Abstract
Background:
Mucinous tubular and spindle-cell carcinoma (MTSCC) is a rare kidney cancer subtype with limited cases reported in the literature. We report on outcomes of 25 patients with this variant who were managed at our institution.
Materials and Methods:
The institution database was queried, and clinical data extracted for patients with MTSCC. Molecular features examined included next generation sequencing with MSK-IMPACT and allele-specific copy number analysis using FACETS algorithm in a subset of patients.
Results:
All patients underwent primary tumor-directed therapy including (Nephrectomy=23, cryoablation=2). Metastases were diagnosed in 6 patients (24%), 3 of which had de novo metastatic disease. Five of 6 patients with metastatic disease had high grade histological features compared to 0 of 19 non-metastatic patients (83% vs 0%, p < 0.001, Fisher’s exact test). 3-year overall survival from diagnosis was 84.8% (95% CI: 59.6, 94.9) with a median follow-up time of 3.9 years (range:1 months, 10.3 years). Three deaths occurred, all from metastatic disease. Four patients received systemic therapy with time to treatment failure ≤ 6 months across different agents with the exception of one patient with prolonged response on sunitinib (30.6 months). Most frequent molecular alterations were NF2 mutations (n=2, 40%), germline alterations (n=2, 40%) including CHEK2 and BRCA2 mutations, multiple chromosomal copy number losses and mismatch repair deficiency in one patient.
Conclusions:
MTSCC is characterized by localized tumors treated successfully with primary tumor directed therapy. However, patients with high grade histological features were more likely to develop metastatic disease with limited responses to standard therapies.
Keywords: Genomics, Mucinous tubular and spindle cell carcinoma, Non-clear cell renal cell carcinoma, Survival
MICRO ABSTRACT:
We studied cancer specifics outcomes of 25 patients with mucinous tubular and spindle-cell carcinoma of the kidney and we found that the majority of patients presented with localized tumor that were treated successfully with primary tumor directed therapy. However, those with high grade histological features were more likely to develop metastatic disease with limited responses to standard therapies.
Introduction
Renal cell carcinoma (RCC) encompasses a group of heterogeneous malignancies each with unique pathological, molecular and clinical features. The taxonomy of RCC has expanded over the last 3 decades and the most recent 2016 WHO classification included 12 recognized subtypes with several emerging entities1. Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney is a rare subtype which was first described under a variety of terms including RCC with unusual differentiation2, low-grade collecting duct carcinoma3, unusual RCC with prominent spindle cell change possibly related to the loop of Henle4, and subsequently recognized as a distinct RCC entity in the WHO 2004 classification of renal tumors5. It has a wide of age distribution with female predominance in most of the reported case series6,7, and generally appreciated as a tumor of low malignant potential and low risk of metastasis6-8. Few metastatic cases have been reported mainly in association with high grade and sarcomatoid features7,9-11 and the evidence on how treat those cases remains limited.
Histologically characterized by the presence of an admixture of low grade bland tubules and spindle cells set in a mucinous stroma. However, it was realized that these tumors show resemblance to type 1 papillary RCC with both overlapping morphologic and immunohistochemistry features12,13. At the molecular levels, a multi institutional study of 22 patients with MTSCC who underwent whole exome and transcriptome sequencing demonstrated that recurrent Hippo pathway alterations, low mutational burden and recurrent chromosomal loss represent the dominant molecular features of these tumors14.
In this retrospective study, we identified patients with MTSCC managed at our institutions between 2004 to 2018. The objectives of our study are to report on clinical presentations, genomic profile, survival and treatment outcomes of those patients.
Materials and methods
Study population:
Approval by the institutional review board was obtained to retrospectively identify patients with a diagnosis of MTSCC between 01/01/2004 to 06/01/2018 based on pathology reports within the institution database. In view of the rarity of this subtype and overlapping features with papillary RCC, all identified cases were submitted for further pathological confirmation by a dedicated genitourinary pathologist (Y.C.).
Data Collection:
Clinical data were extracted through individual retrospective chart reviews including patients’ demographics, clinical features, treatment details and survival outcomes. International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk scores were calculated for patients with metastatic disease.
