Abstract
Purpose:
Despite high tumor mutation burden, immune checkpoint blockade has limited efficacy in small cell lung cancer (SCLC). We hypothesized that poly (ADP-ribose) polymerase (PARP) inhibition could render SCLC more susceptible to immune checkpoint blockade.
Methods:
Single-arm, phase II trial () enrolled relapsed SCLC patients who received durvalumab 1500 mg q 4 weeks and olaparib 300 mg BID. The primary outcome was objective response rate (ORR). Correlative studies included mandatory pre and on-treatment biopsies which were assessed to define SCLC immune-phenotypes: desert (CD8+ T cell prevalence low), excluded (CD8+ T cells in stroma immediately adjacent/within tumor) or inflamed (CD8+ T cells in direct contact with tumor).
Results:
Twenty patients enrolled. The median age was 64 years and most patients (60%) had platinum-resistant/refractory disease. Of 19 evaluable patients, confirmed partial or complete responses were observed in 2 patients (10.5%), including a patient with EGFR-transformed SCLC. Clinical benefit was observed in four (21.1%; 95% CI: 6.1-45.6%) patients with confirmed responses or prolonged stable disease (8 months+). Most common treatment-related adverse events were anemia (80%), lymphopenia (60%), and leucopenia (50%). Nine of 14 (64%) tumors exhibited an excluded phenotype; 21% and 14% of tumors exhibited inflamed and desert phenotypes, respectively. Tumor responses were observed in all instances when pretreatment tumors showed an inflamed phenotype. Of the five tumors without an inflamed phenotype at baseline, no on-treatment increase in T-cell infiltration or PD-L1 expression on tumor infiltrating immune cells was observed.
Conclusions:
The combination did not meet the preset bar for efficacy. Pre- and on-treatment biopsies suggest that tumor-immune phenotypes may be relevant for SCLC responses to immune checkpoint blockade combinations. The predictive value of pre-existing CD8+ T-cell infiltrates observed in this study needs to be confirmed in larger cohorts.
Keywords: Small cell lung cancer, PARP inhibitors, immune checkpoint blockade, tumor immune phenotype, DNA repair
INTRODUCTION
Small cell lung cancer (SCLC) is the most aggressive and lethal form of lung cancer. It represents 15% of all lung cancers with an annual incidence of over 34,000 cases in the United States. SCLC is characterized by rapid doubling time, high growth fraction and early and widespread metastatic involvement1. Although response rates to first-line platinum-based chemotherapy are exceptionally high, tumor usually recurs in months. Additional chemotherapy is usually ineffective at relapse and fewer than 5% of patients with extensive-stage SCLC survive two years.
Despite recent advances in immune checkpoint blockade, only a minority of SCLC patients benefit from these therapies. Nivolumab, an anti-programmed death 1 (PD-1) antibody yielded objective response rates (ORR) of 10% and median overall survival (OS) of 4.4 months in previously treated SCLC patients2. Although efficacy is better in programmed death-ligand 1 (PD-L1)-positive SCLC and with combined immune checkpoint blockade, these approaches are applicable in only a limited number of patients and are associated with substantial toxicities2-4.
One approach to augment the clinical activity of immune checkpoint inhibitors is to modulate the DNA damage response (DDR)5. Poly (ADP-ribose) polymerase 1 (PARP1) is highly expressed in SCLC and PARP inhibitors have shown anti-tumor activity in both SCLC preclinical models and patients6-9. Preclinical observations suggest that combination of immune checkpoint blockade with PARP inhibition may be an effective therapeutic strategy10-12. PARP inhibition potentiated the anti-tumor effect of PD-L1 blockade and augmented cytotoxic T-cell infiltration in multiple immunocompetent SCLC models13. Accumulating evidence also suggest that DNA double-strand break (DSB) and cytosolic DNA can induce PD-L1 expression via mechanisms including a stimulator of interferon genes (STING)-mediated innate immune response 10,14-16.
Durvalumab (MEDI4736) is a selective, high-affinity human IgG1 monoclonal antibody that blocks PD-L1 binding to PD-1 and CD80, thereby enhancing the function of tumor-directed T-cells17 Durvalumab is approved for metastatic urothelial carcinoma and unresectable stage III NSCLC after chemoradiation. The PARP inhibitor olaparib blocks the DNA repair function of these enzymes and traps inactivated PARP onto single-strand DNA breaks, preventing repair an generating a DNA replication block, leading to DNA DSB18. Olaparib is approved for epithelial ovarian cancers and germline BRCA-mutated metastatic breast and ovarian cancers. We previously established the safety and tolerability of a combination of durvalumab and olaparib in a phase I trial19. No dose-limiting toxicities were observed and most common adverse events (AE) were hematologic, which were manageable with supportive care.
