Abstract
Background
Viral bronchiolitis is a common respiratory infection that often affects term, otherwise healthy infants. A small literature suggests maternal stress during pregnancy is associated with bronchiolitis. However, the association between maternal exposure to lifetime traumatic stress, including traumatic events occurring in childhood or throughout the life-course, and bronchiolitis has not been studied previously.
Objectives
To investigate the association between maternal exposure to total lifetime and childhood traumatic stress events and infant bronchiolitis.
Methods
We studied mother-infant dyads enrolled in a prospective prenatal cohort, recruited 2006–2011, and Tennessee Medicaid. During pregnancy, we assessed maternal lifetime exposure to types of traumatic events by questionnaire. We captured bronchiolitis diagnoses in term, non-low birthweight infants’ first 12 months using linked Medicaid data. In separate models, we assessed the association of maternal lifetime traumatic events (0 to 20 types) and a subset of traumatic events that occurred during childhood (0 to 3: family violence, sexual and physical abuse) and infant bronchiolitis using multivariable log-binomial models.
Results
Of 629 women, 85% were African-American. The median count (interquartile range) of lifetime traumatic events was 3 (2, 5); 42% reported ≥1 childhood traumatic event. Among infants, 22% had a bronchiolitis diagnosis (0 to 2 lifetime traumatic events: 24%; 3 events: 20%; 4 to 5 events: 18%; 6 or more events: 24%). Total maternal lifetime traumatic events were not associated with bronchiolitis in multivariable analyses. For maternal childhood traumatic events, the risk of infant bronchiolitis increased with number of event types reported: adjusted Risk ratios were 1.12 (95% confidence interval [CI] 0.80, 1.59), 1.31 (95% CI 0.83, 2.07), and 2.65 (95% CI 1.45, 4.85) for 1, 2 and 3 events, respectively, versus none.
Conclusions
Infants born to women reporting multiple types of childhood trauma were at higher risk for bronchiolitis. Further research is needed to explore intergenerational effects of traumatic experiences.
Keywords: Intergenerational, Psychological Trauma, Trauma and Stressor Related Disorders, Infant, Respiratory Tract Diseases
Background
Viral bronchiolitis is a common lower respiratory infection in early childhood, often with preceding upper respiratory symptoms and progression to symptoms of lower airway obstruction.1,2 In the first year of life, viral lower respiratory tract infections, primarily bronchiolitis, affect approximately 20–30% of children.3,4 Additionally, approximately 3% of infants are hospitalized with bronchiolitis, particularly those younger than 5 months of age.5 Bronchiolitis during infancy is strongly associated with morbidities later in life, including recurrent wheeze and childhood asthma.6 The public health burden of bronchiolitis is substantial, and although prematurity and lung disease are well established risk factors for bronchiolitis and increased severity, many affected infants are born at term, without existing lung or cardiac disease.3,5 Accordingly, there is a need to understand prenatal and early life factors which may contribute to viral bronchiolitis, including maternal factors such as exposure to psychosocial and traumatic stress.
Prenatal immune system and lung development are orchestrated through the interaction between immune, neural and endocrine systems which may be susceptible to disruption from exposures such as maternal stress.7,8 An aberrant maternal stress response during pregnancy has been shown to adversely influence fetal development, including fetal immune functioning which can be linked to respiratory outcomes after birth.9,10 Maternal stress during pregnancy has been associated with decreased time to first infection in infancy,11 increased risk for bronchiolitis and respiratory illness in infancy,12–14 and wheezing and asthma later in childhood.15 Recent evidence suggests that this association between maternal stress and child respiratory health is relevant not only to stressors that occur during an index pregnancy, but also stressors that may have occurred prior to pregnancy that may yield prolonged exposure to stress correlates (e.g., disruption in cortisol production or inflammation during the index pregnancy).16–18 In particular, traumatic stress, or stress involving threat or serious harm, can induce physiologic responses long after exposure to the traumatic event, particularly if those events occurred during sensitive developmental periods such as a woman’s own childhood 19 or if they occurred in multiple instances over the life-course.20
The public health burden of viral bronchiolitis is well understood, and while the importance of maternal psychosocial factors on infant and child respiratory outcomes are increasingly recognized,7 the effect of lifetime maternal traumatic stress on infant bronchiolitis has not been studied. Here, our primary objective is to examine the association between maternal traumatic stress and infant bronchiolitis using maternal report of lifetime and childhood traumatic life events (TLEs), ascertained during pregnancy in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) cohort study, and infant bronchiolitis identified using Tennessee Medicaid (TennCare) administrative data.