Clinical and statistical endpoints:
The entry point for analysis was from the date of first pathological diagnosis of MTSCC histology. Time to recurrence (TTR) with metastatic disease was calculated for patients who developed metastatic disease after undergoing nephrectomy in curative intent. Systemic therapy outcomes were described using time to treatment failure (TTF) analysis, defined as the interval between the date of initiating systemic therapy and the date of radiographic progression, drug cessation, or death, whichever occurred first, or censored at the last follow-up date. Radiographic progression was based on the interpretation of individual patient’s radiological reports by the treating Oncologist. Overall survival (OS) was calculated for all patients from the date of first pathological diagnosis of MTSCC histology until death, and patients who were still alive at the data cutoff were censored at the date of last follow-up. Median follow-up time was calculated from date of diagnosis among censored patients.
All statistical tests were 2-tailed, and P value <.05 was considered to indicate statistical significance. All analyses were performed with SAS version 9.3 software (SAS Institute, Cary, NC).
Molecular analysis
Next generation sequencing (NGS) was performed in 5 cases using the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT™), briefly this is a hybridization capture technique of exons and introns of tumor with matched normal samples across 341, 441 and 468 genes which corresponds to the first three versions of the assay15. IHC for mismatch repair (MMR) proteins was performed in one case with underlying MLH1 deep deletion. Estimates of genome-wide total, allele-specific, and integer DNA copy number, were inferred from IMPACT sequencing data using the FACETS (Fraction and Allele-Specific Copy Number Estimates from Tumor Sequencing) algorithm (v0.5.6)16.
Results
Between 2004 to 2018, a total of 28 patients were initially identified from the pathology reports at our institution with a diagnosis of MTSCC. After further pathological confirmation, 3 patients were subsequently excluded as the diagnosis was inconclusive of MTSCC. The final data set included 25 patients and their baseline characteristic are summarized on Table 1. The median age at first pathological diagnosis with MTSCC was 58 years (range: 21, 74), with a female predominance (n=16, 64%). Symptoms at presentation were available for 21 patients, and most patients were asymptomatic at presentation (n=13, 62%) with one patient presenting with systemic symptoms who subsequently developed metastatic disease.
Table 1.
Patient characteristics (N=25)
| N | Median (range) or Frequency (%) |
Quartile range for continuous variables |
|
|---|---|---|---|
| Age at diagnosis, years | 25 | 58 (21, 74) | 13.1 |
| Female gender | 16 | 64% | |
| Primary tumor size, cm | 22 | 5.7 (1.3, 16.2) | 6 |
| Primary stage | 22 | ||
| pT1 | 11 | 50% | |
| pT2 | 8 | 36% | |
| pT3a | 2 | 9% | |
| pT3b | 1 | 5% | |
| Symptoms at presentation | 21 | ||
| None | 13 | 62% | |
| Localized | 7 | 33% | |
| Systemic | 1 | 5 | |
| Sarcomatoid features | 2 | 8% | |
| High grade features | 5 | 25% | |
| Primary tumor directed therapy | 25 | ||
| Radical nephrectomy | 7 | 28% | |
| Partial nephrectomy | 16 | 64% | |
| Ablation | 2 | 8% | |
| Metastatic disease | 6 | 24% | |
| Sites of metastases | |||
| Bone | 4 | 67% | |
| Lung | 3 | 50% | |
| Lymph nodes | 2 | 33% | |
| Brain | 1 | 17% | |
| Cutaneous | 1 | 17% |
All patients underwent primary tumor-directed therapy including nephrectomy in 23 patients (Radical, n=7, Partial, n=16) and cryoablation in 2 patients who both presented with small sized tumors on imaging (1.7 and 1.9 cm). Primary tumor pathological staging details were available in 22 out of the 23 patients who underwent nephrectomy. Most patients had small tumors with pathological T1 and T2 primary tumor staging in 11 patients (50%) and 8 patients (36%) respectively. Pathological T3 primary tumor staging was recognized in 3 patients who were also diagnosed with metastatic disease. High grade histological features were identified in 5 cases (20%) and 2 of those 5 patients also had evidence of sarcomatoid dedifferentiation.