We hypothesized that PARP inhibition could render SCLC more susceptible to immune checkpoint blockade and expanded the phase II trial of durvalumab and olaparib to enroll a cohort of SCLC patients. The primary objective was to determine anti-tumor activity in relapsed SCLC patients. Mandatory fresh biopsies were obtained at baseline and on-treatment to assess the dynamic changes in T-cell infiltration and PD-L1 expression before and during treatment. Here we report the efficacy, safety and biomarker results from SCLC patients treated with durvalumab plus olaparib.
PATIENTS AND METHODS
Eligible patients had histologically confirmed SCLC and ≥ 1 platinum-based chemotherapy treatment. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 and adequate organ/bone marrow function. Previous therapy with an immune checkpoint inhibitor was not an exclusion. Patients with both platinum sensitive (progression ≥ 90 days from last platinum dose) and resistant/refractory (< 90 days) disease were eligible. Key exclusion criteria were previous treatment with PARP inhibitors, symptomatic brain metastases, autoimmune disease requiring steroids, pneumonitis and/or interstitial lung disease, or inflammatory bowel disease. The trial was conducted under a National Cancer Institute (NCI) Center for Cancer Research–sponsored investigational new drug application with institutional review board approval. Written informed consent was obtained from all patients. ClinicalTrials.gov: .
Study Design and Treatment
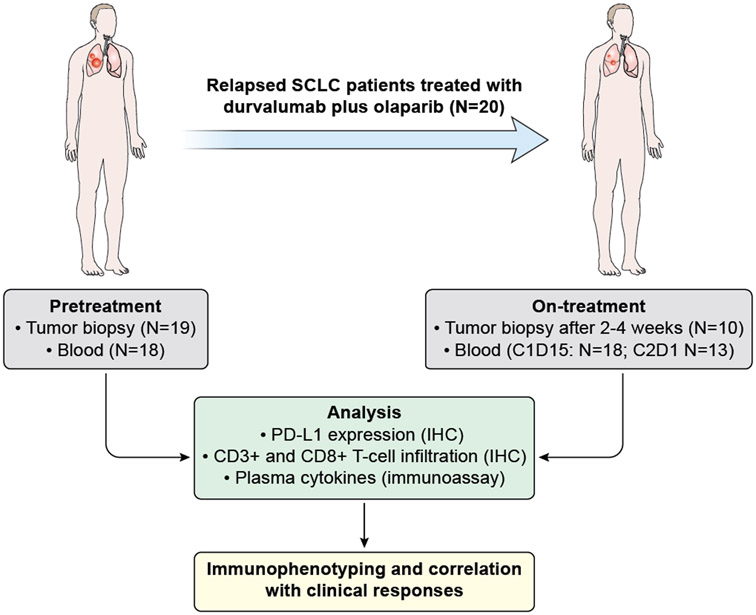
This was an open-label, single-arm phase II study of a combination of durvalumab and olaparib in patients with relapsed SCLC (Figure 1A). Treatment cycles were 28 days long. Durvalumab (1500 mg IV) was administered every 28 days. Olaparib tablets (300 mg twice daily) were administered continuously.
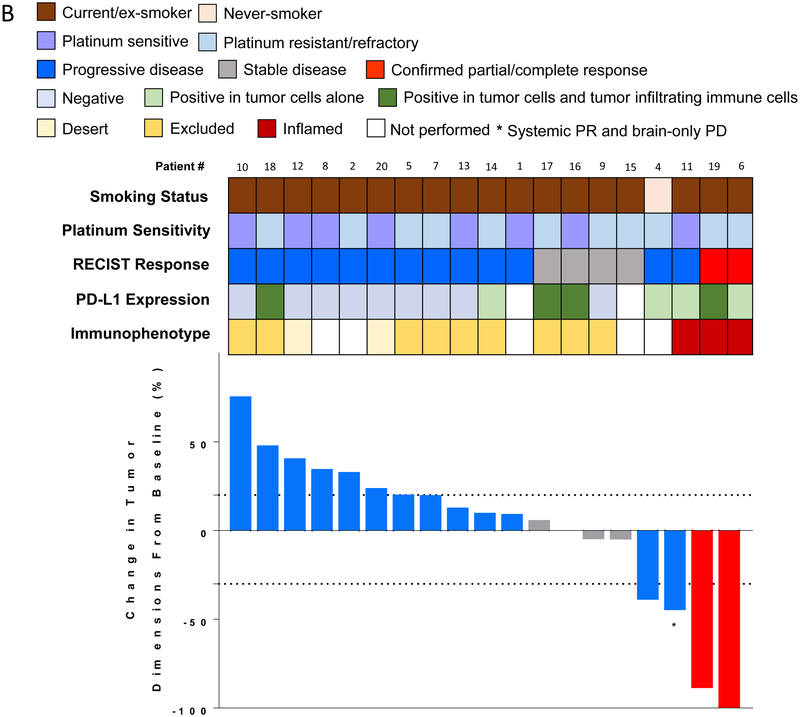
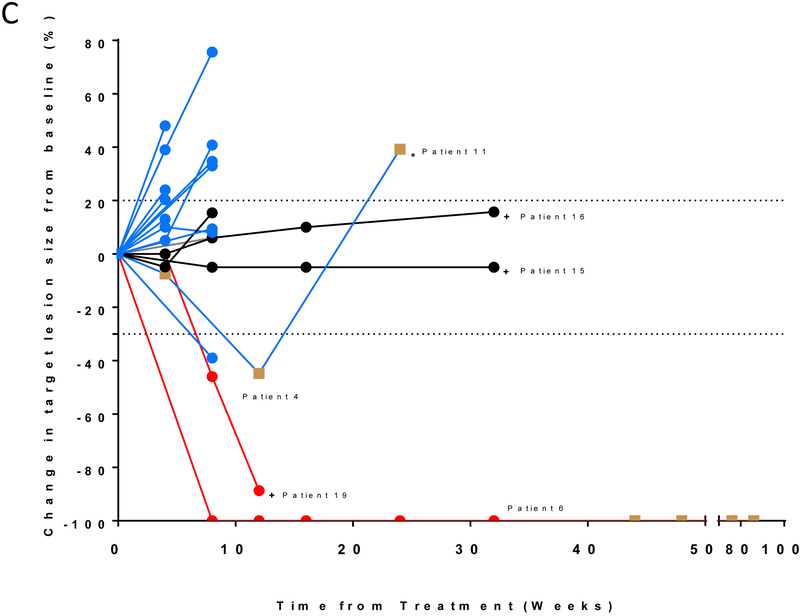
Figure 1.
Efficacy of durvalumab plus olaparib in relapsed SCLC. (A) Study schema and biomarker analyses. (B) Waterfall plot showing change of tumor burden from baseline (investigator assessed; N = 19). One patient was not evaluable for response due to rapidly progressive disease before first restaging scans. Bar length represents decrease/increase in target lesion size. Bar color is the best overall response (according to RECIST 1.1). Indicated by asterisk is a patient who came off treatment after one cycle for brain-only disease progression but who had a partial response in the systemic disease-sites that lasted six months with no additional systemic therapy. Boxes above the waterfall plot indicate the smoking status, platinum sensitivity, RECIST response, PD-L1 expression and immunophenotype of pre-treatment tumors. (C) Spider plot of change in the sum of unidimensional tumor measurements