Methods
Study design and population
We studied mother-infant dyads who were enrolled prenatally (2006–2011) in CANDLE, a prospective cohort of mother-infant dyads based at the University of Tennessee Health Science Center21,22 and were also enrolled in TennCare. The cohort was recruited from Shelby County (Memphis), TN and includes a predominately low-income, urban, African-American population, with 59% of women reporting Medicaid insurance at enrollment.21 Women who were pregnant (16–28 weeks gestation; singleton, low-risk pregnancies), 16 to 40 years of age, English speaking, and residents of Shelby County planning to deliver at designated study health care settings were eligible. Low-risk pregnancies excluded those with certain chronic health conditions (e.g., chronic hypertension requiring therapy, acute or chronic hepatitis, human immunodeficiency virus) or severe pregnancy complications (e.g., oligohydramnios, placenta previa).21 CANDLE did not exclude women with asthma. For this study, we included CANDLE dyads in which infants were also continuously enrolled in Tennessee Medicaid during the first year of life (no more than 90 days of non-enrollment) in order to ascertain bronchiolitis diagnoses in Medicaid claims data.22 We excluded infants with low birthweight (<2500 grams), preterm birth (<37 weeks estimated gestational age), International Classification of Diseases (ICD-9) diagnoses that indicated high risk conditions (chronic lung disease, congenital heart disease, congenital upper airway anomaly, other congenital anomaly), or receipt of respiratory syncytial virus immunoprophylaxis in order to investigate associations among infants without pre-existing lung or cardiac disease.3,22 We further limited analyses to dyads with African-American and white women because other races were too few to study (~1%). CANDLE and TennCare records were linked using probabilistic linkage.23 Informed consent was obtained by women at enrollment. This study was approved by Institutional Review Boards of Vanderbilt University, University of Tennessee Health Science Center and Tennessee Department of Health, and by representatives of the Bureau of TennCare.
Maternal traumatic stress
During the third trimester study visit, we assessed maternal traumatic life events using the Traumatic Life Events Questionnaire (TLEQ), a self-report instrument for assessing exposure to types of traumatic events (yes/no) over one’s lifetime,24 as previously described.25 The TLEQ administered addressed exposure to 20 potentially traumatic events, such as those involving accidents, violence, abuse, death, and illness. We defined a total lifetime TLE score and a childhood TLE score. The total lifetime TLE score, ranging from 0–20, was derived by summing the number of endorsed event types over the mother’s lifetime. The childhood TLE score (0–3) was based on three TLEQ questions that asked whether events related to family violence, physical abuse, and/or sexual abuse occurred “while growing up” or “before [her] 13th birthday.” Because maternal adverse childhood experiences have the potential to influence health across the lifecourse and also influence the developing fetus, our investigation of the association of maternal childhood traumatic life events and infant bronchiolitis was determined a priori.
Infant bronchiolitis
Our outcome was a bronchiolitis diagnosis during the first postnatal year.3,22 We identified bronchiolitis healthcare visits using International Classification of Diseases-9 codes for bronchiolitis (466.1) or respiratory syncytial virus pneumonia (480.1) captured during hospitalizations, 23-hour observations, emergency department and clinic visits.3,22 Infants were classified as having at least one bronchiolitis visit or none.
Statistical Analyses
Descriptive characteristics were compared across categories of TLEQ (quartiles) and childhood TLEQ scores using Kruskal-Wallis and Pearson chi-squared tests. We assessed associations between 1) lifetime TLEs (quartiles and continuous) and 2) childhood TLEs (categorical: 1, 2, or 3 vs. 0) and the dichotomous outcome of bronchiolitis using generalized linear models with log function for the link between independent variables and the probability of the outcome. We used the robust sandwich error estimation for the calculation of standard errors and corresponding 95% confidence intervals (CIs). For childhood TLE models, the Poisson link was specified to achieve model convergence. The non-linear association between continuous TLEs and bronchiolitis was assessed using restricted cubic splines. For childhood TLEs, we tested for a linear trend by modeling exposure frequencies as an ordered continuous variable. We included maternal age at delivery (years), race (African-American/white), smoking during pregnancy (yes/no), asthma (yes/no), education (≤high school, >high school), Social Support Questionnaire score (average number of people available to offer an individual support across a range of situations26), and infant sex (male/female) and birthweight (grams) as covariates. We assessed for interaction between continuous lifetime TLEs and infant sex. In sensitivity analyses, we removed birthweight and maternal smoking from multivariable models since they may be on a causal pathway between maternal traumatic stress and infant health. To address potential bias due to missing TLEQ data (7% of eligible subjects, Figure 1), we first assessed whether cohort characteristics were associated with missingness. Next multiple imputations (MI) were used to account for the missing TLEQ values. We used Hmisc and Amelia packages in R to take random draws from imputation models (200 imputations).27,28 Finally, we calculated E-values to assess the robustness of our findings to unmeasured confounding.29 We used SAS version 9.1 (SAS Inc., Cary, NC, US) for data management and R version 3.4.0 for analyses.
