Abstract
Background:
Polymorphisms in adrenergic signaling affect the molecular function of adrenergic receptors and related proteins. The β1 adrenergic receptor (ADRB1) Arg389Gly, G-protein receptor kinase type 5 (GRK5) Gln41Leu, G-protein β-3 subunit (GNB3) 825 C/T, and α2c deletion affect adrenergic tone, impact heart failure outcomes and differ in prevalence by ethnicity. Their combined effect within black cohorts remains unknown.
Methods and Results:
We analyzed subjects from the African American Heart Failure Trial (A-HeFT) by assessing event-free survival, quality of life, and gene coinheritance. Significant coinheritance effects on survival included GRK5 Leu41 among subjects coinheriting GNB3 825 C alleles (n = 166, 90.4% vs 69.0%, p < 0.001). By contrast, the impact of ADRB1 Arg389Arg genotype was magnified among subjects with GNB3 825 TT genotype (n = 181, 66.3% vs 85.7%, p = 0.002). The lack of the α2c deletion (i.e., insertion) led to a greater impact of the ARG389Arg genotype (n = 289, 76.4% vs86.1%, p = 0.007).
Conclusions:
Polymorphisms in adrenergic signaling affects outcomes in black subjects with heart failure. Coinheritance patterns in genetic variation may help determine heart failure survival.
Keywords: heart failure, gene polymorphism, adrenergic receptor, adrenergic signaling
INTRODUCTION
The pathophysiology of heart failure with reduced ejection fraction (HFrEF) includes regulatory responses to catecholamine stimulation of the failing heart. Important adrenergic receptor activity features feedback mechanisms that determine cardiac function (1,2). Researchers have studied polymorphisms in the genes affecting adrenergic signaling to understand the causal effects of genetic background on heart failure outcomes (3). Much of the clinical heterogeneity in heart failure outcomes can be associated with genetic variability (4), findings that overlap with variation in disease progression by race or ethnicity (5).
Work initially addressing the genomic risk of hypertension has previously determined that allelic frequencies of genes involved in adrenergic signaling differ significantly in black and white cohorts (3). Several adrenergic polymorphisms common in black cohorts have been suggested to impact heart failure outcomes including the β1 adrenergic receptor (ADRB1) Arg389Gly(6), G-protein receptor kinase type 5 (GRK5) Leu41Gln (3,5,7), G-protein β-3 subunit (GNB3) 825 T/C(8), and the α2C deletion (9). Three of these genes, ADRB1, GRK5, and GNB3, interact at the level of the G-protein complex. The fourth, the α2c receptor, helps regulate release of norepinephrine from cardiac sympathetic nerves. A polymorphism of the α2c receptor gene is common in black subjects and involves deletion of amino acids 322-325, leading to the loss of normal negative feedback and, therefore increased release of norepinephrine (9). While these genes are predicted to interact on a molecular level during heart failure pathogenesis, the genetic interaction of their functional variants on heart failure outcomes has not been previously studied.
Allelic frequencies of these adrenergic polymorphisms differ in black and white cohorts (3). The α2c deletion is more common in black cohorts but rare in white cohorts (minor allele frequency of 0.42 vs 0.06, respectively). In a similar fashion, the GRK5 Leu41 variant is more common in black subjects with a minor allele frequency of 0.23 but very rare in white subjects (minor allele 0.013) (3). The GNB3 825T allele is the major allele in black cohorts (allele frequency of 0.72 (10) with roughly 50% of black subjects being homozygous, while in white subjects it is the minor allele (frequency 0.42) with only 10-15% being homozygous (3,11). The GNB3 T allele is tightly linked to a splicing variant and a truncated GNB3 subunit which results in increased adrenergic tone. The increased prevalence of this variant in black cohorts has been extensively studied and is one of the mechanisms by which subjects of African genomic ancestry are speculated to have a higher prevalence of hypertension than those with European genomic ancestry (12). The β1 receptor Arg389 variant is an exception as it is the major allele compared with Gly389 and Arg389 is actually slightly less common in black subjects compared with white subjects (allele frequency 0.56 vs 0.75, respectively (12). Analysis of the impact of single gene mutations of GRK5 Leu41 and ADRB1 Arg389 polymorphisms on heart failure outcomes has led to variable results (7,9,13–17). Potential explanations for the inconsistent findings may be that single genetic variants do not explain complex clinical outcomes and polygenic analysis accounting for genetic interactions with other important adrenergic polymorphisms is required. This may be particularly pertinent for black heart failure cohorts with relatively increased gene frequencies of several potentially pathologic adrenergic variants. To address this, we evaluated the impact on heart failure outcomes of functional variants of ADRB1, GRK5, GNB3, and α2c, and their coinheritance, by examining participants in GRAHF (Genetic Risk Assessment of Heart Failure in African Americans) the genetic sub-study of the African American Heart Failure Trial (A-HeFT).
METHODS
Study population
A-HeFT was conducted between 2001-2004 in 161 centers across the United States and evaluated mortality benefit of fixed dose combination of isosorbide dinitrate and hydralazine (FDC I/H) compared with usual heart failure care (18). At the 6-month follow up visit, a subset of 350 subjects enrolled for GRAHF1 after providing additional informed consent. The study protocol was reviewed and approved by the institutional review board at each of the 64 enrolling sites. Subjects were followed to an endpoint of death or heart failure hospitalization. Subjects had an assessment of quality of life (QOL) using the Minnesota living with heart failure questionnaire (MLHFQ) at entry and at six months post-randomization. The A-HeFT composite score (CS) was the primary endpoint of the A-HeFT trial. The A-HeFT CS incorporates survival (alive = 0, dead = −3), heart failure hospitalization (no = 0, yes = −1) and change in QOL score (−2 to +2, depending on the degree of change in the MLHFQ raw score) into a single score, which can range from −6 to +2.
