Abstract
Varying degrees of cognitive deficits affect over half of all HIV-1 infected patients. Because of antiretroviral treatment (ART) regimens, the HIV-1 patient population is increasing in age. Very few epidemiological studies have focused on sex-specific differences in HIV-1-associated neurocognitive disorders (HAND). The purpose of this study is to examine any possible differences between male and female mice in the progression of cognitive dementia during persistent low-level HIV-1 protein exposure, mimicking the typical clinical setting in the post- ART era. Eight to ten-month old HIV-1 Tg26(+/−) transgenic mice were utilized to assess for specific learning and memory modalities. Initial physiological screening and fear conditioning assessments revealed that Tg26 mice exhibited no significant differences in general behavioral function, contextual fear conditioning, or cued fear conditioning responses when compared to their wild-type (WT) littermates, regardless of sex. However, Barnes maze testing revealed significantly impaired short and long-term spatial memory in males, while females had impaired spatial learning abilities and short-term spatial memory. The potential cellular mechanism underlying these sex-specific neurocognitive deficits was explored with hippocampal neurogenic analysis. Compared to WT mice, both male and female Tg26(+/−) mice had fewer quiescent neural stem cells and neuroblasts in their hippocampi. Male Tg26(+/−) mice had a more robust reduction of the quiescent neural stem cell pool than female Tg26(+/−) mice. While female WT mice had a higher number of neural progenitor cells than male WT mice, only female Tg26(+/−) mice exhibited a robust reduction in the number of neural progenitor cells. Altogether, these results suggest that middle-aged male and female Tg26(+/−) mice manifest differing impairments in cognitive functioning and hippocampal neurogenesis. This study emphasizes the importance of understanding sex related differences in HAND pathology, which would aid in designing more optimized therapeutic regimens for the treatment of HAND.
Keywords: HIV-1, HAND, Tg26 Mouse, Neurogenesis, Neural Stem Cells, Differentiation, Fear Conditioning, Barnes Maze, Biological Sex
INTRODUCTION
HIV-1 associated neurocognitive disorders (HAND) are a sequela of HIV-1 infection currently afflicting approximately 36.9 million people worldwide. HAND manifests in over 50% of HIV-1 infected patients, even while adhering to the antiretroviral treatment (ART) regimens (Ferrell and Giunta, 2014; Saylor et al., 2016). Specifically, in the post-ART era, milder forms of HAND continue to plague the HIV-1 patient population (Ellis et al., 1997; Heaton et al., 2010; Kaul et al., 2001; Kaul et al., 2005). Dysregulation of the blood-brain barrier and the “Trojan Horse” mechanism of neuronal damage (Gonzalez-Scarano and Martin-Garcia, 2005; Liu et al., 2002; Williams and Hickey, 2002) are commonly regarded as potential contributors to the pathogenesis and progression of HAND. However, the comprehensive cellular and molecular mechanisms underlying HAND pathogenesis remain poorly understood.
To better understand HIV-1/HAND pathogenesis, several animal models have been developed throughout the years. For example, the SIV-infected rhesus macaque models have been utilized to characterize HAND disease progression and validate ART regimens (Bissel et al., 2018; Calascibetta et al., 2016; Williams et al., 2008). Additionally, the HIV-1 transgenic rat has high levels of HIV-1 transcript expression in the lymph nodes, kidney, thymus, spleen and brain (McLaurin et al., 2018a; Reid et al., 2001; Reid et al., 2016). Initial neurological characterization revealed that these HIV-1 transgenic rats develop hind-limb paralysis and circling-like behavior (Reid et al., 2001). Further neurocognitive tests such as brainstem auditory evoked potentials, signal detection operant tasks, and Morris water maze assessments demonstrated significant impairments in temporal processing (McLaurin et al., 2018a; Moran et al., 2013a), sustained attention (McLaurin et al., 2017; McLaurin et al., 2019), flexibility/inhibition (McLaurin et al., 2019; Roscoe et al., 2014), motivation (Bertrand et al., 2018), learning, and memory (Lashomb et al., 2009; McLaurin et al., 2019; Vigorito et al., 2015; Vigorito et al., 2007).
Several HIV-1 transgenic mouse models have been developed for the characterization of neurological dysfunction in HAND, most notably the doxycycline-inducible glial fibrillary acidic protein (GFAP)-Tat transgenic mouse (Fan et al., 2016) and the GFAP-gp120 transgenic mouse (Lee et al., 2011; Toggas et al., 1994). The doxycycline-inducible GFAP-Tat transgenic mouse and the GFAP-gp120 transgenic mouse only present the effects of a single viral protein expressed in a single cellular type (astrocytes and/or neural stem cells). Similar to the HIV-1 transgenic rat model, the HIV-1 Tg26 transgenic mouse model can recapitulate the effects of the entire HIV-1 proviral genome on physiological and cognitive function (Dickie et al., 1991; Putatunda et al., 2018). This mouse line contains a replication-deficient truncated HIV-1 NL4–3 genome in all cellular types (Dickie et al., 1991). Consequently, only 7 out of the 9 viral proteins are expressed, such as Tat, gp120 (env), Nef, Gag (p17), Vpr, Vpu and Rev (Carroll et al., 2016; Kopp et al., 1992; Lu et al., 2006). Because the replication-deficient HIV-1 proviral DNA randomly integrates into the host genome, and proviral transcription is spontaneously driven by the HIV-1 long terminal repeats (LTRs) or in response to niche factor stimulation; these HIV-1 Tg26 mice serve as a clinically-relevant model for the study of the latent HIV-1 provirus (aviremia) during the ART era. Our recent study demonstrated that HIV-1 Tg26 mice have early and late-stage neurogenic deficits, when compared to their wild-type (WT) littermates (Putatunda et al., 2018).
While there have been significant advances in the understanding of HAND pathogenesis, sex differences in HIV-1 infection and subsequent neurocognitive dysfunction have been largely underrepresented and inconsistent in clinical studies (Burlacu et al., 2018; Griesbeck et al., 2016; McLaurin et al., 2017; McLaurin et al., 2018b; Qiao et al., 2019; Scully, 2018; Ziegler and Altfeld, 2016; Ziegler and Altfeld, 2017). This gap in knowledge is significant, as over 51% of the HIV-1 patient population worldwide are women (McLaurin et al., 2017). For example, only 23% of HIV-1 patients enrolled in the CHARTER clinical study were female (Heaton et al., 2010), and the Multicenter AIDS Cohort Study (MACS) focused solely on the evolution of HAND in male HIV-1 patients (Becker et al., 2015). Given that the two largest epidemiological studies on HAND focus mainly on male HIV-1 seropositive individuals (McLaurin et al., 2017), it is imperative that sex-specific differences in HAND progression be further examined in pre- clinical and clinical studies (Maki et al., 2018; Royal et al., 2016; Scully, 2018). So far, the effects of biological sex on dopaminergic neuronal function (Bertrand et al., 2018; Javadi-Paydar et al., 2017), frontal-subcortical synaptic connectivity (McLaurin et al., 2018b), and signal detection (McLaurin et al., 2017) have been characterized in HIV-1 transgenic rats.
As described above, two labs reported the effects of persistent HIV-1 proteins on learning and memory, and their sex dependence in HIV-1 transgenic rats. However, there are no reports on potential neurocognitive impairments in HIV-Tg26 mice. In this study, we aimed to test whether HIV-1 Tg26 mice have impairments in learning and memory, as well as reduced hippocampal neurogenesis. We also determined whether these potential changes are significantly different between male and female mice. A comprehensive analysis on general behavioral functioning, cognitive abilities, and adult neurogenesis was performed in male and female middle-aged HIV- 1 Tg26(+/−) mice and their wild-type (WT) littermates. The middle-aged mice may better model human condition because older HIV-1 patients are at a higher risk for cognitive decline than younger HIV-1 positive patients (Fazeli et al., 2014; Maartens et al., 2014; Valcour et al., 2004). Specifically, HIV-1 patients exhibit precocious aging by an average of about 5 years compared to their peers (Gross et al., 2016). Our studies demonstrate sex-related differences in the types and severities of cognitive decline and neurogenic deficits in HIV-1 Tg26(+/−) mice, compared to WT littermates.