over time. The red lines represent confirmed responders; blue lines represent patients with progressive disease; grey lines represent patients with stable disease. Light brown squares indicate the time points when patients came off treatment due to progressive disease in the brain. + indicates patients who are receiving treatment at data cut-off. * indicates the patient who came off treatment after one cycle for brain-only disease progression but who had a PR in the systemic disease-sites. One patient who was not evaluable is not included.
Efficacy and safety evaluations
History and physical examination were conducted at baseline and prior to each cycle. Complete blood counts with differential and serum chemistries were performed at baseline, 2 weeks later and prior to each cycle. Radiographic evaluation was performed at baseline and every 2 cycles and tumor response assessed based on Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Toxicities were graded using NCI Common Terminology Criteria for Adverse Events (CTCAE 4.0). Given reports of neurological immune-related adverse events (irAEs) in SCLC patients receiving immune checkpoint inhibitors2, patients underwent baseline and follow up neurological exams as needed by a neuromuscular disorders’ expert.
Tumor biopsies and correlative studies
Patients underwent mandatory pretreatment and optional on-treatment biopsies (2-4 weeks after treatment from the same location) (Figure 1A). Five-micron sections of formalin fixed paraffin embedded (FFPE) tissue were assessed for PD-L1 expression and T cell infiltration by immunohistochemistry (IHC) using the following antibodies and detection methods: PD-L1 (1:3 dilution, clone SP142, Springer Biosciences; Leica Bond), CD3 (predilute, clone 2GV6, Ventana; Ventana BenchMark Ultra), CD8 (1:25, clone CD8/144B, Dako; Ventana BenchMark Ultra) following manufacturer’s protocol.
The location of tumor-infiltrating T-cells was noted as follows: intratumoral (within the mass of tumor cells, with direct proximity between cancer and immune cells), stromal (in the surrounding connective tissues and blood vessels) or peritumoral (around the tumor at the advancing margin of the tumor, in the stroma or the tissues adjacent to the tumor). Based on the presence of CD3+ and CD8+ T cells and the pattern of infiltration with respect to tumor cells, tumors were categorized into immunophenotypes as described previously20: “desert” when the prevalence of CD8+ T cells was low, “excluded” if CD8+ T cells were exclusively seen in stroma immediately adjacent to or within the tumor, “inflamed” if CD8+ T cells were seen in direct contact with tumor cells either in the form of spillover of stromal infiltrates into tumor cell aggregates or of diffuse infiltration of CD8+ T cells in aggregates or sheets of tumor cells.
