance abuse disorders, as well as pervasive developmental disorder, were exclusionary due to concerns that they would not be able to participate in the RFT.
2.2. Procedures
Participants underwent a full clinical evaluation using the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version [KSADS-PL; (Kaufman et al., 1997)] by either a board-certified child/adolescent psychiatrist or a licensed clinical psychologist trained in administration of the KSADS-PL. The final clinical report was discussed between the Principal Investigator, a licensed child/adolescent psychiatrist, and the assessor in order to ensure diagnostic accuracy. While only validated for use through age 17 (Kaufman et al., 1997), the KSADS-PL was used for all participants up to age 20 for consistency and because the age range of ‘adolescence’ is still debated and may extend into the twenties due to continued frontal lobe development (Jaworska and MacQueen, 2015).
Participants also underwent a fasting blood draw (for plasma cytokines) in the morning, immediately prior to an fMRI scan that took place that same day and included both structural and functional imaging. The fasting blood draw was conducted in a child research clinic in a hospital setting by a nurse trained to draw blood in pediatric patients. A parent or guardian was allowed to be present during the blood draw in order to provide comfort to the child and alleviate stress. All procedures were approved by the Institutional Review Boards of the Icahn School of Medicine at Mount Sinai and the Nathan S. Kline Institute of Psychiatric Research. Signed consent was obtained by participants 18 years and older; those under 18 provided signed assent, and a parent or legal guardian provided signed consent.
2.3. Behavioral assessments
In addition to the full clinical evaluation using the KSADS-PL, depression severity was assessed using the clinician-rated Children’s Depression Rating Scale-Revised [CDRS-R; (Poznanski et al., 1985; Poznanski et al., 1984; Poznanski and Mokros, 1996)]. Anhedonia was quantified using the self-rated Snaith-Hamilton Pleasure Scale [SHAPS; (Snaith et al., 1995)], and anxiety was assessed using the self-rated multidimensional anxiety scale for children [MASC; (March et al., 1997)]. Lastly, suicidality was assessed using the self-rated Beck Scale for Suicide Ideation [BSS; (Beck et al., 1979)].
2.4. Multiplex analysis of immune biomarkers
Blood samples for all participants were collected in the morning after an overnight fast and processed within 20 min of collection; samples were stored at −80 °C. Sera cytokine profiles were measured using a Luminex-200 system and the XMap Platform (Luminex Corporation), and xPONENT software was used to analyze acquired fluorescence data. Forty-one peripheral inflammation markers (see Table 1 for a full list, abbreviations, and descriptive statistics) were determined in duplicate 25 μL volumes of plasma or serum using the multiplex cytokine panel (Multiplex High Sensitivity Human Cytokine Panel, Millipore Corp.). Using this system, multiple targets can be measured and analyzed simultaneously from a single sample. A bead-based multiplex system is used in which all 41 cytokines are measured in a single well using microsphere beads coated with analyte specific antibodies. Microspheres are individually examined in a fast flowing fluid stream as they pass through the Luminex analyzer. High-speed digital signal processing quantifies the reaction and classifies microspheres based on their spectral addresses.
Table 1.
Cytokines, Abbreviations, and Descriptive Statistics.
| Cytokines | Abbreviation | Mean (SD) | Range |
|---|---|---|---|
| Epidermal growth factor | EGF | 317.91 (380.71) | 17.00–1599.25 |
| Eotaxin | Eotaxin | 120.08 (136.27) | 19.00–861.50 |
| Fibroblast growth factor-2 | FGF-2 | 22.11 (10.49) | 9.25–60.75 |
| FMS-like tyrosine kinase 3-ligand | Flt3-L | 27.91 (9.11) | 10.00–46.50 |
| Fractalkine | Fractalkine | 20.51 (6.92) | 8.50–38.50 |
| Granulocyte-macrophage colony-stimulating factor | GM-CSF | 26.41 (12.31) | 10.50–62.00 |
| Granulocyte colony-stimulating factor | G-CSF | 30.41 (18.77) | 7.00–85.50 |
| Growth regulated oncogene | GRO | 2610.93 (3612.34) | 25.00–10883.50 |
| Interferon-alpha 2 | IFN-α2 | 29.10 (22.26) | 10.00–156.50 |
| Interferon-gamma | IFN-γ | 47.40 (51.05) | 11.00–278.50 |
| Interleukin-1 alpha | IL-1α | 37.93 (22.28) | 12.00–122.00 |
| Interleukin-1 beta | IL-1β | 151.48 (800.53) | 11.50–5457.50 |
| Interleukin-1 receptor antagonist | IL-1Rα | 84.03 (165.07) | 15.00–989.00 |
| Interleukin-2 | IL-2 | 52.42 (25.63) | 11.50–142.00 |
| Interleukin-3 | IL-3 | 25.66 (10.01) | 11.00–61.50 |
| Interleukin-4 | IL-4 | 30.32 (30.06) | 11.50–169.00 |
| Interleukin-5 | IL-5 | 47.10 (102.15) | 8.00–655.75 |
| Interleukin-6 | IL-6 | 234.68 (1159.85) | 12.00–7864.50 |
| Interleukin-7 | IL-7 | 21.42 (8.72) | 9.75–43.50 |
| Interleukin-8 | IL-8 | 647.21 (2278.01) | 30.50–11219.00 |
| Interleukin-9 | IL-9 | 35.27 (26.69) | 13.00–140.00 |
| Interleukin-10 | IL-10 | 31.53 (31.23) | 11.50–194.75 |