Genotyping
We isolated DNA from peripheral blood by leukocyte centrifugation and cell lysis using the PureGene DNA purification kit (Gentra Systems, Minneapolis, Minnesota). We assessed the ADRB1 gene position 1165 Arg389Gly G/C polymorphism using a TaqMan SNP Genotyping Assay (Assay ID: C__8898494_10, Applied Biosystems Inc., Foster City, CA), the GRK5 Gln41Leu polymorphism position 41 A/T polymorphism using a TaqMan SNP Genotyping Assay (Assay ID: C__15852506_10, Applied Biosystems Inc., Foster City, CA), and the GNB3 825 C/T polymorphism using a TaqMan SNP Genotyping Assay (Assay ID: C__2184734_10, Applied Biosystems Inc., Foster City, CA) with tagged primers (reporter 1 tagged dye = VIC; reporter 2 tagged dye = FAM). We read the products with the Applied Biosystems 7000 (ABI, Foster City, CA).
For genotyping of the α2c deletion polymorphism, genomic DNA was amplified using the following primers (19): sense 5’-AGCCCGACGAGAGCAGCGCA-3’ and antisense 5’-AGGCCTCGCGGCAGATGCCCTACA -3’, in PCR reactions consisting of 100 ng genomic DNA, 20 ul 5× buffer (Invitrogen), 5 pmol of each primer, 0.8 nM dNTPs, 10% DMSO, and 2.5 units Platinum Taq DNA polymerase (GIECO/BRL) in a 20ul reaction volume. PCR cycling started at 94 for 5 minutes, followed by 94” for 30 seconds, 63 for 30 seconds, and 72” for 2 minutes for 40 cycles, and a final extension of 72” for 10 minutes. 15 ul was then loaded onto 3% agarose gel and run overnight at 45V.
Statistical analysis
We evaluated the agreement of observed genotypes for Hardy-Weinberg equilibrium by χ2 analysis comparing observed genotypes percentages with expected for the polymorphisms (20). All other analyses of genotypes were based on two group comparisons. For GNB3, ADBR1, and GRK5, subjects homozygous for the major allele were compared to all subjects with the minor allele (subjects homozygous for the minor allele combined with heterozygous subjects). For α2C, subjects homozygous for the minor allele (α2C deletion) were compared to all subjects heterozygous or homozygous for the wild type receptor. Student’s t and Fisher’s exact tests compared continuous and categorical variables by genotype, respectively. The impact of GNB3 TT genotype on heart failure outcomes has been previously evaluated (11). Event-free survival (survival free from heart failure hospitalization) using Kaplan-Meier survival and log rank analysis for ADRB1Arg389Arg, GRK5 Leu41 (Leu41Leu and Gln41Leu combined), and α2C deletion/deletion first in the overall cohort and then separately based on GNB3 genotype subsets (GNB3 825 TT or GNB3 C (TC and CC genotypes combined)). An alpha of 0.05 determined statistical significance except where adjustments were required. For the impact on survival of variants of the four adrenergic genes investigated, a Bonferroni correction for multiple comparisons resulted in a significance level of p = 0.0125 (0.05 divided by four comparisons). For the analysis of survival for three adrenergic variants (ADRB1 Arg389Arg, GRK5 Leu41 (Leu41Leu and Gln41Leu combined), and α2C deletion homozygous subjects) within the two GNB3 genotype subsets (GNB3TT and GNB3 C), the Bonferroni correction for six comparisons led a significance level of p = 0.0083 (0.05 divided by six comparisons). We evaluated interactions between genotypes for their impact on event-free survival with Cox regression analysis. We analyzed two group comparisons for QOL and CS by medical therapy within genotype subset using Student’s paired t tests. We performed statistical analyses using SPSS version 24 and Stata version 15.
RESULTS
Baseline demographics
A total of 350 participants from GRAHF were genotyped for ADRB1 Arg389Gly, the GRK5 Gln41Leu, GNB3 825 C/T, and α2C deletion/insertion polymorphisms. Genotype data were unavailable for three subjects for ADRB1, two subjects for GRK5, and six subjects for α2C deletion/insertion. Table 1 shows descriptive statistics and baseline characteristics. The GRAHF cohort was 60% male with a mean age = 57 years ± 13. The mean left ventricular ejection fraction was 0.26 ± 0.06. Twenty-five percent of participants had ischemic cardiomyopathy. All participants had New York Heart Association class III or IV symptoms (% class III/IV = 97/3).
TABLE 1.