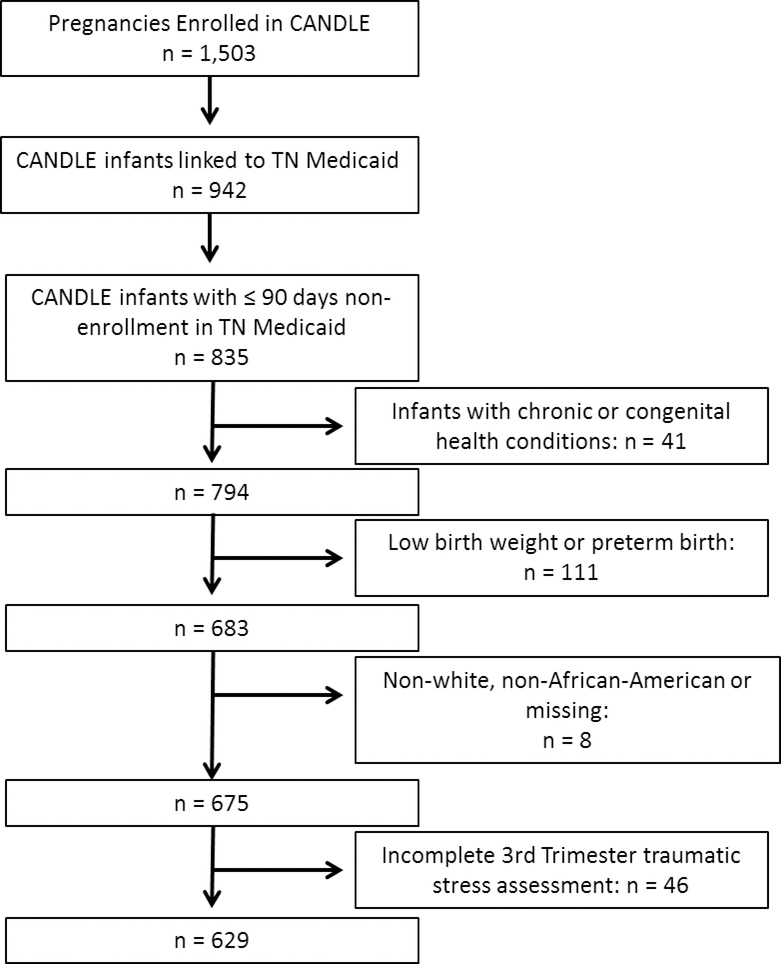
Figure 1.
Flow diagram for study entry.
Results
Of 835 CANDLE dyads that met TennCare continuous enrollment criteria, 629 met additional eligibility criteria and were included in this study (Figure 1). Most women were African-American (85%) and had a high school education or less (78%) (Table 1). Overall, 13% of women reported smoking during pregnancy and 11% reported a history of asthma. Infants had a median estimated gestational age of 39 weeks (IQR 38, 40 weeks), a median birthweight of 3235 g (IQR 2995, 3510 g), and 51% were male. We identified 136 infants (22%) with a bronchiolitis healthcare visit in the first year of life.
Table 1.