MATERIALS AND METHODS
Transgenic Mice
HIV-1 Tg26 transgenic mice backcrossed onto a pure C57BL/6J background were utilized in this study (Dickie et al., 1991). Previous studies have demonstrated that Tg26 mice generated on the FV/B background developed aberrant kidney pathology. As a result, most mice were moribund between 2 and 6 months of age (Dickie et al., 1991; Kopp et al., 1992). Since Tg26 mice on the C57BL/6J background do not develop kidney disease and have longer life expectancies (Gharavi et al., 2004; Mallipattu et al., 2013; Zhong et al., 2005), we generated Tg26 mice on a complete C57BL/6J background by backcrossing FVB/N-Tg(HIV)26Aln/PkltJ mice (Jackson Lab, #022354) with C57BL/6J mice (Jackson Lab, #000664) for at least 8 generations. This same backcrossing procedure was performed in a similar manner with HIV-1 transgenic rats to prolong their life expectancies (McLaurin et al., 2018a). Throughout the study, the Tg26 mice were maintained as heterozygotes (+/−), since Tg26 homozygous (+/+) mice rarely survive to weaning (Dickie et al., 1991). For this study, the Tg26(+/−) mice and their wild-type (WT) littermates were aged out to 8–10 months old, since most patients on ART have longer life expectancies, and are more susceptible to the milder forms of HAND (Smit et al., 2015; Smith et al., 2012). Both sexes were analyzed for behavioral and neurogenic changes. All procedures involving mice were in compliance with the 2018 American Veterinary Medical Association Guidelines and the Temple University Institutional Animal Care and Use Committee (IACUC).
Mouse Neurobehavioral Testing
SHIRPA Screen:
The SHIRPA (SmithKline Beecham, Harwell, Imperial College, Royal London Hospital Phenotype Assessment) was developed in 1997 as a quick screen for any abnormal phenotypes in mice and rats. There are three specific screens for these mice, each with increasing levels of complexity (Brooks and Dunnett, 2009; Rogers et al., 1997). The first behavioral screen examined superficial behavioral and morphological mouse characteristics. The second screen examined specific sensorimotor responses, and the third screen assessed responsiveness to external stimuli as well as more complex motor functions (Brooks and Dunnett, 2009).
Contextual and Cued Fear Conditioning:
Fear conditioning studies were conducted in a previously described manner (Di Meco et al., 2016; Joshi et al., 2013; Joshi et al., 2014). All testing was conducted in a fear conditioning chamber with black methacrylate walls, a speaker, and a metal grid floor (Startle and Fear Combined System, Harvard Apparatus, Holliston, MA, USA), and was carried out over 2 days. On day 1, mice were placed in the conditioning chamber for a total of 6 minutes. Two minutes into the testing, a 100-dB sound was administered for thirty seconds before the mouse received a 0.5-mA foot shock for 10 seconds. This sound/foot shock administration was repeated at the 3 and 4-minute marks. After the 6 minutes elapsed, the mouse was taken out of the chamber. The next day, the mice were tested for contextual and cued recall. During the contextual recall phase of the test, the mouse was placed back into the testing chamber for 5 minutes, during which no sound tones or foot shocks were administered. The cued recall phase of the test took place two hours later. During this time, the wall colors were changed from black to white, and a vanilla extract scent was placed in the chamber. The mouse was placed back into this chamber for 6 minutes. The same 100-dB tone was administered for 30 seconds at the 2, 3, and 4-minute marks without any foot shocks. The percentage of time the mouse spent freezing was used as a metric for analyzing the contextual and cued response to these aversive stimuli.
Barnes Maze:
The Barnes Maze was developed as a dry-land alternative to the Morris Water Maze to assess spatial memory acquisition and retention in both rats and mice (Barnes, 1979; Rosenfeld and Ferguson, 2014). Because there is no strong aversive stimulus on the rodents that could confound the results (eg. swimming), this is a more ideal alternative to spatial memory testing for our purposes (Holscher, 1999; Miyakawa et al., 2001). A commercially available Barnes maze with 20 holes around the periphery was utilized (Stoelting Co. Cat.# 60170). The maze itself was isolated from the rest of the room with black curtains to exclude any external cues which could confound the results (Barbe et al., 2016). Before the 4 days of acquisition training, a habituation phase took place, during which the mouse was placed onto the maze and given 1 minute to find the escape hole. If the mouse failed, the experimenter gently guided the mouse to the escape hole and sequestered it there for 1 minute. The acquisition trials were performed after the habituation phase. During these trials, the mouse was placed on the maze, and given 5 minutes to explore. If the mouse found the escape hole before 5 minutes elapsed, the trial was ended. After the mouse found the escape hole, it was kept there for 1 minute, while the maze was cleaned with 70% isopropanol. These trials were performed 4 times per day with each mouse (Harrison et al., 2006), with an inter-trial interval of 3 minutes (Barbe et al., 2016). On day 5, the short-term memory retention test was performed. During this time, the escape hole was blocked, and the mouse explored the maze for 5 minutes. The long-term memory retention test was performed 13 days after the habituation day, which was considered the day 14 retention. Mouse movements were tracked by an overhead CCD monochrome camera and analyzed with ANY-Maze Tracking Software (RRID: SCR_014289) as described previously (Barbe et al., 2016).
Immunohistochemistry/Hippocampal Neural Lineage Analysis
For immunohistochemistry, mice were euthanized with an overdose of pentobarbital solution and transcardially perfused with 4% paraformaldehyde. The brains were dissected, post-fixed overnight in the same fixative, and cryopreserved with buffered 30% sucrose. Each coronal brain section at a 40 μm thickness was collected in a serial manner, and stored at −20°C. Then standard immunofluorescent staining was performed. Briefly, free-floating brain sections were washed 3 times with 0.5% Triton X-100/TBS and blocked for 30 minutes in blocking buffer containing 2% BSA in TBS with 0.5% Triton X-100. Then, primary antibodies were added and the sections were incubated overnight at 4°C. After 3 washes, the brain sections were treated with the corresponding Alexa fluorophore-conjugated secondary antibodies in blocking buffer for 1 hour at room temperature. After washing and DAPI counterstaining (Sigma Aldrich Cat. # D9542), brain sections were mounted onto glass microscope slides and coverslipped with Fluoroshield (Sigma Aldrich Cat. # F6182). Image acquisition and analysis was performed with the Leica SP8 confocal system. The following primary antibodies were used: chicken anti-Glial fibrillary acidic protein (GFAP, 1:500, Aves Labs Cat. # GFAP, RRID: AB_2313547), Goat Sox2 (1:250, Santa Cruz Biotechnology Cat. # sc-54517, RRID: AB_2195807), rabbit anti-Ki67 (1:500, Abcam Cat. # ab92353, RRID: AB_2049848), and goat anti-Doublecortin (DCX, 1:500, Santa Cruz Biotechnology Cat. # sc-8066, RRID: AB_2088494).
To assess in vivo hippocampal neurogenic dynamics, specific antibody combinations were used to label different cellular types. Generally, GFAP and Sox2 co-localization is used to histologically delineate neural stem cells (NSCs) while Sox2 immunoreactivity only labels neural progenitor cells (NPCs) (Beckervordersandforth et al., 2017). Additional Ki67 expression would help distinguish quiescent NSCs from the actively dividing NSCs (Boldrini et al., 2018; Encinas et al., 2011; von Bohlen Und Halbach, 2007). Doublecortin (DCX) is a microtubule- associated protein that is prominently expressed in neuroblasts, and becomes lost once the neuroblast terminally differentiates into a neuron, which usually expresses NeuN and βIII- tubulin. Quiescent NSCs (qNSCs), actively proliferating NSCs (aNSCs), NPCs, and neuroblasts were quantified in an unbiased stereological manner as described previously (Beckervordersandforth et al., 2017; Fan et al., 2016; Lee et al., 2011).
Statistical Analysis
Sample sizes and sexes for all mice used in these studies are listed in either the results, figures, or the figure legends. The SHIRPA data, fear conditioning data, and hippocampal neurogenesis data sets were analyzed with a one-way ANOVA, followed by a Tukey multiple comparison post-hoc test for statistical significance. For the Barnes Maze behavioral data sets, a two-way ANOVA with linear mixed-effects models were used to compare genotype differences by fitting all repeated measures by sex. The Barnes maze statistical analyses were separated by the acquisition phases (to analyze learning ability), and the retention probe phases (to analyze short and long-term memory). All statistical tests were performed using GraphPad Prism 6.0 Software (RRID: SCR_002798) and SAS 9.4 (SAS Institute Inc., Cary, NC). Thresholds for statistical significance were set at p < 0.05, < 0.01, and < 0.001.