Plasma cytokines were assessed before treatment, cycle 1 day 15 and cycle 3 day 1. Circulating free-DNA (cfDNA) was assessed in selected patients. Methodological details are provided in the Data Supplement.
Statistical Methods
The primary endpoint was objective response rate (ORR). Progression-free survival (PFS), defined as time from start of treatment to time of progression or death, and safety were secondary endpoints. Identification of biomarkers of response was an exploratory endpoint. The trial was conducted using an optimal two-stage phase II trial design to rule out an unacceptably low ORR rate of 15% in favor of an improved response rate of 35% with alpha=0.10 and beta=0.10. Futility was defined as 0-3 responses in the first 19 patients; accrual would continue to 33 patients if there were 4 or more responses in the first stage. PFS, overall survival (OS) and duration of response were calculated using Kaplan-Meier method.
RESULTS
Patient Demographics
Between April 2016 and June 2018, 20 patients with extensive-stage SCLC were enrolled (Table 1). The median age of patients was 64 years (range, 42-76). All patients had received prior chemotherapy and had disease progression at enrollment. Ten (50%) patients had two or more prior lines of therapy. Most patients (60%) had platinum resistant/refractory disease. All patients had received platinum plus etoposide as first-line treatment, 30% had received second-line or later treatment with topotecan, temozolomide or paclitaxel and 15% had received prior immune checkpoint blockade.
Table 1.
Patient characteristics
| Characteristic | N = 20 (%) |
|---|---|
| Gender | |
| Female | 11 (55) |
| Male | 9 (45) |
| Median (range) age in years | 64 (42-76) |
| ECOG performance status | |
| 0 | 1 (5) |
| 1 | 18 (90) |
| 2 | 1 (5) |
| Race | |
| White | 18 (90) |
| Asian | 1 (5) |
| Black | 1 (5) |
| Type of prior therapy* | |
| Chemotherapy | 20 (100) |
| Radiotherapy | 9 (45) |
| Immunotherapy | 3 (15) |
| Surgery | 1 (5) |
| Investigational agents | 4 (5) |
| No. of lines of prior systemic therapy | |
| 1 | 10 (50) |
| 2 | 7 (35) |
| 3 | 3 (15) |
| Prior chemotherapy type | |
| Cisplatin/carboplatin plus etoposide | 20 (100) |
| Topotecan | 3 (15) |
| Paclitaxel | 2 (10) |
| Temozolomide | 1 (5) |
| Sensitivity to first-line therapy | |
| Platinum-sensitive | 8 (40) |
| Platinum-resistant/refractory | 12 (60) |
Patients could have received ≥1 type of prior therapy
All patients were evaluable for safety. A median of two cycles of treatment was administered (range, 1-12 cycles). Nineteen patients were evaluable for response. One patient was not evaluable for response due to rapidly progressive disease before first restaging scans. At the time of data cut-off on 10/1/2018, the median follow-up was 11.1 months (range, 4.0-29.8). Three patients remain on treatment.
Efficacy
Of 19 evaluable patients, one patient each had a confirmed complete response (CR) and a partial response (PR) and four patients had stable disease (SD), including two instances of prolonged SD (8 months+). (Figure 1B, C, Table S1). Thirteen patients had progressive disease (PD). This included a patient who came off treatment after one cycle for brain-only disease progression but who had a PR in the systemic disease-sites that lasted six months with no additional systemic therapy as well as another patient who had a PR at the first restaging, but PD on the confirmatory scan. The investigator assessed ORR was 10.5% (95% CI, 1.3-33.1%). The median PFS was 1.8 months (95% CI, 0.9-2.4 months) and 6-month PFS probability 20.0% (95% CI, 6.2-39.3%) (Figure S1). The median OS was 4.1 months (95% CI, 2.4-9.2 months) and 6-month OS rate 37.1% (95% CI, 16.3-58.2%) (Figure S2). Taken together, clinical benefit was observed in four of 19 (21.1%; 95% CI: 6.1-45.6%) evaluable patients who either had confirmed CR, PR or prolonged SD (8 months+).
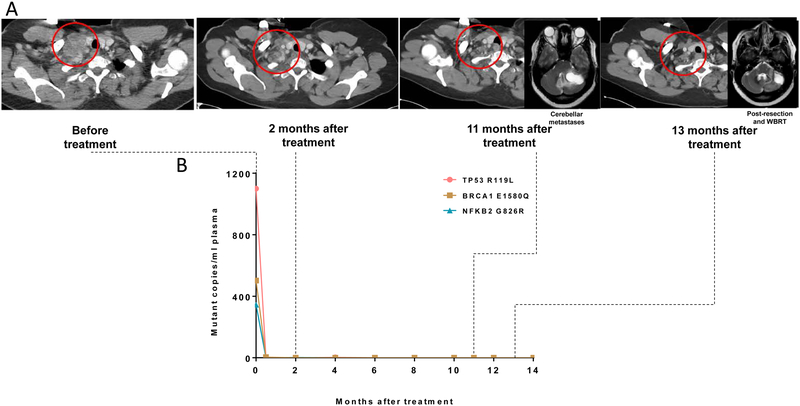
The patient with the CR (patient #6) had platinum-refractory disease that harbored a deleterious BRCA1 mutation. The rapid clinical improvement in this patient was accompanied by a steep decline in cfDNA (Figure 2A, B). The patient came off treatment after 11 months for relapse limited to the brain. At the last follow-up, 21 months after starting on treatment, the patient continues to have no evidence of systemic disease.