Characteristics of mother-child dyads by maternal lifetime traumatic events, % or median (interquartile range)
| Maternal lifetime traumatic events (count) | |||||
|---|---|---|---|---|---|
| 0–2 (n = 232) | 3 (n = 96) | 4–5 (n = 153) | 6+ (n = 148) | Total (n = 629) | |
| Maternal Characteristics | |||||
| Race (%) a | |||||
| African American | 91 | 88 | 82 | 78 | 85 |
| White | 9 | 12 | 18 | 22 | 15 |
| Education (%) | |||||
| ≤ High school | 84 | 76 | 72 | 78 | 78 |
| >High school | 16 | 24 | 29 | 22 | 22 |
| Asthma (% yes) a | 8 | 4 | 11 | 19 | 11 |
| Prenatal smoking (% yes) b | 9 | 11 | 10 | 22 | 13 |
| Age (years), median b | 22 (20, 26) | 22 (20, 27) | 23 (20, 27) | 25 (21, 29) | 23 (20,27) |
| Social Support Score, median c | 2.7 (1.7, 3.8) | 2.7 (1.7, 4.3) | 3.0 (2.0, 4.2) | 2.8 (2.0,4.0) | 2.8 (1.8,4.0) |
| Infant Characteristics | |||||
| Bronchiolitis (% yes) | 24 | 20 | 18 | 24 | 22 |
| Sex (% male) | 53 | 51 | 50 | 51 | 51 |
| Birth weight (kg), median | 3.2 (3.0, 3.5) | 3.3 (3.0, 3.5) | 3.3 (3.0, 3.5) | 3.3 (3.1, 3.6) | 3.2 (3.0, 3.5) |
| Gestational age (weeks), median | 39 (38, 40) | 39 (38, 40) | 39 (39, 40) | 39 (39, 40) | 39 (38, 40) |
p<0.01
p<0.001 as determined by Kruskal-Wallace and Pearson chi-squared statistical tests.
The Social Support Score was generated from the Social Support Questionnaire and equals the average number of people available to offer an individual support across a range of situations.
Maternal Lifetime Trauma
The distribution of lifetime TLEs ranged from 0–15 (median 3; IQR 2, 5), with 92% of women experiencing at least one TLE. The most commonly reported lifetime TLEs included having a loved one experience a sudden death (71%) or having a loved one survive a life-threatening illness (45%). Approximately 25% reported experiencing physical abuse from an intimate partner. Women with higher lifetime TLE scores were more likely to be white than women with lower scores (6+ TLEs 22% white versus 0–2 TLEs 9% white; Table 1). Women with higher TLEQ scores also tended to be older, have a history of asthma, and report a history of smoking during pregnancy than those with lower scores.
The proportion of infants with bronchiolitis did not differ across quartiles of lifetime TLEQ score. In the multivariable model, the adjusted risk ratios (RR) were non-significant using both continuous models (adjusted RR 1.02, 95% confidence interval [CI] 0.96, 1.07) per one unit increase in TLE) and categorical models (e.g., RR 1.11, 95% CI 0.75, 1.64) comparing those with 6+ TLEs to those with 0–2 TLEs; Table 2), with no apparent departure from linearity. Adjusted results did not appreciably change when birthweight and prenatal smoking were removed from the model (data not shown). The interaction between infant sex and continuous lifetime TLEQ score was not statistically significant (Pinteraction=0.15).
Table 2.
Risk ratios (95% confidence intervals) for the association between maternal traumatic life events and infant bronchiolitis
| Maternal lifetime traumatic events | Unadjusted RR | Adjusted RRa |
|---|---|---|
| 0–2 | 1.00 (Reference) | 1.00 (Reference) |
| 3 | 0.83 (0.52, 1.33) | 0.91 (0.57, 1.45) |
| 4–5 | 0.74 (0.49, 1.12) | 0.85 (0.57, 1.29) |
| 6+ | 1.00 (0.69, 1.44) | 1.11 (0.75, 1.64) |
Multivariable log-binomial regression analysis adjusted for maternal race, age, education, asthma, social support and smoking; infant sex, and birth weight. RR: risk ratio
Missing data
To assess potential bias due to missing data, we examined characteristics of subjects with and without missing TLEQ data (eTable 1). Compared to those without missing data, subjects with missing data were less likely to report bronchiolitis (9% versus 22%) and were more likely to have less than high school education (30% versus 18%); no other notable differences were observed. Conclusions from the multiple imputation sensitivity analysis were consistent with primary findings. Point estimates from the multiple imputation models were marginally attenuated relative to the primary models but did not differ with respect to statistical significance (eTable 2).
Maternal Childhood Trauma
At least one traumatic childhood event was reported by 42% of women (Table 3). Of the three childhood TLEs evaluated, exposure to family violence was most commonly reported (32%), followed by sexual abuse (18%) and physical abuse (9%). Three percent of women reported exposure to all three categories of childhood TLEs. The proportion of women who were white varied by number of childhood TLEs (13%, 13%, 27% and 15% for none, one, two and three events, respectively, p = 0.03), as did th e number reporting tobacco use (10%, 12%, 21% and 45% for none, one, two and three events, respectively, p <0.01). We observed that an increasing number of reported childhood TLEs was associated with increasing risk of infant bronchiolitis (Table 3). For women’s childhood experiences, when contrasting maternal report of a single childhood TLE with no childhood TLEs, there was no increase in risk of bronchiolitis (RR 1.12, 95% CI 0.80, 1.59), however, adjusted risk ratios increased with report of two and three childhood TLEs (two TLEs: RR 1.31, 95% CI 0.83, 2.07) and three TLEs: RR 2.65, 95% CI 1.45, 4.85). The test for linear trend supported a linear dose-response relationship (p = 0.02). Results remained consistent when infant birthweight and maternal smoking were removed from the model (data not shown) and when obtained through multiple imputation analyses (eTable 2). The E-values corresponding to the association between three maternal childhood TLEs and infant bronchiolitis (versus no childhood TLEs) were 4.74 (RR) and 2.26 (lower bound of 95% CI).