Metastatic disease was identified in 6 patients, including 3 who presented with de novo metastatic disease and they all underwent cytoreductive nephrectomy. High grade histological features or sarcomatoid dedifferentiation were diagnosed in 5 of 6 patients with metastatic disease compared to 0 of 19 non-metastatic patients (83% vs 0%, p< 0.001, Fisher’s exact test). Most common sites of metastases included the bone (n=4, 67%) and lung (n=3, 50%). TTR with metastatic disease for the 3 patients who did not present with de novo metastatic disease and underwent nephrectomy in curative intent was 6.7, and 9.9 months in 2 patients with high grade histological features and 15.5 months in 1 patient who didn’t have any high grade histological features. IMDC risk parameters were available for 5 of the six metastatic case and were all in intermediate risk category.
Survival and treatment outcomes:
Survival outcomes for the entire cohort were favorable, with 3-year OS from first pathological diagnosis with MTSCC of 84.8% (95% CI: 59.6, 94.9) with a median follow-up time for survivors of 3.9 years (range:1 months, 10.3 years) (Figure 1). Three deaths occurred which were all from metastatic disease at 3.7, 15.0 and 29.2 months.
Figure 1. Overall survival of patients with MTSCC from first pathological diagnosis.
3-year overall survival from first pathological diagnosis with MTSCC was 84.8% (95% CI: 59.6, 94.9) for all patients with a median follow-up time for survivors of 3.9 years (range: 1 months, 10.3 years). Three deaths occurred which were all from metastatic disease at 3.7, 15.0 and 29.2 months.
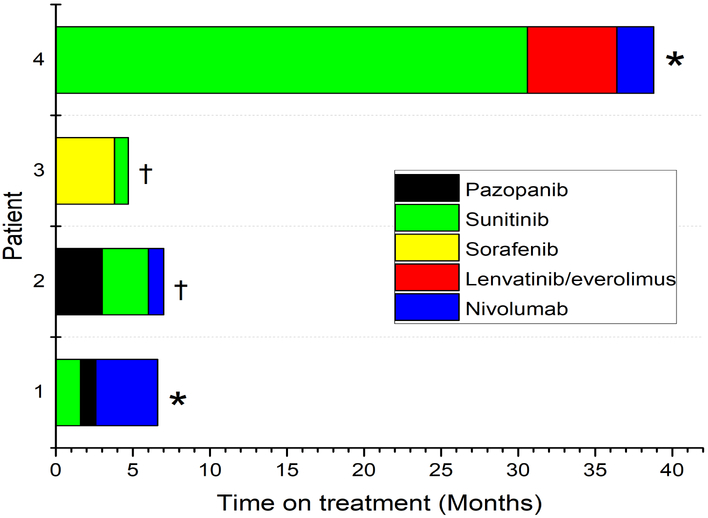
Systemic therapy was initiated in 4 patients, details on duration of therapy of all lines of therapy are summarized on Figure 2. All 4 patients received first-line vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI) with sunitinib (n=2), pazopanib (n=1) and sorafenib (n=1), with a TTF of 30.6, 1.6, 3.0 and 3.8 months respectively. 3 patients received Anti-PD-1 checkpoint inhibitor therapy with single agent nivolumab in the third line setting, with TTF of 1.0, 2.4 and 4 months which were all discontinued due to progression of disease without any radiological disease response. Two of 4 metastatic patients who received systemic therapy are still alive and being considered for the next line of therapy, while the 2 other patients died from metastatic disease. Two other patients with metastatic disease didn’t receive any systemic therapy, including one patient who was managed with metastasectomy (right hemiarthroplasty and scalp lesion resection), and another patient who was unwell on presentation with brain metastasis and died at 3.7 months from diagnosis. Metastatic cases clinical features and treatment outcomes are detailed in the Supplementary Table.
Figure 2. Swimmers plot for treatment durations on all lines of therapy.
Details on all lines of systemic therapy per individual patients by coded by different colors. (*) Death from metastatic disease (†) Drug stopped due to disease progression.