Baseline demographic and clinical characteristics of 350 participants in the GRAHF Cohort
| All n = 350 | ADRB1 | GRK5 | GNB3 | α2c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Arg389Gly+Gly389Gly n = 236 | Arg389Arg n = 111 | Gln41Gln n = 192 | Leu41Leu+Gln41Leu n = 156 | CC+TC n = 166 | TT n = 184 | ins/ins+ins/del n = 291 | del/del n = 53 | ||
| Age (years) ± SD | 57 ± 13 | 57 ± 13 | 56 ± 13 | 58 ± 13 | 56 ± 12 | 58 ± 13 | 57 ± 12 | 58 ± 13 | 58 ± 14 |
| Female (%) | 40 | 38 | 44 | 43 | 36 | 40 | 40 | 40 | 40 |
| NYHA class (%/III/IV) | 97/3 | 97/3 | 96/4 | 96/4 | 97/3 | 97/3 | 97/3 | 97/3 | 98/2 |
| Ischemic (%) | 25 | 24 | 27 | 23 | 28 | 26 | 24 | 25 | 28 |
| LVEF ± SD | 0.24 ± 0.06 | 0.24 ± 0.06 | 0.25 ± 0.07 | 0.24 ± 0.06 | 0.24 ± 0.06 | 0.23 ± 0.07 | 0.24 ± 0.06 | 0.24 ± 0.06 | 0.25 ± 0.06 |
| BP systolic (mmHg)± SD | 127 ± 17 | 127 ± 17 | 127 ± 17 | 128 ± 17 | 125 ± 17 | 126 ± 18 | 128 ± 17 | 127 ± 17 | 124 ± 16 |
| BP diastolic (mmHg)± SD | 77 ± 11 | 76 ± 11 | 78 ± 10 | 77 ± 10 | 77 ± 11 | 76 ± 11 | 77 ± 11 | 77 ± 10 | 75 ± 11 |
| ACE inhibitor or ARB (%) | 94 | 93 | 97 | 93 | 96 | 95 | 94 | 94 | 94 |
| Aldosterone receptor antagonist (%) | 36 | 36 | 36 | 38 | 34 | 37 | 35 | 36 | 34 |
| β-blocker (%) | 84 | 81 | 92* | 82 | 86 | 83 | 85 | 84 | 81 |
| FDCI/H (%) | 47 | 44 | 51 | 47 | 46 | 48 | 46 | 47 | 43 |
ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; BP: blood pressure; del: deletion polymorphism of α2c; FD I/H: fixed dose isosorbide dinitrate and hydralazine; ins: insertion (wild type) of α2c; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart Association; SD: standard deviation.
Percent of Arg389Arg subjects on β-blockers significantly higher than Gly389 subjects, p = 0.007. All other comparisons by genotype not statistically significant.
Gene frequencies
For ADRB1 (n = 347), 111 (32%) subjects were Arg389Arg, 180 (51%) were Arg389Gly, and 56 (16%) were Gly389Gly. For GRK5 genotyping, (n = 348) 192 (55%) subjects were Gln41Gln, 134 (39%) were Gln41Leu, and 22 (6%) were Leu41Leu. For GNB3 genotyping (n = 350), 184 (55%) subjects were TT, 137(39%) were TC, and 29(8%) were CC. For α2c deletion (n = 344), 135 (39%) subjects were homozygous wild type, 156 (45%) were heterozygous for wildtype and deletion, and 53 (15%) were homozygous for the deletion. All polymorphisms were in Hardy-Weinberg equilibrium. For ADRB1 Arg398Gly observed = 111/180/56; expected= 116/169/61; χ2 = 1.43, p = ns. For GRK5 Gln41Leu observed = 192/134/22; expected = 192/132/23, χ2 = 0.0024, p = ns. For GNB3 825 C/T, observed = 184/137/29; expected = 182/14½7; χ2 = 0.24, p = ns. For α2c deletion, observed = 135/156/53; expected = 131/165/52, χ2 = 0.93, p = ns.
Event-free survival by genotype
There were 12 (3.9%) deaths and 61 heart failure hospitalizations (18.9%) and the event-free survival at one year for the overall cohort was 80%. Event-free survival was similar in women and men (p = 0.29). There was no impact of gender on survival by genotype. Comparison of survival by genotype revealed that poorer survival was evident for subjects with the GRK5 Leu41 variant with an event-free survival at one year of 77.4% for GRK5 Leu41 vs 82.6% for Gln41Gln, p = 0.046 (figure 1). Subjects with the ADRB1 Arg389Arg genotype had worse survival compared with those with the Gly389 variant with an event-free survival at one year 74.1% for Arg389Arg vs 83.7% for Gly389, p = 0.015 (figure 2). When adjusted for multiple comparisons, the p value for the overall impact of GRK5 Leu41 was no longer significant, while the value for the impact of Arg389Arg was borderline. There was no difference in heart failure event-free survival for subjects with the GNB3 TT genotypes compared with subjects with the GNB3 C allele (event-free survival at one year 80% vs 81%, p = 0.43) or for the α2c deletion compared with the insertion (event-free survival at one year 74% vs 83%, p = 0.23).
1. Event-free survival by ADRB1 Arg389Gly genotype.
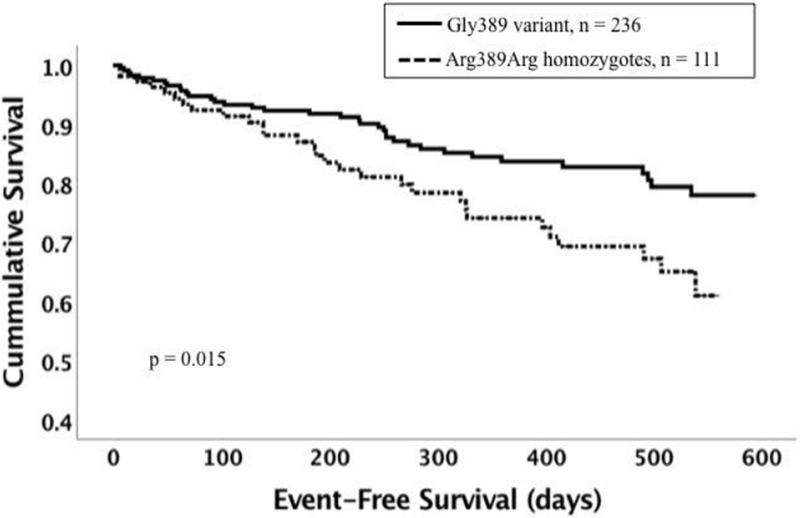
Dotted line represents subjects with the Arg389Arg variant (n = 111). Solid line represents subjects with the Gly389 variant (n = 236, homozygotes and heterozygotes combined). The Arg389Arg variant was associated with worse event-free survival (p = 0.015)
