Dear Editor,
Kovacs and Podda1 present a case series of Hurley Stage 3 Hidradenitis Suppurativa patients responsive to Guselkumab (a monoclonal IL-23 antagonist) over a 12-week period as measured by the IHS4 outcome measure. A phase 2 trial of Guselkumab in HS2 is currently underway, utilizing the HiSCR outcome measure in line with the PIONEER studies3. Both outcome measures have been appropriately validated both psychometrically and clinically3,4. HiSCR is defined as a 50% reduction in inflammatory abscess and nodule count from baseline3; whereas the IHS4 is a differentially weighted outcome measure, the total calculated by nodule count (weighted x1) abscess count (weighted x2) and draining fistulae count (weighted x4)4.
Comparing results of biologic clinical trials in HS requires consistent outcome measures and acknowledgement of differences in patient characteristics, in order to draw appropriate conclusions regarding external validity. Whilst the HiSCR has the advantage of being widely used and reported in multiple RCTs, it does not consider the draining fistulae of advanced disease. This may have implications on responsiveness, particularly in the presence of low inflammatory nodule counts which can occur in highly cicatricial disease.
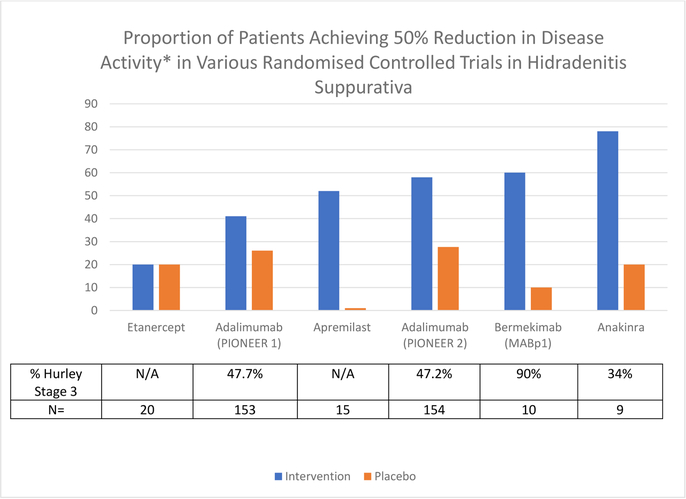
Disparate response rates to Adalimumab are seen in Hurley Stage 2 and Stage 3 in PIONEER data (PIONEER 1: 44.6% vs 38.6% and PIONEER 2: 62.4% vs 55.1%)3. It is unclear whether this is due to the low median baseline inflammatory nodule counts (PIONEER 1: 11.5 (10.92), PIONEER 2: 8.6 (6.92))3 or inherent differences in the disease processes in stage 2 and stage 3 patients. If disease and pathogenic heterogeneity is implicated5, this could have impacts upon the interpretation of other RCTs due to differing ratios of stage 2 and stage 3 patients6–9 (Figure 1).
Figure 1:
Graphical representation of proportion of patients achieving HiSCR (50% reduction of inflammatory nodules and abscesses at Week 12 compared to Baseline) in various RCTs in Hidradenitis Suppurativa – Intervention Group versus Placebo.
(NB: Apremilast Study results are from Week 16, all other results from Week 12.)
*50% reduction in disease activity defined by HiSCR or calculated based upon raw data of abscess and nodule count.
Understanding pathogenic heterogeneity in HS may lead to identification of clinical or biochemical variables which may predict clinical response to specific therapeutics. Anecdotal evidence suggests that differential response may occur between moderate (primarily inflammatory disease) and severe (fistulizing and cicatricial) disease, however subgroup analysis of existing trials have included cohorts too small to demonstrate statistical significance. Comparison of the efficacy rates of existing clinical trials along with the proportions of stage 2 and 3 patients are suggestive of this differential response (Figure 1).
The underlying inflammatory mechanisms differentiating Hurley Stage 2 and Stage 3 patients are proposed to involve feed-forward mechanisms of keratinocyte-derived pro-inflammatory mediators including IL-1α, IL-1β, ICAM-1 and TGF-β instigating aberrant wound healing responses, scarring and fistulae formation. The inflammatory cascade in moderate disease is more in line with the TNF-alpha and Th-17 mediated inflammatory cascade10.
Kovacs and Podda’s report1 demonstrating response to Guselkumab in Hurley stage 3 HS supports the concept of upstream blockade of the Th17 cascade as well as leucocyte and dendritic cell mediated keratinocyte/fibroblast stimulation in advanced disease10, however in the absence of HiSCR and RCT data it is difficult to determine where Guselkumab may fit upon the efficacy spectrum in HS. Whilst HiSCR is the accepted primary outcome measure for clinical trials in HS for the forseeable future, the IHS4 has a complementary role, particularly in the assessment of advanced disease where the HiSCR may suffer from decreased responsiveness.
The open reporting and availability of de-identified individual patient data from randomized clinical trials of new biologic agents in HS may allow for the retrospective collation of IHS4 and HiSCR statistics to allow direct comparison between studies. This would provide the greatest utility in determining the cause of variation in response rates to biologic therapies in advanced HS, whether this be a product of the outcome measure used, or a possible signal of disease heterogeneity.
Funding:
Supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare
References:
- 1.Kovacs M and Podda M Guselkumab in the treatment of severe Hidradenitis Suppurativa J Eur Acad Dermatol Venereol 2018; doi: 10.1111/jdv.15368 [DOI] [PubMed]
- 2.ClinicalTirals.gov [Internet] Bethesda MD: National Library of Medicine (US) 2018. November 29 Identifier: NCT03628924. A Study to Evaluate the Efficacy, Safety and Tolerability of Guselkumab for the Treatment of Participants with Moderate to Severe Hidradenitis Suppurativa (HS) (NOVA) Available from https://www.clinicaltrials.gov/ct2/show/NCT03628924 [Google Scholar]
- 3.Kimball AB, Okun MM, Williams DA, Gottlieb AB Papp KA Zouboulis CC et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med 2016;375:422–434 [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS Severity. [DOI] [PubMed]
- 5.Riis PT, Saunte DM, Benhadou F, del Marmol V, Guillen P, El-Domyati M et al. Low and High body mass index in hidradenitis suppurativa patients – different subtypes? J Eur Acad Dermatol Venereol 2018;32:307–312 [DOI] [PubMed] [Google Scholar]
- 6.Adams D, Yankura JA, Fogelberg AC, Anderson BE Treatment of Hidradenitis Suppurativa with Etanercept Injection. Arch Dermatol 2010;146:501–504 [DOI] [PubMed] [Google Scholar]
- 7.Vossen ARJV van Doorn MBA van der Zee HH Prens EP Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial JAAD 2018; DOI: 10.1016/j.jaad.2018.06.046 [DOI] [PubMed]
- 8.Kanni T, Argyropoulou M, Spyridopoulos T, Pistiki A, Stecher M et al. MABp1 Targeting IL-1alpha for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Ramdomized Study J Invest Dermatol 2018;138:795–801 [DOI] [PubMed] [Google Scholar]
- 9.Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa A Randomized Clinical Trial JAMA Dermatol. 2016;152:52–59. [DOI] [PubMed] [Google Scholar]
- 10.Frew JW, Hawkes JE , Krueger JG A Systematic Review and Critical Evaluation of Inflammatory Cytokine Associations in Hidradenitis Suppurativa F1000Res 2018;(In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]