| Interleukin-12p40 | IL-12p40 | 30.13 (23.45) | 11.50–116.50 |
| Interleukin-12p70 | IL-12p70 | 23.36 (16.69) | 7.50–114.25 |
| Interleukin-13 | IL-13 | 88.61 (214.86) | 11.50–1326.75 |
| Interleukin-15 | IL-15 | 29.83 (23.40) | 11.75–131.00 |
| Interleukin-17A | IL-17A | 50.24 (44.66) | 18.75–207.00 |
| Interferon gamma-induced protein-10 | IP-10 | 375.83 (357.06) | 60.75–2031.50 |
| Monocyte chemotactic protein-1 | MCP-1 | 870.82 (1865.50) | 49.50–12727.00 |
| Monocyte chemotactic protein-3 | MCP-3 | 174.45 (524.36) | 10.00–3385.25 |
| Macrophage-derived chemokine | MDC | 526.23 (270.40) | 175.50–1081.50 |
| Macrophage inflammatory protein-1 alpha | MIP-1α | 415.11 (2197.20) | 10.00–14768.75 |
| Macrophage inflammatory protein-1 beta | MIP-1β | 289.68 (1427.25) | 17.50–9729.25 |
| Platelet-derived growth factor-AA | PDGF-AA | 4070.61 (4939.07) | 268.25–15780.50 |
| Platelet-derived growth factor-AB/BB | PDGF-AB/BB | 932.22 (1527.65) | 21.25–6248.50 |
| Regulated on activation, normal T cell expressed and secreted | RANTES | 6493.20 (5570.48) | 520.25–19376.25 |
| Soluble cluster of differentiation 40 ligand | sCD40L | 1039.70 (1628.91) | 32.25–6037.00 |
| Transforming growth factor-alpha | TGF-α | 33.51 (27.37) | 13.00–152.50 |
| Tumor necrosis factor-alpha | TNF-α | 160.85 (513.62) | 25.00–3510.00 |
| Tumor necrosis factor-beta | TNF-β | 85.13 (195.36) | 10.00–1241.25 |
| Vascular endothelial growth factor | VEGF | 36.35 (46.84) | 12.50–325.00 |
In the statistical analyses, when bead counts were low (i.e., below 20), samples were deemed undetectable and excluded. Only GCSF had multiple samples (n = 10) with low bead counts, and thus this cytokine was left out of all analyses due to 22% of participants not having data for this biomarker. Eight of the cytokines (i.e., fractalkine, IFN-α2, IL-7, IL-9, IL-13, MCP-1, MIP-1α, PDGF-AB/BB) had one sample with a low bead count; in these cases, mean imputation was used to allow these cytokines to be included in the fMRI regression models. Staff at Mount Sinai’s Human Immune Monitoring Center (HIMC) who were blind to participants’ clinical designation performed these assays. Median fluorescence intensity (MFI) values of all 41 cytokines were used in the final statistical models rather than absolute concentration values. This decision was made because concentration values can introduce bias for samples that have very low or very high values in relation to the standard curves (Breen et al., 2015; Breen et al., 2016). Lastly, intra-assay coefficients of variability (CV) were calculated using assay duplicates. The average was 4.63%. The range was between 0.22% and 12.10%, with the majority < 10%. The average inter-assay CV was 16% (minimum to maximum quartile range 12–45%). Data showed reproducibility above the limit of detection over 8 consecutive assays. Analytes with CV greater than 20% were nearly all for analytes with the lowest detection range in these samples, close to the limit of detection, thereby introducing greater standard deviation over average.
2.5. Reward Flanker Task (RFT)
The RFT has been fully described previously in a published pilot study (Bradley et al., 2016). In brief, participants were tasked to identify a target letter surrounded by four flanker letters using a hand-held button box in the MRI scanner and received a monetary reward if they chose correctly. Each trial consisted of a monetary cue [low reward (“10¢”), high reward (“50¢”), no reward (“0¢”), and unknown reward (“?”)] that informed subjects how much a trial would be worth if a correct response was made to flanker stimuli (to index reward anticipation). After making a response, subjects received feedback (value of the attained or unattained reward; Fig. 1). In total, 120 trials were presented in a pseudo-random event-related design over 4 equal runs.
Fig. 1.
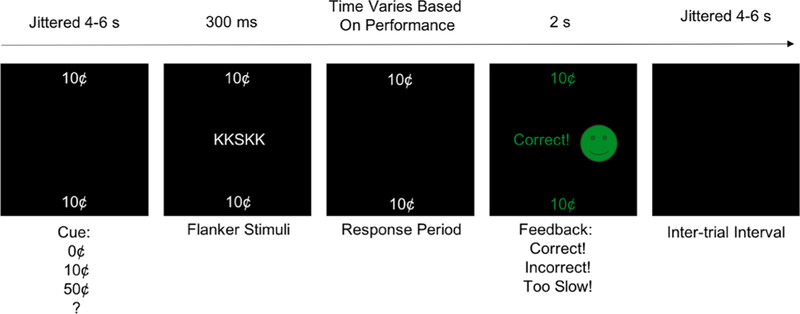
Reward Flanker Task (RFT). This figure depicts an example trial of the RFT.
2.6. MRI acquisition and analysis
2.6.1. Image acquisition
Imaging data were acquired after the fasting blood draw on a 3 Tesla Skyra scanner with a 16 + 4 head-neck coil using a previously described protocol (Bradley et al., 2016). A magnetization prepared rapid acquisition gradient echo sequence was used to acquire high-re-solution T1-weighted anatomical images [repetition time (TR) = 2400 ms, echo time (TE) = 2.06 ms, flip angle = 8°, field of view (FOV) = 256 mm × 256 mm, 224 sagittal slices 0.9 mm thick, inplane resolution = 0.9 mm × 0.9 mm]. During the RFT, T2*-weighted gradient echo multiband echo planar images were acquired over the 4 runs with alternating left-right and right-left phase-encoding directions [TR = 1000 ms, TE = 31.4 ms, flip angle = 60°, FOV = 624 mm × 720 mm, 374 transverse slices 2.3 mm thick, in-plane resolution = 2.3 mm × 2.3 mm]. Field maps with opposing right-left and left-right phase-encoding directions were also acquired [TR = 6150 ms, TE = 57 ms, flip angle = 80°, FOV = 624 mm × 720 mm, 2 transverse slices 2.3 mm thick, in-plane resolution = 2.3 mm × 2.3 mm].
