Abstract
Purpose
To evaluate the feasibility of ferumoxytol (FE)-enhanced ultra-short echo time (UTE)-MRA for depiction of the pulmonary vascular and non-vascular structures.
Methods
Twenty healthy volunteers underwent contrast-enhanced pulmonary MRA at 3.0T at two visits, separated by ≥4 weeks:
Visit 1: MRA started with a conventional multi-phase 3D-T1-weighted breath-held spoiled gradient echo (SGRE) MRA before and after the injection of 0.1mmol/kg GD. Subsequently, free-breathing GD-UTE-MRA was acquired as a series of three flip angles (FA: 6°, 12°, 18°) to optimize T1 weighting.
Visit 2: After the injection of 4mg/kg FE, MRA was performed during the steady state, starting with a conventional 3D T1-weighted breath-held SGRE-MRA, and followed by free-breathing FE-UTE-MRA, both at four different FA (6°, 12°, 18°, 24°).
Optimal FA for best T1 contrast was evaluated. Image quality at the optimal FA was compared between methods on a 4-point ordinal scale, using multi-phase GD-cMRA as standard of reference (SOR).
Results
FA in the range of 18–24° resulted in best T1-contrast for FE-cMRA and both UTE-MRA techniques (p>0.05).
At optimized FA, image quality of the vasculature was good/excellent with both FE-UTE-MRA and GD-cMRA (98% vs. 97%; p=0.51). Both UTE-techniques provided superior depiction of non-vascular structures compared to either gadolinium- or ferumoxytol-enhanced cMRA (p<0.001). However, GD-UTE-MRA showed the lowest image quality of the angiogram due to low image contrast.
Conclusion
Free-breathing UTE-MRA using ferumoxytol is feasible for simultaneous assessment of the pulmonary vasculature and non-vascular structures. Patient studies should investigate the clinical utility of free-breathing UTE-MRA for assessment of pulmonary emboli.
Keywords: ultra-short echo time, magnetic resonance angiography, ferumoxytol, gadobenate dimeglumine, pulmonary MRA
Introduction
Conventional contrast enhanced pulmonary MRA (cMRA) has emerged as an attractive alternative to computed tomography angiography (CTA) for diagnosis of pulmonary embolism (PE) (1–3) without the use of ionizing radiation. However, cMRA shows inferior depiction of the lung parenchyma (1–3) and may be limited in dyspneic patients due to the need for breath-holding (3). Further, gadolinium-based contrast agents (GBCA), despite their outstanding safety profiles, are contraindicated in some patients, such as pregnant women or patients with gadolinium allergies. To overcome the limitations of cMRA, free-breathing MR-techniques and alternative contrast agents that facilitate adequate acquisition timing may be beneficial for the diagnosis of PE.
Ultra-short echo time (UTE) MRA is a new technique that has shown high diagnostic yield for PE in animal experiments and can be acquired during free-breathing (4). Unlike cMRA, UTE-MRA has also shown promise for visualization of the lung parenchyma, thus allowing for simultaneous assessment of the pulmonary vasculature and pulmonary parenchyma. However, free-breathing UTE techniques require the use of a blood pool contrast agent due to longer scan times (4). Currently, there are no gadolinium-based blood pool contrast agents available on the market.
Ferumoxytol (FE) is an ultra-small superparamagnetic iron oxide (USPIO) that can be used as an off-label MR-contrast agent with favorable T1-shortening properties (5,6) and a blood pool half-life of 10–14 hours. It is approved by the US Food and Drug Administration (FDA) for intravenous treatment of iron deficiency anemia in adults with chronic kidney disease (7,8). Recently, ferumoxytol has drawn increasing interest as an MR contrast agent due to the growing safety concerns of gadolinium-based contrast agents with respect to nephrogenic systemic fibrosis (NSF) and gadolinium deposition in the brain (9,10). Ferumoxytol enhanced MRA has been shown to be feasible for a variety of vascular indications, including imaging of the aorta, coronary and pulmonary arteries, intracranial and peripheral vasculature or 4D flow techniques (11–13). Therefore, pulmonary MRA with iron-based ferumoxytol could be an alternative approach in pregnant women, in which CTA is undesirable and GBCAs are contraindicated. However, the different magnetic properties of ferumoxytol compared to conventional GBCAs require adjustment of scan parameters such as echo time (TE), repetition time (TR), and flip angle (FA).