RESULTS
No Overt Morphological and Reflexive Differences Were Observed in HIV-1 Tg26(+/−) Mice
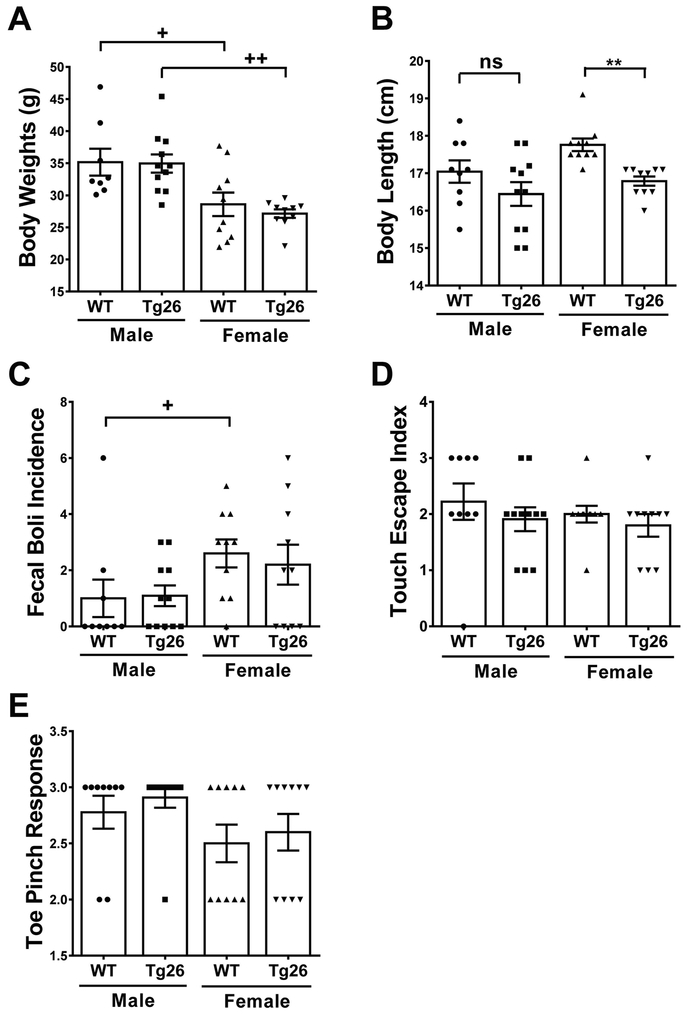
To assess any potential differences in general behavioral functions between WT and Tg26(+/−) mice, a SHIRPA screening was performed when the mice were at the age of 8 months old. Morphological screening showed that male mice had significantly higher body mass than female mice, regardless of genotype (F3,35 = 7.39, p = 0.0006) (Figure 1A). A significant difference was observed in body lengths among male and female WT and Tg26(+/−) mice (F3,36 = 5.43, p = 0.0035) (Figure 1B). Further post-hoc analysis revealed that only female Tg26(+/−) mice had significant reductions in body length, when compared to their female WT littermates (q = 3.993, p < 0.05). These results suggest that the Tg26(+/−) genotype had no effect on body weights in both sexes, but reduced body lengths in female mice. Further screening for physiological function showed that male and female Tg26(+/−) mice exhibited similar gastrointestinal motilities, since the number of fecal boli produced in a span of three minutes was similar between genotypes (F3,36 = 1.991, p = 0.13) (Figure 1C). Finally, male and female Tg26(+/−) mice had similar touch escape responses (F3,36 = 0.60, p = 0.62) (Figure 1D) and toe pinch responses (F3,36 = 1.69, p = 0.62) (Figure 1E) when compared to their sex-matched WT littermates.
Figure 1. HIV-1 Tg26(+/−) transgenic mice do not manifest any significant abnormalities in physiological and reflexive functions.
Before learning and memory testing, wild-type (WT) and Tg26(+/−) mice were screened for any morphological, sensorimotor, and reflexive differences. Male mice had a larger body mass than female mice (A), yet only female Tg26(+/−) mice had diminished body length (B) when compared to their female WT littermates. Defecation incidence (C), touch escape responses (D), and toe pinch responses (E) remained similar, between genotype and biological sex. Data is presented as the Mean ± SEM from 8 to 11 mice per genotype/sex. ** p < 0.01 indicates significant decrease compared to corresponding WT littermate. + p < 0.05 and ++ p < 0.01 indicate significant changes compared to the corresponding males.
Altogether, these sets of data demonstrate that male mice weighed more than female mice, regardless of genotype, and that only female Tg26 mice had diminished body lengths. The relative similarities in gastrointestinal motility and reflexive testing demonstrate that any confounding effects on subsequent neurocognitive testing would be minimized.
HIV-1 Tg26 Mice Exhibited No Differences in Contextual and Cued Fear Responses when Compared to WT Mice
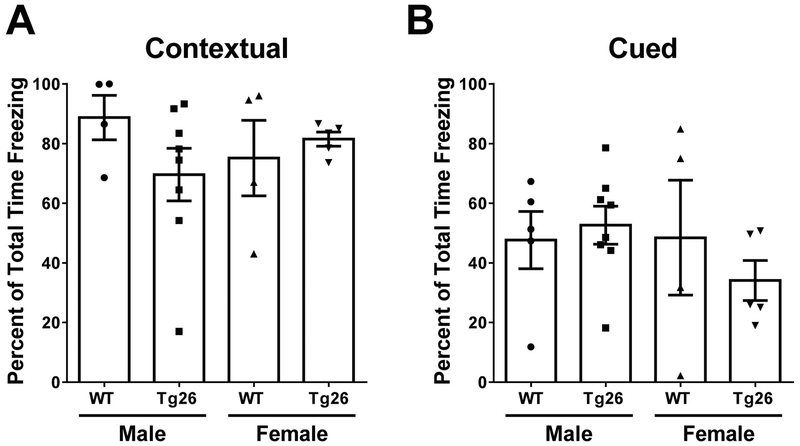
Episodic memory is impaired during HIV-1 infection, affecting up to 60% of all HIV-1 patients (Rippeth et al., 2004). In fact, deficits in episodic memory function have been shown to be one of the most sensitive predictors of HAND progression (Carey et al., 2004). To model any changes in episodic memory in our Tg26(+/−) mouse model, a contextual and cued fear conditioning paradigm was employed (Maren et al., 2013) on middle-aged (8 months old) WT and Tg26(+/−) mice. During the contextual and cued fear conditioning tests, percent of time spent freezing was used as a metric for determining how the mice learned in their environment (Di Meco et al., 2016; Joshi et al., 2013; Joshi et al., 2014). There were no observable differences in contextual fear conditioning responses (F3,17 = 0.89, p = 0.47) (Figure 2A) or cued fear conditioning responses (F3,18 = 0.69, p = 0.57) (Figure 2B) between male or female WT and Tg26(+/−) mice. These results suggest that Tg26(+/−) mice do not show any significant abnormalities in episodic memory function compared to WT mice.
Figure 2. Tg26(+/−) mice exhibit similar contextual and cued fear conditioning responses as WT mice.
Eight-month old male and female mice were tested for percent of total time spent on freezing during a contextual (A) and cued (B) fear conditioning paradigm. Data is represented as the Mean ± SEM from 4 to 8 mice per group.
HIV-1 Tg26(+/−) Mice Demonstrated Sex-Specific Deficits in Spatial Memory Acquisition, Short Term Memory Retention, and Long-Term Memory Retention
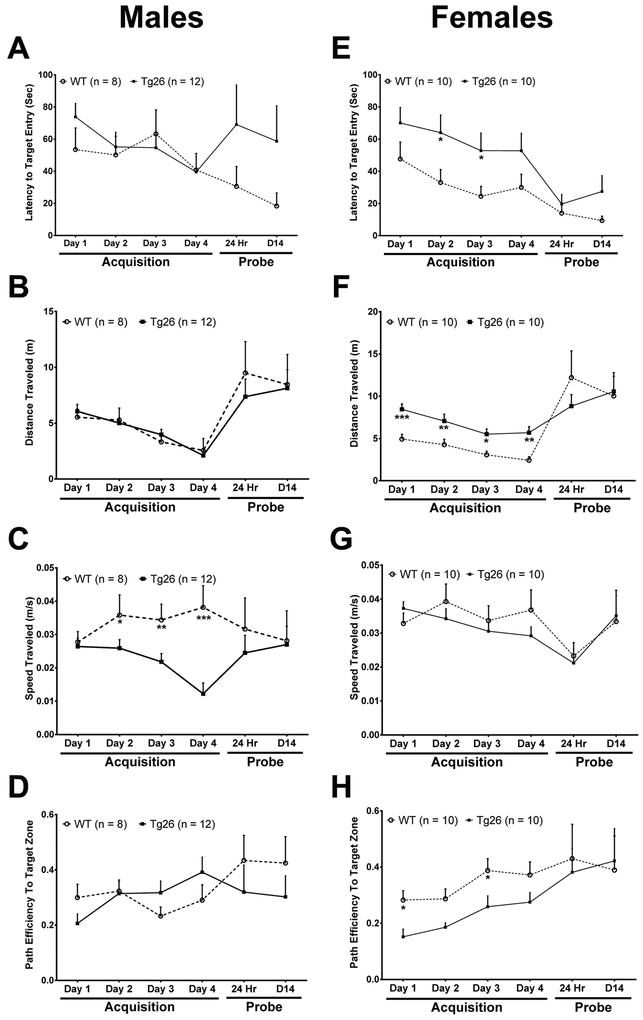
In addition to episodic memory, spatial learning and memory functions have been linked to hippocampal neurogenesis (Deng et al., 2010). To assess any possible changes in spatial learning and memory in middle-aged (9–10 month old) Tg26(+/−) mice, the Barnes maze testing paradigm was employed (Barbe et al., 2016). During the acquisition phase, male Tg26(+/−) mice did not show any statistically significant differences in the primary latency to enter the target hole (Figure 3A) (F1,278 = 0.26; p = 0.61) as compared with male WT littermates. The distance traveled on the maze also did not differ between WT and Tg26(+/−) mice (Figure 3B) (F1,310 = 0.06; p = 0.81). Interestingly, male Tg26(+/−) mice traveled significantly slower on the maze during the acquisition period than male WT mice (Figure 3C) (F1,267 = 18.07, p < 0.0001). Further assessment of the path efficiency to the target zone validated the similarities between WT and Tg26(+/−) mice during the acquisition phase of testing (Figure 3D) (F1,233 = 0.44; p = 0.51). These sets of data demonstrate that male Tg26(+/−) mice did not show any significant differences in primary latency to initial target entry, total distance traveled, or path efficiency to target entry during the memory acquisition phase. However, the observation that male Tg26(+/−) mice moved significantly slower raises the possibility that male Tg26(+/−) mice exhibit a higher level of uncertainty when deciding to enter the target hole.