Molecular features:
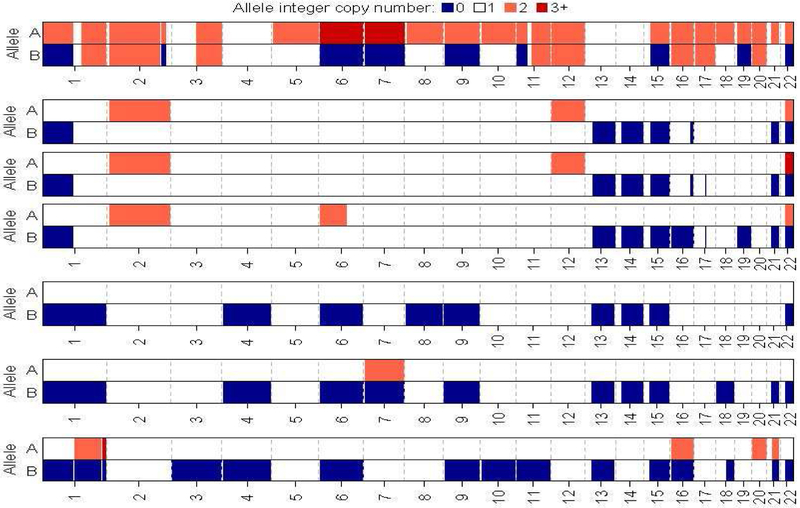
NGS with IMPACT™ was performed in 5 patients including 4 with metastatic disease. The tumor mutational burden was 0.0, 1.89, 4.10, 7.55 and 9.89 mutation/megabase. The most frequently altered gene was NF2 in 2 patients (40%) which is consistent with previous reports of common alterations in the Hippo pathway (Figure 3). Germline alterations were detected in 2 patients (40%) including CHEK2(T367Mfs*15) and BRCA2(S1982Rfs*22) mutation. The BRCA2 mutation was identified in a female patient of Ashkenazi Jewish descent and none of the 2 patients with germline mutations were diagnosed with another malignancy. One patient had evidence of MLH1 deep deletion on NGS, and subsequently IHC staining for MMR proteins showed loss of MLH1 and PMS2 expression consistent with deficient MMR status (dMMR) (Picture 1). The tumor of the patient who achieved long lived response on sunitinib harbored a total of 8 mutations including BAP1(I696T) missense mutation. FACETS analysis of 7 samples from 5 patients who underwent NGS identified loss of heterozygosity (LOH) of chromosomes 1,15 and 22 in 100% of cases and 6, 9 and 13 in 80% of cases and 14 in 60% of cases (Figure 4).
Figure 3.
Oncoprint illustrating most common Genomic alterations changes detected by NGS using MSK- IMPACT analysis across 5 patients with columns representing individual patients
Picture 1:
Immunohistochemistry staining for mismatch repair (MMR) proteins for one patient in the series with deficient.MMR status. Note for the loss of MLH1 and PMS2 in tumor cell.
Figure 4.
Allelic integer copy number as estimates by FACETS of 7 samples from 5 patients. HETLOSS (LOH) A=1; B=0, HOMDEL A=0, B=0, Gain A=2, B=1, Tetraploid A=2, B=2, AMP (or if broader high copy gain) A=3+, B=1, Copy number neutral LOH (CNLOH) A=2, B=0, Reciprocal LOH (RLOH) A=3+, B=0
Discussion:
MTSCC is a rare subtype of kidney cancer which was first recognized as a distinct entity in the WHO 2004 classification of renal neoplasms5. Multiple reports have described it as an indolent subtype with low grade histological features, low risk of metastases and favorable outcomes6,8. Similar to other subtypes of RCC, cases with high grade histological and sarcomatoid dedifferentiation were previously reported and mostly in association with metastatic disease 7,9,10. In light of the rarity of this subtype, limited cases were reported in the literature including ~ 13 metastatic cases and therefore it still remains not well defined. In this retrospective study, we studied outcomes of 25 patients with MTSCC managed at our institution and we found that the majority of patients presented with asymptomatic, low grade and localized tumors which were successfully treated with primary tumor directed therapy and overall achieved favorable survival outcomes. However, high grade histological features including high grade nuclear and sarcomatoid dedifferentiation were present in 20% of patients, and the presence of those features was strongly associated with development of metastatic disease (p< 0.001, Fisher’s exact test) with limited responses to standard metastatic RCC systemic therapy with the exception of one patient who achieved a long-lived response on sunitinib. Consistent with previous work most frequent molecular features included Hippo pathway mutations (40%) and multiple chromosomal losses14.