Figure 2.
Representative responses. (A) CT images and (B) dynamic changes in circulating free DNA at the corresponding time points in a patient (#6) with a complete response. The right supraclavicular lymph node is indicated by red circles.
The patient with the confirmed PR (#19) had EGFR-mutant adenocarcinoma transformed to SCLC and remains on treatment, with the response maintained for five months at the time of data cut off. Two patients each of whom had previously received immune checkpoint inhibitors (#15 and #16 who previously had PD after 4 and 6 months of immune checkpoint inhibitor-combination respectively), had prolonged SD ongoing at eight months each. Finally, an additional patient (#11) came off treatment after one cycle for brain-only disease progression. This patient later had a systemic response which was maintained for 6 months until disease progression.
Safety
Treatment-related AEs are listed in Table 2. The most common AEs were anemia (80%), lymphopenia (60%), leucopenia (50%), fatigue and thrombocytopenia (45% each). Nine patients (45%) had grade 3 or 4 treatment-related AEs: anemia, lymphopenia, thrombocytopenia and hypophosphatemia. No neurological or Ir-AEs were observed except for elevated thyroid-stimulating hormone (25%), which was asymptomatic in all cases. One patient needed dose reduction of olaparib for grade 3 anemia. No additional dose modifications were required, and no patient discontinued treatment due to AEs.
Table 2.
Treatment-Related Adverse Events (maximum grade, all cycles, N=20 patients)
| Adverse Event | Grades | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total (%) | |
| Anemia | 7 | 7 | 2 | 16 (80) | |
| Lymphocyte count decreased | 3 | 4 | 3 | 2 | 12 (60) |
| White blood cell decreased | 6 | 4 | 10 (50) | ||
| Platelet count decreased | 7 | 1 | 1 | 9 (45) | |
| Fatigue | 7 | 2 | 9 (45) | ||
| Hypothyroidism | 5 | 5 (25) | |||
| Vomiting | 4 | 4 (20) | |||
| Nausea | 3 | 3 (15) | |||
| Diarrhea | 3 | 3 (15) | |||
| Neutrophil count decreased | 2 | 2 (10) | |||
| Anorexia | 2 | 2 (10) | |||
| Constipation | 2 | 2 (10) | |||
| Gastroesophageal reflux disease | 2 | 2 (10) | |||
| Hypophosphatemia | 1 | 1 | 2 (10) | ||
| Alanine aminotransferase increased | 1 | 1 (5) | |||
| Alkaline phosphatase increased | 1 | 1(5) | |||
| Dysgeusia | 1 | 1(5) | |||
| Dyspepsia | 1 | 1(5) | |||
| Headache | 1 | 1(5) | |||
| Hypomagnesemia | 1 | 1(5) | |||
PD-L1 expression and tumor-infiltrating immune cells
Except for one patient who was considered high-risk for biopsy due to clinical deterioration since screening, 19 patients underwent fresh pre-treatment core biopsies. Eight of 18 patients (44%) with adequate tissue for evaluation had quantifiable PD-L1 expression in tumor cells or tumor-infiltrating immune cells (Table S2)- in most cases limited to <20% cells. An inflamed phenotype was usually accompanied by PD-L1 expression. PD-L1 was expressed in tumor or stroma in both the patients with confirmed responses (#6, 19), and in a patient with systemic response and brain-only PD (#11). However, PD-L1 expression was also noted in several tumors that did not respond. In contrast, tumor responses were observed in all instances when pretreatment tumors showed an inflamed phenotype (#6, 19 and 11) (Figure 1A). None of the non-inflamed tumors responded to treatment.