We hypothesized that contrast-enhanced free-breathing UTE MRA with optimized scan parameters would be feasible for adequate depiction of the pulmonary vasculature and improved depiction of the lung parenchyma. Furthermore, we hypothesized that UTE-MRA benefits from the use of a blood pool contrast agent like ferumoxytol compared to a conventional multi-purpose GBCAs that show an average blood half-life of only about 1–2 hours. Therefore, the purpose of this study was to evaluate the feasibility of ferumoxytol-enhanced UTE-MRA for depiction of the pulmonary vasculature. A secondary purpose was to evaluate the utility of this approach to visualize non-vascular structures of the thorax.
Methods
This prospective single-site HIPAA-compliant feasibility study was approved by the local institutional review board (IRB). Subjects were recruited from a database of healthy volunteers. Subjects with general contraindications to MRI, including a history of allergic reactions to GBCAs, ferumoxytol or other intravenous iron products were not included. All subjects underwent two visits that were separated by at least four weeks.
MR-Imaging
Imaging was performed on a 3.0T MRI-system (MR750, GE Healthcare, Waukesha, WI) using a 32-channel phased-array torso coil (Neocoil, Pewaukee, WI). Figure 1 summarizes the protocol setup. Table 1 summarizes MR-acquisition parameters. A 20-gauge antecubital intravenous catheter was placed in each subject.
Figure 1.

Protocol setup for Visit 1 and Visit 2. FA, flip angle, UTE: ultra-short echo time.
Table 1:
MR-acquisition parameters
| Visit 1 (Gadobenate dimeglumine) | Visit 2 (Ferumoxytol) | |||
|---|---|---|---|---|
| Breath-held 3D SGRE MRA | free-breathing 3D UTE-MRA | Breath-held 3D SGRE MRA | free-breathing 3D UTE-MRA | |
| Acquisition type | Cartesian | radial | Cartesian | radial |
| Slab excitation orientation | sagittal | axial | sagittal | axial |
| TE [ms] | 1.1 | 0.25 | 1.1/1.0/1.0/1.0 | 0.25 |
| TR [ms] | 3.3 / 3.4 / 3.3 | 3.6 | 3.2/2.7/2.8/2.8 | 3.6 |
| FOV (LR x AP x SI) [cm] | 30.4×32.4×38 | 36×36×28 | 30.4×32.4×38 | 36×36×28 |
| Acquisition matrix | 200×162×256 | 280×280×216 | 200×162×256 | 280×280×216 |
| Resolution | 1.9×2.0×1.5 | 1.3×1.3.x1.3 | 1.9×2.0×1.5 | 1.3×1.3.x1.3 |
| Flip angle [degrees] | 28 | 6/12/18 | 24/18/12/6 | 24/18/12/6 |
| Receiver BW [±kHz] | 111.1 | 125 | 111.1 | 125 |
| Number of slab/slices | 162 | 216 | 162 | 216 |
| Slice thickness [mm] | 2 | 1.3 | 2 | 1.3 |
| Number of radial spokes | NA | 95000 | NA | 95000 |
| Parallel imaging acceleration factor | 2 × 2 | NA | 2 × 2 | NA |
| Acquisition time [min] | 0:20 | 5:45 | 0:18 | 5:45 |
Visit 1: GD-enhanced cMRA vs. UTE-MRA
At visit one, pulmonary MRA was performed using a conventional multi-phase 3D T1-weighted breath-held spoiled gradient echo (SGRE) sequence (cMRA) with elliptical-centric Cartesian k-space sampling before, during, and after the injection of 0.1 mmol/kg body weight gadobenate dimeglumine (GD) (MultiHance, Bracco Diagnostics Inc., Princeton, NJ) (Figure 1, Table 1). The r1 relaxivity of GD in plasma at 37°C has previously been reported to be 5.5 (5.2–5.8) s−1mM−1 at 3.0T (14). Per our standard-of-care clinical protocol, GD was diluted with saline up to a total volume of 30 ml and injected with a power injector (Spectris Solaris, MedRad Inc., Warendale, PA) at a flow rate of 1.5 ml/s (15), followed by a 35 ml saline flush injected at the same rate.
Immediately following the multi-phase cMRA acquisition, free-breathing GD-enhanced UTE-MRA was acquired as a series of three different flip angles (FA: 6°, 12°, 18°) to determine the optimal T1 weighting that maximizes image quality. The order of the FA was changed randomly between subjects to either ascending (6°, 12°, 18°) or descending (18°, 12°, 6°) (Figure 1, Table 1).