Figure 3. Female Tg26(+/−) mice exhibit more robust spatial learning impairments during a Barnes maze testing paradigm.
The primary latency (A, E) to enter the target hole was measured during the acquisition and probe phases of testing to assess for spatial memory acquisition and retention between middle-aged (9–10 months old) WT and Tg26(+/−) mice. Additionally, total distance traveled on the maze (B, F), running speed (C, G) and path efficiency (D, H) to initial target hole entry was measured during the acquisition and probe phases of testing. Data is presented as the Mean ± SEM of 8–12 animals per genotype/sex. *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant changes compared to the corresponding WT littermate.
In contrast, female Tg26(+/−) mice exhibited a robust deficit in the primary latency to enter the target hole during the acquisition phase (Figure 3E) (F1,287 = 14.79; p = 0.0001). Further post hoc linear mixed-effects modeling testing revealed a significant increase in their latency to enter the target on day 3 (t86 = 2.04, p = 0.04), while on day 2 (t86 = 1.94 p = 0.06), trending tendencies were observed. The robust deficits in latency to target entry during the acquisition phase of training coincided with a significantly longer distance traveled on the maze (Figure 3F) (F1,316 = 8.13; p < 0.0001). Linear mixed-effect modeling revealed that female Tg26(+/−) mice traveled longer distances than their female WT counterparts on day 1 (t92 = 3.11, p = 0.0025), 2 (t92 = 2.82, p = 0.006), 3 (t92 = 2.23, p = 0.03), and 4 (t92 = 2.95, p = 0.004) of acquisition training. However. female Tg26(+/−) mice traveled at similar speeds as female WT mice on the maze (Figure 3G) (F1,316 = 1.12, p = 0.29). With similar movement speeds on the maze, female Tg26(+/−) mice demonstrated significant decreases in path efficiency to the target zone (Figure 3H) (F1,285 = 5.24; p = 0.0016). On day 1 (t84 = −2.16, p = 0.03) and 3 (t84 = −2.1, p = 0.04), significant deficits in path efficiency to the target zone were the most robust, while insignificant trends were observed on acquisition day 2 (t84 = −1.92, p = 0.06) and 4 (t84 = −1.85, p = 0.07) (Figure 3H). Altogether, these sets of data demonstrate that female Tg26(+/−) mice have robust deficits in spatial learning ability, which was not observed with male Tg26(+/−) mice.
During the short (24 hour) and long-term (14 day) probe phases of testing, there were no differences in the latency to enter the target hole, total distance traveled, and path efficiencies for both male and female Tg26(+/−) mice, when compared to their WT littermates. Male Tg26(+/−) mice showed slight, yet statistically insignificant differences in short and long-term memory retention during the probe testing session (Figure 3A) (F1,32 = 3.41; p = 0.07). This similarity in primary latency correlated with similar distances traveled on the maze as well (Figure 3B) (F1,36 = 0.34; p = 0.56). Finally, male Tg26(+/−) mice and male WT mice moved at around the same speed on the maze (Figure 3C) (F1,36 = 0.35, p = 0.56), and demonstrated similar path efficiencies to the target zones during the probe phase of testing (Figure 3D) (F1,30 = 1.64; p = 0.21). Like the male Tg26(+/−) mice, female Tg26(+/−) mice revealed statistically insignificant differences in latency to initial target entry during the short or long-term probe phases of the test (Figure 3E) (F1,41 = 3.17; p = 0.08). Additionally, no significant differences in the amount of distance traveled on the Barnes maze (Figure 3F) (F1,42 = 0.40; p = 0.53), speed traveled (Figure 3G) (F1,37 = 0, p = 0.97) or the target path efficiencies (Figure 4H) (F1,30 = 1.64; p = 0.21) were observed between female WT and Tg26(+/−) mice.
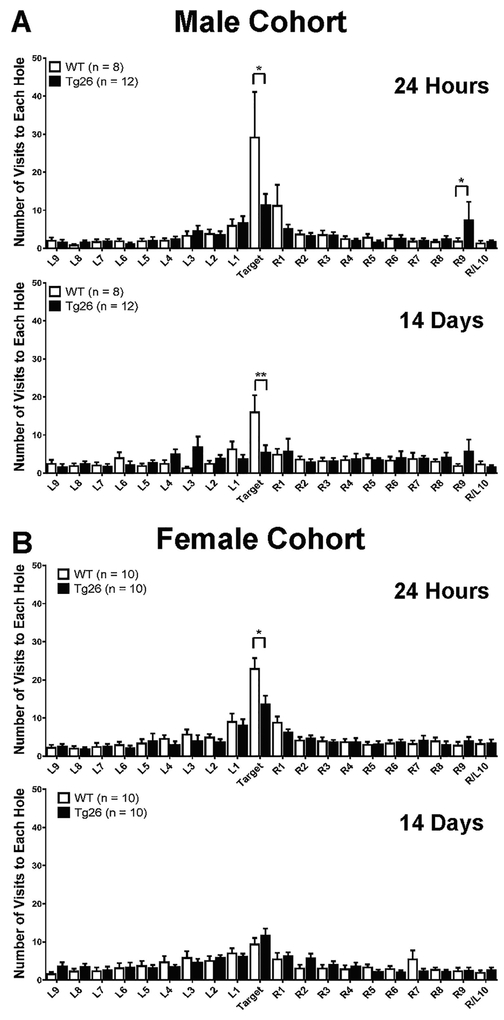
Figure 4. Male Tg26(+/−) mice demonstrate short-term and long-term memory retention deficits (A), while female Tg26(+/−) mice specifically manifest short-term memory retention impairments (B).
The number of times WT or Tg26(+/−) mice (9–10 month old) visited each hole on the Barnes maze was recorded during the 24-hour probe phase and the 14-day probe phase to assess whether the mice could remember where the target hole was located. Data is presented as the Mean ± SEM of 8–12 animals per genotype/sex. * p < 0.05, ***p < 0.001 indicate significant changes compared to the corresponding WT littermate.