As described above, the overall survival for the entire cohort was favorable with 3-year overall survival rate of 84.8% over a median follow up time of 3.9 years (range:1 months, 10.3 years). However, we observed a distinct difference in outcomes between MTSCC of low and high grade histological features. Patients with low grade histological features presented with localized tumors with only 1 of 20 patients developing recurrent metastatic disease. While all 5 patients who had underlying high grade histological features developed or presented with metastatic disease. Most of the reported large series in the literature indicated that MTSCC is characterized by low grade, small sized and asymptomatic tumors 6,7 although those series included ≤ 2 metastatic cases compared to 6 patients with metastatic disease in our study. Large studies demonstrated that the presence of high grade histological features in the common subtypes of RCC portends poorer prognosis 17-19 which is consistent with our findings in this study.
With regards to systemic therapy for metastatic cases, 4 patients were treated with standard metastatic RCC systemic therapy with TTF of ≤ 6.0 months across different lines of therapy with the exception of one patient who achieved a long-lived response on first line sunitinib and progressed after staying on drug for 30.6 months. Three patients received nivolumab in the third line setting with TTF of ≤ 4.0 months with no disease response on imaging. Larkin et al 20 previously reported a case with metastatic MTSCC treated with first line sunitinib and observed disease response at 12 weeks with continued clinical benefit from therapy at 24 weeks. As for nivolumab activity in this subtype, a series of 41 patients with metastatic non clear cell RCC who were treated with nivolumab included one patient with metastatic MTSCC who didn’t achieve radiological disease response and progressed after 6 months of therapy21. In view of the limited number of nivolumab treated metastatic cases with MTSCC and the lack of robust biomarkers of response it remains unclear whether nivolumab is an active treatment option for this disease. In light of the current available evidence, sunitinib seems to be an appropriate first line therapy in patients with advanced disease.
In our study, NGS with MSK-IMPACT™ was performed in 5 cases including 4 with metastatic disease both at the somatic and germline setting. Alterations in NF2 gene a member of the Hippo pathway was the most frequent somatic alteration in our cohort in 40% of patients. Using FACETS analysis on 5 cases who underwent NGS analysis, we observed consistent LOH mostly involving chromosomes 1, 6, 9, 13, 14, 15 and 22. Rakozy et al 8first observed unique and consistent chromosomal copy number losses among 6 MTSCC tumors involving chromosomes 1, 4, 6, 8, 9, 13, 14, 15, and 22. In an effort to define the molecular features of these tumors further, a multi-institutional cohort study analyzed tumors from 22 patients with MTSCC with both whole exome and transcriptome sequencing and identified that recurrent Hippo pathway alterations, low mutational burden and recurrent chromosomal loss represents the dominant features of these tumors14. However, both studies included non-metastatic cases while most patients in our study who underwent NGS had metastatic and high grade disease with similar genomic findings. Other interesting finding is the identification of germline alterations in 2 patients (40%) including CHEK2(T367Mfs*15) and BRCA2(S1982Rfs*22) mutations. Carlo et al22, recently reported on germline alterations across 254 patients with advanced RCC and demonstrated that 16.1% of patients harbored germline alterations including 10.5% in non–RCC-associated genes. In particular, CHEK2 germline mutations were overexpressed in patients with RCC compared to the general population suggestive of a potential role in the pathogenesis of RCC. The patient with germline BRCA2 mutation was of Ashkenazi Jewish origin and the significance of this finding in MTSCC is unknown. Although, we noted that on a previous reported case series of 15 patients with MTSCC one patient was diagnosed with both MTSCC and breast cancer6. Another unique finding is the identification of dMMR status in one patient based on IHC testing which is a very rare finding in RCC, and it could be prove to be of therapeutic significance in this patient as pembrolizumab is FDA approved for patients with unresectable or metastatic dMMR solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options23. Specific alterations including mutations in KDM5C and PBRM1 were shown to be associated with favorable outcomes on VEGF-TKI agents in patients with advanced clear cell RCC24,25. The patient in our study who achieved an extreme response to sunitinib harbored a BAP1 mutation which is thought to have adverse outcomes on VEGF-TKI agents25. Taken together, these findings support that patient with metastatic or high grade MTSCC could be considered for somatic and germline molecular genomic testing.