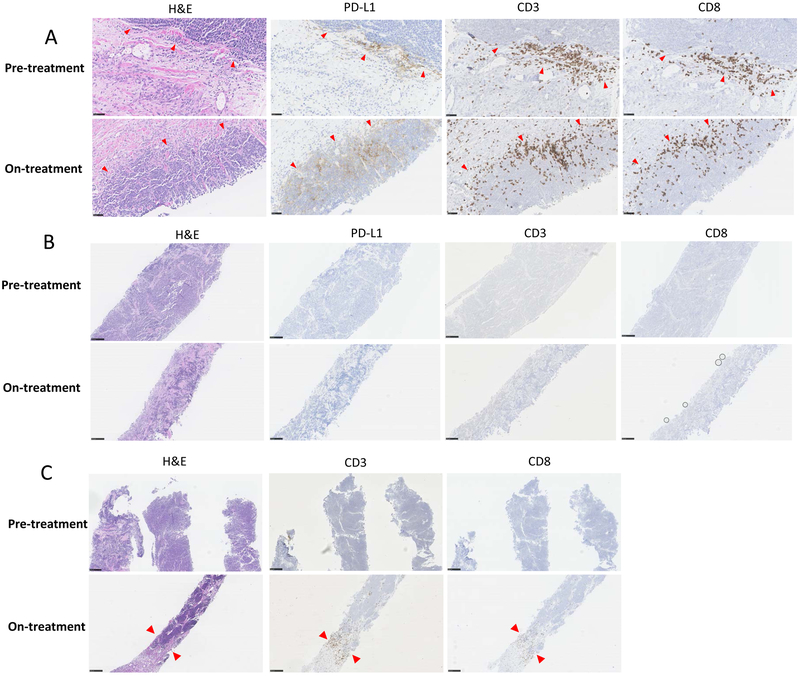
To characterize the immunological events associated with tumor response or progression, on-treatment tumor biopsies were performed in ten patients, of which nine provided adequate material (Table S2). Responding tumors (#11 and 19) displayed a dense T-cell infiltrate in clusters and aggregates extending deeply into the tumor with extensive tumor cell necrosis, accompanied by increased PD-L1 expression on tumor and infiltrating immune cells (Figure 3A). In contrast, non-responding tumors showed a lack of PD-L1 expression and displayed either an immune-desert (minimal or no T-cell infiltration) (Figure 3B) or immune-excluded pattern (T-cells solely around the outer edge of the tumor) (Figure 3C).
Figure 3.
Biomarker status and responses. SCLC immunophenotypes visualized on pre- and on-treatment (2-4 weeks later) biopsies stained immunohistochemically for the presence of PD-L1, CD3+ and CD8+ T cells (A) Immune inflamed phenotype in a patient with transformed SCLC (#19) and an ongoing partial response. Pre- and on-treatment biopsies stained immunohistochemically for the presence of PD-L1, CD3+ and CD8+ T cells (40X magnification). (B) Immune desert phenotype in a patient (#20) who had progressive disease. Pre- and on-treatment tumors show no T cell infiltration or PD-L1 expression (10X magnification). (C) Immune-excluded phenotype in a patient (#9) with arrows indicating the tumor-stroma margin with T cell infiltration post-treatment (10X magnification).
Changes in PD-L1 expression and T cell infiltration are described in Table S2. Of five tumors with no pre-treatment PD-L1 expression, two cases (#9 and 12) remained negative for PD-L1 expression after treatment; in three cases (#10, 13, 20), PD-L1 expression was seen on tumor cells post-treatment with no expression on tumor infiltrating immune cells. In all five cases, no significant changes in the T cell infiltration pattern was observed; T cells were limited to the stroma and peritumoral area both before and after treatment. Of four tumors with pre-treatment PD-L1 expression on tumor cells or tumor infiltrating immune cells, three cases showed substantial increase in the number and intensity of PD-L1 positive cells, which in two instances were associated with clinical response (#11 and 19).
Cytokines
Treatment resulted in raised concentrations of inflammatory cytokines, notably IFN-γ, interleukin (IL)-6 and IL-10, indicative of a systemic immune activation (Figure S3). Cytokine levels at baseline and change with treatment were not significantly associated with clinical response.
DISCUSSION
Patients with SCLC continue to have one of the worst survival rates of all patients with cancer. Response to immune checkpoint blockade is relatively low despite a high tumor mutational burden. This study was conducted on the basis of preclinical data suggesting a beneficial interaction between DDR inhibition and immune checkpoint blockade and an extension of our previous work that defined the safety and tolerability of the combination of durvalumab and olaparib19. To our knowledge, this is the first published report to evaluate immune checkpoint inhibitors with PARP inhibitor in patients with relapsed SCLC.
The study did not meet its primary endpoint and the objective response rate of 10.5% failed to reject the null hypothesis of 35%. The confirmed ORR is similar to that of the PD-1 inhibitor nivolumab alone in this setting2. Clinically meaningful antitumor activity was observed in 21% of patients (confirmed CR, PR or prolonged SD lasting 8+ months) and no unexpected safety signals were detected. Two of three patients who had previously received immune checkpoint inhibitors have prolonged stable disease and remain on treatment at 8 months at the time of data cut-off.