2.6.2. Image analysis
Neuroimaging analyses utilized a combination of Human Connectome Project (HCP) minimal pre-processing scripts (Glasser et al., 2013) and Statistical Parametric Mapping (SPM) processes, version 12 (Wellcome Trust Centre for Neuroimaging, London, UK), running on a Matlab 2015a platform (The MathWorks, Inc., Natick, MA, USA). Gradient non-linearity and echo planar image distortion correction were performed using HCP scripts, while realignment, coregistration of the functional images to the anatomical images, normalization to a standard Montreal Neurological Institute (MNI) template, and spatial smoothing with a 6 mm full width at half maximum (FWHM) Gaussian kernel, were all conducted in SPM. Motion plots from realignment were examined and runs with more than 5 mm of translation or rotation were eliminated from analyses; if more than one run of data did not meet motion requirements, that participant was excluded due to an insufficient number of trials for analysis. In total, 9 participants were excluded due to motion. The participants excluded due to motion were slightly younger than those included [mean age of excluded = 14.50, SD = 2.68; mean age of included = 16.43, SD = 2.24; Mann-Whitney U = 109, p = .026], but this is to be expected given that younger participants have greater difficulty holding still through lengthy scans. However, there were no other significant differences in demographic characteristics between those included and excluded, including sex [χ2 = 0.004, p = .95], BMI [Mann-Whitney U = 230, p = .52], ethnicity [χ2 = 1.26, p = .74], or in diagnostic clinical makeup [i.e. psychiatric symptoms vs. no symptoms; χ2 = 0.059, p = .81].
At the first-level, a general linear model included 17 regressors convolved with the canonical hemodynamic response function: 11 task-based regressors [reward anticipation (high, low, no reward, and unknown reward cues), reward attainment (high, low, and no reward feedback on correct trials, separately for known and unknown cues), error feedback (incorrect trials)] and 6 motion parameters from realignment. First-level contrasts of interest included anticipation of known rewards (10¢ and 50¢ cues) versus an implicit baseline and attainment of known rewards (10¢ and 50¢) versus an implicit baseline. Unknown reward conditions, including those involved in calculating positive prediction error (PPE), were not of primary interest in this investigation and thus not included; they will be reported separately.
Three separate second-level analyses were conducted. The first utilized a more exploratory data-reduction approach whereby a factor analysis of the multiplex cytokine panel was done in order to examine the association between a reduced number of specific ‘inflammatory factors’ with whole-brain activation during the RFT. The second examined the association between CRP and whole-brain activation during the RFT. The third utilized a ROI approach to extract the activation signal from a priori selected regions in order to correlate all individual cytokine levels and CRP with activation in previously established reward-related fronto-striatal regions.
2.6.2.1. Factor analysis.
A principle component analysis (PCA) was conducted on 40 of the baseline cytokine levels across the entire participant sample; granulocyte colony-stimulating factor (G-CSF) was left out of the factor analysis due to missing values in 10 participants (22% of the sample) due to failure of these samples to pass quality assurance procedures as described above. This more exploratory technique allowed the data to inform us of patterns within the 40 cytokines rather than presumptively picking only a few cytokine levels to examine in relation with brain activity. Given the complexity of the immune system and the unknown relations between inflammatory markers and reward processing in the brain, this factor analysis allowed the results to be data-driven.
The factor analysis used orthogonal rotation. Initially, only factors that showed eigenvalues greater than 1 were retained. From this initial PCA, 7 ‘inflammatory factors’ had eigenvalues greater than 1. However, only 4 of the factors explained at least 5% of the variance and were thus retained; visual inspection of the scree plot confirmed retention of 4 factors. Each of these 4 factors was included as predictors in separate second-level regression models that examined whole-brain activation for both reward anticipation (10¢ + 50¢ cues) versus an implicit baseline and reward attainment (10¢ and 50¢) versus an implicit baseline in relation to inflammation; age, sex, and BMI were also included as regressors of no interest in these models to control for the effects of factors known to impact inflammation and neuroimaging measures.
2.6.2.2. CRP analysis.
CRP was included as a predictor in a second-level regression model that examined whole-brain activation for both reward anticipation (10¢ + 50¢ cues) versus an implicit baseline and reward attainment (10¢ and 50¢) versus an implicit baseline in relation to inflammation; age, sex, and BMI were included as regressors of no interest to control for the effects of these factors. Four participants did not have CRP data and thus were excluded from the analyses.
All correlational neuroimaging analyses used Threshold-Free Cluster Enhancement (TFCE) as implemented in PALM (Winkler et al., 2014), family-wise error (FWE) corrected for multiple comparisons (p < .05).
2.6.2.3. ROI analysis.
Four a priori ROIs were created using the WFU Pickatlas (Maldjian et al., 2003), including the left and right ACC/MCC, and the left and right basal ganglia (including the dorsal and ventral striatum). Marsbar in SPM (Brett et al., 2002) was used to extract parameter estimates from each of these ROIs in two contrasts of interest. Specifically, at the group level, one-sample t-tests that included age as a covariate examined whole-brain activation for reward anticipation (10¢ and 50¢ cues) versus an implicit baseline and reward attainment (10¢ and 50¢ outcomes) versus an implicit baseline for the full sample of subjects. Parameter estimates were extracted in each ROI (left basal ganglia, right basal ganglia, left ACC/ MCC, right ACC/MCC) from the raw data from each of these contrasts of interest. Statistical analyses were conducted using SPSS, version 23 (IBM Corp., Armonk, NY). Descriptive statistics were used to describe the sample and examine the distribution and normality of each of the experimental variables. Due to non-normal data, rank-based partial correlations between extracted parameter estimates in each of the ROIs and all 40 usable cytokines and CRP were examined in the total sample and the group with psychiatric symptoms only, controlling for age, sex, and BMI, with significance false discovery rate (FDR) corrected for multiple comparisons (p < .05) in SPSS (IBM Corp., Armonk, NY).
3. Results
3.1. Demographics and clinical characteristics of the sample
The final sample consisted of 46 participants between the ages of 12 and 20 years old, 34 of whom had psychiatric symptoms. Of the 34 participants with psychiatric symptoms, 19 had a depressive disorder, 19 had an anxiety disorder, 3 had a bipolar spectrum disorder, 10 had attention-deficit/hyperactivity disorder (ADHD), and 6 had a behavior disorder. Twelve youth did not exhibit symptoms of any psychiatric disorder and had no history of mental illness. Demographic and clinical characteristics of the sample are presented in Table 2.