2. Event-free survival by GRK5 Gln41Leu genotype.
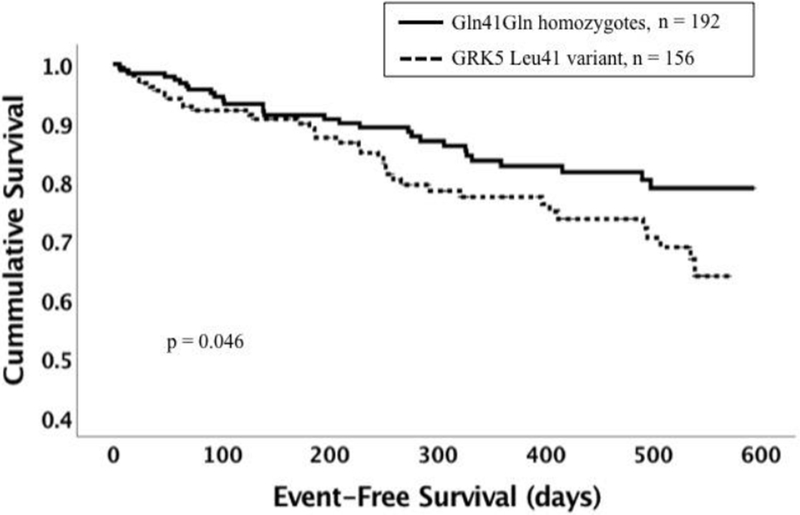
Dotted line represents subjects with the GRK5 Leu41 variant (n = 156, homozygotes and heterozygotes combined). Solid line represents Gln41Gln homozygous subjects (n = 192). The Leu41 variant was associated with worse event-free survival (p = 0.046)
GENE-GENE INTERACTIONS
GRK5 and GNB3
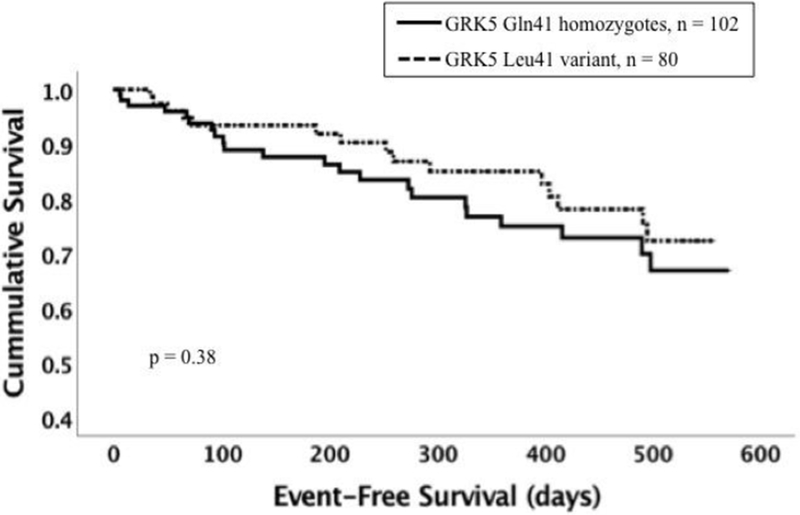
To evaluate the interactions of GRK5 and GNB3 genotypes we examined the impact of the GRK5 Leu41 variant separately in subsets with and without the GNB3 TT genotype. The adverse impact of GRK5 Leu41 was not evident in subjects with the GNB3 TT genotype (n = 182, % event-free survival at one year for Gln41Gln vs Leu41 = 75% vs 85% p = 0.38, figure 3B), but was dramatically magnified in subjects with the GNB3 C allele (n = 166, event-free survival at one year 90% vs 69%, p<0.001, figure 3A). A significant gene-gene interaction was present wherein the detriment to event-free survival was only evident with coinheritance of GRK5 Leu41 with GNB3 825 C allele (significance of the interaction of GNB3 genotype and GRK5 Leu41 variant p = 0.001, hazard ratio = 6.38). When adjusted for multiple comparisons, the impact of GRK5Leu within the GNB3C subset remained statistically significant.