To assess whether Tg26(+/−) mice recognized the target hole in relation to the other holes on the maze, the number of times the mice entered each hole was measured during the short term (24 hours) and long term (14 days) probe sessions. This reference error metric is a more robust and reliable method to assess hippocampal-dependent spatial memory (Attar et al., 2013; Harrison et al., 2006). During the 24-hour probe testing session, two-way ANOVA analysis revealed a statistically significant interaction between genotype and the hole visited (Figure 4A) (F19,360 = 7.03; p = 0.0068) for the male cohort of mice. Further post hoc linear mixed-effects modeling analysis showed that Tg26(+/−) mice entered the target hole significantly less than WT mice, indicating a deficiency in short-term recognition of the target hole (t342 = 2.43, p = 0.02). Interestingly, male Tg26(+/−) mice entered the R9 hole more frequently than their male WT littermates (t342 = 2.31, p = 0.02), indicating that male Tg26(+/−) mice made more errors in trying to recognize the target hole. During the 14-day probe testing session, there was a marginally significant interaction between genotype and the hole visited (F19,359 = 1.5; p = 0.08) for the male cohort of mice (Figure 4B). Further linear mixed-effects modeling revealed that male Tg26(+/−) mice entered the target hole much less than male WT mice (t323 = −3.28, p = 0.0012), further supporting the conclusion that male Tg26(+/−) mice demonstrated significant impairments in long-term memory. Likewise, female Tg26(+/−) mice had a statistically significant deficit only in the short-term memory to recognize the target hole compared to female WT mice (Figure 4C) (F1,338 = 4.36; p = 0.04; post hoc linear mixed-effects modeling t342 = −2.11, p = 0.04). No overt long-term memory retention impairments were observed between female WT and Tg26(+/−) mice, as they seemed to recognize and enter the target hole in a relatively similar manner (Figure 4D) (F19,340 = 0.81; p = 0.69).
Altogether, the Barnes Maze test results demonstrate that female, but not male, Tg26(+/−) mice manifested spatial memory acquisition deficits (indicative of a learning disability). Male Tg26(+/−) mice demonstrated short and long-term memory deficits, while only female Tg26(+/−) mice had short-term memory deficits.
Sex-Specific Hippocampal Neurogenic Deficits Correlated to Cognitive Abnormalities
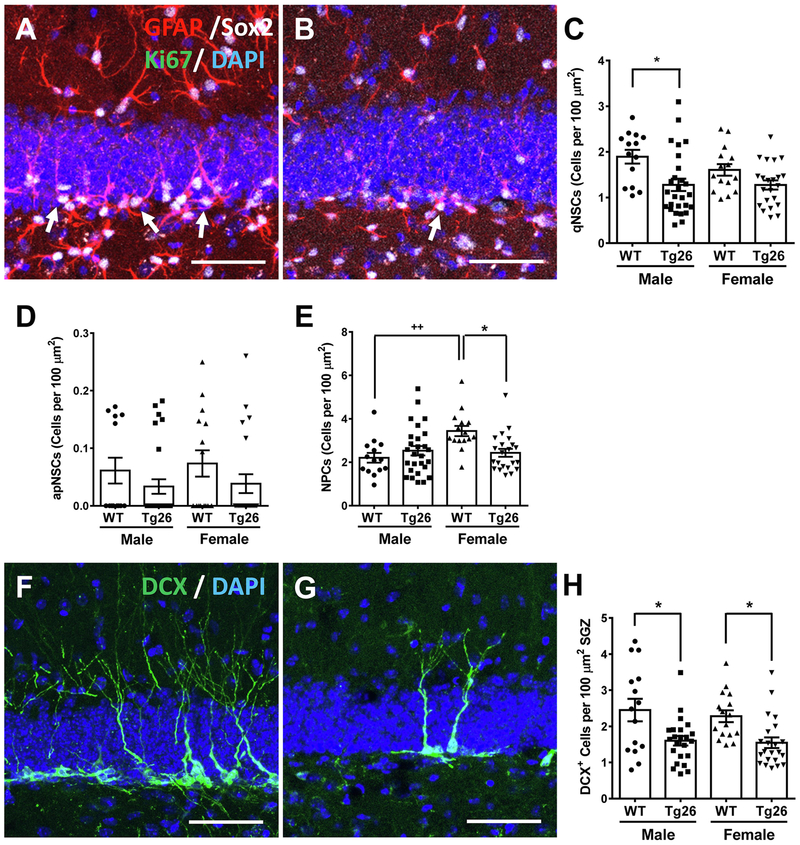
The results from our behavioral testing battery demonstrated differences in the degrees of cognitive decline between male and female Tg26(+/−) mice, when compared to WT mice. These changes could possibly be attributed to differing hippocampal neurogenic dynamics. To assess this potential correlation, in vivo hippocampal neurogenic analysis was performed in mice that were tested in the Barnes Maze. Immunohistochemistry with GFAP, Sox2, and Ki67 was performed in both WT (Figure 5A) and Tg26(+/−) (Figure 5B) mice to assess the levels of qNSCs, aNSCs, and NPCs (Boldrini et al., 2018; Encinas et al., 2011). Interestingly, the number of qNSCs was significantly different between the four groups of mice compared (F3,74 = 4.53, p = 0.0057) (Figure 5C). Further analysis revealed that only male Tg26(+/−) mice had significant reductions in qNSCs (q = 4.56, p < 0.05). Female Tg26(+/−) mice had a trending, but statistically insignificant decrease in qNSCs (q = 2.39, no significance). Both male and female Tg26(+/−) mice had low, but similar levels of proliferating NSCs (F3,73 = 1.13, p = 0.34) (Figure 5D). NPC analysis revealed a statistically significant difference between groups (F3,74 = 4.77, p = 0.0043) (Figure 5E). Further post-hoc analysis revealed that female WT mice had more NPCs than male WT mice (q = 4.84, p < 0.01). However, the Tg26(+/−) genotype only had an effect on female mice (q = 4.34, p < 0.05), and not on male mice (q = 1.44, no significance).
Figure 5. Middle-aged Tg26(+/−) mice exhibit sex-specific deficits in hippocampal neurogenesis.
A-B, Representative confocal images showing more GFAP+/Sox2+ qNSCs (white arrows) in WT SGZs (A) than Tg26(+/−) SGZs (B), with the corresponding stereological analysis for qNSCs, (C), aNSCs (D), and NPCs (E). Representative confocal images showing more DCX+ neuroblasts in WT SGZs (F) than Tg26(+/−) SGZs (G), with the stereological quantification for neuroblasts (H). Data is presented as the Mean ± SEM of indicated positive cells per SGZ from 2 to 4 mice per genotype/sex, with 6 to 8 hippocampal sections per mouse being utilized for quantification. *p < 0.05, ++ p < 0.01 indicates significant increase in female mice compared to the corresponding male mice. Scale bars in A, B, F, G: 50 μm.
To assess neuronal differentiation in these aged mice, subgranular zones (SGZs) from WT (Figure 5F) and Tg26(+/−) (Figure 5G) mice were analyzed for DCX immunoreactivity (specific for neuroblasts and immature neurons). Quantification revealed a significant difference in the number of neuroblasts formed (F3,72 = 6.17, p = 0.0009) (Figure 5H). Further statistical analysis revealed that both male Tg26(+/−) mice (q = 4.56, p < 0.05) and female Tg26(+/−) mice (q = 3.98, p < 0.05) had deficits in neuroblast formation. Taken together, these results suggest that chronic HIV-1 infection may induce an accelerated exhaustion of the NSC pools, and cause significant deficits in neuronal differentiation during adult neurogenesis. However, only female Tg26(+/−) mice had an impairment in NPC formation.
DISCUSSION
In the ART era, HIV-1 infection continues to elicit mild to moderate forms of HAND (Ellis et al., 1997; Heaton et al., 2010). Several mechanisms have been characterized that explain some of the pathogenesis underlying HAND. Recently, compromised adult neurogenic changes have been proposed as a newer mechanism for HIV-induced central nervous system (CNS) injury (Balinang et al., 2017; Fan et al., 2016; Ferrell and Giunta, 2014; Putatunda et al., 2018; Saylor et al., 2016). Here, our studies demonstrate that middle-aged Tg26(+/−) mice have sex-specific deficits in several behavioral modalities. Specifically, the highlights include: (1) male Tg26(+/−) mice demonstrated mild short and long-term spatial memory deficits; (2) female Tg26(+/−) mice manifested spatial learning and mild short-term spatial memory impairments; (3) accelerated depletion of the hippocampal NSC pool and the neuroblast pool in middle-aged male and female Tg26(+/−) mice; and (4) male Tg26(+/−) mice displayed an increased exhaustion of the NSC pool, while female Tg26(+/−) mice exhibited a dramatic reduction of the pre-existing higher levels of NPCs. To our knowledge, these studies are the first of their kind to characterize sex- specific neurobehavioral deficits in a novel mouse model of chronic stress from multiple HIV-1 viral proteins at an older age. These findings highlight the importance of biological sex differences in HIV-1 pathogenesis and therapeutic evaluation (Burlacu et al., 2018; Griesbeck et al., 2016; McLaurin et al., 2017; McLaurin et al., 2018b; Qiao et al., 2019; Scully, 2018; Ziegler and Altfeld, 2016; Ziegler and Altfeld, 2017).