3.2. Whole-brain factor analysis
The factor analysis yielded 4 ‘inflammatory factors.’ The four-component solution from the PCA explained 76.4% of the total variance. The complete component loadings and communalities of the rotated solution can be seen in Table 3.
Table 3.
Factor Loadings for Principle Component Analysis (PCA).
| Cytokine | Components | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Communalities | |
| EGF | 0.332 | 0.840 | 0.830 | ||
| Eotaxin | 0.882 | 0.794 | |||
| FGF-2 | 0.548 | 0.679 | 0.853 | ||
| Flt3-L | 0.546 | 0.451 | |||
| Fractalkine | 0.535 | 0.659 | 0.754 | ||
| GM-CSF | 0.559 | 0.736 | 0.874 | ||
| GRO | 0.937 | 0.890 | |||
| IFN-α2 | 0.341 | 0.140 | |||
| IFN-γ | 0.448 | 0.676 | 0.715 | ||
| IL-10 | 0.779 | 0.499 | 0.912 | ||
| IL-12P40 | 0.332 | 0.784 | 0.311 | 0.822 | |
| IL-12P70 | 0.816 | 0.518 | 0.952 | ||
| IL-13 | 0.873 | 0.809 | |||
| IL-15 | 0.384 | 0.831 | 0.926 | ||
| IL-17A | 0.478 | 0.568 | 0.589 | ||
| IL-1α | 0.515 | 0.712 | 0.349 | 0.894 | |
| IL-1β | 0.980 | 0.968 | |||
| IL-1Rα | 0.373 | 0.886 | 0.937 | ||
| IL-2 | 0.606 | 0.539 | 0.731 | ||
| IL-3 | 0.330 | 0.559 | 0.445 | ||
| IL-4 | 0.518 | 0.327 | |||
| IL-5 | 0.953 | 0.919 | |||
| IL-6 | 0.983 | 0.976 | |||
| IL-7 | 0.360 | 0.322 | 0.556 | 0.502 | 0.795 |
| IL-8 | 0.741 | 0.557 | |||
| IL-9 | 0.822 | 0.357 | 0.842 | ||
| IP-10 | 0.762 | 0.335 | 0.721 | ||
| MCP-1 | 0.958 | 0.939 | |||
| MCP-3 | 0.957 | 0.924 | |||
| MDC | 0.346 | 0.147 | |||
| MIP-1α | 0.981 | 0.972 | |||
| MIP-1β | 0.979 | 0.970 | |||
| PDGF-AA | 0.954 | 0.929 | |||
| PDGF-AB/BB | 0.856 | 0.739 | |||
| RANTES | 0.666 | 0.469 | |||
| sCD40L | 0.941 | 0.897 | |||
| TGF-α | 0.661 | 0.533 | 0.834 | ||
| TNF-α | 0.982 | 0.982 | |||
| TNF-β | 0.981 | 0.969 | |||
| VEGF | 0.567 | 0.374 | |||
Note: Table displays the rotated structure matrix for PCA with varimax rotation and 4 components. Major loadings for each cytokine are bolded.
Abbreviations: EGF = epidermal growth factor; FGF = fibroblast growth factor; Flt3-L = FMS- like tyrosine kinase 3-ligand; GM-CSF = granulocyte-macrophage colony-stimulating factor; GRO = growth regulated oncogene; IFN = interferon; IL = interleukin; IP = interferon gamma-induced protein; MCP = monocyte chemotactic protein; MDC = macrophage-derived chemokine; MIP = macrophage inflammatory protein; PDGF = platelet-derived growth factor; RANTES = regulated on activation, normal T cell expressed and secreted; sCD40L = soluble cluster of differentiation 40 ligand; TGF = transforming growth factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
3.2.1. Reward anticipation
Across all participants, only factor 3 (FGF-2, Flt3-L, fractalkine, GM-CSF, IFN-α2, IFN-γ, IL-3, IL-4, IL-7, IL-17A, MDC, and VEGF) was negatively correlated with activity in 3 clusters within the bilateral pre- cuneus/PCC (k = 255 voxels, MNI X = 4, Y = −74, Z = 24; k = 15 voxels, MNIX =14, Y = −74, Z = 12; k = 2 voxels, MNIX = 10, Y = −84, Z = 2) during reward anticipation when results were FWE corrected for multiple comparisons (p < .05). See Fig. 2 for visual representation. While only a single much smaller cluster was significant, results remained unchanged with respect to region when healthy individuals with no psychiatric symptoms were excluded from the analyses (k = 3 voxels; MNI X = 2, Y = −74, Z = 24).
Fig. 2.
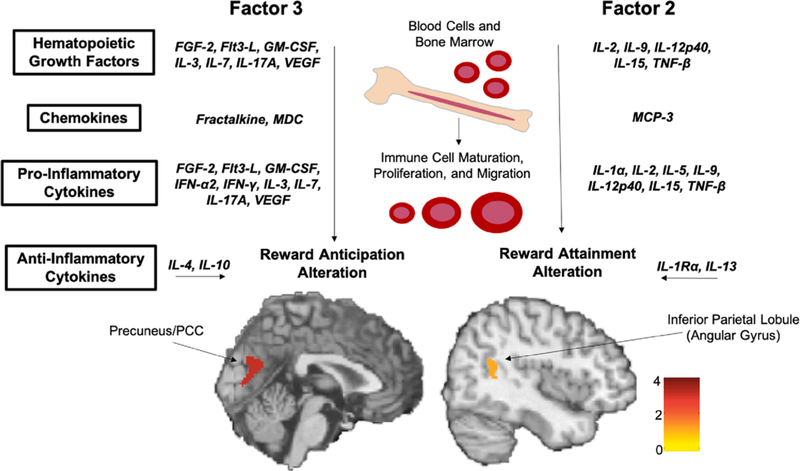
Peripheral Inflammation and Neural Activation. This figure depicts the results of the whole-brain principle component analysis. Factor 3 was negatively correlated with activation of the bilateral precuneus/posterior cingulate cortex (PCC) during reward anticipation [k = 255 voxels, MNI X = 4, Y = −74, Z = 24; k = 15 voxels, MNI X = 14, Y = −74, Z = 12; k = 2 voxels, MNI X = 10, Y = −84, Z = 2]. Factor 2 was negatively correlated with activation of the right angular gyrus during reward attainment [k = 128 voxels; MNI X = 48, Y = −54, Z = 18]. Abbreviations: FGF = fibroblast growth factor; Flt3-L = FMS-like tyrosine kinase 3- ligand; GM-CSF = granulocyte-macrophage colony-stimulating factor; IFN = interferon; IL = interleukin; MCP = monocyte chemotactic protein; MDC = macrophage-derived chemokine; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
3.2.2. Reward attainment
Across all participants, only factor 2 (eotaxin, IL-12p40, IL-13, IL-15, IL-1α, IL-1Ra, IL-2, IL-5, IL-9, MCP-3, and TNF-β) was negatively correlated with activation in a single cluster within the right angular gyrus (k = 128 voxels; MNI X = 48, Y = −54, Z = 18) during reward attainment when results were FWE corrected for multiple comparisons (p < .05). See Fig. 2 for visual representation. While the cluster was smaller, results again remained unchanged when the individuals with no psychiatric symptoms were excluded from the analyses (k = 10 voxels; MNI X = 50, Y = −54, Z = 18).