Table 3.
Maternal childhood traumatic events and infant bronchiolitis (n = 629)
| Number of maternal childhood traumas | Bronchiolitis (n) | No Bronchiolitis (n) | Total (n) | Unadjusted RR (95% CI) | Adjusted RR (95% CI)a |
|---|---|---|---|---|---|
| 0 | 72 | 291 | 363 | 1.00 (Reference) | 1.00 (Reference) |
| 1 | 39 | 141 | 180 | 1.09 (0.77, 1.54) | 1.12 (0.80, 1.59) |
| 2 | 16 | 50 | 66 | 1.22 (0.76, 1.96) | 1.31 (0.83, 2.07) |
| 3 | 9 | 11 | 20 | 2.27 (1.34, 3.84) | 2.65 (1.45, 4.85) |
Multivariable log-binomial regression analysis adjusted for maternal race, age, education, asthma, social support and smoking; infant sex, and birth weight. RR: risk ratio
Comment
Principal findings
In this study, we investigated the association between maternal lifetime and childhood trauma and infant bronchiolitis in a well-characterized prenatal cohort of women and their infants also enrolled in TennCare. Our study sample was limited to generally healthy, term, non-low birthweight infants to study maternal traumatic stress in a population heavily affected by bronchiolitis but without preexisting lung or cardiac disease. Consistent with our previous report,3 approximately 20% of infants obtained a bronchiolitis diagnosis in the first postnatal year. Almost all women reported at least one traumatic life event, with 63% reporting three (median) or more, and 43% reporting at least one childhood TLE. We did not observe a significant association between maternal lifetime traumatic stress and infant bronchiolitis. We did observe a positive association between maternal childhood trauma and infant bronchiolitis, with an over two-fold increase in risk of infant bronchiolitis among women who reported experiencing childhood family violence, physical abuse and sexual abuse, compared to those who were unexposed to all three. These findings suggest maternal childhood traumatic stress may be a risk factor for bronchiolitis among otherwise healthy infants. However, the number of subjects reporting multiple types of childhood trauma was small, and therefore our results should be interpreted with caution.
Strengths of the study
Our study has several strengths. We eliminated the potential for recall bias by assessing lifetime maternal traumatic stress during pregnancy and objectively assessing infant bronchiolitis using linked administrative records. The CANDLE cohort is well-characterized, so we were able to assess and adjust for multiple confounding factors. Through sensitivity analyses, we were able to determine that control for potential intermediate factors such as birthweight and maternal smoking did not influence the associations detected.
Limitations of the data
There are limitations to consider. There may be unmeasured adverse pregnancy-related health behaviors or other factors that play a role in the associations studied here. We did address prenatal smoking, which is one such behavior that is associated with both maternal traumatic stress and infant bronchiolitis.30 However, in our sensitivity analyses, we found no evidence that prenatal smoking influenced our results. We also determined that unmeasured confounding would need to be substantial in order to alter our conclusions: such a confounder would have to be associated with both maternal childhood trauma and infant bronchiolitis by risk ratios of 4.74-fold or greater in order to explain away the association observed among women reporting three childhood traumas, or 2.26-fold or greater to explain away the lower confidence limit. Next, CANDLE excluded women with certain pre-pregnancy chronic health conditions (e.g., hypertension), therefore our cohort represents a population that is healthier than the overall Medicaid population. However, our generalizability may still be limited given that Medicaid enrollment criteria apply. Third, while our method of ascertaining bronchiolitis diagnoses has been widely used to study bronchiolitis epidemiology,3,6,31–34 we acknowledge that we may not capture mild cases or other instances where medical care was not sought. If case ascertainment was poorest among the most highly traumatized women, possibly due to failure to seek care, then we may be underestimating true associations. However, we expect that avoidance of medical care would be less likely in this cohort, given that women demonstrated willingness to engage in our study. Additionally, our assessment of lifetime TLEs did not distinguish between events occurring during pregnancy or prior to pregnancy, or the frequency of events. We were able to study certain events that occurred during childhood. However, the number of women experiencing multiple childhood TLEs was small, limiting our power and precision, and therefore our results should be interpreted with caution. Finally, the childhood events reported for our study were particularly severe, including experiences of family violence, physical abuse and sexual abuse. While we have attributed our findings to potential increased susceptibility to trauma in childhood, we acknowledge that experiences at this level of severity may be associated with adverse outcomes if experienced in adolescence or adulthood. However, we were not able to assess this in our data.