Currently, most studies assessing neurocognitive dysfunction induced by HIV-1 Tat or gp120 in GFAP-driven transgenic mouse models have focused on the males (Fitting et al., 2014; Hahn et al., 2015; Kesby et al., 2015; Maung et al., 2014). One study in GFAP-Tat transgenic mice demonstrated that male mice display decreased forelimb grip strength and increased anxiety-like behavior when compared to their female counterparts. Female GFAP-Tat transgenic mice also had less astrogliosis and preserved dendritic spine density compared to males, implying that male GFAP-Tat transgenic mice are more vulnerable to CNS damage than female mice (Hahn et al., 2015).
Our initial SHIRPA screen revealed that both male and female Tg26(+/−) mice did not show any overt gastrointestinal or reflexive deficits, when compared to their WT biological sex-matched littermates. This preservation in overall physiological function was consistent with the findings in both male and female HIV-1 transgenic rats (McLaurin et al., 2017; Moran et al., 2012; Moran et al., 2013b; Roscoe et al., 2014). This allowed us to confidently proceed with neurobehavioral testing since other physiological processes which could confound subsequent analysis were likely minimized.
Episodic memory has been shown to be impaired due to HIV-1 infection (Carey et al., 2004; Rippeth et al., 2004). To model possible changes in episodic memory in the Tg26(+/−) model, contextual and cued fear conditioning was employed. We did not observe any significant deficits in fear conditioning in HIV-1 Tg26(+/−) mice, regardless of age or sex. These results are in direct contrast to the data reported in male and female HIV-1 transgenic rats (McLaurin et al., 2018a) and in male doxycycline-inducible GFAP-Tat transgenic mice (Fitting et al., 2013). The discrepancy between our results in the Tg26 mouse and the HIV-1 transgenic rat may be attributed to the differences in the behavioral paradigms utilized. For example, the HIV-1 transgenic rats were assessed for episodic memory function by intrasession habituation in an open field chamber (McLaurin et al., 2018a), which may elicit differing degrees of responses when compared to a contextual and cued fear conditioning paradigm. Additionally, rats and mice with the same transgene may have differences in behavioral responses due to intrinsic species- specific differences (Bonthuis et al., 2010). Finally, doxycycline induction in the GFAP-Tat mice may induce higher levels of Tat expression in the CNS than that observed in HIV-1 patients undergoing ART. Therefore, it is not surprising that doxycycline-inducible GFAP-Tat transgenic mice exhibit more robust fear conditioning deficits than Tg26(+/−) mice.
Further behavioral testing with a Barnes maze paradigm demonstrated that male and female Tg26(+/−) mice showed differing learning and memory impairments, which is consistent with sex-specific behavioral differences in the HIV-1 transgenic rat (McLaurin et al., 2018a). First, female Tg26(+/−) mice exhibited robust deficits in memory acquisition, while male Tg26(+/−) mice showed relatively preserved learning abilities. Second, female Tg26(+/−) mice had no deficits in long-term memory in contrast to male Tg26(+/−) mice, although male and female Tg26(+/−) mice demonstrated short-term impairments in finding the specific target hole. To date, most neurobehavioral studies assessing fear conditioning or spatial learning/memory function in doxycycline-inducible GFAP-Tat mice, GFAP-gp120 mice, and HIV-1 transgenic rats have only been conducted using male rodents (Carey et al., 2012; Fitting et al., 2014; Kesby et al., 2015; Maung et al., 2014; Vigorito et al., 2015). Given that sex-specific differences in HIV-1/HAND incidence have been documented (Griesbeck et al., 2016; Hagen and Altfeld, 2016; Qiao et al., 2019; Scully, 2018), it is imperative that any possible sex-specific differences in learning and memory deficits be fully elucidated in HIV-1 animal models (McLaurin et al., 2018a).
The cellular mechanism behind the sex-specific neurobehavioral deficits caused by HIV-1 or viral proteins remains poorly understood. Here, we explored the potential contribution of adult neurogenesis, because this is a process affected in HIV-1 infection (Balinang et al., 2017; Fan et al., 2016; Kaul, 2008; Lee et al., 2011; Putatunda et al., 2018; Schwartz et al., 2007). Hippocampal neurogenesis is strongly linked to spatial learning and memory function (Epp et al., 2013; van Praag et al., 1999). The behavioral paradigms utilized in this study were mainly dependent on hippocampal neurogenic function. For example, contextual and cued fear conditioning tests have been extensively utilized to correlate adult hippocampal neurogenic impairments with potential neurocognitive and emotional disorders (Kitamura et al., 2009; Seo et al., 2015). Sex-specific differences in adult neurogenesis and its correlation with cognition have been extensively documented (Galea et al., 2013; Heberden, 2017; Ponti et al., 2018; Yagi and Galea, 2019), although the overall conclusion remains controversial. Generally, males outperform females on hippocampus-dependent tasks (Chow et al., 2013; Yagi et al., 2016), and females are more likely to experience greater cognitive decline in Alzheimer’s disease and depression (Yagi and Galea, 2019). Some studies showed significant sex-dependent effects on NSC proliferation (Tzeng et al., 2014) but others failed to find sex differences in mice (Amrein et al., 2004; Lagace et al., 2007). For the survival of adult newborn neurons in the hippocampus, no significant sex differences were identified in most studies (Amrein et al., 2004; Barker and Galea, 2008; Lagace et al., 2007; Lee et al., 2014), though some studies showed better survival of immature neurons in males (Dalla et al., 2009; Hillerer et al., 2013). In this current study, male Tg26(+/−) mice exhibited more prolonged exhaustion of quiescent NSC pool than female mice while significant reduction of NPCs occurred only in female Tg26(+/−) mice.
Several explanations could account for the sex-specific differences in HIV-induced neurocognitive impairment and neurogenic deficits (McLaurin et al., 2018a; McLaurin et al., 2017; Scully, 2018). For example, subtle differences in the levels of neuroinflammation between males and females may account for differences in behavioral phenotypes (Potter et al., 2019; Scully, 2018). Genetic differences in sex chromosome-encoded genes (Dunford et al., 2017; van Lunzen and Altfeld, 2014) and host restriction factors (Szaniawski et al., 2019) may also potentially affect HAND pathogenesis. Different levels of sex hormones during the estrous cycle could possibly influence the observed sex difference in HIV-1 Tg26(+/−) mice. Although the cycling of sex hormones may mainly affect HIV-1 infection due to morphological changes of vaginal mucosa, higher estrogen levels may induce higher levels of HIV-1 transcriptional suppression or interferon-alpha production (Scully, 2018). Tracking estrous cycles or ovariectomies in HIV-1 Tg26(+/−) mice may help to delineate the effects of sex hormones on neurocognition and neurogenesis.
While the HIV-1 Tg26(+/−) mouse is an elegant model that closely mimics ART-controlled and aviremic HIV-1 patients, several caveats remain. For example, proviral gene expression is ubiquitous across all cellular types (Dickie et al., 1991; Gharavi et al., 2004; Kopp et al., 1992). However, many studies have demonstrated that HIV-1 infection and viral gene integration is limited to only a few cellular reservoirs, notably CD4+ T-lymphocytes and macrophages (Saylor et al., 2016). Additionally, anywhere between 10 and 20 copies of the replication-deficient proviral genome are present in each cell, which is much higher than the 1–4 proviral copies per cell found in HIV-1 infected patients (Jung et al., 2002). However, even though the virus is replication-deficient in Tg26(+/−) mice, behavioral deficits, renal disease, and cardiovascular pathologies have manifested themselves (Bryant et al., 2018; Cheung et al., 2019; Cheung et al., 2015; Johnstone et al., 2013; Kearns et al., 2017). These results have all been corroborated in the HIV-1 transgenic rat (Lashomb et al., 2009; McLaurin et al., 2019; Vigorito et al., 2015; Vigorito et al., 2007) and the EcoHIV-infected mouse (Gorantla et al., 2012; Gu et al., 2018; Kim et al., 2019; Olson et al., 2018).
In conclusion, our results demonstrate the presence of sex specific cognitive and neurogenic deficits in HIV-1 Tg26(+/−) mice. These findings are consistent with the sex-specific behavioral deficits seen in HIV-1 transgenic rats (McLaurin et al., 2018a; McLaurin et al., 2017) and HIV-1 infected patients (Burlacu et al., 2018). Further studies are needed to assess whether in vivo excision of the HIV-1 proviral genome (Yin et al., 2017) could possibly reverse the cognitive and hippocampal neurogenic deficits observed in HIV-1 Tg26(+/−) mice. This would further solidify the potential link between hippocampal neurogenesis and behavioral outcomes.
Highlights.
