Abstract
The data in the present article are related to the previous article entitled “Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary” (Chen et al., 2013). The data describe the characteristic localization of coxsackievirus and adenovirus receptor (CAR), a junctional adhesion molecule involved in the regulation of cell–cell interactions, migration, proliferation, and growth (Coyne and Bergelson, 2005, Matthaus et al., 2017, Raschperger et al., 2006, Schiestl and Gietz, 1989) and in the stem/progenitor cell niche in the embryonic rat pituitary gland (Chen et al., 2013, Yoshida et al., 2016). Immunohistochemical analyses of CAR showed frequent colocalization with SOX2 in the embryonic rat brain, except for choroid plexus cells. CAR showed distinct apical and basolateral polarity. These data contribute to our understanding of prenatal brain development.
Specifications table
| Subject area | Biology |
| More specific subject area | Developmental biology |
| Type of data | Immunohistochemistry |
| Data format | Figures and table |
| How data was acquired | Immunofluorescence images were obtained by fluorescence microscopy (Keyence BZ-9000). |
| Experimental factors | Immunohistochemistry was performed for CAR, S100β, and the stem/progenitor markers (SOX2, E-cadherin, Nestin, and Vimentin). |
| Experimental features | Sections were prepared from embryonic rat tissues fixed with 4% (w/v) paraformaldehyde. After immuno-reaction with primary antibodies, fluorescein isothiocyanate-, Cy3-, or Cy5-conjugated secondary antibodies were used for detection. |
| Data source location | Kawasaki, Kanagawa, Japan |
| Data accessibility | Data are within the present article |
| Related research article | M. Chen, T. Kato, M. Higuchi, S. Yoshida, H. Yako, N. Kanno, Y. Kato, Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary, Cell Tissue Res., 354, 2013, 823–836. http://www.ncbi.nlm.nih.gov/pubmed/24057874[1]. |
Value of the data
|
1. Data
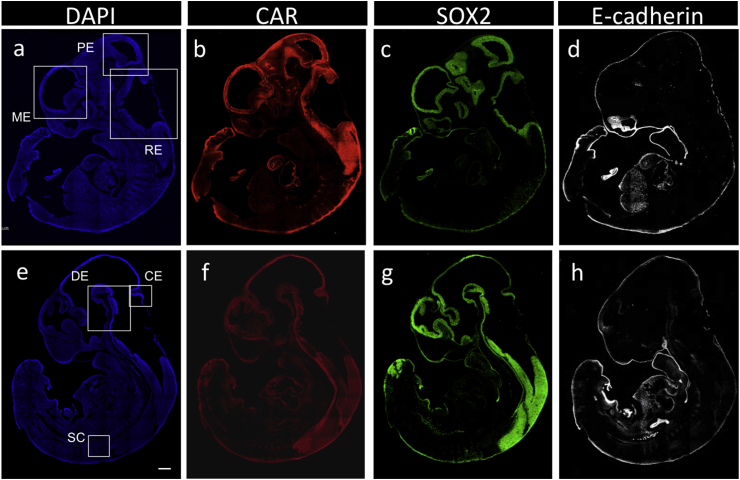
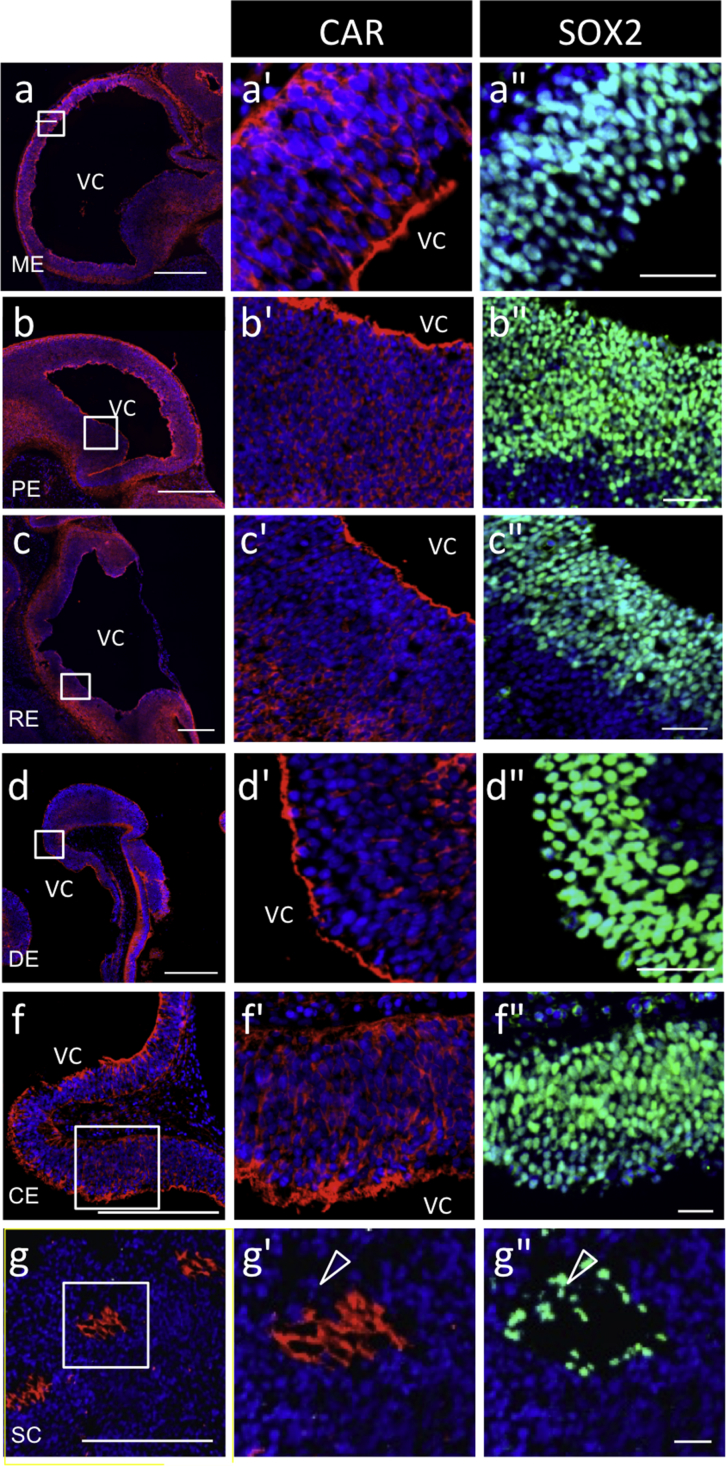
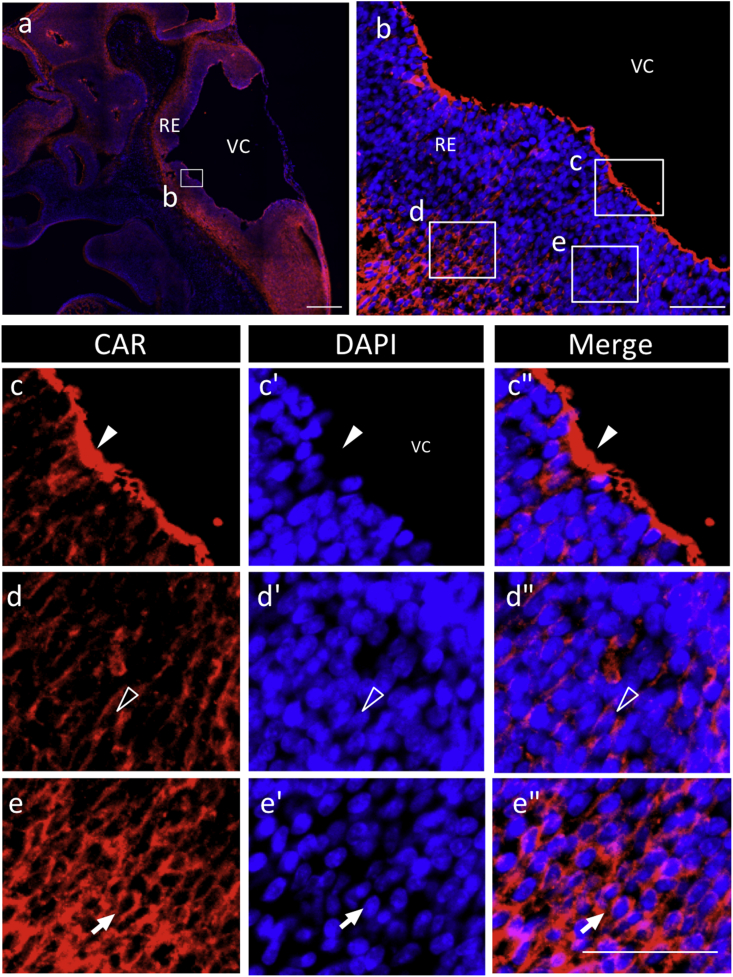
Using whole bodies on embryonic day 13.5 (E13.5), immunohistochemical analyses showed extensive CAR, which plays multifold functions [1], [2], [3], [4], [5], [6], and SOX2 signals in the embryonic brain, while E-cadherin was mostly negative (Fig. 1). Enlarged merged images with DAPI showed the colocalization of CAR and SOX2, including the spinal cord (Fig. 2). Characteristic apical, basolateral, and pericellular localization of CAR in the hindbrain were observed (Fig. 3).
