Abstract
Cystic brain lesions are a common clinical dilemma facing infectious disease providers. A broad differential diagnosis is required in the proper evaluation and care of patients facing such an illness. Here the authors describe the case of a 29-year-old woman who presented with seizures and was found to have multiple cystic brain lesions, with risk factors for neurocysticercosis. Ultimately, she was found to have a metastatic neuroendocrine malignancy. The authors review the ideal imaging and testing modalities in the diagnosis and exclusion of neurocysticercosis. This case serves as guidance for clinicians caring for patients with cystic brain lesions that may be infectious or non-infectious in etiology.
Keywords: Neurocysticercosis, Cysticercosis, Cystic brain lesion
Introduction
Relatively few infectious pathologies result in multiple cystic brain lesions, with the primary differential including neurocysticercosis, toxoplasmosis, echinococcosis, paragonimiasis, and bacterial or fungal brain abscesses [[1], [2], [3], [4]]. Neurocysticercosis, caused by the disseminated larvae of the tapeworm Taenia solium, is the most common infectious cause of cystic brain lesions and the most common cause of seizure disorder in the developing world [5]. In neurocysticercosis the cystic lesions can be seen throughout the cerebral cortex [6]. These cystic central nervous system (CNS) infections are generally uncommon in the immunocompetent host in non-endemic areas.
When cystic brain lesions are encountered, careful planning and interpretation of diagnostic testing, including imaging, serologic assays, and histopathology, is required. Non-infectious etiologies should also be considered. Clinicians should be aware of the strengths and limitations of available infectious testing modalities to make an expedient and accurate diagnosis.
Case report
A 29-year-old female presented with new onset generalized tonic-clonic seizure. She described a three year history of intermittent bilateral temporal headaches. Past medical history was remarkable only for prior injection drug use, from which she had been abstinent for three years. Her only medication was daily buprenorphine-naloxone. She was born and raised in rural Vermont, and had never travelled outside the state. She worked in a manufacturing facility where she often shared lunch prepared by co-workers, many of whom had recently emigrated from South Asia. She was a 15 pack-year smoker and drank minimal alcohol. She had one cat who was treated for an intestinal worm infection two years prior. The family history was notable for breast cancer in her maternal aunt at age 40.
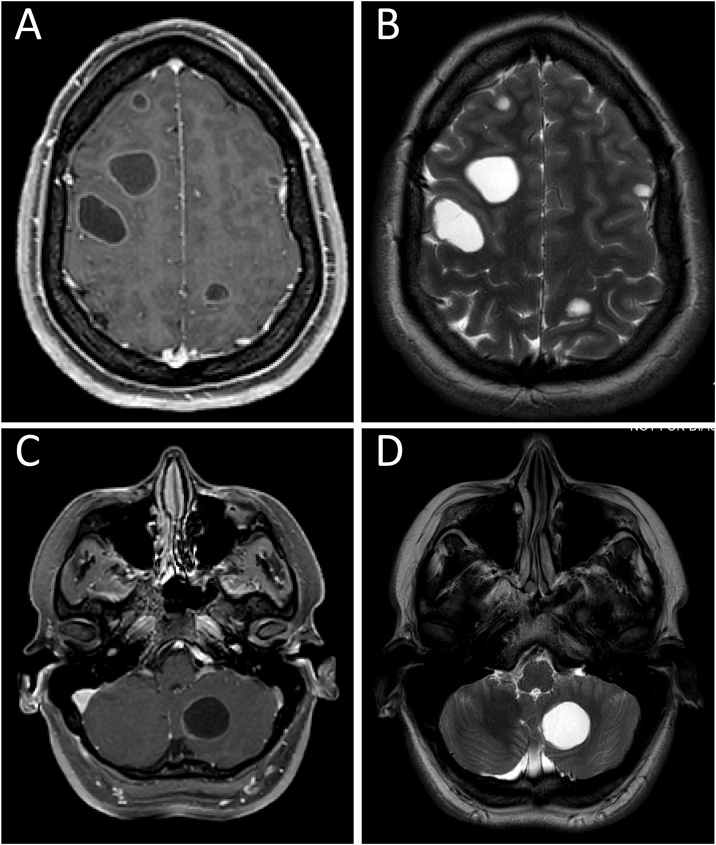
Upon presentation for care after the seizure, the patient was afebrile with normal vital signs. Aside from an initial post-ictal state, physical and neurologic examinations were unremarkable. Initial laboratory results showed a white blood cell count of 7.2 × 103 cells/μL, hemoglobin of 13.3 g/dL, platelets of 270 × 103/μL, and serum creatinine of 0.6 mg/dL. A computed tomography (CT) scan of the brain was notable for numerous cystic lesions. A subsequent magnetic resonance imaging (MRI) study of the brain with gadolinium contrast showed approximately 35 smoothly circumscribed cystic structures throughout the supra- and infra-tentorial brain parenchyma (Fig. 1). The cystic lesions ranged in size from 2 to 33 mm, the largest located in the left temporal lobe. Notably, mass effect, perilesional edema, and restricted diffusion were absent on MRI. A lumbar puncture was performed and cerebrospinal fluid (CSF) analysis demonstrated no white blood cells, normal glucose and protein, and negative bacterial, fungal, and mycobacterial stains and cultures. Chest X-ray was normal.