Reduced spatial learning ability in female, but not male Tg26(+/−) mice
Short-term and long-term memory deficits in male Tg26(+/−) mice
Short-term, but not long-term memory deficits in female Tg26(+/−) mice
Sex-specific adult neurogenic impairments in Tg26(+/−) mice
Acknowledgements
This work was supported by National Institutes of Health (R01DK075964 and R01AI145034 to W.H., T32MH079785 to R.P., P30MH092177 to M.F.B. and R01NS086570–01 to S.H.R). This study utilized services offered by core facilities of the Comprehensive NeuroAIDS Center (CNAC, P30MH092177) at Temple University Lewis Katz School of Medicine.
Abbreviations:
- ART
Antiretroviral Therapy
- GFAP
Glial Fibrillary Acidic Protein
- HAND
HIV-1-Associated Neurocognitive Disorders
- LTR
Long Terminal Repeats
- Tg26
HIV-1 Viral DNA Transgenic Founder Line 26
- WT
Wild Type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP, 2004. Marked species and age- dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus 14, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Attar A, Liu T, Chan WT, Hayes J, Nejad M, Lei K, Bitan G, 2013. A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS One 8, e80355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balinang JM, Masvekar RR, Hauser KF, Knapp PE, 2017. Productive infection of human neural progenitor cells by R5 tropic HIV-1: opiate co-exposure heightens infectivity and functional vulnerability. AIDS 31, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Krueger JJ, Loomis R, Otte J, Gordon J, 2016. Memory deficits, gait ataxia and neuronal loss in the hippocampus and cerebellum in mice that are heterozygous for Pur-alpha. Neuroscience 337, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LA, 2008. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience 152, 888–902. [DOI] [PubMed] [Google Scholar]
- Barnes CA, 1979. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, Martin E, Miller EN, Munro CA, Ragin A, Sacktor N, Selnes OA, 2015. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol 44, 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Ebert B, Schaffner I, Moss J, Fiebig C, Shin J, Moore DL, Ghosh L, Trinchero MF, Stockburger C, Friedland K, Steib K, von Wittgenstein J, Keiner S, Redecker C, Holter SM, Xiang W, Wurst W, Jagasia R, Schinder AF, Ming GL, Toni N, Jessberger S, Song H, Lie DC, 2017. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 93, 560–573 e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand SJ, Mactutus CF, Harrod SB, Moran LM, Booze RM, 2018. HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Sci Rep 8, 7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Gurnsey K, Jedema HP, Smith NF, Wang G, Bradberry CW, Wiley CA, 2018. Aged Chinese-origin rhesus macaques infected with SIV develop marked viremia in absence of clinical disease, inflammation or cognitive impairment. Retrovirology 15, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ, 2018. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF, 2010. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol 31, 341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB, 2009. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 10, 519–529. [DOI] [PubMed] [Google Scholar]
- Bryant JL, Guda PR, Asemu G, Subedi R, Ray S, Khalid OS, Shukla V, Patel D, Davis H, Nimmagadda VKC, Makar TK, 2018. Glomerular mitochondrial changes in HIV associated renal injury. Exp Mol Pathol 104, 175–189. [DOI] [PubMed] [Google Scholar]
- Burlacu R, Umlauf A, Luca A, Gianella S, Radoi R, Ruta SM, Marcotte TD, Ene L, Achim CL, 2018. Sex-based differences in neurocognitive functioning in HIV-infected young adults. AIDS 32, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calascibetta F, Micci L, Carnathan D, Lawson B, Vanderford TH, Bosinger SE, Easley K, Chahroudi A, Mackel J, Keele BF, Long S, Lifson J, Paiardini M, Silvestri G, 2016. Antiretroviral Therapy in Simian Immunodeficiency Virus-Infected Sooty Mangabeys: Implications for AIDS Pathogenesis. J Virol 90, 7541–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP, 2012. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, Grant I, Heaton RK, Group H, 2004. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol 18, 234–248. [DOI] [PubMed] [Google Scholar]
- Carroll VA, Lafferty MK, Marchionni L, Bryant JL, Gallo RC, Garzino-Demo A, 2016. Expression of HIV-1 matrix protein p17 and association with B-cell lymphoma in HIV-1 transgenic mice. Proc Natl Acad Sci U S A 113, 13168–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JY, Gordon J, Wang J, Song J, Zhang XQ, Prado FJ, Shanmughapriya S, Rajan S, Tomar D, Tahrir FG, Gupta MK, Knezevic T, Merabova N, Kontos CD, McClung JM, Klotman PE, Madesh M, Khalili K, Feldman AM, 2019. Mitochondrial dysfunction in human immunodeficiency virus-1 transgenic mouse cardiac myocytes. J Cell Physiol 234, 4432–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JY, Gordon J, Wang J, Song J, Zhang XQ, Tilley DG, Gao E, Koch WJ, Rabinowitz J, Klotman PE, Khalili K, Feldman AM, 2015. Cardiac Dysfunction in HIV-1 Transgenic Mouse: Role of Stress and BAG3. Clin Transl Sci 8, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C, Epp JR, Lieblich SE, Barha CK, Galea LA, 2013. Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory. Psychoneuroendocrinology 38, 1236–1250. [DOI] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ, 2009. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci U S A 106, 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH, 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meco A, Joshi YB, Lauretti E, Pratico D, 2016. Maternal dexamethasone exposure ameliorates cognition and tau pathology in the offspring of triple transgenic AD mice. Mol Psychiatry 21, 403–410. [DOI] [PubMed] [Google Scholar]
- Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N, Notkins AL, 1991. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185, 109–119. [DOI] [PubMed] [Google Scholar]
- Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, Sullivan TJ, Hess JM, Gimelbrant AA, Beroukhim R, Lawrence MS, Getz G, Lane AA, 2017. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet 49, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, Heaton RK, Marcotte TD, McCutchan JA, Nelson JA, Abramson I, Thal LJ, Atkinson JH, Wallace MR, Grant I, 1997. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol 54, 416–424. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G, 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Chow C, Galea LA, 2013. Hippocampus-dependent learning influences hippocampal neurogenesis. Front Neurosci 7, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Gao X, Chen J, Liu Y, He JJ, 2016. HIV Tat Impairs Neurogenesis through Functioning As a Notch Ligand and Activation of Notch Signaling Pathway. J Neurosci 36, 11362–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE, 2014. Cognitive Functioning in Adults Aging with HIV: A Cross-Sectional Analysis of Cognitive Subtypes and Influential Factors. J Clin Res HIV AIDS Prev 1, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell D, Giunta B, 2014. The impact of HIV-1 on neurogenesis: implications for HAND. Cell Mol Life Sci 71, 4387–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF, 2013. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Knapp PE, Zou S, Marks WD, Bowers MS, Akbarali HI, Hauser KF, 2014. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na(+) influx, mitochondrial instability, and Ca(2)(+) overload. J Neurosci 34, 12850–12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK, 2013. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 25, 1039–1061. [DOI] [PubMed] [Google Scholar]
- Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D’Agati VD, Klotman PE, Lifton RP, 2004. Mapping a locus for susceptibility to HIV-1- associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci U S A 101, 2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J, 2005. The neuropathogenesis of AIDS. Nat Rev Immunol 5, 69–81. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Poluektova L, Gendelman HE, 2012. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci 35, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck M, Scully E, Altfeld M, 2016. Sex and gender differences in HIV-1 infection. Clin Sci (Lond) 130, 1435–1451. [DOI] [PubMed] [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF, Licon K, Jepsen KL, Khosroheidari M, Morsey BM, Swindells S, Shen H, Ng CT, Flagg K, Chen D, Zhang K, Fox HS, Ideker T, 2016. Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Mol Cell 62, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Kim BH, Chao W, Arancio O, Suh J, Polsky B, McMillan J, Edagwa B, Gendelman HE, Potash MJ, Volsky DJ, 2018. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog 14, e1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen S, Altfeld M, 2016. The X awakens: multifactorial ramifications of sex-specific differences in HIV-1 infection. J Virus Erad 2, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE, 2015. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct 220, 605–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP, 2006. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem 13, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberden C, 2017. Sex steroids and neurogenesis. Biochem Pharmacol 141, 56–62. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Couillard-Despres S, Aigner L, Slattery DA, 2013. Sex-dependent regulation of hippocampal neurogenesis under basal and chronic stress conditions in rats. Hippocampus 23, 476–487. [DOI] [PubMed] [Google Scholar]
- Holscher C, 1999. Stress impairs performance in spatial water maze learning tasks. Behav Brain Res 100, 225–235. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Roscoe RF Jr., Denton AR, Mactutus CF, Booze RM, 2017. HIV-1 and cocaine disrupt dopamine reuptake and medium spiny neurons in female rat striatum. PLoS One 12, e0188404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone DB, Ikizler O, Zhang J, Holzman LB, 2013. Background strain and the differential susceptibility of podocyte-specific deletion of Myh9 on murine models of experimental glomerulosclerosis and HIV nephropathy. PLoS One 8, e67839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Chu J, Pratico D, 2013. Knockout of 5-lipoxygenase prevents dexamethasone-induced tau pathology in 3xTg mice. Aging Cell 12, 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Giannopoulos PF, Chu J, Sperow M, Kirby LG, Abood ME, Pratico D, 2014. Absence of ALOX5 gene prevents stress-induced memory deficits, synaptic dysfunction and tauopathy in a mouse model of Alzheimer’s disease. Hum Mol Genet 23, 6894–6902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jung A, Maier R, Vartanian JP, Bocharov G, Jung V, Fischer U, Meese E, Wain-Hobson S, Meyerhans A, 2002. Recombination: Multiply infected spleen cells in HIV patients. Nature 418, 144. [DOI] [PubMed] [Google Scholar]