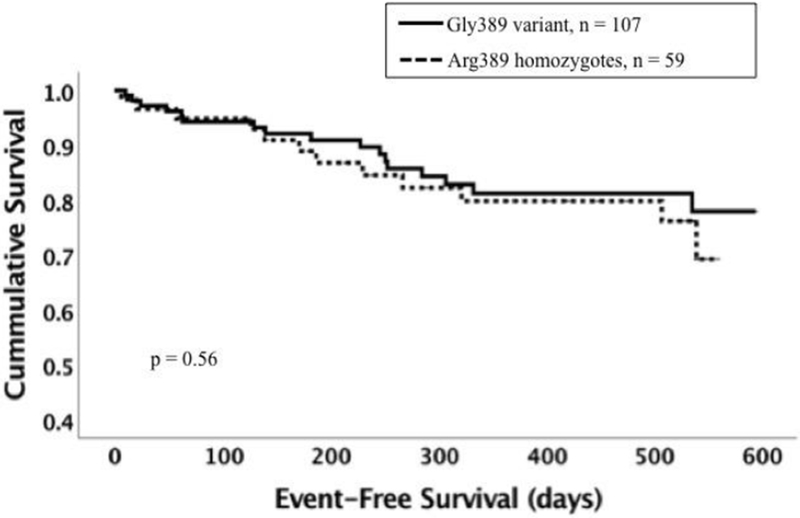
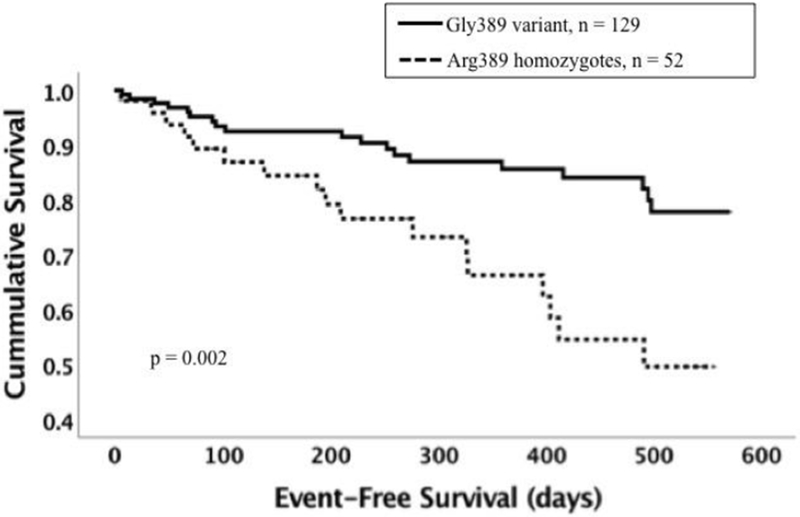
3. Effect of coinheritance of the GNB3 825 TT genotype with GRK5 Leu41 or ADRB1 variants on survival.
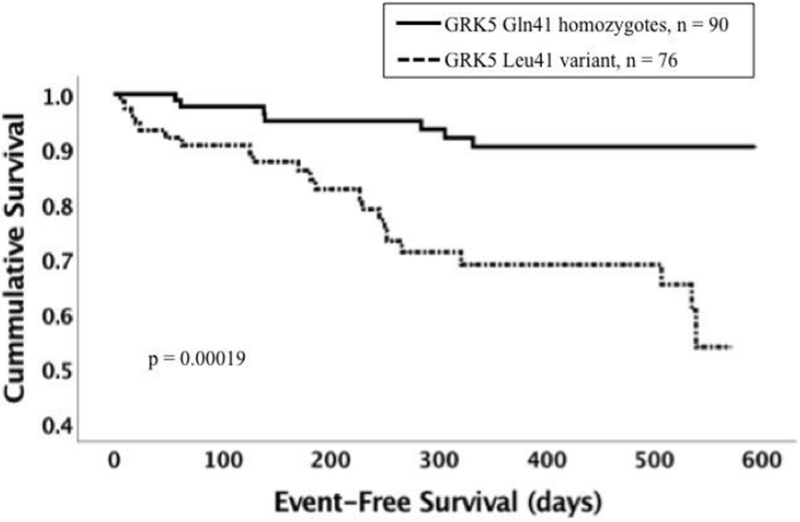
Panel A: Event-free survival for subjects with GRK5 Leu41 vs Gln41Gln among subjects (n = 166) with the GNB3 C allele (GNB3 CC and TC genotypes combined). Dotted line represents subjects with the GRK5 Leu41 variant (homozygotes and heterozygotes combined, n = 76). Solid line represents Gln41Gln subjects (n = 90). The Leu41 variant associated with poor event-free survival (p < 0.001).
Panel B: Event-free survival for GRK5 Leu41 vs Gln41Gln among subjects with GNB3 TT (n = 182). Dotted line represents subjects with the GRK5 Leu41 variant (n = 80). Solid line represents Gln41Gln subjects (n = 102). The Leu41 variant was not associated with worse event-free survival (p = 0.38).
Panel C: Event-free survival for ADRB1 Arg389Arg vs Gly389 among subjects with the GNB3 C allele (n = 166). Dotted line represents subjects with the Arg389Arg genotype (n = 59). Solid line represents subjects with the Gly389 variant (homozygotes and heterozygotes combined, n = 107). The Arg389Arg genotype was not associated with worse survival (p = 0.56).
Panel D: Event-free survival for ADRB1 Arg389Arg vs Gly389 among subjects with GNB3 TT subset (n = 181). Dotted line represents subjects with the Arg389Arg genotype (n = 52). Solid line represents subjects with the Gly389 variant (homozygotes and heterozygotes combined, n = 129). The Arg389Arg genotype was associated with poor event-free survival (p = 0.002).
ADRB1 and GNB3
The Arg389Arg genotype had a markedly adverse impact on event-free survival for subjects coinheriting the GNB3 TT genotype (n = 181, % event-free survival at one year for Arg389Arg vs Gly389 = 66% vs 86%, p = 0.002, figure 3D). In contrast, there was no impact of the ADRB1 Arg389Arg genotype evident in the subset with the GNB3 C allele (n = 166, % event-free survival at one year for Arg389Arg vs Gly389 = 80% vs 81% p = 0.56, figure 3C). When adjusted for multiple comparisons, the impact of Arg389Arg within the GNB3TT subset remained statistically significant. The analysis by Cox regression of the interaction between ADRB1 Arg389Gly and GNB3 825 TT genotypes in determining event-free survival failed to reach statistical significance (p = 0.10, hazard ratio = 2.28).
α2C and GNB3
By Cox regression analysis there was no interaction between the α2c deletion/deletion and GNB3 TT genotypes as determinants of event free survival (p = 0.32). The impact of the α2C deletion/deletion was not significantly associated with poor outcomes for either the GNB3 TT subset (p = 0.84) or within the GNB3 C subset (p = 0.15).