The objective responses in this study occurred in patients with identifiable genomic alterations. The patient with a CR had a deleterious somatic BRCA1 mutation. It is possible that the DDR defect sensitized the tumor to PARP inhibition, yet the depth and durability of tumor regression suggest a contribution from an immune-mediated response. An association between DDR gene alterations and improved responses to immune checkpoint inhibitors has been reported in urothelial cancers20 and melanoma21. The frequency of DDR alterations in SCLC and whether there is an association between DDR alterations and immune checkpoint inhibitor-response need further study. The prolonged ongoing response in a patient with EGFR-mutant NSCLC that transformed to SCLC is notable in light of a recent report that found no responses among 17 transformed SCLC patients who received immune checkpoint inhibitor alone22. The results of this study are in line with the findings from phase II basket study which administered olaparib as monotherapy for 4 weeks followed by combination with durvalumab in relapsed SCLC patients at least 12 weeks after platinum-based therapy23. Among 38 patients, 2 patients had responses (5%) and the 12-week disease control rate was 29%.
Defining predictive biomarkers of response in SCLC is challenging since this disease is generally not biopsied at relapse and biopsies usually provide limited tissue for analyses. Further, the relevance of the tumor-immune phenotype to the response of SCLC was previously unknown. Consistent with the published literature, we observed that PD-L1 expression in most cases was limited to a minority of tumor cells and or tumor-infiltrating immune cells24. In our cohort, a large proportion of tumors (64%) exhibited the excluded phenotype; by contrast, only 21% and 14% of tumors exhibited the inflamed and desert phenotypes, respectively. Tumor responses were observed in all cases where pre-treatment tumors were T-cell inflamed. Tumor CD8+ T-cell infiltration has been linked to antitumor activity in patients with advanced melanoma25 and NSCLC26 treated with immune checkpoint inhibitors. Our observation extends these findings to relapsed SCLC and suggests that a pre-existing CD8+ T-cell response may be predictive of benefit from immune checkpoint inhibitor-based therapies in SCLC. If confirmed in larger cohorts, these findings may help identify SCLC patients who are most likely to benefit from immune checkpoint inhibitor-based therapies.
To our knowledge this study is the first to assess immune phenotypes in serial SCLC on-treatment biopsies. Biopsies were obtained from the same lesion to minimize biological variability. Several observations are worth highlighting: first, the regressing lesions had a preexisting immune response and after treatment showed dense T-cell infiltration, suggesting that pre-existing immunity is further amplified during treatment when responses do occur. Second, several non-responding tumors had minimal or no tumor-infiltrating immune cells before and after treatment, likely reflecting the absence of preexisting tumor-specific T-cells. Downregulation of human leukocyte antigen class I (HLA-I), which reduces the number of T-cell targets among the potential pool of aberrantly expressed tumor antigens, has been documented in SCLC27 and perhaps may be operational here. Third, some of the non-responding tumors showed a pattern wherein the immune cells did not penetrate the tumor but instead were restricted to the stroma, likely reflecting the presence of immunosuppressive mechanisms within the tumor. Finally, treatment with the combination did not result in substantial induction of PD-L1 in the non-responding phenotypes or induction of an immune response where there was no preexisting immune response. It is unlikely that the lack of PD-L1 induction was due to sampling timepoints as patients received continuous twice daily dosing of olaparib that should have resulted in continuous PARP inhibition.
Potential limitations of this trial are its small sample size and its single-arm design with no monotherapy comparator groups, thereby preventing direct comparison of the combination with either agent alone. Lack of a comparator group also limits our ability to attribute the relative contribution of both drugs to the clinical and biomarker response. The relevance of the genomic profile to responses cannot be assessed in this study since such information is not available for non-responding patients. Conclusions regarding the tumor microenvironment are limited by the sample size and the core biopsy size which limits our understanding of the heterogeneity of immune responses among tumor sites. Nevertheless, the findings highlight the importance of serial biological profiling of SCLC over time at different treatment-decision points. The combination of PARP inhibition and immune checkpoint blockade is being investigated in multiple tumor types, including prostate, ovarian, NSCLC and breast cancers (, , , ). Preliminary results suggest that the improved combinatorial activity may be tissue-specific28.
In conclusion, although the trial did not meet its primary endpoint, examination of pre- and on-treatment biopsies provides important insights into SCLC patient selection for immune checkpoint inhibitor-based approaches. Rational development of immunotherapy combinations remains a challenge, particularly so in SCLC where tissue availability presents a major obstacle to progress. Optimal immunotherapy in SCLC may need to not only enhance T-cell function, but also antigen presentation and target the immunosuppressive mechanisms in the tumor microenvironment29.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the efforts of the following individuals in the design and conduct of this clinical trial: Linda Sciuto, research nurse; Alexis Dennis patient care coordinator; Alejandra Chipana Cordero and Rashmita Singh data managers; David Kohler, NIH Clinical Center pharmacist. Both olaparib and durvalumab were supplied under a Collaborative Research and Development Agreement between the CCR, NCI and AstraZeneca/MedImmune.