Visit 2: FE-enhanced cMRA vs. UTE-MRA
At visit two, subjects received 0.07 mmol/kg (4 mg Fe/kg) body weight ferumoxytol (FE) (Feraheme, AMAG, Waltham, MA) diluted with saline to 60 ml and injected as a slow infusion over 15 minutes (0.1 ml/s) (16). This injection was followed by a saline flush of 35 ml. The r1 relaxivity of FE in plasma at 37°C has previously been reported to be 10.0 ± 0.3 s−1mM−1 at 3.0T (5). Pulmonary MRA was then performed during the steady state phase of ferumoxytol, starting with a conventional 3D T1-weighted breath-held SGRE sequence (FE-cMRA) as a series of four different FA (6°, 12°, 18°, 24° in fixed order) to optimize T1 weighting (Figure 1, Table 1). Subsequently, free-breathing FE-enhanced UTE-MRA was acquired as a series of four different FA (6°, 12°, 18°, 24° in fixed order) to optimize T1 weighting (Figure 1, Table 1).
Image Analysis
All image analyses were performed on a PACS workstation (McKesson Radiology Station, Version 12.3, San Francisco, CA). Multi-phase GD-cMRA served as the reference standard for the comparison with the different flip angle GD-UTE-MRA, FE-cMRA, and FE-UTE-MRA.
Determining Best Flip Angle for Optimal T1-weighting
The differences in image quality between the acquired flip angles of GD-UTE-MRA, FE-cMRA, and FE-UTE-MRA were anticipated to be subtle. To determine the optimal FA for relative T1 contrast between vasculature and PE, regions of interest (ROIs) were drawn in the left and right main and lower lobe pulmonary arteries, as well as paraspinal muscle in the same imaging slice as an internal reference. The relative contrast ratio between the vessel lumen and the paraspinal muscle was determined as follows:
| [1] |
where SI is the average signal intensity of the ROI.
Image Quality Rating at Best Flip Angle
In a second step, two radiologists (S.N. and M.S.), each with 11 years of experience in interpreting pulmonary MRAs, performed a direct side-by-side comparison consensus reading to assess subjective image quality of the three investigational MRA techniques (GD-UTE-MRA, FE-UTE-MRA, FE-cMRA) at their previously determined optimal FA, using multi-phase GD-cMRA as the SOR.
Image quality of vascular structures (overall image quality of the angiogram, lobar pulmonary arteries, segmental pulmonary arteries, subsegmental pulmonary arteries, ascending aorta, aortic arch, descending thoracic aorta) and non-vascular structures (pleura, lung periphery, central lung, lobar bronchi, segmental bronchi, subsegmental bronchi, mediastinum, esophagus, bone, thoracic soft tissue) were rated on a 4-point ordinal scale (0=nondiagnostic; 1=fair, limited diagnostic value; 2=good, diagnostic; 3=excellent, diagnostic with high degree of confidence).
Statistical analysis
For the determination of the best FA for optimal T1 contrast, a linear mixed-effects model was used to determine which FA resulted in the highest signal intensity and highest relative contrast between pulmonary vasculature and paraspinal muscle.
Results from the consecutive image quality consensus reading were grouped into four criteria:
Overall image quality of the angiogram,
Image quality of the pulmonary arteries (treating ordinal scale results from the lobar pulmonary arteries, segmental pulmonary arteries, and subsegmental pulmonary arteries as independent variables),
Image quality of the thoracic aorta (independent variables: ascending aorta, aortic arch, descending thoracic aorta), and
Image quality of all other non-vascular structures (independent variables: pleura, lung periphery, central lung, lobar bronchi, segmental bronchi, subsegmental bronchi, mediastinum, esophagus, bone, thoracic soft tissue).
Image quality results from the four criteria are presented as percentages. The Wilcoxon signed-rank test was used to compare ordinal scale results between the four MRA techniques. Two-sided p-values < 0.05 were regarded as statistically significant. Statistical analyses were conducted using R (R Foundation for Statistical Computing, version 3.4.2, Vienna, Austria).
Results
Study population
No adverse events were observed after injection of either GD or FE. A total of 22 healthy volunteers were included. Two subjects were unable to participate in Visit 2 and were therefore excluded from the final analysis. The remaining subjects returned for the second visit 47±10 days after the first visit with at least 35 days between visits. Therefore, the final study cohort consisted of 20 subjects (10 females : 10 males; mean age: 34.0 ± 12.0 years; mean height: 172.2 ± 10.7 cm; mean weight: 74.7 ± 14.8 kg; mean BMI: 25.1 ± 3.9). Representative image examples for all four MRA techniques are shown in Figure 2 and 3.
Figure 2.