Fig. 1.
Magnetic resonance imaging. A: Axial contrast-enhanced T1-weighted image demonstrates faint, uniform, peripheral enhancement of the lesions in the frontal and parietal lobes. B: Axial T2-weighted image demonstrates multiple round, circumscribed, T2 hyperintense lesions in the frontal and parietal lobes, without surrounding edema. C: Axial contrast-enhanced T1-weighted image of the lesion in the left cerebellum demonstrates faint, uniform, peripheral enhancement. D: Axial T2-weighted image of the posterior fossa demonstrates a 25 mm round, circumscribed, T2 hyperintense lesion in the left cerebellum without any surrounding edema.
The initial working diagnosis was neurocysticercosis, with consideration given to tuberculosis, toxoplasmosis, cryptococcus, histoplasmosis, blastomycosis, echinococcus, nocardia, and malignancy. Negative results included cysticercosis serum and CSF IgG enzyme-linked immunosorbent assay (ELISA), interferon gamma release assay for tuberculosis, toxoplasma CSF polymerase chain reaction and serum IgG serology, CSF cryptococcal antigen, urine histoplasma and blastomyces antigens, serum echinococcus serology, and fourth generation human immunodeficiency virus assay. No empiric antimicrobial agent was given.
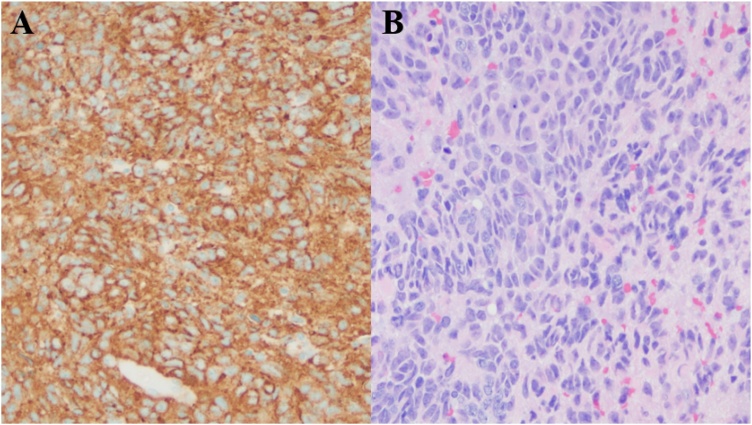
Repeat MRI four weeks later showed a slight increase in the size of the cystic lesions, up to 35 mm. Six weeks after initial presentation, a brain biopsy of the left posterotemporal cystic lesion was performed, with pathology demonstrating metastatic neuroendocrine tumor (Fig. 2). A CT chest then showed a 3 cm irregular mass in the lower lobe of the left lung, not seen on the prior chest X-ray. A positron emission tomography scan showed fluoro-deoxy-glucose uptake in the lung lesion, the cystic brain lesions, and the T8 vertebral body. She was diagnosed with lung neuroendocrine tumor with cystic brain metastases, and treated with carboplatin, pemetrexed, and pembrolizumab. Subsequent imaging has shown a decrease in lesion size and no further metastases.
Fig. 2.
A: Diffusely positive chromogranin immunohistochemical stain (a neuroendocrine tumor marker) of the left posterotemporal cystic brain lesion biopsy tissue. B: Hematoxylin-eosin (H&E) staining of the left posterotemporal cystic brain lesion biopsy specimen shows small epithelioid cells with abundant mitoses infiltrating into glial tissue, consistent with invasive malignancy.
Discussion
The imaging appearance of neurocysticercosis is widely variable as it is dependent on the parasite’s life stage, the number and location of lesions, the host’s inflammatory response, and in the setting of ventricular disease the degree of CSF obstruction. Kimura-Hayama et al provide an excellent review of the radiographic and pathologic presentation of neurocysticercosis [7]. The first radiographic stage is non-cystic, and is often invisible on brain imaging. The second stage is vesicular, where cysts are typically 10–20 mm in size with a thin, smooth wall, the scolex of the parasite is usually visible, and there is minimal perilesional edema. The third stage is colloidal vesicular, where the cysts are more inflammatory and there is pericystic enhancement and edema. The fourth stage is granular nodular, which shows even more edema and thicker rim enhancement than the colloidal vesicular stage. In the fifth and final radiographic stage, calcified nodular, the lesions are calcified, do not enhance, and have no perilesional edema. It should be noted that, unlike in the case of metastatic lung neuroendocrine tumor presented here, neurocysticercosis lesions are typically less than 20 mm. The cyst size up to 33 mm on the initial MRI of our patient suggests an alternative diagnosis. Additionally, the imaging characteristics in this case are not entirely consistent with any of the described radiographic stages.
The diagnosis of neurocysticercosis is facilitated by serologic analysis. Enzyme-linked immunotransfer blot (EITB) assay is the test of choice, and is available from the Centers for Disease Control and Prevention (CDC) and some reference laboratories. