Abstract
Objectives:
To determine 1) whether higher maternal body mass index (BMI) and Cesarean (C) Section mode of delivery are associated with neonatal hypoglycemia (NH) and 2) whether timing of NH onset differs by risk factors.
Study Design:
Retrospective cohort study (n=4602) to determine the odds of NH, NH requiring IV dextrose and timing of NH onset among infants with established and plausible (BMI and C-section) risk factors.
Result:
Infants born to class III obese mothers had higher odds of NH (OR 1.3, 95% CI 1.0–1.8) and of requiring IV dextrose (OR 2.2, 95% CI 1.2–3.9). Infants born via C-section had higher odds of requiring IV dextrose (OR 1.4, 95% CI 1.1–1.9). Infants who were delivered to high BMI mothers and by C-section developed NH earlier than the reference group.
Conclusion:
Determining the predictors and timing of NH onset may help develop tailored evaluation and management strategies for at-risk neonates.
Introduction
Neonatal hypoglycemia (NH) is estimated to affect 10–15% of newborns and is associated with neurodevelopmental sequelae (1). NH is easily treatable but identifying prenatal risk factors for NH is critical to ensuring appropriate screening and timely treatment. There is a normal physiologic transition of glycemic control that occurs in the hours following birth, when maternal glucose supply is eliminated. Maternal and infant factors can perturb this transition, causing lower than typical neonatal glucose concentrations. Established risk factors for NH include maternal factors such as gestational diabetes and maternal use of beta blockers and beta agonists (terbutaline). Other established risk factors for NH are infants who are small- or large- for gestational age (SGA or LGA), preterm or postdates infants, infants with a history of perinatal stress (pre/eclampsia, birth asphyxia, meconium aspiration syndrome, hypothermia), infants with a midline syndrome and a family history of hypoglycemia (2). Infants who fall into these categories should receive blood glucose screening in the first 24 hours of life and are treated with dextrose and feeding if these values are lower than the expected norm for their age.
There has been a rapid upward trend in maternal pre-pregnancy body mass index (BMI) in the past 20 years. Currently, one of every three mothers enter pregnancy obese. National guidelines do not include maternal obesity as an independent risk factor for NH, although there is biologic plausibility and early evidence to suggest that maternal obesity may result in subclinical insulin resistance and predispose the infant to NH. Two cohort studies have reported that maternal obesity is an independent risk factor for NH, after controlling for maternal comorbidities (3,4). Another emerging risk factor is Cesarean section (c-section) delivery, which occurs in nearly 31.9% of all births in the United States (5). Infants born by c-section may have less time for hormonal transitions to occur than infants who are delivered after labor. In addition, postnatal measures to prevent NH such as skin-to-skin and early feeding post birth are challenging to initiate in the operating room setting. Lastly, lactogenesis can be delayed after c-section, potentially delaying provision of milk (6). However, there is minimal available data examining the association between c-section delivery and risk of NH.
Understanding the timing of onset and duration of NH is also critical to devise informed practice guidelines. Data on the optimal timing for glucose screening initiation is limited. Screening guidelines could be improved with data to inform when to initiate glucose measurement and how long to continue to screen, based on which prenatal risk factor is present. Currently, per the American Academy of Pediatrics (AAP) guidelines, SGA and preterm infants are screened for a longer period of time compared to LGA infants and infants of diabetic mothers (IDM)(7). For all at-risk infants, NH screening is initiated 30 min after the first feed, which should ideally take place within one hour of birth. However, data regarding timing of onset NH by risk factor is sparse.
Using a large sample of at-risk infants born at a single, academic, high-volume birth hospital in the United States, the objectives of this study were two-fold: 1) to determine whether maternal obesity and c-section are independent risk factors for NH and for NH requiring IV dextrose treatment, and 2) to assess the relationship between risk factors for NH and timing of NH onset.
Methods
Subjects
This was a retrospective study of asymptomatic infants who were screened for NH within the first 48 hours of life per institutional NH screening protocol from January 2016 to March 2018 at Brigham and Women’s Hospital in Boston, MA. Using a data extract report from the electronic health record (EHR), we first identified all infants born during this period who had a blood glucose measurement within 48 hours of birth. This included infants who did and did not develop NH. Timing of all variables were provided by a date and time stamp in this data extract report. Infants were excluded from this analysis if they were less than 35 weeks gestation at birth, a multiple gestation, received IV dextrose for non-NH related conditions, or developed NH after 48 hours of life. This project was undertaken as a quality improvement initiative at Brigham and Women’s Hospital and per institutional policy, was not formally supervised by the Institutional Review Board.
Summary of clinical NH screening guideline
At our institution, infants are screened for NH if they have one of the following established risk factors: infants of diabetic mothers (IDM), SGA or LGA infants, maternal beta blocker or terbutaline administration, preterm or post-term infants, infants with perinatal stress (Apgar score <7 at 5 min of life, respiratory distress >1hr, family history of hypoglycemia or a midline syndrome). If one or more of the screening criteria are met, bedside nurses feed the infant and measure a point-of-care blood glucose within the first hour of life. The goal blood glucose for infants <48 hours old is ≥ 45mg/dL. Infants are provided dextrose gel up to three times and then intravenous (IV) dextrose if their blood sugar concentrations remain below the treatment threshold.
Statistical analysis
NH Demographics
Maternal and infant demographic and clinical characteristics were summarized overall and by NH status using descriptive statistics that included means and standard deviations for continuous variables, and number and percentages for categorical variables. Differences among these variables by NH status, as well as by NH with IV dextrose status, were assessed using nonparametric Mann Whitney U, given the non-normality of the data, or χ2 test for continuous and categorical variables, respectively.
Risk factors for NH outcomes
The association between NH outcomes (NH and NH with IV dextrose treatment) and both already-established and plausible risk factors for NH was examined using multiple logistic regression. The already-established risk factors were (1) gestational age (preterm < 37 weeks, term 37 – 41 weeks (reference), postdates > 41weeks); (2) Infant of a diabetic mother (IDM) vs. no gestational diabetes (reference) (3) preeclampsia, as a surrogate for beta blocker exposure (data on maternal medications were not available through our data extract report) vs. no preeclampsia (reference); and (4) size for gestational age - SGA (<10th percentile), AGA (10th – 90th percentile, reference) and, LGA (>90th percentile) - derived using Fenton growth reference curves. Plausible risk factors for NH were (1) maternal BMI categorized per WHO guidelines (Normal 18.5–24.9 kg/m2 (reference), Overweight 25–29.9 kg/m2, Obese Class I 30–34.9 kg/m2, Obese Class II 35–39.9 kg/m2, Obese Class III ≥40 kg/m2) and (2) mode of delivery (c-section vs. vaginal delivery (reference). The multiple logistic regression models included all risk factors as categorical variables to provide the odds of NH outcomes for each risk factor (compared to its reference group), adjusted for the other NH risk factors. The analytic sample for this analysis included all infants in the total sample, n = 4602; those infants having 1 ≤ NH risk factor (established or plausible) along with infants having none of the maternal risk factors listed but screened for NH given their having non-maternal risk factors for NH, including perinatal and respiratory stress, based on Pediatric Endocrine Society recommendations (2).
Association between risk factors for NH and NH onset: Time to event analysis
We utilized the Kaplan-Meier survival function estimator to determine the time in minutes since birth when infants developed NH for each risk factor. The event of interest was determined as the minutes of life since birth when the first dextrose gel was administered (i.e. time of first hypoglycemic episode). The Breslow test was used to determine whether the survival distribution curves differed. We further explored whether median (in minutes) timing of onset of NH differed by risk factor. For survival analyses, the analytic sample was based on those infants who received gel 1708 (37.1%).
Established risk factors:
For this analysis, we only included participants among those who received gel with one or zero of the established risk factors for NH; subjects with more than one risk factor were excluded. The reference, or “none” group for this analysis consisted of infants who had glucose screens for reasons other than presence of any of the aforementioned risk factors (see Risk factors for NH outcome). We used the Kruskal Wallis test, given non-normality of data, followed by post-hoc pairwise comparison with Bonferroni correction to determine whether time to first dextrose gel differed by risk factor.
Plausible risk factors:
These analyses were based on infants who received gel with one or more risk factors, since infants were not screened solely based on the plausible risk factors of interest (maternal BMI and mode of delivery). To determine whether maternal BMI and mode of delivery affected time to NH, we used Mann Whitney U test for comparison by maternal BMI (<30 (ref) vs ≥30 kg/m2) and by mode of delivery (vaginal (ref) vs. c-section). Given the higher risk of c-section among obese women (3), as an exploratory analysis, we further assessed the modification by maternal BMI on the relationship between mode of delivery and NH onset by evaluating the survival functions of four groups: (1) vaginal delivery and BMI < 30 (reference), (2) vaginal delivery and BMI ≥30, (3) caesarean delivery and BMI < 30, (4) caesarean delivery and BMI ≥30. For this analysis, we defined BMI<30 kg/m2 and vaginal delivery as the reference group because this group was considered the least at risk for NH. We also used the Kruskal Wallis test, given non-normality of data, followed by post-hoc pairwise comparison with Bonferroni correction to determine whether time to first dextrose gel differed by delivery method and BMI group.
We conducted all analyses with SPSS Statistical Software version 24. Statistical significance was designated to be p< 0.05.
Results
Participant characteristics
Out of the 4602 infants who were screened for NH, 40.1% (n=1846) experienced NH and 4.9% (n=227) received IV dextrose to treat NH. In univariate analyses, infants who were preterm, SGA, LGA, born via C-section, whose mothers had a BMI ≥30kg/m2 were more likely to develop NH and require IV dextrose for NH treatment (p<0.005). Infants whose mothers had gestational diabetes (GDM) were more likely to develop NH only, and those with preeclampsia were more likely to require IV dextrose only (Table 1, p<0.005).
Table 1.
Maternal and Infant Characteristics among 4602 at-risk infants for NH
| Maternal Characteristics | All (n=4602) | Hypoglycemic Event (n=1846) | No Hypoglycemic Event (n=2756) | p-value |
|---|---|---|---|---|
| Parity: n(%) | 0.491 | |||
| Primiparity | 2251 (49.0) | 892 (48.4) | 1359 (49.4) | |
| Multiparity | 2341 (51.0) | 951 (51.6) | 1390 (50.6) | |
| Race: n(%) | 0.574 | |||
| White | 2397 (52.1) | 972 (52.7) | 1425 (51.7) | |
| Asian | 507 (11.0) | 188 (10.2) | 319 (11.6) | |
| Black/African American | 771 (16.7) | 306 (16.6) | 465 (16.9) | |
| Hispanic/Latino | 321 (7.0) | 127 (6.9) | 194 (7.0) | |
| Other | 606 (13.2) | 253 (13.7) | 353 (12.8) | |
| Delivery BMI: n(%) | 0.003* | |||
| Normal (18.5–24.9 kg/m2) | 506 (12.4) | 200 (12.2) | 306 (12.5) | |
| Overweight (25–29.9 kg/m2) | 1477 (36.1) | 553 (33.7) | 924 (37.7) | |
| Obese, Class I (30–34.9 kg/m2) | 1140 (27.9) | 455 (27.7) | 685 (28.0) | |
| Obese, Class II (35–39.9 kg/m2) | 567 (13.9) | 243 (14.8) | 324 (13.2) | |
| Obese, Class III (≥40 kg/m2) | 403 (9.8) | 192 (11.7) | 211 (8.6) | |
| Preeclampsia: n(%) | 0.112* | |||
| Yes | 121(2.6) | 57 (3.1) | 64 (2.3) | |
| No | 4481 (97.4) | 1789 (96.9) | 2692 (97.7) | |
| Gestational Diabetes: n(%) | <0.001 | |||
| Yes | 623 (13.5) | 299 (16.2) | 324 (11.8) | |
| No | 3979 (86.5) | 1547 (83.8) | 2432 (88.2) | |
| Mode of Delivery: n(%) | 0.003* | |||
| Vaginal Delivery | 2914 (63.3) | 1122 (60.8) | 1792 (65.0) | |
| C-section | 1688 (36.7) | 724 (39.2) | 964 (35.0) | |
| Infant Characteristics | ||||
| Birth Weight: mean (SD) | 3279.0 (631.2) | 3226.3 (684.9) | 3314.4 (589.9) | <0.001 |
| Sex: n(%) | 0.041* | |||
| Female | 2156 (46.8) | 831 (45.0) | 1325 (48.1) | |
| Male | 2446 (53.2) | 1015 (55.0) | 1431 (51.9) | |
| Gestational Age: n(%) | <0.001* | |||
| Late Preterm (35–36.9) | 603 (13.1) | 340 (18.4) | 263 (9.6) | |
| Term (37–41) | 3453 (75.0) | 1316 (71.3) | 2137 (77.5) | |
| Post Dates (>41) | 546 (11.9) | 190 (10.3) | 356 (12.9) | |
| Centile: n(%) | <0.001* | |||
| SGA (<10%) | 717 (15.7) | 360 (19.6) | 357 (13.1) | |
| AGA | 32153 (70.3) | 1164 (63.3) | 2049 (75.0) | |
| LGA (>90%) | 642 (14.0) | 315 (17.1) | 327 (12.0) | |
| Received Dextrose Gel: n(%) | <0.001* | |||
| Yes | 1708 (37.1) | 1708 (92.5) | 0 (0.0) | |
| No | 2894 (62.9) | 138 (7.5) | 2756(100.0) | |
| Received Dextrose IV: n(%) | <0.001 | |||
| Yes | 227 (4.9) | 227 (12.3) | 0 (0.0) | |
| No | 4375 (95.1) | 1619 (87.7) | 2756 (100) | |
indicates characteristics that differed in infants who received IV dextrose for NH treatment
Risk factors for a hypoglycemic event
In the adjusted model, IDM, preterm, SGA, and LGA infants had higher odds than the respective reference groups of developing NH (Table 2). In addition to these established risk factors, infants born to mothers with Class III obesity (BMI ≥40 kg/m2) also had higher odds of developing NH than infants born to normal BMI mothers (18.5 ≤ BMI < 25 kg/m2), after adjustment for other risk factors (Table 2).
Table 2.
Pregnancy and delivery risk factors associated with infant hypoglycemia among 4066* infants at-risk for NH
| Pregnancy and Delivery Characteristics | Hypoglycemia ORADJ (95% CI)1 | IV Dextrose ORADJ (95% CI)1 |
|---|---|---|
| Gestational Diabetes | 1.7 (1.4–2.1) | 1.2 (0.8–1.8) |
| Preeclampsia | 0.9 (0.6–1.4) | 1.6 (0.8–3.0) |
| Gestational Age (term as ref) | ||
| Preterm | 2.4 (2.0–2.9) | 2.4 (1.7–3.4) |
| Post-Date | 1.1(0.9–1.4) | 0.6 (0.3–1.2) |
| Size for Gestational Age (appropriate for gestational age as ref) | ||
| Small for Gestational Age, SGA | 2.1 (1.7–2.5) | 2.6 (1.8–3.8) |
| Large for Gestational Age, LGA | 2.0 (1.7–2.5) | 2.7 (1.9–3.9) |
| Delivery BMI, Maternal (Normal (18.5 < 25) as ref) | ||
| Overweight (25< 30) | 1.0 (0.8–1.2) | 0.8 (0.4–1.3) |
| Obese, Class I (30 < 35) | 1.1 (0.9–1.3) | 1.3 (0.8–2.2) |
| Obese, Class II (35< 40) | 1.2 (0.9–1.5) | 1.4 (0.8–2.5) |
| Obese, Class III and above (≥40) | 1.3 (1.0–1.8) | 2.2 (1.2–3.9) |
| Delivery Method, C-section (vaginal as ref) | 1.1 (0.9–1.2) | 1.4 (1.1–1.9) |
Estimates are based on multiple logistic regression models for NH and NH requiring IV dextrose. Models include all maternal risk factors identified from univariate analysis as significant risk factors for infant hypoglycemia and/or IV dextrose administration.
Due to missing data for 536 infants, the total analytic sample for this analysis is 4066.
Risk factors for IV dextrose for NH treatment
In the adjusted model, infants had higher odds of requiring IV dextrose to treat NH if they were born preterm, SGA, or LGA compared to the respective reference groups (Table 2). Infants also had higher odds of requiring IV dextrose to treat NH if they were born to mothers with Class III obesity (BMI ≥40kg/m2) compared to infants born to normal BMI mothers (18.5 ≤ BMI < 25 kg/m2) or if they were born via C-section compared to those born vaginally (Table 2).
Time to event analysis
Established risk factors:
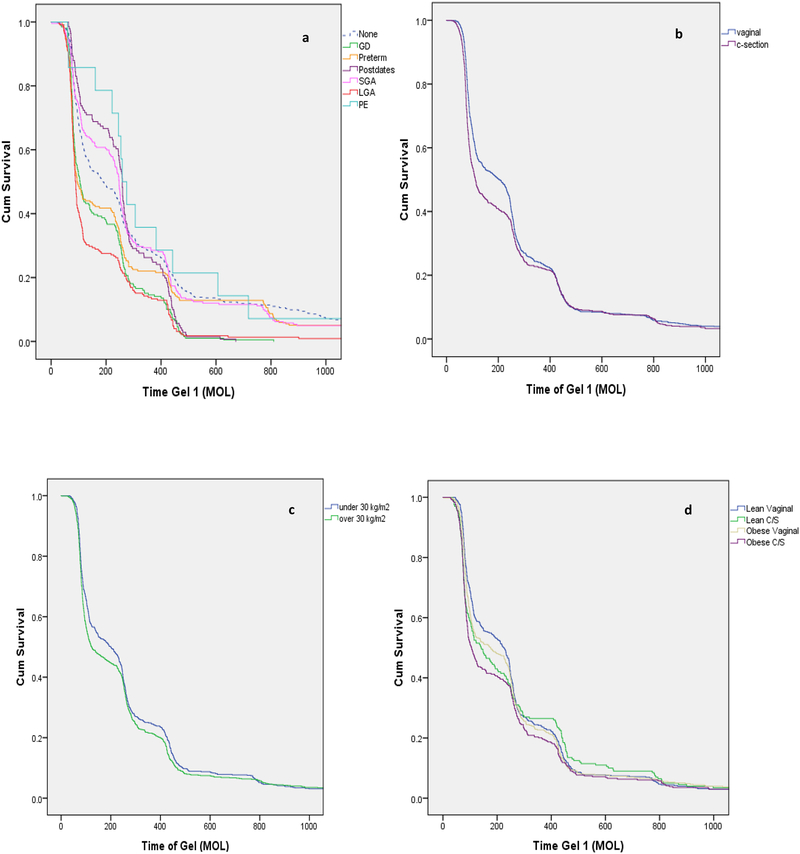
We first examined if established risk factors differentially influenced the timing of NH onset. Timing of NH onset was not equal among the risk factor groups in this analysis (Breslow p<0.001) (Figure 1a). Infants who were born to mothers with GDM, preterm or LGA developed NH sooner (median onset between one and two hours of life; Table 3) than the reference group (no established risk factors, or “none”, p<0.001). Infants who were SGA, postdates or born to mothers with preeclampsia did not have a significant difference in the timing of NH onset compared to the reference group and developed NH later with median onset, after two hours of life (Table 3).
Figure 1:
Timing of NH by established and plausible risk factors. 1a. Timing on NH onset for infants with non-overlapping established risk factors. The “none” group had blood glucose measurements for perinatal stress or respiratory distress. 1b. Timing of NH onset for infants delivered vaginally and by c-section. 1c. Timing of NH onset for infants born to non-obese (BMI <30kg/m2) and obese mothers (BMI ≥30kg/m2). 1d. Timing of NH onset for infants categorized by delivery method and maternal BMI.
Table 3.
Time to first gel by risk factor among 1708 infants who received gel
| Established Risk Factors+ | N | Time to First Gel Median (IQR) | p-value* |
|---|---|---|---|
| None (ref)** | 455 | 189 (323) | 1 |
| Large for gestational age | 225 | 89 (172) | 0.000 |
| Preterm | 218 | 95 (207) | 0.000 |
| Gestation diabetes | 199 | 105 (183) | 0.000 |
| Small for gestational age | 242 | 247 (322) | 0.575 |
| Postdates | 141 | 259 (270) | 0.165 |
| Preeclampsia | 14 | 267 (277) | 0.259 |
| Adjunct Risk Factors++ | |||
| Delivery Method | |||
| Vaginal Delivery (ref) | 1043 | 205 (250) | 1 |
| C-section | 642 | 111.5 (221) | 0.000 |
| Maternal BMI | |||
| Under 30 kg/m2(ref) | 675 | 201 (269) | 1 |
| Over 30 kg/m2 | 818 | 123 (218) | 0.013 |
| Adjunct Risk Factors Grouped by Maternal BMI and Delivery Method++ | |||
| Under 30 and Vaginal Delivery (ref) | 475 | 225 (245) | 1 |
| Under 30 and C-Section | 200 | 141 (343) | 0.034 |
| Over 30 and Vaginal Delivery | 454 | 176 (224) | 0.135 |
| Over 30 and C-Section | 364 | 105.5 (209) | 0.000 |
Subjects in the established risk factor analysis only had one or zero risk factors (≤ 1) of interest
Subjects in these analyses had one or more risk factors
p value determined by Kruskal-Wallis Test or Mann-Whitney U test.
The none group is comprised of babies who were screened for non-maternal indications such as perinatal stress and respiratory distress, based on Pediatric Endocrine Society recommendations (2).
Plausible risk factors:
Infants at-risk for NH delivered via C-section (Figure 1b) had earlier onset of NH compared to those delivered via vaginal delivery (p<0.001; Table 3). Similarly, infants at-risk for NH born to mothers with a BMI ≥30kg/m2 (Figure 1c) had earlier onset of NH than those born to mothers with BMI<30kg/m2 (p=0.013; Table 3). Both plausible risk factors were associated with development of NH, with median onset 90 min earlier than their reference groups (i.e., normal BMI and vaginal delivery, respectively) (Table 3). When further stratified by both mode of delivery and maternal BMI, compared to infants born vaginally to mothers with a BMI <30kg/m2, infants born via c-section to mothers with a BMI ≥30 kg/m2 had the earliest onset of NH followed by infants born via c-section to mothers with BMI <30 kg/m2 (Table 3). Infants born via vaginal delivery to a mother with a BMI≥30kg/m2 and infants born via vaginal delivery to mothers with BMI<30kg/m2 (Breslow p<0.001 Figure 1d) followed these first two groups. Infants delivered via c-section to a mother with a BMI ≥ 30 kg/m2 developed NH at a median onset of 105.5 minutes of life, compared to 225 minutes for an infant born via vaginal delivery to a mother with a BMI<30kg/m2 (p<0.001; Table 3).