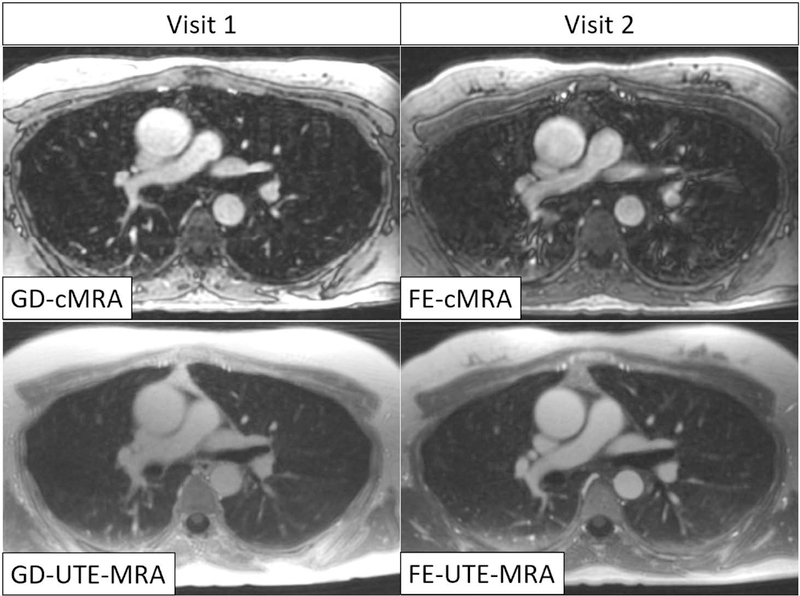
Representative image example of one subject at the height of the pulmonary truncus. Both UTE-MRA techniques (lower row) show superior depiction of non-vascular structures (lung, bone, bronchi, muscle) compared to conventional MRA using a gadolinium-based contrast agent (top left). Furthermore, FE-UTE-MRA also showed excellent image quality of the angiogram, comparable to GD-cMRA. GD-UTE-MRA suffers from low image contrast. Vascular structures appeared blurry in FE-cMRA.
GD-cMRA: gadobenate dimeglumine enhanced conventional MRA, FE-cMRA: ferumoxytol enhanced conventional MRA, GD-UTE-MRA: gadobenate dimeglumine enhanced ultra-short echo time MRA, FE-UTE-MRA: ferumoxytol enhanced ultra-short echo time MRA.
Figure 3.

Coronal images through the thorax and mediastinum from one subject (different from Figure 2). Both UTE-MRA techniques (lower row) show superior depiction of non-vascular structures (lung, bone, bronchi, muscle) compared to conventional MRA using a gadolinium-based contrast agent (top left) or ferumoxytol (top right). FE-UTE-MRA also showed excellent image quality of the angiogram, comparable to GD-cMRA. GD-UTE-MRA suffers from low image contrast.
GD-cMRA: gadobenate dimeglumine enhanced conventional MRA, FE-cMRA: ferumoxytol enhanced conventional MRA, GD-UTE-MRA: gadobenate dimeglumine enhanced ultra-short echo time MRA, FE-UTE-MRA: ferumoxytol enhanced ultra-short echo time MRA.
Best Flip Angle for Optimal T1-weighting
Signal intensities in GD-UTE-MRA, FE-cMRA, and FE-UTE-MRA showed a tendency to decrease at higher flip angles, especially in muscle tissue, while contrast between the pulmonary vasculature and muscle tissue increased at higher flip angles (Table 2, Figure 4). For FE-UTE-MRA, the best relative contrast between the pulmonary arteries and muscle was achieved at a FA of 24°, (p<0.001) Table 2, Figure 4B.
Table 2:
Relative contrast between pulmonary arteries and paraspinal muscle at different flip angles
| Visit | Method | Flip angle | Rel. contrast (pulm. artery/muscle) | P-Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | GD-UTE-MRA | 6 | 1.31 | 0.0107 | <0.001 | ||||
| 12 | 1.52 | 0.083 | |||||||
| 18 | 1.70 | ||||||||
| 2 | FE-cMRA | 6 | 2.09 | <0.001 | <0.001 | <0.001 | |||
| 12 | 2.84 | 0.002 | <0.001 | ||||||
| 18 | 3.10 | 0.485 | |||||||
| 24 | 3.34 | ||||||||
| FE-UTE-MRA | 6 | 1.82 | <0.001 | <0.001 | <0.001 | ||||
| 12 | 3,14 | <0.001 | <0.001 | ||||||
| 18 | 4.02 | <0.001 | |||||||
| 24 | 4.40 | ||||||||
Relative contrast (means across subjects) between the pulmonary arteries and muscle (surrogate for pulmonary emboli) increases at higher flip angles. The spread of mean relative signal intensity values for pulmonary arteries and muscle is illustrated in Figure 4A.