3.3. Whole-brain CRP analysis
3.3.1. Reward anticipation
Contrary to our expectation, there were no correlations between whole-brain neural activation during reward anticipation and CRP levels, either in the total sample or the participants with psychiatric symptoms alone, when results were FWE corrected for multiple comparisons (p < .05).
3.3.2. Reward attainment
There were also no significant correlations between whole-brain activation during reward attainment and CRP levels, both in the total sample and the psychiatric subgroup, when results were FWE corrected for multiple comparisons (p < .05).
3.4. ROI analysis
3.4.1. Reward anticipation
Only one cytokine was correlated with neural reward activation during reward anticipation in the total sample. Eotaxin was negatively correlated with activation in the right ACC/MCC (rho (ρ) = −0.57, p < .0005) and right basal ganglia (rho (ρ) = −0.52, p < .0005) during reward anticipation. None of the other correlations between ROI activation and cytokine or CRP levels in the total sample or the subgroup with psychiatric symptoms were significant when results were FDR corrected for multiple comparisons (p < .05). Complete correlation results are presented in the Supplementary Materials, Tables S1-S4.
3.4.2. Reward attainment
There were no significant correlations between activation during reward attainment in the 4 ROIs and any of the cytokines or CRP levels in the total sample, when results were FDR corrected for multiple comparisons (p < .05). However, when analyses were restricted to only participants with psychiatric symptoms, several correlations were significant. Activation in the left basal ganglia during attainment was negatively correlated with FGF-2 (rho (ρ) = −0.55, p < .005) and Flt- 3L (rho (ρ) = −0.57, p < .0005). Activation in the right ACC/MCC during attainment was negatively correlated with Flt-3L (rho (ρ) = −0.56, p < .005). Lastly, activation in the right basal ganglia during attainment was negatively correlated with EGF (rho (ρ) = −0.49, p = .01), FGF-2 (rho (ρ) = −0.52, p < .005), Flt-3L (rho (ρ) = −0.62, p < .0005), IL-2 (rho (ρ) = −0.55, p < .005), IL-13 (rho (ρ) = −0.50, p = .01), IL-15 (rho (ρ) = −0.48, p = .01), IL-1Ra (rho (ρ) = −0.56, p < .005), and MCP-3 (rho (ρ) = −0.49, p = .01). There were no significant correlations between activation during reward attainment in the 4 ROIs and CRP levels. Results are presented in Supplementary Materials, Tables S1-S4.
4. Discussion
To our knowledge, this is the first study to examine the relationship between peripheral inflammation and brain activation during a fMRI reward task in a diverse sample of psychotropic medication-free youth with and without psychiatric symptoms. Specifically, we used the fMRI RFT to examine neural activation during two reward processes, reward anticipation and attainment, in relation to both specific (i.e., cytokine levels) and general (i.e., CRP) markers of peripheral inflammation. Overall, our primary hypothesis that increased peripheral inflammation would be associated with reduced reward-related brain activation during the RFT was partially supported. When we utilized an exploratory whole-brain PCA approach that clustered specific cytokines into ‘inflammatory factors,’ inverse relationships between inflammation and brain activation during both anticipation and attainment were found in posterior default mode network (DMN) brain regions such as the precuneus/PCC and angular gyrus, respectively. ROI analyses further confirmed that reduced activation of fronto-striatal brain regions during reward attainment were associated with increased levels of multiple cytokines in youth with diverse psychiatric symptoms. However, when we examined the relationships between a more general marker of peripheral inflammation (i.e., CRP) and neural activation during the RFT, our hypothesis was not supported; there were no significant relationships between this general marker of inflammation and neural activation during either reward anticipation or attainment. These results suggest the importance of investigating more specific relationships between functional components of the immune system and reward processing in youth. These findings are discussed in detail below.
4.1. Immune function and reward processing
Through both data-driven whole-brain PCA, as well as a priori selected fronto-striatal ROI analyses, we found that a wide variety of cytokines were associated with neural activation during both anticipatory and consummatory reward processing. This is significant, as conservative multiple comparison corrections were applied and analyses controlled for factors such as age, sex, and BMI, which are known to impact both inflammation and neuroimaging markers. Using whole-brain PCA, factor 3, in which 12 cytokines loaded (FGF-2, Flt3-L, fractalkine, GM-CSF, IFN-2α2, IFN-γ, IL-3, IL-4, IL-7, IL-17A, MDC, and VEGF), was associated with activation in the precuneus/PCC during reward anticipation, while factor 2, in which 11 cytokines loaded (eo-taxin, IL-1α, IL-1Rα, IL-2, IL-5, IL-9, IL-12P40, IL-13, IL-15, MCP-3, and TNF-β), was associated with activation in the angular gyrus during reward attainment. While factors 2 and 3 were associated with different regions of brain activation during separate phases of reward processing, it is important to note that the cytokines that load onto each of these two factors are similar in that the immune functions of both factors are overlapping; for example, the cytokines are comparably dispersed into hematopoietic growth factors, chemokines, proinflammatory cytokines, and anti-inflammatory cytokines in both factors 2 and 3 (see Fig. 2).