Funding: This study was supported by the Center for Cancer Research, the Intramural Program of the NCI (ZIA BC 011793). Both olaparib and durvalumab were supplied under a Collaborative Research and Development Agreement between the CCR, NCI and AstraZeneca/MedImmune.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no potential conflicts of interest
DISLCOSURES:
Anish Thomas MD reports grants from AstraZeneca to NCI/NIH, during the conduct of the study.
Christopher Trindade MD owns stock in Gilead, Celgene, Exelixis, Clovis, and Trevena. He is an employee of the Food and Drug Administration, outside the submitted work.
Yuanbin Chen MD reports personal fees from AstraZeneca, personal fees from Genentech, personal fees from Brystol-Myers Squibb, personal fees from Merck, personal fees from Norvatis, personal fees from Takeda, personal fees from Eli-Lilly, personal fees from Guardant Health, personal fees from Pfizer, personal fees from Array Biopharma, grants from AstraZeneca, grants from ISPEN, grants from Roche, grants from Brystol-Myers Squibb, outside the submitted work.
Vamsidhar Velcheti MD reports personal fees from BMS, personal fees from Genentech, personal fees from Astrazenca, personal fees from Merck, personal fees from Celgene, personal fees from Foundation Medicine, personal fees from Taekeda Oncology, personal fees from Reddy Labs, personal fees from Alkermes, personal fees from Novartis, outside the submitted work.
Yves Pommier MD, PhD reports grants from AstraZeneca to NCI during the conduct of the study.
Jung-Min Lee MD reports grants from AstraZeneca to NCI/NIH during the conduct of the study.
NOTHING TO DISCLOSE:
Faye Yin MD
Seth M. Steinberg PhD
Andy Mammen MD
Howard A. Young PhD
Eva Szabo MD
Nitin Roper MD
Rebecca Erwin-Cohen PhD
Javed Khan MD
Liqiang Xi PhD
Rasa Vilimas BSN
Mark Raffeld MD
Elliot Levy MD
Venkatesh Krishnasamy MD
Stephen Hewitt MD, PhD
Jane B. Trepel
Samantha Nichols MS
REFERENCES
- 1.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17(12):725–737. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. [DOI] [PubMed] [Google Scholar]
- 3.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. Journal of Clinical Oncology. 2017;35(34):3823–3829. [DOI] [PubMed] [Google Scholar]
- 4.Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell. 2018;33(5):853–861 e854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas A, Pommier Y. Small cell lung cancer: Time to revisit DNA-damaging chemotherapy. Sci Transl Med. 2016;8(346):346fs312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2(9):798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bono J, Ramanathan RK, Mina L, et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017;7(6):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietanza MC, Waqar SN, Krug LM, et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J Clin Oncol. 2018:JCO2018777672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owonikoko TK, Dahlberg SE, Sica G, et al. Randomized trial of cisplatin and etoposide in combination with veliparib or placebo for extensive stage small cell lung cancer: ECOG-ACRIN 2511 study. Journal of Clinical Oncology. 2017;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouw KW, Goldberg MS, Konstantinopoulos PA, D'Andrea AD. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discovery. 2017;7(7):675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao SP, Xia WY, Yamaguchi H, et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clinical Cancer Research. 2017;23(14):3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Wang L, Cong Z, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(−/−) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463(4):551–556. [DOI] [PubMed] [Google Scholar]
- 13.Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes anti-tumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitai Y, Kawasaki T, Sueyoshi T, et al. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING- Dependent Pathway and Reinforce Antitumor Immunity. J Immunol. 2017;198(4):1649–1659. [DOI] [PubMed] [Google Scholar]
- 15.Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-Dependent Innate Immune Signaling By S-Phase-Specific DNA Damage in Breast Cancer. Jnci-J Natl Cancer I. 2017;109(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato H, Niimi A, Yasuhara T, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017;8(1):1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart R, Morrow M, Hammond SA, et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol Res. 2015;3(9):1052–1062. [DOI] [PubMed] [Google Scholar]
- 18.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72(21):5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women's Cancers: A Dose-Escalation, Phase I Study. J Clin Oncol. 2017;35(19):2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Turley SJ, Nickles D, et al. TGF beta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016; 165(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. Journal of Clinical Oncology. 2019;37(4):278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs M RK, Kim S et al. An Open-Label, Multitumor Phase II Basket Study of Olaparib and Durvalumab (MEDIOLA): Results in Patients with Relapsed SCLC. Journal of Thoracic Oncology. 2017;Volume 12, Issue 11, Supplement 2, November 2017, Pages S2044–S2045. [Google Scholar]
- 24.Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 Expression in Lung Cancer. J Thorac Oncol. 2016;11(7):964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle A, Martin WJ, Funa K, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985;161(5):1135–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart RA, Pilie PG, Yap TA. Development of PARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 29.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.