Figure 4.

Contrast between pulonary arteries and muscle increases at higher flip angles. Best image contrast between was achieved at a flip angle of 18° for GD-UTE-MRA, 18° or 24° for FE-cMRA, and 24° for Fe-UTE-MRA, respectively (p<0.05). Box plots on the left (A) show signal intensities obtained from ROI-measurements of pulmonary arteries and muscle; box plots on the right (B) show relative contrast between pulmonary arteries and muscle. Gadobenate dimeglumine-enhanced ultra-short echo-time (GD-UTE-MRA), ferumoxytol-enhanced conventional Cartesian MRA (FE-cMRA), ferumoxytol-enhanced UTE-MRA (FE-UTE-MRA).
For GD-UTE-MRA, the best relative contrast was achieved at FA of 12° or 18° respectively (p=0.083) whereas FA of 18° or 24° resulted in best relative contrast for FE-cMRA (p=0.083). Lower flip angles resulted in significantly lower relative signal intensity and relative contrast between pulmonary arteries and muscle Table 2, Figure 4B.
Based on these results, we chose the following flip angles for the subsequent image quality rating: GD-UTE-MRA: FA 18°; FE-cMRA: FA 24°; FE-UTE-MRA: 24°.
Image Quality Rating
Results from image quality ratings are summarized in Figure 5 and Table 3.
Figure 5.

Both UTE techniques were advantageous compared to the conventional MRA regarding the depiction of non-vascular structures (pleura, lung, bronchi, mediastinum, esophagus, and bones). Image quality of FE-UTE was overall excellent and significantly better than GD-MRA regarding the visualization of non-vascular structures (p>0.001). No significant difference was found between FE-UTE and GD-MRA regarding image quality of the overall angiogram, the pulmonary arteries or the aorta. Gadobenate dimeglumine (GD), ferumoxytol (FE). Asterisk* mark significant differences, using Wilcoxon Signed-Ranks test (p<0.05). P-values are listed in Table 3.
Table 3:
P-Values for Figure 5 (Image quality rating between different MRA-techniques)
| P-Value | ||||
|---|---|---|---|---|
| Method | Overall Angiogram | Pulmonary Arteries | Aorta | Non-vascular Structures |
| GD-cMRA vs. GD-UTE-MRA | 0.12 | <0.001*** | <0.001*** | <0.001*** |
| GD-cMRA vs. FE-cMRA | 0.089 | 0.006** | 0.003** | 0.015* |
| GD-cMRA vs. FE-UTE-MRA | 0.53 | 0.506 | 0.79 | <0.001*** |
| GD-UTE-MRA vs. FE-cMRA | 0.802 | 0.002** | <0.001*** | <0.001*** |
| GD-UTE-MRA vs. FE-UTE-MRA | 0.024* | <0.001*** | <0.001*** | 0.008** |
| FE-cMRA vs. FE-UTE-MRA | 0.059 | 0.005** | 0.049* | <0.001*** |
Asterisk mark significant differences, using Wilcoxon Signed-Ranks test (p<0.05).
GD-cMRA: gadobenate dimeglumine enhanced conventional MRA, GD-UTE-MRA: gadobenate dimeglumine enhanced ultra-short echo time MRA, FE-cMRA: ferumoxytol enhanced conventional MRA, FE-UTE-MRA: ferumoxytol enhanced ultra-short echo time MRA.
Overall image quality of the pulmonary angiogram was predominantly good or excellent for all four MRA techniques. FE-UTE-MRA was overall rated best, although without significant differences to FE-cMRA (p=0.06) and GD-cMRA (p=0.53, SOR). GD-UTE-MRA showed the worst image quality of the angiogram due to low image contrast, Figure 5 and Table 3.
When examining the image quality of the pulmonary arteries, both FE-UTE-MRA and GD-cMRA (SOR) showed predominantly good or excellent image quality (98% and 97%, respectively; p=0.51), Figure 5. Both techniques (FE-UTE-MRA and GD-cMRA) were rated significantly better than FE-cMRA (p=0.005 and p=0.006, respectively) and GD-UTE-MRA (both p < 0.001), Figure 5 and Table 3.
Similarly, when examining the image quality of the thoracic aorta, both FE-UTE-MRA and GD-cMRA (SOR) showed predominantly good or excellent image quality (96% and 98%, respectively; p=0.79), Figure 5. Both techniques (FE-UTE-MRA and GD-cMRA) were rated significantly better than FE-cMRA (p=0.049 and p=0.003, respectively) and GD-UTE-MRA (both p<0.001), Figure 5 and Table 3.