Other interactions
There was no clear interaction between the ADRB1 Arg389Gly and the GRK5 Gln41Leu polymorphisms in terms of event free survival (p = 0.67). There was no statistical interaction between the GRK5 Gln41Leu and α2C deletion/insertion polymorphisms (p = 0.32). There did appear to be an interaction between the α2c deletion/insertion and ADRB1 Arg389Gly. The presence of the α2c insertion enhanced the Arg389Arg mutation. Among subjects with the α2c insertion (e.g., wild type) the adverse impact of Arg389Arg lead to 76% one-year survival compared with 86% for Gly389 variants (p = 0.007; HR = 2.14). On the other hand, for the subset of subjects homozygous for the α2c deletion, the Arg389Arg genotype did not have significantly worse survival (p = 0.80). Adjusting for multiple comparisons, the impact of Arg389Arg within the α2c wild type subset remained statistically significant. Cox regression analysis did not demonstrate a statistical interaction (p = 0.18)
Medical therapy
Isosorbide dinitrate and hydralazine therapy
Of the GRAHF cohort, 164 subjects were randomized to FDC I/H and 186 to placebo. We have previously reported an interaction of GNB3 genotype with therapy with greater impact of drug on event-free survival, CS, and change in QOL for GNB3 TT subjects (11). There was no clear association with FDC I/H therapy effects on CS and event-free survival by GRK5 or α2C genotypes. In contrast, for the ADRB1 Arg389Gly polymorphism, the impact of FDC I/H on CS was significant among subjects with Arg389Arg genotype (CS for FDC I/H = 0.40±1.50 vs placebo −0.26 ± 1.96, p = 0.047), but not among subjects with the Gly389 variants (p-value = 0.59). However, this differential impact of FDC I/H on CS was primarily based on a greater impact of FDC I/H on QOL assessment for ADRB1 Arg389Arg subjects (QOL for FDC I/H = 0.68±1.32 vs placebo 0.20±1.59, p = 0.09), as treatment was not associated with any difference in event-free survival for this subset (p-value = 0.36).
β-blocker therapy
The vast majority of participants (n=293, 84%) were on β-blocker therapy (Table 1). β-blocker therapy did not appear to eliminate the adverse impact of the ADRB1 Arg389Arg genotype on event-free survival. The subset of subjects on β-blockers with this genotype demonstrated worse event-free survival than those with the ADRB1 Gly389 variants (% one-year event-free survival for Arg389Arg vs Gly389 = 75% vs 85%, p = 0.018). This survival difference by genotype was similar in magnitude for the subset not on β-blockers, though not significant (65% vs 77%, p = 0.45). In contrast, for the GRK5 Gln41Leu polymorphism the adverse impact of Leu41 appeared greater for subjects not on β-blockers. In this small subset, subjects with the GRK5 Leu41 variant did significantly worse than Gln41Gln homozygotes not on β-blockers (% one year event-free survival Leu41 vs Gln41Gln = 59% vs 85%, p = 0.04). There was no significant difference between survival for subjects on β-blockers by GRK5 Leu41 genotype (80% vs 82%, p = 0.19). There was no significant difference in event-free survival by GNB3 or α2C genotypes based on β-blocker use.
DISCUSSION
This investigation from the genetic sub-study of A-HeFT demonstrates that genetic polymorphisms affecting adrenergic receptor sensitivity impact heart failure survival in black subjects with HFrEF. The GRK5 Leu41 variant, which decreases β-receptor sensitivity, and the ADRB1 Arg389Arg genotype, which increases β-receptor sensitivity, show trends toward worse event-free survival. The GNB3 TT genotype, associated with increased adrenergic tone, did not impact overall event-free survival. However, the impact of Arg389Arg was markedly increased by coinheritance of the GNB3 TT genotype, while the impact of GRK5 Leu41 variant was dramatically increased by the absence of GNB3 TT. This study demonstrates the effect of inheritance patterns in multiple related genes determining overall adrenergic signaling, which ultimately affects heart failure outcomes.
GRK5 expression is associated with clinical heart failure severity and left ventricular size, indicating a role in advanced heart failure (21,22). In vitro studies have shown that GRK5 Leu41 polymorphism augments β-adrenergic receptor phosphorylation and desensitization (3,5–7,22), in essence decreasing the activity of the β-adrenergic receptor. GRK5 Leu41 has been theorized to have a protective role in dilated cardiomyopathy (23,24). Initial reports suggested that the Leu41 variant was associated with better heart failure outcomes and less benefit from exogenous β-blocker use (7). A larger follow up investigation of Leu41 suggested no impact for subjects not treated with β-blockers and surprisingly poorer survival with Leu41 for subjects treated with β-blockers (5). In the current study we also found that participants with the GRK5 Leu41 genotype had worse outcomes compared with those with the Gln41Gln genotype. We did not find evidence that this adverse impact is due to attenuated β-blocker response.
Subjects with the ADRB1 Arg389 variant have a gain of function of the β-1 adrenergic receptor, which is more responsive to adrenergic stimulation than the Gly389 variant (6,25–27). Our investigation found that subjects with Arg389Arg had significantly worse outcomes than those with the ADRB1 Gly389 variant. The adverse impact in our investigation did not appear to be diminished by treatment with β-blockers. Biolo and colleagues also showed worse survival for subjects with the Arg389 variant compared with Gly389Gly, a finding they attributed to less ventricular arrhythmias in the Gly389Gly group (28). However, their investigation suggested that β-blocker use was associated with significantly improved outcomes in Arg389 allele carriers. Sehnert and colleagues evaluated impact of the Arg389Gly polymorphism on survival in a predominantly white heart failure cohort on β-blockers and did not demonstrate a significant effect (29). Notably, coinheritance of the GNB3 TT genotype, which facilitates the adverse impact of Arg389Arg in the current study would be uncommon in the Sehnert cohort given the lower prevalence of the GNB3 T allele in whites.