Characteristics for the reference group can be found in Supplemental Table 1
Discussion
In our population, we found that in addition to the established risk factors, high maternal BMI and c-section delivery were associated with increased odds of developing NH and needing IV dextrose for the treatment of NH compared with infants of mom with normal BMI and delivered vaginally, respectively. Infants at risk for NH born to mothers with high BMI and/or via c-section delivery were also more likely to develop NH earlier compared to infants of mothers with normal BMI and delivered vaginally, respectively.
We report that high maternal BMI is a risk factor for NH, after adjustment for other comorbid risk factors often associated with maternal BMI. Other studies have had similar findings. In 2016, Suk et al reported in a retrospective cohort study of 1,736 infants that infants of mothers with high BMI were more likely to be admitted to the NICU for NH, independent of maternal diabetes diagnosis (4). Similarly, Neumann et al conducted a retrospective cohort study of 11,939 mother-infant dyads between 2001–2011 and reported that maternal obesity was a risk factor for NH (OR 1.8, 95% CI 1.0, 3.0) (3). This population had a much lower prevalence of obesity (approximately 10%) than the general U.S. population and thus, was not able to examine maternal obesity sub-classes. These studies did not adjust for infant characteristics such as gestational age and fetal growth status, which are known to be associated with BMI and NH risk. Furthermore, neither study examined the interaction of maternal BMI with mode of delivery. Given that high BMI women are more likely to deliver by C-section, an understanding of both factors allows for a more complete understanding of NH risk.
Obesity before and during pregnancy is characterized by insulin resistance, inflammation and dyslipidemia (8). Insulin sensitivity decreases over the course of a healthy pregnancy. Overweight and obese women have decreased insulin sensitivity as compared with lean or average weight women throughout pregnancy, but particularly in the third trimester (8). Obese women are 2.6 times more likely to be diagnosed with GDM than lean women, but the vast majority (up to 95%) of obese women do not meet the clinical threshold for diagnosis of GDM during pregnancy (9,10). Maternal metabolic dysregulation, characterized by elevated pro-inflammatory cytokines and lipid concentrations, is also associated with the longitudinal changes in insulin sensitivity in pregnant women (11–13). Physiologically, obesity is associated with increased inflammation and dyslipidemia, which have been shown to cumulatively contribute to adverse fetal outcomes, such as macrosomia (14). Current screening criteria during pregnancy only include one component of metabolic dysregulation, namely overt glucose intolerance. There is biologic plausibility that sub-clinical metabolic dysregulation in pregnant women with high BMI may be on the causative pathway of NH.
Evidence linking C-section delivery to NH is sparse. There are no published studies that we could find that address the risk of NH by mode of delivery. However, there is reason to suspect that infants delivered via C-section are at increased risk for NH. Potential mechanisms that might mediate this association include delayed lactogenesis, delayed skin-to-skin and impaired thermoregulation (6,15,16). These results lay the groundwork for future studies to better understand the factors that link C-section delivery to NH and more importantly, studies that will test interventions aimed at preventing NH following c-section delivery. In short, these results could also inform future practice during the immediate post-delivery care of infants delivered via C-section.
The timing of onset of NH differed based on risk category. IDM and LGA infants, whose NH physiology is characterized by hyperinsulinemia paired with truncated supply of maternal glucose at birth, seemed to develop NH earlier (2,7,17). SGA infants and infants exposed to beta blockers (i.e. maternal pre-eclampsia) developed NH later. They are at risk for NH due to low glycogen stores or inability to mobilize these stores, respectively (2,17,18). The underlying physiology of NH may contribute to the timing of onset yet, there is no published information that describes the difference in NH onset based on risk factor. This data can help provide individualized screening and feeding practice recommendations for infants based on risk factor and should be considered in the development of screening and treatment guidelines.