Hematopoietic growth factors (IL-2, IL-3, IL-7, IL-9, IL-12p40, IL-15, IL-17α, FGF-2, Flt3-L, VEGF, GM-CSF, TNF-β) typically induce proliferation and maturation of blood cells and assist in the initiation of an immune response in the bone marrow. Alternately, chemokines, such as fractalkine, MDC, and MCP-3, were also involved and may initiate cell migration during an immune reaction. Lastly, both pro-inflammatory (IL-1α, IL-2, IL-3, IL-7, IL-5, IL-9, IL-12p40, IL-15, IL-17α, FGF-2, Flt3-L, VEGF, G-CSF, GM-CSF, TNF-β, IFN-γ, IFN-α2) and anti-inflammatory (IL-4, IL-10, IL-1Rα, IL-13) cytokines were involved, with these cyto-kines initiating or inhibiting an immune response, respectively. Therefore, it seems that more than one functional component of the immune system is related to neural reward processing—during both anticipation and attainment. This finding suggests that the relationships between inflammation and reward dysfunction may be more diverse and complicated than previously thought.
In line with the whole-brain findings, the ROI analyses in general also showed that reduced reward-related brain activation is associated with increased levels of a functionally diverse set of immune components. Importantly, all but one (i.e., EGF) of the immunological markers that were associated with fronto-striatal ROI activation were also the same as those that loaded onto both significant factors from the whole- brain PCA analyses, factor 2 (IL-1Rα, IL-2, IL-13, IL-15, and MCP-3) and factor 3 (Eotaxin, FGF-2, Flt-3L). However, unlike the whole-brain results, most of the ROI findings were restricted to the subgroup of participants with diverse psychiatric symptoms and the attainment phase of reward processing. It is possible that these ROI findings were more restricted and showed fewer cytokines from both factors 2 and 3 as significant in part due to the very stringent multiple comparisons corrections that controlled for all cytokines in the multiplex panel, thus suggesting a need for larger, stronger powered studies.
4.2. Inflammation and default mode network activation
While the ROI findings linking decreased fronto-striatal activation with increased inflammation were expected given previous literature (Eisenberger et al., 2010; Felger and Lotrich, 2013; Felger et al., 2016), the whole brain PCA analyses yielded surprising findings documenting associations between activation of posterior reward-related brain regions that function as part of the default mode network (DMN) and cytokine levels. Specifically, we found that factor 3 (comprised of FGF- 2, Flt3-L, fractalkine, GM-CSF, IFN-α2, IFN-γ, IL-3, IL-4, IL-7, IL-17A, MDC, and VEGF) was negatively correlated with activation of the bilateral precuneus/PCC during reward anticipation. Additionally, factor 2 (comprised of eotaxin, IL-1α, IL-1Rα, IL-2, IL-5, IL-9, IL-12p40, IL-13, IL-15, MCP-3, and TNF-β) was negatively correlated with activation of the right angular gyrus/inferior parietal lobule (IPL) during reward attainment. These results suggest that increased peripheral inflammation is associated with reduced activation in what is postulated to be the “core hub” of the DMN during both anticipatory and consummatory reward processing.
It has been suggested that the “core hub” of the DMN consists of the mPFC, as well as the PCC/precuneus and inferior parietal lobule (IPL) (Buckner et al., 2008; Fransson and Marrelec, 2008; Utevsky et al., 2014), the latter both of which we see activated less in association with increased inflammation. The PCC/precuneus is thought to play an especially pivotal role mediating activity within the DMN, as this region has been previously found to be the only node within the DMN that directly interacts with all other nodes in the network (Fransson and Marrelec, 2008). Typically, the default mode network is thought to be activated during periods of rest, with these same regions deactivated during tasks requiring cognitive effort (Zhang and Li, 2012). However, this is not always the case, as some studies have shown task-related increases in DMN regions (Zhang and Li, 2012). Despite these conflicting findings, it is clear that alterations in both resting state and task-based PCC/precuneus activity and connectivity have been implicated in reward dysfunction in youth, including research from our laboratory. Previously, we documented that resting-state intrinsic functional connectivity of the striatum with the precuneus and IPL were correlated with anhedonia severity in depressed adolescents (Gabbay et al., 2013). More recently, we found that resting state connectivity of the precuneus with the dorsal ACC was positively correlated with kynurenic acid (KA) levels and negatively correlated with kynurenine pathway (KP) activity, indexed by the kynurenine (KYN)/tryptophan (TRP) ratio (DeWitt et al., 2018).
Studies in adults similarly support our findings. For example, in adults with obsessive-compulsive disorder (OCD), patients with OCD were found to exhibit decreased activation of the PCC/precuneus during monetary reward attainment but increased connectivity between the PCC bilaterally and with the mPFC (Koch et al., 2018). The authors suggested that decreased PCC/precuneus activity during reward appraisal could indicate lower levels of arousal and responsivity to reward feedback, while the increased connectivity of this region with the PFC could indicate more internally focused thought patterns due to these regions role in mentation and self-thought (Koch et al., 2018). These results are in line with our own that documented decreased activation of the PCC/precuneus in association with higher inflammation and together suggest that individuals exhibiting reward dysfunction may show a reduced level of arousal in response to both the anticipation and attainment of a reward.