Regarding imaging quality of non-vascular structures, both UTE-techniques demonstrated high rates of good or excellent image quality (FE-/GD-UTE-MRA: both 86%) and were significantly better than the conventional MRA techniques with either FE (both p < 0.001) or GD (both p < 0.001). Mean image quality of FE-UTE-MRA was significantly better than GD-UTE-MRA (p = 0.008).
A more detailed overview of the image quality results for non-vascular structures can be found in the Figure 6 . Both UTE-MRA techniques were significantly better than either cMRA method regarding the depiction of the pleura, peripheral and central lung, lobar and segmental bronchi, the mediastinum, esophagus and bone. All four techniques demonstrated good results for the visualization of thoracic soft tissue. However, all techniques (cMRA and UTE-MRA) failed to adequately depict subsegmental bronchi.
Figure 6.

Graphical sub-analysis from image quality reading of non-vascular structures. Both UTE techniques were advantageous compared to the conventional MRA regarding the depiction of non-vascular structures, particularly pleura, peripheral and central lung parenchyma, segmental and lobar bronchi, mediastinum, esophagus and bone. None of the four techniques could adequately depict subsegmental bronchi. All MRA techniques showed good or excellent results for the depiction of soft tissue.
GD-cMRA: gadobenate dimeglumine enhanced conventional MRA, GD-UTE-MRA: gadobenate dimeglumine enhanced ultra-short echo time MRA, FE-cMRA: ferumoxytol enhanced conventional MRA, FE-UTE-MRA: ferumoxytol enhanced ultra-short echo time MRA.
Discussion
This study showed the feasibility of ferumoxytol-enhanced ultra-short echo-time MR angiography as an alternative to conventional gadolinium-enhanced MRA of the pulmonary vasculature. We evaluated three alternative approaches for pulmonary MRA including gadolinium-enhanced UTE-MRA, ferumoxytol-enhanced UTE-MRA, and ferumoxytol-enhanced conventional MRA, using gadolinium-enhanced conventional MRA as the reference. We found that all three approaches are feasible and adequately depicted the pulmonary vasculature. Secondarily, we optimized the T1 weighting by identifying the flip angle that provided the greatest image contrast. We found that the optimal flip angle was 18–24° for gadolinium-enhanced and ferumoxytol-enhanced UTE-MRA. Using these optimized flip angles, we found that both free-breathing UTE-techniques provided superior depiction of non-vascular structures compared to conventional MRA. With UTE-MRA, the angiogram image quality benefitted from the use of ferumoxytol as an alternative contrast agent with a longer blood half-life. Gadolinium-enhanced UTE-MRA generally suffered from lower image contrast.
Conventional pulmonary MRA with GBCAs is of high diagnostic yield and generally has high technical success using modern acquisition strategies (2,3,17). However, in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) III study 25% of patients demonstrated insufficient image quality with, mainly due to dyspnea, coughing, or faulty timing of contrast material administration (3). The results of our study indicate that UTE-MRA, particularly with ferumoxytol, is feasible to overcome these limitations. The radial sampling of the UTE-MRA technique helps mitigate motion-related artifacts, thus allowing its acquisition under free-breathing. The short echo-time minimizes susceptibility-related artifacts, improving assessment of non-vascular structures, particularly pulmonary parenchyma. Image acquisition of UTE-MRA can be performed during the steady-state phase due to the long blood half-life of ferumoxytol of 10–14 h, avoiding the need for contrast agent timing. Although gadobenate dimeglumine is a high-relaxivity GBCA, its elimination half-life is only approximately 90 minutes (18). Free-breathing UTE-MRA of the thorax however requires longer acquisition times of approximately 5–6 minutes (4). Due to the faster wash-out of gadolinium from the blood pool, GD-UTE-MRA suffered from lower contrast of the pulmonary vasculature. Ferumoxytol-enhanced UTE-MRA on the other hand resulted in excellent image quality of the pulmonary vasculature compared to GD-cMRA and superior depiction of non-vascular structures such as pleura, lung tissue, mediastinum and bone.
The results of our study therefore support results from previous animal studies by Bannas et al. that demonstrated high diagnostic yield of FE-UTE-MRA for PE and confirm that image quality of UTE-MRA benefits from a blood pool contrast agent (4). So far, however, few clinical studies have investigated the feasibility of different MRA techniques for assessment of PE.