Our study had several strengths. Given the prevalence of high maternal BMI and C-sections in our population, we were able to stratify by maternal BMI class and examine the intersection of maternal BMI and mode of delivery. We had detailed information on treatment timing and maternal and infant characteristics. We were limited by the measure of maternal BMI at delivery and the lack of detailed early pregnancy information. Thus, we were not able to examine weight gain during pregnancy as a covariate in this analysis. We also did not have information on maternal beta blocker usage and utilized maternal preeclampsia as a surrogate for this. Given that this analysis was conducted within a quality improvement project, we screened infants based on the established risk factors, i.e., we were not able to examine the role of the plausible risk factors alone in the absence of the established risk factors. However, we conducted thorough statistical adjustment to account for confounding, although there is always the possibility to residual confounding. Future studies should prospectively enroll participants to understand the independent role of the plausible risk factors (maternal BMI and C-section delivery) on odds of NH.
Here we report that both high maternal BMI and delivery by C-section are associated with higher odds of developing NH than lower BMI and vaginal delivery. This lays the groundwork for future investigation of these potential risk factors, which affect over one-third of US infants. In addition, we describe that the timing of NH onset differs by risk factor, which may be useful for clinical guideline development.
Supplementary Material
Funding Source:
Dr. Sen is supported by K23 HD 074648.
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
References:
- 1.McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of Neonatal Glycemia With Neurodevelopmental Outcomes at 4.5 Years. JAMA Pediatr. 2017;(Journal Article). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. JPediatr. 2015;167(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann K, Indorf I, Hartel C, Cirkel C, Rody A, Beyer DA. C-Section Prevalence Among Obese Mothers and Neonatal Hypoglycemia: a Cohort Analysis of the Department of Gynecology and Obstetrics of the University of Lubeck. Geburtshilfe Frauenheilkd. 2017;77(5):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suk D, Kwak T, Khawar N, Vanhorn S, Salafia CM, Gudavalli MB, et al. Increasing maternal body mass index during pregnancy increases neonatal intensive care unit admission in near and full-term infants. JMaternFetalNeonatal Med. 2016;29(20):3249–53. [DOI] [PubMed] [Google Scholar]
- 5.National Vital Statistics Reports Volume 67, Number 1, January 31, 2018. :55. [Google Scholar]
- 6.Hobbs AJ, Mannion CA, McDonald SW, Brockway M, Tough SC. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy and Childbirth. 2016. April 26;16(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamkin D Clinical Report—Postnatal Glucose Homeostasis in Late-Preterm and Term Infants [Internet]. 2011. Available from: http://pediatrics.aappublications.org/content/pediatrics/early/2011/02/28/peds.2010-3851.full.pdf [DOI] [PubMed]
- 8.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. JClinEndocrinolMetab. 2002;87(9):4231–7. [DOI] [PubMed] [Google Scholar]
- 9.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes. 1999. April;48(4):848–54. [DOI] [PubMed] [Google Scholar]
- 10.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier J-C, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002. July;51(7):2207–13. [DOI] [PubMed] [Google Scholar]
- 11.Catalano P, Ehrenberg H. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–33. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa E, Oakley L, Seed PT, Doyle P, Oteng-Ntim E. Maternal BMI and diabetes in pregnancy: Investigating variations between ethnic groups using routine maternity data from London, UK. PLOS ONE. 2017. June 22;12(6):e0179332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988. August;67(2):341–7. [DOI] [PubMed] [Google Scholar]
- 14.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti MG, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005. January;22(1):21–5. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LW, Crenshaw JT, Gilder RE. Influence of Immediate Skin-to-Skin Contact During Cesarean Surgery on Rate of Transfer of Newborns to NICU for Observation. Nurs Womens Health. 2017. March;21(1):28–33. [DOI] [PubMed] [Google Scholar]
- 16.Nolan A, Lawrence C. A pilot study of a nursing intervention protocol to minimize maternal-infant separation after Cesarean birth. J Obstet Gynecol Neonatal Nurs. 2009. August;38(4):430–42. [DOI] [PubMed] [Google Scholar]
- 17.Rozance PJ, Hay WW. Describing hypoglycemia - definition or operational threshold? Early Hum Dev. 2010. May;86(5):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, et al. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiology and Drug Safety. 2011;20(2):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.