4.3. Clinical significance of immune/reward relations
The current findings, which display the diverse complexity of the associations between inflammation and neural reward processing in youth, are an extension of a recently published study from our laboratory examining the link between these same markers of peripheral inflammation and clinical symptomatology. In this study of a larger, partially overlapping sample of youth, our laboratory found that levels of 19 cytokines were positively correlated with anhedonia severity, but not other clinical symptoms, such as depression severity, anxiety, fatigue, or suicidality in adolescents with diverse psychiatric symptoms (Freed et al., 2018). Remarkably, 16 out of the 19 cytokines identified in this larger study as being associated with a clinical measure of an-hedonia severity (FGF-2, Flt3-L, fractalkine, GM-CSF, IL-1α, IL-2, IL-3, IL-4, IL-7, IL-9, IL-12p40, IL-15, IL-17α, MCP-3, TNF-β, VEGF) (Freed et al., 2018) overlapped with the results of either the data-driven whole-brain PCA or ROI analyses in the current investigation that showed an inverse association between inflammation and neural reward processing. Only 3 additional cytokines (IL-10, IL-12p70, and G-CSF) were identified by Freed et al. (2018) as showing an association with anhedonia severity. Together, these findings display the clinical significance of the associations between inflammation and neural reward activation. Here, increased inflammation was associated with reduced activation of key reward-related brain regions, which in combination with the findings of Freed et al. (2018), may also suggest that this neural dysfunction manifests clinically as anhedonia, even across diagnostic categories. Our findings implicating inflammation in the development of anhedonia add to the current notion that the pathophysiology of depression is complex, involving not only monoamine dysfunction but also the hypothalamic-pituitaryadrenal axis stress response, changes in neuroplasticity, mitochondrial dysfunction, and neuroimmunological pathways (see Ferrari and Villa, 2017 for a review of the neurobiology of depression). Further examining specific symptoms dimensionally across diagnostic categories using similar RDoC methods may facilitate our understanding of heterogeneous psychiatric disorders such as depression as a whole.
4.4. Mechanisms of immune/reward dysfunction
There is extensive evidence to support the notion that peripheral inflammation could extend to the brain and affect neural circuits and subsequent behavioral patterns. Cytokines are thought to cross the blood-brain barrier (BBB) through multiple pathways, such as by passive transport at circumventricular sites lacking a BBB, by binding to cerebral vascular endothelium and inducing secondary messengers such as prostaglandins and nitric oxide, through carrier-mediated transport across the BBB, and by activation of peripheral afferent nerve terminals (Cserr et al., 1992; Dantzer, 2009; Dantzer et al., 1998; Pollmacher et al., 2002; Watkins et al., 1995). Furthermore, recent evidence of a central nervous system (CNS) lymphatic system capable of shuttling cytokines and kynurenine pathway (KP) metabolites across the BBB (Louveau et al., 2015) gives further support for the idea that peripheral inflammation could extend to the brain. Specifically, Louveau et al. (2015) discovered lymphatic vessels that line the dural sinuses and are connected to cervical lymph nodes, which are capable of shuttling immune cells to cerebrospinal fluid. Previously, it has been hypothesized that the neuroimmune KP is the mechanism through which inflammation impacts reward processing in the brain, and the discovery of this CNS lymphatic system further supports the viability of this hypothesis.
The KP is activated by the enzyme indoleamine 2,3-dioxygenase (IDO), which is induced by pro-inflammatory cytokines and metabolizes tryptophan (TRP), the precursor of serotonin, into either neuro-trophic (e.g., kynurenic acid) or neurotoxic factors (e.g., quinolinic acid). Both neuroimaging and clinical behavioral work have supported the specific link between the KP and reward dysfunction. For example, in depressed adults, IDO levels were related to striatal volumes (Savitz et al., 2015a). Additionally, neurotoxic KP metabolites were related to mPFC thickness (Meier et al., 2016) in depressed adults and to amygdala and hippocampal volumes in bipolar and depressed adults (Savitz et al., 2015a; Savitz et al., 2015b). Furthermore, our own laboratory documented multiple relationships between the KP and reward dysfunction in youth. For example, we found increased IDO levels (kynurenine [KYN]/tryptophan ratio) in melancholic, depressed adolescents (Gabbay et al., 2010), as well as positive correlations between IDO levels (KYN/TRP) and anhedonia severity in depressed adolescents (Gabbay et al., 2012). Moreover, we found that IDO levels (KYN/TRP) were related to suicidality, specifically in depressed adolescents (Bradley et al., 2015), and KA and the KA/QUIN ratio were related to resting state connectivity of reward-related brain regions such as the anterior cingulate cortex (ACC), precuneus, medial prefrontal cortex (mPFC), and inferior temporal gyrus in youth (DeWitt et al., 2018).
Despite that all of these studies provide strong support for a link between inflammatory processes and neural reward dysfunction via the KP, it is important to note that causal mechanisms cannot be extrapolated from these correlational associations. It is also possible that increased inflammation could instead be a consequence of neural dysfunction in individuals with psychiatric symptoms rather than the cause of it. This issue requires further investigation to better understand whether inflammation induces neural alterations or rather that inflammation is a consequence of neural alterations that are the result of psychiatric illness.
4.5. Limitations
Despite the novelty and importance of the current investigation, the findings should be interpreted in light of several limitations. The relatively small sample size (n = 46), along with the use of stringent multiple comparisons corrections in all analyses may have prevented smaller associations between inflammation and neural activation from being detected. Additionally, due to the relatively small sample size, not all biological and health factors that affect inflammation could be controlled (e.g., menstrual cycle stage, exercise, sedentary behavior, diet, stress, sleep, etc.). Lastly, due to higher motion, some of our very young adolescents had to be excluded due to lack of high quality imaging data for analysis. Larger replication studies of adolescents displaying a wide range of anhedonia and reward deficits are thus necessary to further examine the relationship between inflammation and reward processing, while controlling for additional health and lifestyle factors.
5. Conclusion
In conclusion, this is the first study that examined the relationship between peripheral inflammation and neural activation during distinct phases of reward processing in psychotropic medication-free youth with diverse psychiatric symptoms. Importantly, we found evidence for an inverse relationship between both fronto-striatal and more posterior DMN activation during reward processing and inflammation. Given the large number of cytokines from different functional classes, including pro-inflammatory cytokines, anti-inflammatory cytokines, chemokines, and hematopoietic growth factors, that were associated with patterns of neural activation, this study provides additional evidence linking multiple functional components of the immune system to reduced neural activation of the posterior core hub of the DMN during both anticipatory and consummatory phases of reward processing in youth, which may suggest lower levels of arousal and reward responsivity in these individuals. These findings thus underscore the need for future studies that use similar data-driven modeling techniques to better understand the mechanisms underlying the relationships between the many functional components of the immune system and patterns of neural activation and connectivity that contribute to reward dysfunction in youth.
Supplementary Material
Acknowledgment
We would like to thank Lushna Mehra for research coordination and assistance preparing a figure for the manuscript.