Fig. 1.
Overall localization of CAR, SOX2, and E-cadherin in embryos on E13.5. Nuclei staining with DAPI (blue,a and e) and immunohistochemical analyses of CAR (Cy3, red, b and f), SOX2 (FITC, green, c and g), and E-cadherin (Cy5, white, d and h) were performed for lateral (a–d) and medial (e–h) regions. Boxed regions in a and e are enlarged in Fig. 2, Fig. 3. PE; prosencephalon, ME; mesencephalon, RE; rhombencephalon, DE; diencephalon, CE; cerebellum, SC; spinal cord. Scale bar 500 μm.
Fig. 2.
Localization of CAR in embryos on E13.5. Color codes are the same as those in Fig. 1. Each boxed area in a–g is enlarged in a'–g' (CAR) and a''–g'' (SOX2) as merged images with DAPI. Open arrowheads indicate a SOX2-single positive cell in the ependymal zone of the spinal cord. VC; ventricular cavity, ME; mesencephalon, PE; prosencephalon, RE; rhombencephalon, DE; diencephalon, CE; cerebellum, SC; spinal cord. Scale bars 500 μm (a–g), or 50 μm (a''–g'').
Fig. 3.
Localization of CAR-positive cells in the rhombencephalon of embryos on E13.5. Color codes are the same as those in Fig. 1. Boxed areas in b, part of the rhombencephalon (RE) of the rat embryo (boxed in a), are further enlarged (c–c''; ependymal zone/ventricular zone, d–d''; mantle zone/intermediate zone, e–e''; marginal zone). Arrowheads, open arrowheads, and arrows indicate the polarized localization of CAR; apical, basolateral, and round localization, respectively. VC; ventricular cavity. Scale bars 500 μm (a), or 50 μm (b–e'').