In the Small et al. study of heart failure risk, the presence of Arg389Arg genotype increased the risk of heart failure, but only in subjects homozygous for the α2c deletion (9), a genotype rare in white cohorts, but more common in black cohorts. Both the current study and this earlier study of heart failure risk, suggest the impact of Arg389 is magnified by coinheritance of α-adrenergic variants. The four genes analyzed in the current report work together in the molecular mechanisms of cardiac sympathetic activation, and the genetic interactions likely reflect their cellular relationships (figure 4). Adrenergic receptors are G protein coupled receptors (GPCRs) that all have a similar structure with seven transmembrane domains, an amino terminus domain in the extracellular region, and a carboxy terminus domain in the intracellular region (30). A large subset of G proteins are heterotrimeric, with α, β, and γ subunits (figure 4), and GNB3 encodes for a ubiquitous βsubunit. Once a ligand binds to a GPRC, a conformational change results in separation of the β-γ subunit from the α subunit, and the GPRC is then potentially phosphorylated and desensitized by a G protein receptor kinases such as GRK5 (1). Sympathetic innervation of the heart releases norepinephrine as a ligand to cardiac β receptors. A2c receptors on the nerve ending are a critical negative feedback loop, being activated by released norepinephrine and limiting subsequent additional release.
4. Adrenergic receptor and heterotrimeric G protein complex.
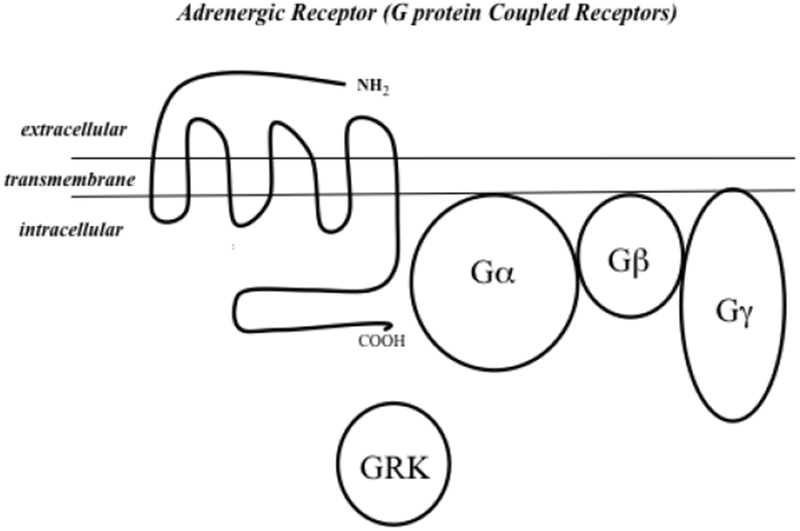
Adrenergic receptors are G protein coupled receptors (GPCRs) with seven transmembrane domains, an amino acid terminus in the extracellular region, and a carboxy domain in the intracellular region (30). Many GPCRs are heterotrimeric, with a α, β, and γ subunit. GNB3 encodes for a ubiquitous β subunit. Sympathetic innervation of the heart releases norepinephrine as a ligand to cardiac β receptors, and α2c receptors on the nerve ending as a critical negative feedback loop, limiting additional release of norepinephrine (31). Once norepinephrine binds, the β-γ subunit separates from the α subunit, and the GPCR is then potentially phosphorylated and desensitized by a G protein receptor kinase such as GRK5 (1).
The GNB3 TT genotype diminished the impact of GRK5 Leu41 but enhanced the impact of the Arg389Arg genotype. The fact that the GNB3 TT genotype, which increases adrenergic signaling, enhances the adverse impact of the Arg389Arg genotype (which increases β-adrenergic signaling), but diminishes the impact of the GRK5 Leu41 variant (which decreases β-receptor responsiveness), appears consistent with the genetic variants’ distinctly opposite physiologic effects on β-receptor responsiveness. Similarly, the lack of α2c interaction with GRK5 and GNB3 likely reflects a difference in effect based on intracellular (G-protein related compounds) vs extracellular (α2c) locations.
Our study has some limitations. First, the cohort size of 350 subjects limits the power of subset analysis by genotype. Secondly, the majority of participants were on β-blocker therapy, which was not randomized, thus limiting the conclusions of this exploratory pharmacogenetic analysis. Finally, our study evaluated outcomes in self-described black participants. Without analysis of ancestral genomic heritage, we are unable to determine the extent that overall African genomic ancestry may influence the current results. The physiology of the adrenergic system and the differing impact of functional variants must be considered in concert. Together, these assessments will clearly add to the complexity of any polygenic risk determination.
CONCLUSIONS
This investigation demonstrates that genetic polymorphisms of adrenergic functional variants impact event-free survival in black subjects with HFrEF. Interactions with the GNB3 TT genotype were far more important modifiers of the adverse effect than pharmacogenetic interactions with either β-blockers or FDC I/H. Interaction between genetic polymorphisms affecting adrenergic signaling must be analyzed together to understand their impact on clinical outcomes. Whether differences in the frequencies of these variants in subjects of African genomic descent compared with those of European descent underlie racial variation in heart failure outcomes requires further study.
Acknowledgements:
Funding Sources: Study supported by NIH contracts MD009118 and HL69912
Abbreviations used
- GRK5
G-protein receptor kinase type 5
- ADRB1
β1 adrenergic receptor
- GNB3
G-protein β-3 subunit
- FDC I/H
fixed dose combination of isosorbide dinitrate and hydralazine
- MLHFQ
Minnesota living with heart failure questionnaire
- CS
composite score
- QOL
quality of life
Footnotes
Disclosures: The authors have no industry relationships to disclose.