Funding
This study was supported by grants from the National Institute of Mental Health (NIMH) to VG (grant numbers MH095807, MH101479). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIMH. The NIMH had no role in the design, collection, analysis, or interpretation of data, or in the preparation of the manuscript.
Footnotes
Declaration of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2019.04.014 .
References
- APA, 2000. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC. [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the scale for suicide ideation. J. Consult. Clin. Psychol. 47, 343–352. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Freed RD, Stern ER, Gabbay V, 2016. Neural correlates of RDoC reward constructs in adolescents with diverse psychiatric symptoms: a Reward Flanker Task pilot study. J. Affect Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Case JA, Khan O, Ricart T, Hanna A, Alonso CM, Gabbay V, 2015. The role of the kynurenine pathway in suicidality in adolescent major depressive disorder. Psychiatry Res. 227, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EJ, Polaskova V, Khan A, 2015. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine 71, 188–198. [DOI] [PubMed] [Google Scholar]
- Breen EJ, Tan W, Khan A, 2016. The statistical value of raw fluorescence signal in Luminex xMAP based multiplex immunoassays. Sci. Rep. 6, 26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J, 2002. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Harling-Berg CJ, Knopf PM, 1992. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2, 269–276. [DOI] [PubMed] [Google Scholar]
- Dantzer R, 2009. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am 29, 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW, 1998. Cytokines and sickness behavior. Ann. N. Y. Acad. Sci. 840, 586–590. [DOI] [PubMed] [Google Scholar]
- De La Garza R 2nd, 2005. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci. Biobehav. Rev. 29, 761–770. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A, 2012. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt SJ, Bradley KA, Lin N, Yu C, Gabbay V, 2018. A pilot resting-state functional connectivity study of the kynurenine pathway in adolescents with depression and healthy controls. J. Affect. Disord. 227, 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, 2011. The developmental psychopathology of motivation in adolescence. Dev. Cogn. Neurosci. 1, 414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, 2017. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticos-triatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE, 2013. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246, 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F, Villa RF, 2017. The neurobiology of depression: an integrated overview from biological theories to clinical evidence. Mol. Neurobiol. 54, 4847–4865. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G, 2008. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage 42, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Freed RD, Mehra LM, Laor D, Patel M, Alonso CM, Kim-Schulze S, Gabbay V, 2018. Anhedonia as a clinical correlate of inflammation in adolescents across psychiatric conditions. World J. Biol. Psychiatry 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Coffey BJ, Guttman LE, Gottlieb L, Katz Y, Babb JS, Hamamoto MM, Gonzalez CJ, 2009a. A cytokine study in children and adolescents with Tourette’s disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L, 2012. The possible role of the kynurenine pathway in anhedonia in adolescents. J. Neural Transm. (Vienna) 119, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP, 2013. Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 52, 628–641.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ, 2009b. Immune system dysregulation in adolescent major depressive disorder. J. Affect. Disord. 115, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ, 2009c. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J. Child Adolesc. Psychopharmacol. 19, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L, 2010. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J. Child Psychol. Psychiatry 51, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, 2017. Adolescence, brain maturation and mental health. Nat. Neurosci. 20, 503–504. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, 2011. Chapter 11: neuroanatomy of reward: a view from the ventral striatum In: Gottfried JA (Ed.), Neurobiology of Sensation and Reward. CRC Press/Taylor & Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Hagele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, Stoy M, Strohle A, Wittchen HU, Dolan RJ, Heinz A, 2015. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl.) 232, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL, 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI, 2015. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain. Behav. Immun. 44, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska N, MacQueen G, 2015. Adolescence as a unique developmental period. J. Psychiatry Neurosci. 40, 291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL, 1990. Manual for the Kaufman Brief Intelligence Test. American Guidance Service, Circle Pines, MN. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Koch K, Reeß TJ, Rus OG, Gürsel DA, Wagner G, Berberich G, Zimmer C, 2018. Increased default mode network connectivity in obsessive-compulsive disorder during reward processing. Front. Psychiatry 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R, 2002. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 25, 154–159. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B, 2012. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 10, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH, 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK, 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 554–565. [DOI] [PubMed] [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic ky-nurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain. Behav. Immun. 53, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Portnoff LC, Armstrong CC, Keenan-Miller D, Breen EC, Muscatell KA, Eisenberger NI, Irwin MR, 2016. Inflammatory cytokines and nuclear factorkappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 241, 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC, 2013. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety 30, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RH, Goldstein BI, 2014. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J. Am. Acad. Child Adolesc. Psychiatry 53, 274–296. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI, 2016. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain. Behav. Immun. 57, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O, 2013. Neuroinflammation and psychiatric illness. J. Neuroinflam. 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R, 2002. Low levels of circulating inflammatory cytokines-do they affect human brain functions? Brain. Behav. Immun. 16, 525–532. [DOI] [PubMed] [Google Scholar]
- Poznanski E, Freeman L, Mokros H, 1985. Children’s depression rating scale-revised. Psychopharmacol. Bull. 21, 979–989. [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R, 1984. Preliminary studies of the reliability and validity of the children’s depression rating scale. J. Am. Acad. Child Psychiatry 23, 191–197. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros H, 1996. Children’s Depression Rating Scale-Revised (CDRS-R). Western Psychological Services, Los Angeles, CA. [Google Scholar]
- Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, Ford BN, Morris HM, Bodurka J, Teague TK, Drevets WC, 2015a. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology 62, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R, 2015b. Putative neuroprotective and neurotoxic ky-nurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M, 2015. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE, 2010. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. PNAS 107, 14817–14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Swardfager W, Rosenblat JD, Benlamri M, McIntyre RS, 2016. Mapping inflammation onto mood: inflammatory mediators of anhedonia. Neurosci. Biobehav. Rev. 64, 148–166. [DOI] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Meda S, Xu X, Ayoub R, Medina O, Pearlson GD, Krystal JH, Abi-Dargham A, 2012. Imaging human reward processing with positron emission tomography and functional magnetic resonance imaging. Psychopharmacology (Berl.) 221, 67–77. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA, 2014. Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE, 1995. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 57, 1011–1026. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. NeuroImage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J, 2001. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 24, 531–544. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C-SR, 2012. Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front. Psychol. 3 172–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J, 2013. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord. 151, 531–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