Furthermore, our results are in good agreement with previous reports, attributing free-breathing UTE-MRI high sensitivity for the depiction of non-vascular thoracic structures that outperformed conventional 3D GRE techniques (19–21). The concept of using MRI as an alternative to contrast-enhanced CT for detection of PE remains appealing, especially in young or pregnant patients in whom exposure to ionizing radiation is undesirable. 24% of patients have contraindications for CTA (3). Furthermore, pulmonary CTA also requires patients to hold their breath. The FE-UTE-MRA approach could therefore be an attractive alternative for detection of PE under free-breathing, while simultaneously avoiding ionizing radiation and allowing for assessment of non-vascular structures. Further investigations are needed to determine the clinical utility of this new approach in patients with known or suspected PE.
The current work was designed as a feasibility study, which is associated with several limitations. All MRA techniques were acquired in healthy volunteers without PE and without difficulty complying with the instructions of regular and calm breathing. The excellent image quality results of the free-breathing UTE-MRA approach could be degraded in agitated patients due to the longer scan times. Since our subjects did not have PE, we had to use a reference tissue other than thrombotic material for the optimization of T1-weighting. Paraspinal muscle tissue has been used in previous studies as a surrogate for non-enhancing lesions (22). However, muscle does show mild delayed enhancement that reaches a plateau approximately 3–4 minutes after contrast injection. Since GD-UTE-MRA was always performed after the multi-phase GD-cMRA, this might have negatively influenced the results of the relative vasculature-to-muscle contrast of GD-UTE-MRA. Similarly, ferumoxytol-enhanced images were acquired during the steady state phase when muscle tissue may demonstrate some enhancement. We therefore anticipate that the relative contrast between pulmonary vasculature and muscle in our FE-cMRA and FE-UTE-MRA images is lower than the actual contrast between pulmonary arteries and PE. Furthermore, image quality of the three investigated MRA techniques was rated in a non-blinded direct side-by side consensus reading which inevitably introduced some bias.
We also note that the use of ferumoxytol as a contrast agent for MRI is considered off-label. Ferumoxytol has been associated with a very small risk of anaphylaxis, although this is mitigated through the use of slow injections of diluted agent (16). This, however, restricts the use of ferumoxytol for applications that require dynamic phase MR-imaging. Gadobenate dimeglumine is FDA approved in the U.S. for MRA of the kidneys and lower extremities, however pulmonary MRA is currently not included in the label (18). In Europe, GD lost its market approval in 2017 for all indications except MR-imaging of the liver (23). Pulmonary MRA could, however, also be performed using macrocyclic GBCAs. Finally, we not that a direct comparison of the investigated MRA techniques at different field strengths or with CTA was not possible. Such a comparison of the diagnostic performance between free-breathing UTE-MRA, conventional MRA and CTA could be topic for future clinical studies in patients with suspected PE.
In conclusion, free-breathing ferumoxytol-enhanced UTE-MRA is feasible for the evaluation of the pulmonary vasculature as an alternative to gadolinium enhanced conventional MRA. The ultra-short echo-times combined with radial sampling could help mitigate motion-related artifacts and allow simultaneous assessment of non-vascular structures. Larger patient studies are needed to investigate the clinical utility of free-breathing UTE-MRA for assessment of PE.
Acknowledgements
The authors wish to acknowledge support from the Departments of Radiology and Medical Physics, University of Wisconsin, as well as the NIH (T32CA009206 and K24 DK102595). Further, this project was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors also wish to acknowledge GE Healthcare and Bracco Diagnostics, which provide research support to UW-Madison. Further, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.
Tilman Schubert contributed to this work while employed by UW-Madison. T.S. is now an employee of the Department of Radiology, University Hospital Bern, Switzerland.
Footnotes
Scott Reeder has no relevant disclosures. Unrelated disclosures include ownership interest in Elucent Medical, Reveal Pharmaceuticals, Cellectar Biosciences, and Calimetrix.
Scott Nagle has no relevant disclosures. Unrelated disclosures include consulting for Vertex Pharmaceuticals.
Gesine Knobloch has no relevant disclosures. Unrelated disclosures include a current employment at Bayer AG, Berlin, Germany.
References
- 1.Francois CJ, Hartung MP, Reeder SB, Nagle SK, Schiebler ML. MRI for acute chest pain: current state of the art. Journal of magnetic resonance imaging : JMRI 2013;37(6):1290–1300. [DOI] [PubMed] [Google Scholar]
- 2.Schiebler ML, Nagle SK, Francois CJ, Repplinger MD, Hamedani AG, Vigen KK, Yarlagadda R, Grist TM, Reeder SB. Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year. Journal of magnetic resonance imaging : JMRI 2013;38(4):914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr., Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Annals of internal medicine 2010;152(7):434–443, w142–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannas P, Bell LC, Johnson KM, Schiebler ML, Francois CJ, Motosugi U, Consigny D, Reeder SB, Nagle SK. Pulmonary Embolism Detection with Three-dimensional Ultrashort Echo Time MR Imaging: Experimental Study in Canines. Radiology 2016;278(2):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knobloch G, Colgan T, Wiens CN, Wang X, Schubert T, Hernando D, Sharma SD, Reeder SB. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Invest Radiol 2018;53(5):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. Journal of magnetic resonance imaging : JMRI 2015;41(4):884–898. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. American journal of hematology 2010;85(5):315–319. [DOI] [PubMed] [Google Scholar]
- 8.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJ. Ferumoxytol for treating iron deficiency anemia in CKD. Journal of the American Society of Nephrology : JASN 2008;19(8):1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashir MR, Jaffe TA, Brennan TV, Patel UD, Ellis MJ. Renal transplant imaging using magnetic resonance angiography with a nonnephrotoxic contrast agent. Transplantation 2013;96(1):91–96. [DOI] [PubMed] [Google Scholar]
- 10.Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ. Gadolinium-based contrast agents: A comprehensive risk assessment. Journal of magnetic resonance imaging : JMRI 2017;46(2):338–353. [DOI] [PubMed] [Google Scholar]
- 11.Hope MD, Hope TA, Zhu C, Faraji F, Haraldsson H, Ordovas KG, Saloner D. Vascular Imaging With Ferumoxytol as a Contrast Agent. AJR American journal of roentgenology 2015;205(3):W366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Tutton S, Vu AT, Pierchala L, Li BS, Lewis JM, Prasad PV, Edelman RR. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. Journal of magnetic resonance imaging : JMRI 2005;21(1):46–52. [DOI] [PubMed] [Google Scholar]
- 13.Ahlstrom KH, Johansson LO, Rodenburg JB, Ragnarsson AS, Akeson P, Borseth A. Pulmonary MR angiography with ultrasmall superparamagnetic iron oxide particles as a blood pool agent and a navigator echo for respiratory gating: pilot study. Radiology 1999;211(3):865–869. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investigative Radiology 2005;40(11):715–724. [DOI] [PubMed] [Google Scholar]
- 15.Nagle SK, Schiebler ML, Repplinger MD, Francois CJ, Vigen KK, Yarlagadda R, Grist TM, Reeder SB. Contrast enhanced pulmonary magnetic resonance angiography for pulmonary embolism: Building a successful program. European journal of radiology 2016;85(3):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasanawala SS, Nguyen KL, Hope MD, Bridges MD, Hope TA, Reeder SB, Bashir MR. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016;75(5):2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Repplinger MD, Nagle SK, Harringa JB, Broman AT, Lindholm CR, Francois CJ, Grist TM, Reeder SB, Schiebler ML. Clinical outcomes after magnetic resonance angiography (MRA) versus computed tomographic angiography (CTA) for pulmonary embolism evaluation. Emergency radiology 2018;25(5):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA. MultiHance (gadobenate dimeglumine) Labeling-Package Insert Volume 2018 Silver Spring, MD: U.S. Food and Drug Administration; 2018. [Google Scholar]
- 19.Burris NS, Johnson KM, Larson PE, Hope MD, Nagle SK, Behr SC, Hope TA. Detection of Small Pulmonary Nodules with Ultrashort Echo Time Sequences in Oncology Patients by Using a PET/MR System. Radiology 2016;278(1):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauml J, Schiebler ML, François CJ, Johnson KM, Nagle SK. Comparison of Pulmonary Magnetic Resonance Angiography (MRA) and free-breathing Ultra short time to echo (UTE) for the comprehensive evaluation of the vascular and non-vascular anatomy of the chest. International Society for Magnetic Resonance in Medicine Annual Meeting 2016. Singapore2016. Oral Presentation. [Google Scholar]
- 21.Nagle SK, Francois CJ, Poranski M, Bell LC, Johnson KM, Fain SB. 3D Radial UTE MRI Outperforms 3D Cartesian Conventional Echo Time MRI for Evaluation of Cystic Fibrosis Lung Disease. . International Society for Magnetic Resonance in Medicine Annual Meeting Honolulu, HI2017. Oral Presentation. [Google Scholar]
- 22.Frydrychowicz A, Nagle SK, D’Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. Journal of magnetic resonance imaging : JMRI 2011;34(3):585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EMA. Gadolinium-containing contrast agents Volume 2018 London: European Medicines Agency; 2017. [Google Scholar]