Some of the limitations of our study may include the small sized single center retrospective design, the lack of RECIST criteria assessment on patients treated with systemic therapy and the small number of patients within the study cohort who underwent molecular genomic testing.
Conclusions:
Our study supports previous findings of MTSCC as a disease of low grade potential and favorable outcomes. However, cases with high grade features have worse outcomes, therefore referral to centers of expertise and genomic molecular testing are encouraged for this group of patients.
Supplementary Material
Clinical practice points.
MTSCC is a rare subtype of kidney cancer which is poorly defined with limited cases reported in the literature including ~ 13 metastatic cases.
We studied cancer specifics outcomes and genomic features of 25 patients with MTSCC including 6 patients with metastatic disease.
We found that the majority of patients presented with asymptomatic, low grade and localized tumors with favorable survival outcomes with 3-year overall survival rate of 84.8% for the entire cohort.
High grade pathological features were identified in primary tumors from 5 patients (20%) and the presence of those features was strongly associated with metastatic disease (p<0.001, Fisher’s exact test).
Four patients were treated with standard metastatic RCC systemic therapy with time to treatment failure ≤ 6 months across different agents including nivolumab with the exception of 1 patient who achieved long lived response on sunitinib after staying on drug for 30.6 months.
Genomic features were studied in 5 patients including 4 metastatic cases and showed alterations in the hippo pathway in 2 patients which is similar to what is known in the non-metastatic setting. However, other diverse genomic features were identified including germline alterations in 2 patients, multiple chromosomal copy number losses and mismatch repair deficiency in 1 patient.
Acknowledgments
Funding: Tuttle Rare Kidney Cancer Fund, Weiss Family Kidney Research Fund Precision, Center Core Grant P30 CA008748 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
Robert J. Motzer reports receiving commercial research grants from Pfizer, Eisai, Exelixis, Bristol-Myers Squibb, Genentech/Roche, and Novartis, and is a consultant/advisory board member for Pfizer, Merck, Genentech, Exelixis, Eisai, and Novartis.
Martin H. Voss reports receiving commercial research grants from Bristol-Myers Squibb and Genentech/Roche. Honoraria from Novartis. Travel/accommodation from Eisai, Novartis and Takeda. Consultant/advisory board member for- Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis and Pfizer.
Chung-Han Lee reports consulting/advisory role for Exelexis and Eisai.
Maria I. Carlo reports consulting/advisory role for Pfizer.
Darren R. Feldman reports research support from Novartis and Seattle Genetics.