- Kaul M, 2008. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci 13, 2484–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA, 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA, 2005. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12 Suppl 1, 878–892. [DOI] [PubMed] [Google Scholar]
- Kearns A, Gordon J, Burdo TH, Qin X, 2017. HIV-1-Associated Atherosclerosis: Unraveling the Missing Link. J Am Coll Cardiol 69, 3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Markou A, Semenova S, Translational Methamphetamine ARCG, 2015. Cognitive deficits associated with combined HIV gp120 expression and chronic methamphetamine exposure in mice. Eur Neuropsychopharmacol 25, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BH, Kelschenbach J, Borjabad A, Hadas E, He H, Potash MJ, Nedelcovych MT, Rais R, Haughey NJ, McArthur JC, Slusher BS, Volsky DJ, 2019. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HAND in EcoHIV-infected mice. AIDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K, 2009. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827. [DOI] [PubMed] [Google Scholar]
- Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE, 1992. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A 89, 1577–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ, 2007. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17, 175–180. [DOI] [PubMed] [Google Scholar]
- Lashomb AL, Vigorito M, Chang SL, 2009. Further characterization of the spatial learning deficit in the human immunodeficiency virus-1 transgenic rat. J Neurovirol 15, 14–24. [DOI] [PubMed] [Google Scholar]
- Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A, 2011. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis 41, 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT, Wainwright SR, Hill MN, Galea LA, Gorzalka BB, 2014. Sex, drugs, and adult neurogenesis: sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity, and amphetamine sensitization. Hippocampus 24, 280–292. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M, 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol 76, 6689–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TC, He JC, Klotman P, 2006. Animal models of HIV-associated nephropathy. Curr Opin Nephrol Hypertens 15, 233–237. [DOI] [PubMed] [Google Scholar]
- Maartens G, Celum C, Lewin SR, 2014. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 384, 258–271. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Springer G, Seaberg EC, Sacktor N, Miller EN, Valcour V, Young MA, Becker JT, Martin EM, Neuropsychology Working Groups of the Women’s Interagency, H.I.V.S., the Multicenter, A.C.S., 2018. Differences in Cognitive Function Between Women and Men With HIV. J Acquir Immune Defic Syndr 79, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallipattu SK, Liu R, Zhong Y, Chen EY, D’Agati V, Kaufman L, Ma’ayan A, Klotman PE, Chuang PY, He JC, 2013. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 83, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14, 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung R, Hoefer MM, Sanchez AB, Sejbuk NE, Medders KE, Desai MK, Catalan IC, Dowling CC, de Rozieres CM, Garden GA, Russo R, Roberts AJ, Williams R, Kaul M, 2014. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J Immunol 193, 1895–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF, 2018a. Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. J Neurovirol 24, 229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF, Fairchild AJ, 2017. Sex Matters: Robust Sex Differences in Signal Detection in the HIV-1 Transgenic Rat. Front Behav Neurosci 11, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Cook AK, Li H, League AF, Mactutus CF, Booze RM, 2018b. Synaptic Connectivity in Medium Spiny Neurons of the Nucleus Accumbens: A Sex-Dependent Mechanism Underlying Apathy in the HIV-1 Transgenic Rat. Front Behav Neurosci 12, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin KA, Li H, Booze RM, Mactutus CF, 2019. Disruption of Timing: NeuroHIV Progression in the Post-cART Era. Sci Rep 9, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN, 2001. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 11, 763–775. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF, 2012. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res 10, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF, 2013a. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol 8, 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF, 2013b. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol 239, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KE, Bade AN, Namminga KL, Potash MJ, Mosley RL, Poluektova LY, Volsky DJ, Gendelman HE, 2018. Persistent EcoHIV infection induces nigral degeneration in 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine-intoxicated mice. J Neurovirol 24, 398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Farinetti A, Marraudino M, Panzica G, Gotti S, 2018. Sex Steroids and Adult Neurogenesis in the Ventricular-Subventricular Zone. Front Endocrinol (Lausanne) 9, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter OV, Giedraitis ME, Johnson CD, Cox MN, Kohman RA, 2019. Young and aged TLR4 deficient mice show sex-dependent enhancements in spatial memory and alterations in interleukin- 1 related genes. Brain Behav Immun 76, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putatunda R, Zhang Y, Li F, Yang XF, Barbe MF, Hu W, 2018. Adult neurogenic deficits in HIV-1 Tg26 transgenic mice. J Neuroinflammation 15, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X, Lin H, Chen X, Ning C, Wang K, Shen W, Xu X, Xu X, Liu X, He N, Ding Y, 2019. Sex differences in neurocognitive screening among adults living with HIV in China. J Neurovirol. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J, 2001. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A 98, 9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WC, Ibrahim WG, Kim SJ, Denaro F, Casas R, Lee DE, Maric D, Hammoud DA, 2016. Characterization of neuropathology in the HIV-1 transgenic rat at different ages. J Neuroimmunol 292, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, Group H, 2004. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10, 1–14. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE, 1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8, 711–713. [DOI] [PubMed] [Google Scholar]
- Roscoe RF Jr., Mactutus CF, Booze RM, 2014. HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol 9, 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Ferguson SA, 2014. Barnes maze testing strategies with small and large rodent models. J Vis Exp, e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA, 2016. Associations between Cognition, Gender and Monocyte Activation among HIV Infected Individuals in Nigeria. PLoS One 11, e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC, 2016. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12, 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO, 2007. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol 13, 274–283. [DOI] [PubMed] [Google Scholar]
- Scully EP, 2018. Sex Differences in HIV Infection. Curr HIV/AIDS Rep 15, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR, 2015. Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci 35, 11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, de Wolf F, Hallett TB, cohort A.o., 2015. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 15, 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H, 2012. Premature and accelerated aging: HIV or HAART? Front Genet 3, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaniawski MA, Spivak AM, Bosque A, Planelles V, 2019. Sex Influences SAMHD1 Activity and Susceptibility to Human Immunodeficiency Virus-1 in Primary Human Macrophages. J Infect Dis 219, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L, 1994. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367, 188–193. [DOI] [PubMed] [Google Scholar]
- Tzeng WY, Chen LH, Cherng CG, Tsai YN, Yu L, 2014. Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrus. Psychoneuroendocrinology 42, 24–37. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N, 2004. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 63, 822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lunzen J, Altfeld M, 2014. Sex differences in infectious diseases-common but neglected. J Infect Dis 209 Suppl 3, S79–80. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, Connaghan KP, Chang SL, 2015. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun 48, 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL, 2007. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol 2, 319–328. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O, 2007. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res 329, 409–420. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF, 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci 25, 537–562. [DOI] [PubMed] [Google Scholar]
- Williams R, Bokhari S, Silverstein P, Pinson D, Kumar A, Buch S, 2008. Nonhuman primate models of NeuroAIDS. J Neurovirol 14, 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Chow C, Lieblich SE, Galea LA, 2016. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 26, 87–101. [DOI] [PubMed] [Google Scholar]
- Yagi S, Galea LAM, 2019. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Zhang T, Qu X, Zhang Y, Putatunda R, Xiao X, Li F, Xiao W, Zhao H, Dai S, Qin X, Mo X, Young WB, Khalili K, Hu W, 2017. In Vivo Excision of HIV-1 Provirus by saCas9 and Multiplex Single-Guide RNAs in Animal Models. Mol Ther 25, 1168–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zuo Y, Ma J, Fogo AB, Jolicoeur P, Ichikawa I, Matsusaka T, 2005. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int 68, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Ziegler S, Altfeld M, 2016. Sex differences in HIV-1-mediated immunopathology. Curr Opin HIV AIDS 11, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SM, Altfeld M, 2017. Human Immunodeficiency Virus 1 and Type I Interferons-Where Sex Makes a Difference. Front Immunol 8, 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]