Interpretation
To our knowledge, this is the first study to examine maternal lifetime and childhood trauma and early life respiratory infection, specifically bronchiolitis. However, several previous studies have shown maternal prenatal stress and related exposures (e.g., anxiety in pregnancy) are associated with respiratory infection in the first postnatal year. In a large study of first-birth pregnancies among predominantly white, rural women, stress during pregnancy was associated with increased maternal report of infant illness in the first year, including respiratory infection.12 A similar finding was reported in smaller study of well-educated European mothers and their children.13 Lee and colleagues determined that high levels of anxiety during pregnancy were associated with increased risk of bronchiolitis and lower, but not upper, respiratory tract infections.14 Investigators further determined that these risks were modified by genetic factors, including polymorphisms in antioxidant defense genes, suggesting the potential for genetic susceptibility to the effects of stress and anxiety on infant health.
Adverse early life experiences can impact disease risk throughout the lifetime19 and may also have inter-generational consequences if, for example, exposures adversely affect the intrauterine environment and fetal development.35 Maternal stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis can result in increased cortisol during pregnancy, altering immune and oxidative balance.36,37 Similar effects can occur following stress that precedes pregnancy: trauma can increase maternal cortisol levels during pregnancy independent of response symptoms during pregnancy, suggesting such events may result in altered long-term HPA functioning, 17 potentially due to epigenetic modification.38,39 Infants exposed prenatally to elevated cortisol or maternal traumatic stress have been shown to exhibit impaired stress responses themselves, including elevated pre-stress cortisol and decreased stress-response cortisol,40 and impaired cardiorespiratory recovery following stress exposure.41 Elevated cortisol has also been observed in infants of women with exposure to child abuse and comorbid post-traumatic stress.42 HPA responsiveness is important in modulating immune function, and dysregulation may influence an infant’s response to a viral challenge.43
Our findings suggest that childhood trauma may be uniquely associated with respiratory health in offspring, relative to trauma experienced throughout the lifecourse. This finding is broadly consistent with other studies showing that children of mothers who experienced childhood trauma are more likely to have adverse health outcomes, including obesity 44 and behavioral problems.45 Markers of anxiety, such as the startle response, have been shown to be heighted in children of women reporting exposure to child abuse even after adjustment for maternal depression and the child’s own trauma exposure.46 While the mechanisms driving these associations remain speculative, likely involving both behavioral and biological factors,47 the sensitivity of children to trauma is well recognized.48 The long lasting neurobiological effects of traumas during childhood may contribute to the differential associations we observe when compared to total lifetime traumatic experiences.
By focusing on Medicaid recipients living in a large city in the southern United States, we have identified an important risk factor for bronchiolitis in an understudied, low-income population. Our study of this demographic is particularly relevant since low-income urban women experience high rates of traumatic experiences.49 The prevalence of traumatic stress exposure in our study was consistent with previous reports, including a predominantly African-American cohort where 86% of women reported ever having a TLE.50 Identification of childhood trauma as a possible risk factor for infant bronchiolitis in this population informs understanding of risk factors for bronchiolitis and highlights the need for treatment and prevention strategies related to trauma and stress management.
Conclusions
Bronchiolitis is a common and sometimes severe infection during infancy, and the associated burden on healthcare systems is large.3,5 Our study suggests that women with exposure to childhood trauma – particularly multiple traumas - may be at increased risk of having an infant who develops bronchiolitis in their first year of life. While exposure to multiple childhood trauma types was rare in our study (3%), our findings illustrate a potential etiologic relationship between early life trauma and adverse outcomes in offspring that warrants additional investigation. Future studies that incorporate additional measures of childhood trauma and incorporate additional biomarkers of HPA activity or epigenetic measures are needed. Further, strategies that help modify long-term effects of childhood trauma during or prior to pregnancy can also be considered in efforts to lessen the burden of this common illness during infancy.
Supplementary Material
Social Media Quote.
Women who experienced multiple types of traumatic life events during childhood may have an infant with an increased risk bronchiolitis in the first year of life, according to results from a prospective prenatal cohort study linked to Medicaid data.