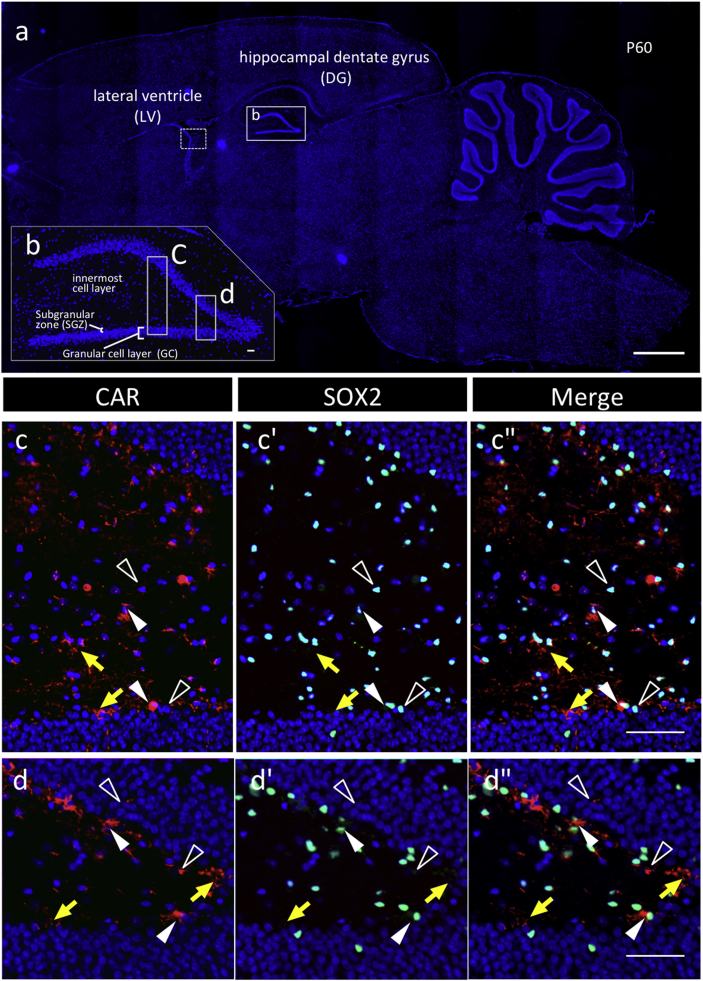
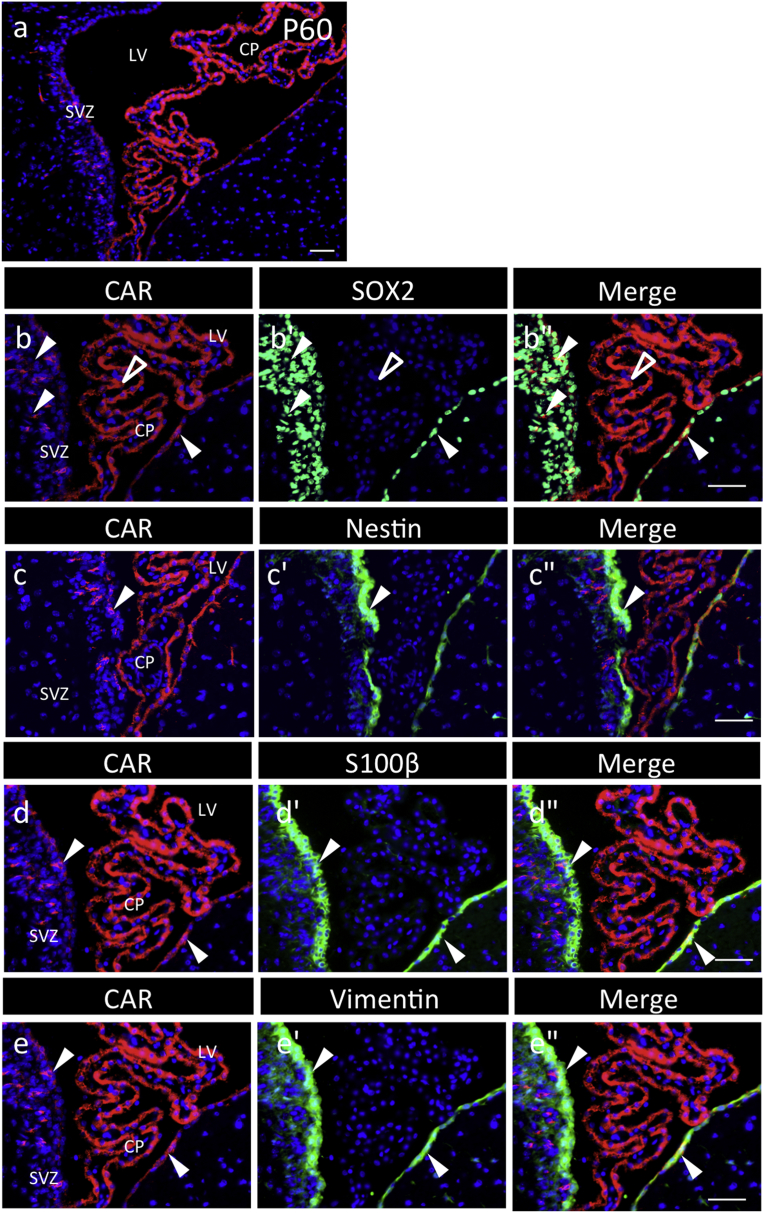
Using the adult brain on postnatal day 60 (p60), CAR localization was examined in the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ), which are known as the stem/progenitor cell niche. CAR-positive signals were found in the SGZ and in the innermost cell layer (Fig. 4); some cells were also positive for Nestin and Vimentin (Fig. 5). In the SVZ, CAR signals were observed in SOX2-positive cells in the ependymal cell layer, but some negatives in those of the choroid plexus, and in multiple cell layers of the parenchyma bordering the ventricular cavity, with polarized localization (Fig. 6). CAR-positive cells of ependymal cells, except for those in the choroid plexus, were also positive for Nestin, S100β, and Vimentin (Fig. 6). Similar profiles were observed in the fourth ventricle (data not shown).
Fig. 4.
Localization of CAR/SOX2-double positive cells in the hippocampal dentate gyrus of P60. Color codes are the same as those in Fig. 1. In the sagittal section of the brain (a), boxed areas and dotted frames are the hippocampal dentate gyrus (DG) and lateral ventricle (LV) and are enlarged in b and in Fig. 6, respectively. Boxed areas in b are enlarged in c–c'' and d–d'', respectively, with images of CAR and SOX2 as merged images with DAPI. Arrowheads and open arrowheads indicate CAR/SOX2-double and CAR-single positive cells, respectively. Yellow arrows indicate the polarized localization of CAR. Scale bars 500 μm (a), or 50 μm (b–d'').