References
- 1.Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiological reviews. 2015;95(2):377–404.1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiological reviews. 2015;95:377–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang ZM, Gold JI, Koch WJ. G protein-coupled receptor kinases in normal and failing myocardium. Frontiers in bioscience (Landmark edition) 2011;16:3047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorn GW 2nd. Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiological reviews 2010;90:1013–62. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro R, Roselli T, Valente F et al. Heart failure: molecular, genetic and epigenetic features of the disease. Minerva Cardioangiol 2012;60:593–609. [PubMed] [Google Scholar]
- 5.Cresci S, Kelly RJ, Cappola TP et al. Clinical and genetic modifiers of long-term survival in heart failure. Journal of the American College of Cardiology 2009;54:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liggett SB, Mialet-Perez J, Thaneemit-Chen S et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proceedings of the National Academy of Sciences of the United States of America 2006;103:11288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liggett SB, Cresci S, Kelly RJ et al. A GRK5 polymorphism that inhibits beta-adrenergic receptor signaling is protective in heart failure. Nature medicine 2008;14:510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meirhaeghe A, Bauters C, Helbecque N et al. The human G-protein beta3 subunit C825T polymorphism is associated with coronary artery vasoconstriction. European heart journal 2001;22:845–8. [DOI] [PubMed] [Google Scholar]
- 9.Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. The New England journal of medicine 2002;347:1135–42. [DOI] [PubMed] [Google Scholar]
- 10.Siffert W, Forster P, Jockel KH et al. Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. Journal of the American Society of Nephrology : JASN 1999;10:1921–30. [DOI] [PubMed] [Google Scholar]
- 11.McNamara DM, Taylor AL, Tam SW et al. G-protein beta-3 subunit genotype predicts enhanced benefit of fixed-dose isosorbide dinitrate and hydralazine: results of A-HeFT. JACC Heart failure 2014;2:551–7. [DOI] [PubMed] [Google Scholar]
- 12.Dorr M, Schmidt CO, Spielhagen T et al. beta-blocker therapy and heart rate control during exercise testing in the general population: role of a common G-protein beta-3 subunit variant. Pharmacogenomics 2010;11:1209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira SB, Velloso MW, Chermont S et al. beta-adrenergic receptor polymorphisms in susceptibility, response to treatment and prognosis in heart failure: implication of ethnicity. Molecular medicine reports 2013;7:259–65. [DOI] [PubMed] [Google Scholar]
- 14.Terra SG, Hamilton KK, Pauly DF et al. Beta1-adrenergic receptor polymorphisms and left ventricular remodeling changes in response to beta-blocker therapy. Pharmacogenetics and genomics 2005;15:227–34. [DOI] [PubMed] [Google Scholar]
- 15.White HL, de Boer RA, Maqbool A et al. An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. European journal of heart failure 2003;5:463–8. [DOI] [PubMed] [Google Scholar]
- 16.Muthumala A, Drenos F, Elliott PM, Humphries SE. Role of beta adrenergic receptor polymorphisms in heart failure: systematic review and meta-analysis. European journal of heart failure 2008;10:3–13. [DOI] [PubMed] [Google Scholar]
- 17.de Groote P, Helbecque N, Lamblin N et al. Association between beta-1 and beta-2 adrenergic receptor gene polymorphisms and the response to beta-blockade in patients with stable congestive heart failure. Pharmacogenetics and genomics 2005;15:137–42. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AL, Ziesche S, Yancy C et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. The New England journal of medicine 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 19.Small KM, Rathz DA, Liggett SB. Identification of adrenergic receptor polymorphisms Methods in Enzymology: Academic Press, 2002:459–475. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. American journal of epidemiology 2009;169:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. European journal of pharmacology 2004;489:167–77. [DOI] [PubMed] [Google Scholar]
- 22.Aguero J, Almenar L, Monto F et al. Myocardial G protein receptor-coupled kinase expression correlates with functional parameters and clinical severity in advanced heart failure. Journal of cardiac failure 2012;18:53–61. [DOI] [PubMed] [Google Scholar]
- 23.Monto F, Oliver E, Vicente D et al. Different expression of adrenoceptors and GRKs in the human myocardium depends on heart failure etiology and correlates to clinical variables. American journal of physiology Heart and circulatory physiology 2012;303:H368–76. [DOI] [PubMed] [Google Scholar]
- 24.Eckhart AD, Duncan SJ, Penn RB, Benovic JL, Lefkowitz RJ, Koch WJ. Hybrid transgenic mice reveal in vivo specificity of G protein-coupled receptor kinases in the heart. Circulation research 2000;86:43–50. [DOI] [PubMed] [Google Scholar]
- 25.Wagoner LE, Craft LL, Zengel P et al. Polymorphisms of the beta1-adrenergic receptor predict exercise capacity in heart failure. American heart journal 2002;144:840–6. [DOI] [PubMed] [Google Scholar]
- 26.Sandilands AJ, Parameshwar J, Large S, Brown MJ, O’Shaughnessy KM. Confirmation of a role for the 389R>G beta-1 adrenoceptor polymorphism on exercise capacity in heart failure. Heart (British Cardiac Society) 2005;91:1613–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodde OE. Beta-1 and beta-2 adrenoceptor polymorphisms: functional importance, impact on cardiovascular diseases and drug responses. Pharmacology & therapeutics 2008;117:1–29. [DOI] [PubMed] [Google Scholar]
- 28.Biolo A, Clausell N, Santos KG et al. Impact of beta1-adrenergic receptor polymorphisms on susceptibility to heart failure, arrhythmogenesis, prognosis, and response to beta-blocker therapy. Am J Cardiol 2008;102:726–32. [DOI] [PubMed] [Google Scholar]
- 29.Sehnert AJ, Daniels SE, Elashoff M et al. Lack of association between adrenergic receptor genotypes and survival in heart failure patients treated with carvedilol or metoprolol. Journal of the American College of Cardiology 2008;52:644–51. [DOI] [PubMed] [Google Scholar]
- 30.Grisanti LA, Schumacher SM, Tilley DG, Koch WJ. Designer Approaches for G Protein-Coupled Receptor Modulation for Cardiovascular Disease. JACC Basic to translational science 2018;3:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 1999;402:181–4. [DOI] [PubMed] [Google Scholar]