Synopsis.
Study question
Using linked prospective cohort and Medicaid data, this study examined the association between maternal childhood and lifetime traumatic stress exposures and infant bronchiolitis
What is already known
Bronchiolitis is a lower respiratory tract infection that commonly affects term, otherwise healthy infants. Maternal stress during pregnancy may influence fetal immune system development, and there is some support that prenatal stress is associated with bronchiolitis in infants.
What this study adds
This study specifically investigates maternal traumatic stress experienced across the life-course (including prior to pregnancy) in relation to infant bronchiolitis. Results suggest that women exposed to multiple traumatic events in childhood are at increased risk of having an infant with bronchiolitis.
Acknowledgements
We acknowledge the contributions of the study research staff and families that enrolled in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood study. The authors are indebted to the Tennessee Division of TennCare (Department of Finance and Administration) and the Tennessee Department of Health (Office of Policy, Planning and Assessment) for providing data for this study.
Funding
This research was supported by the Urban Child Institute (FT) and the National Institutes of Health grants NHLBI HL109977 (KNC) and HL132338 (KNC).
Footnotes
This is the accepted version of the following article: FULL CITE, which has been published in final form at [Link to final article]. This article may be used for non-commercial purposes in accordance with the Wiley Self-Archiving Policy.
References
- 1.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. [DOI] [PubMed] [Google Scholar]
- 2.Meissner HC. Viral Bronchiolitis in Children. The New England Journal of Medicine. 2016;374(18):1793–1794. [DOI] [PubMed] [Google Scholar]
- 3.Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. American Journal of Epidemiology. 1989;129(6):1232–1246. [DOI] [PubMed] [Google Scholar]
- 5.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatric Infectious Disease Journal. 2012;31(1):5–9. [DOI] [PubMed] [Google Scholar]
- 6.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. Journal of Allergy and Clinical Immunology. 2009;123(5):1055–1061, 1061 e1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biological Psychology. 2010;84(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21 Suppl 3:8–14. [DOI] [PubMed] [Google Scholar]
- 9.Lim R, Fedulov AV, Kobzik L. Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014;307(2):L141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. Journal of Allergy and Clinical Immunology. 2002;109(6):923–928. [DOI] [PubMed] [Google Scholar]
- 11.Tegethoff M, Greene N, Olsen J, Schaffner E, Meinlschmidt G. Stress during pregnancy and offspring pediatric disease: A National Cohort Study. Environmental Health Perspectives. 2011;119(11):1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelan AL, DiBenedetto MR, Paul IM, Zhu J, Kjerulff KH. Psychosocial Stress During First Pregnancy Predicts Infant Health Outcomes in the First Postnatal Year. Maternal and Child Health Journal. 2015;19(12):2587–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401–409. [DOI] [PubMed] [Google Scholar]
- 14.Lee E, Chang HY, Lee KS, et al. The effect of perinatal anxiety on bronchiolitis is influenced by polymorphisms in ROS-related genes. BMC Pulmonary Medicine. 2014;14:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Loo KF, van Gelder MM, Roukema J, Roeleveld N, Merkus PJ, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis. European Respiratory Journal. 2016;47(1):133–146. [DOI] [PubMed] [Google Scholar]
- 16.Brunst KJ, Rosa MJ, Jara C, et al. Impact of Maternal Lifetime Interpersonal Trauma on Children’s Asthma: Mediation Through Maternal Active Asthma During Pregnancy. Psychosomatic Medicine. 2017;79(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreier HM, Enlow MB, Ritz T, et al. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress. 2016;19(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Burns VE, Fleming KN. Markers of inflammation in midlife women with intimate partner violence histories. Journal of Women’s Health. 2011;20(12):1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medince. 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 20.Myers HF, Wyatt GE, Ullman JB, et al. Cumulative burden of lifetime adversities: Trauma and mental health in low-SES African Americans and Latino/as. Psychological trauma : theory, research, practice and policy. 2015;7(3):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sontag-Padilla L, Burns RM, Shih RA, et al. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description 2015; https://www.rand.org/pubs/research_reports/RR1336.html. Accessed August 14, 2017.