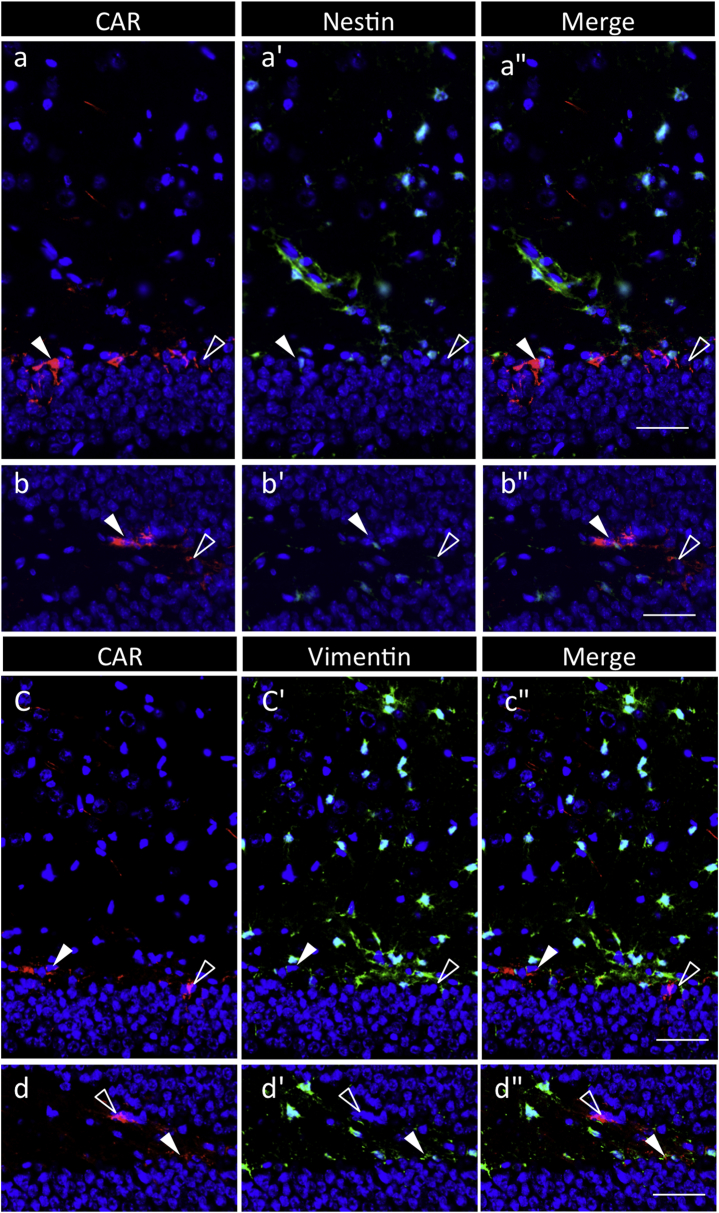
Fig. 5.
Localization of CAR/Nestin-double or CAR/Vimentin-double positive cells in the hippocampal dentate gyrus of rat P60. CAR (red)/Nestin (green)-double (a–a'', b–b'') or CAR/Vimentin (green)-double (c–c'', d–d'') positive cells in the rostral (a–a'', c–c'') and distal (b–b'', d–d'') areas of the hippocampal dentate gyrus are enlarged as merged images with DAPI. Arrowheads and open arrowheads indicate CAR/Nestin (or Vimentin)-double and CAR-single positive cells, respectively. Scale bars 50 μm.
Fig. 6.
Characterization of CAR-positive cells in the subventricular zone of rat P60. Color code is the same as that in Fig. 5. CAR-positive cells in the subventricular zone (SVZ) were characterized by staining together with SOX2 (green, b–b''). Enlarged images (green) of Nestin (c–c''), S100β (d–d''), and Vimentin (e–e'') in part of the SVZ are shown as merged image of CAR and DAPI. Arrowheads and open arrowheads indicate CAR/SOX2 (Nestin, S100β or Vimentin)-double and CAR-single positive cells, respectively. SVZ; subventricular zone, LV; lateral ventricle, CP; choroid plexuses. Scale bars 50 μm.