References
- 1.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. July 2016;70(1):93–105. [DOI] [PubMed] [Google Scholar]
- 2.Ordóñez NG, Mackay B, Swanson DA Renal Cell Carcinoma with Unusual Differentiation. Ultrastructural Pathology. 1996/January/01 1996;20(1):27–30. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan GT, Farrow GM, Bostwick DG. Low-grade collecting duct carcinoma of the kidney: report of 13 cases of low-grade mucinous tubulocystic renal carcinoma of possible collecting duct origin. Urology. November 1997;50(5):679–684. [DOI] [PubMed] [Google Scholar]
- 4.Srigley EJ JR, Grignon DJ, Hartwick RWJ. Unusual renal cell carcinoma (RCC) with prominent spindle cell change possibly related to the loop of Henle. Mod Pathol. 1999;12:107A. [Google Scholar]
- 5.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. May 2006;49(5):798–805. [DOI] [PubMed] [Google Scholar]
- 6.Ferlicot S, Allory Y, Comperat E, et al. Mucinous tubular and spindle cell carcinoma: a report of 15 cases and a review of the literature. Virchows Arch. December 2005;447(6):978–983. [DOI] [PubMed] [Google Scholar]
- 7.Kenney PA, Vikram R, Prasad SR, et al. Mucinous tubular and spindle cell carcinoma (MTSCC) of the kidney: a detailed study of radiological, pathological and clinical outcomes. BJU Int. July 2015;116(1):85–92. [DOI] [PubMed] [Google Scholar]
- 8.Rakozy C, Schmahl GE, Bogner S, Storkel S. Low-grade tubular-mucinous renal neoplasms: morphologic, immunohistochemical, and genetic features. Mod Pathol. November 2002;15(11):1162–1171. [DOI] [PubMed] [Google Scholar]
- 9.Bulimbasic S, Ljubanovic D, Sima R, et al. Aggressive high-grade mucinous tubular and spindle cell carcinoma. Hum Pathol. June 2009;40(6):906–907. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon J, Amin MB, Selbs E, Turi GK, Paner GP, Reuter VE. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid change. Am J Surg Pathol. January 2009;33(1):44–49. [DOI] [PubMed] [Google Scholar]
- 11.Pillay N, Ramdial PK, Cooper K, Batuule D. Mucinous tubular and spindle cell carcinoma with aggressive histomorphology--a sarcomatoid variant. Hum Pathol. June 2008;39(6):966–969. [DOI] [PubMed] [Google Scholar]
- 12.Argani P, Netto GJ, Parwani AV. Papillary renal cell carcinoma with low-grade spindle cell foci: a mimic of mucinous tubular and spindle cell carcinoma. Am J Surg Pathol. September 2008;32(9):1353–1359. [DOI] [PubMed] [Google Scholar]
- 13.Shen SS, Ro JY, Tamboli P, et al. Mucinous tubular and spindle cell carcinoma of kidney is probably a variant of papillary renal cell carcinoma with spindle cell features. Ann Diagn Pathol. February 2007;11(1):13–21. [DOI] [PubMed] [Google Scholar]
- 14.Mehra R, Vats P, Cieslik M, et al. Biallelic Alteration and Dysregulation of the Hippo Pathway in Mucinous Tubular and Spindle Cell Carcinoma of the Kidney. Cancer Discov. November 2016;6(11):1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. May 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic acids research. September 19 2016;44(16):e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. March 2002;26(3):281–291. [DOI] [PubMed] [Google Scholar]
- 18.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. May 2003;27(5):612–624. [DOI] [PubMed] [Google Scholar]
- 19.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. March 2001;25(3):275–284. [DOI] [PubMed] [Google Scholar]
- 20.Larkin J, Fisher R, Pickering L, et al. Metastatic mucinous tubular and spindle cell carcinoma of the kidney responding to sunitinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. October 1 2010;28(28):e539–540. [DOI] [PubMed] [Google Scholar]
- 21.Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. Journal for immunotherapy of cancer. January 29 2018;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ged Y, Knezevic A, Chen Y, et al. Single-center analysis of 109 patients (pts) with metastatic chromophobe renal cell carcinoma (ChRCC): Differences in outcomes by histologic variant. Journal of Clinical Oncology. 2018/May/20 2018;36(15_suppl):4577–4577. [Google Scholar]
- 23.KEYTRUDA® (pembrolizumab) [package insert]. Merck Sharp & Dohme Corp, Whitehouse Station, NJ: 2017. [Google Scholar]
- 24.Hsieh JJ, Chen D, Wang PI, et al. Genomic Biomarkers of a Randomized Trial Comparing First-line Everolimus and Sunitinib in Patients with Metastatic Renal Cell Carcinoma. Eur Urol. Mar 2017;71(3):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss MH, Kuo F, Chen D, et al. Integrated biomarker analysis for 412 renal cell cancer (RCC) patients (pts) treated on the phase 3 COMPARZ trial: Correlating common mutation events in PBRM1 and BAP1 with angiogenesis expression signatures and outcomes on tyrosine kinase inhibitor (TKI) therapy. Journal of Clinical Oncology. 2017;35(15_suppl):4523–4523. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.