- 22.Vereen S, Gebretsadik T, Johnson N, et al. Association Between Maternal 2nd Trimester Plasma Folate Levels and Infant Bronchiolitis. Maternal and Child Health Journal. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper JM, Ray WA, Griffin MR, Fought R, Daughtery JR, Mitchel E Jr. Methodological issues in evaluating expanded Medicaid coverage for pregnant women. American Journal of Epidemiology. 1990;132(3):561–571. [DOI] [PubMed] [Google Scholar]
- 24.Kubany ES, Haynes SN, Leisen MB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychological Assessment.. 2000;12(2):210–224. [DOI] [PubMed] [Google Scholar]
- 25.Slopen N, Roberts AL, LeWinn KZ, et al. Maternal experiences of trauma and hair cortisol in early childhood in a prospective cohort. Psychoneuroendocrinology. 2018;98:168–176. [DOI] [PubMed] [Google Scholar]
- 26.Sarason IG, Levine HM, Basham RB, Sarason BR. Assessing Social Support - the Social Support Questionnaire. Journal of Personality and Social Psychology. 1983;44(1):127–139. [Google Scholar]
- 27.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2 ed: Springer International Publishing; 2015. [Google Scholar]
- 28.Honaker J, King G, Blackwell M. Amelia II: A Program for Missing Data. Journal of Statistical Software. 2011;45(7):1–47. [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of Internal Medicine. 2017;167(4):268–+. [DOI] [PubMed] [Google Scholar]
- 30.Lopez WD, Konrath SH, Seng JS. Abuse-related post-traumatic stress, coping, and tobacco use in pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2011;40(4):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veeranki SP, Gebretsadik T, Dorris SL, et al. Association of folic acid supplementation during pregnancy and infant bronchiolitis. American Journal of Epidemiology. 2014;179(8):938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll KN, Gebretsadik T, Griffin MR, et al. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics. 2007;119(6):1104–1112. [DOI] [PubMed] [Google Scholar]
- 33.Sloan CD, Gebretsadik T, Wu P, Carroll KN, Mitchel EF, Hartert TV. Spatiotemporal patterns of infant bronchiolitis in a Tennessee Medicaid population. Spatial and Spatiotemporal Epidemiology. 2013;6:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloan CD, Gebretsadik T, Rosas-Salazar C, et al. Seasonal Timing of Infant Bronchiolitis, Apnea and Sudden Unexplained Infant Death. PloS one. 2016;11(7):e0158521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? Journal of Internal Medicine. 2007;261(5):453–460. [DOI] [PubMed] [Google Scholar]
- 36.Wright RJ, Fisher K, Chiu YH, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. American Journal of Respiratory and Critical Care Medicine. 2013;187(11):1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wonnacott KM, Bonneau RH. The effects of stress on memory cytotoxic T lymphocyte-mediated protection against herpes simplex virus infection at mucosal sites. Brain, Behavior and Immunity. 2002;16(2):104–117. [DOI] [PubMed] [Google Scholar]
- 38.Weder N, Zhang H, Jensen K, et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):417–424 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dirven BCJ, Homberg JR, Kozicz T, Henckens M. Epigenetic programming of the neuroendocrine stress response by adult life stress. Journal of Molecular Endocrinology. 2017;59(1):R11–R31. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol response to acute stress. Developmental Psychobiolog. 2013;55(2):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enlow MB, Kullowatz A, Staudenmayer J, Spasojevic J, Ritz T, Wright RJ. Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosomatic Medicine. 2009;71(6):607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35(5):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Diaz PV. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117(5):E878–E886. [DOI] [PubMed] [Google Scholar]
- 44.Roberts AL, Galea S, Austin SB, Corliss HL, Williams MA, Koenen KC. Women’s experience of abuse in childhood and their children’s smoking and overweight. American Journal of Preventive Medicine. 2014;46(3):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredland N, McFarlane J, Symes L, Maddoux J. Exploring the Association of Maternal Adverse Childhood Experiences with Maternal Health and Child Behavior Following Intimate Partner Violence. Journal of Women’s Health. 2018;27(1):64–71. [DOI] [PubMed] [Google Scholar]
- 46.Jovanovic T, Smith A, Kamkwalala A, et al. Physiological markers of anxiety are increased in children of abused mothers. Journal of Child Psychology and Psychiatry. 2011;52(8):844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Current Cardiology Reports. 2015;17(10):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herzog JI, Schmahl C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Frontiers in Psychiatry. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lillis TA, Burns J, Aranda F, et al. PTSD Symptoms and Acute Pain in the Emergency Department: The Roles of Vulnerability and Resilience Factors Among Low-income, Inner-city Women. Clinical Journal of Pain. 2018;34(11):1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill JM, Szanton S, Taylor TJ, Page GG, Campbell JC. Medical conditions and symptoms associated with posttraumatic stress disorder in low-income urban women. Journal of Women’s Health. 2009;18(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.