2. Experimental design, materials and methods
2.1. Animals
Wistar-crlj S100β-GFP transgenic strain rats, which express the reporter gene green fluorescent protein (GFP) under the control of the S100β-promoter [7], and Wistar-Imamichi strain rats were used. S100β-GFP transgenic rats were used only for analysis of P60. Breeding of rats and sampling of brains were described in the previous paper [1]. The present experimental design was approved by the Institutional Animal Care and Use Committee, Meiji University and was performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
2.2. Immunohistochemistry
Procedures of an antigen retrieval, fixation and immunostaining were performed as described previously [1] using primary antibodies and secondary antibodies under the conditions listed in Table 1.
Table 1.
| A. List of primary antibodies | ||||
|---|---|---|---|---|
| Primary antibody | Species | Isotype | Working dilution | Vendor (Area) |
| CAR | Rabbit | IgG | 1:500 | Santa Cruz Biotechnology (Dallas, Tex., USA) |
| SOX2 | Goat | IgG | 1:400 | Neuromics (Edina, Minn., USA) |
| GFP | Chicken | IgY | 1:500 | Aves labs (Tigard, Ore., USA) |
| E-cadherin | Mouse | IgG | 1:200 | BD Biosciences (San Jose, Calif., USA) |
| Nestin | Mouse | IgG | 1:250 | BD Bioscience |
| Vimentin | Mouse | IgG | 1:10000 | Sigma (Saint Louis, Mo., USA) |
| B. List of secondary antibodies | ||||
|---|---|---|---|---|
| Secondary antibody | Species/Isotype | Label | Working dilution | Vendor (Area) |
| Anti-rabbit IgG | Donkey/IgG | Cy3 | 1:500 | Jackson ImmunoResearch (West Grove, Pa., USA) |
| Anti-goat IgG | Donkey/IgG | FITC | 1:500 | Jackson ImmunoResearch |
| Anti-chicken IgY | Donkey/IgG | FITC | 1:500 | Jackson ImmunoResearch |
| Anti-mouse IgG | Donkey/IgG | Cy5 | 1:400 | Jackson ImmunoResearch |
| Anti-mouse IgG | Donkey/IgG | FITC | 1:500 | Jackson ImmunoResearch |
Acknowledgments
This work was partially supported by JSPS KAKENHI grants (nos. 21380184 to Y.K. and 24580435 to T.K.), by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018, and by a research grant (A) to Y.K. from the Institute of Science and Technology, Meiji University. This experiment was supported by Meiji University International Institute for BioResource Research (MUIIR).
Footnotes
Transparency document associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2019.103726.
Transparency document
The following is the transparency document related to this article:
References
- 1.Chen M., Kato T., Higuchi M., Yoshida S., Yako H., Kanno N., Kato Y. Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res. 2013;354:823–836. doi: 10.1007/s00441-013-1713-8. http://www.ncbi.nlm.nih.gov/pubmed/24057874 [DOI] [PubMed] [Google Scholar]
- 2.Coyne C.B., Bergelson J.M. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 2005;57:869–882. doi: 10.1016/j.addr.2005.01.007. http://www.ncbi.nlm.nih.gov/pubmed/15820557 [DOI] [PubMed] [Google Scholar]
- 3.Matthaus C., Langhorst H., Schutz L., Juttner R., Rathjen F.G. Cell-cell communication mediated by the CAR subgroup of immunoglobulin cell adhesion molecules in health and disease. Mol. Cell. Neurosci. 2017;81:32–40. doi: 10.1016/j.mcn.2016.11.009. https://www.ncbi.nlm.nih.gov/pubmed/27871939 [DOI] [PubMed] [Google Scholar]
- 4.Raschperger E., Thyberg J., Pettersson S., Philipson L., Fuxe J., Pettersson R.F. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp. Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. http://www.ncbi.nlm.nih.gov/pubmed/16542650 [DOI] [PubMed] [Google Scholar]
- 5.Schiestl R.H., Gietz R.D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida S., Nishimura N., Ueharu H., Kanno N., Higuchi M., Horiguchi K., Kato T., Kato Y. Isolation of adult pituitary stem/progenitor cell clusters located in the parenchyma of the rat anterior lobe. Stem Cell Res. 2016;17:318–329. doi: 10.1016/j.scr.2016.08.016. http://www.ncbi.nlm.nih.gov/pubmed/27596959 [DOI] [PubMed] [Google Scholar]
- 7.Itakura E., Odaira K., Yokoyama K., Osuna M., Hara T., Inoue K. Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology. 2007;148:1518–1523. doi: 10.1210/en.2006-1390. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17234709 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.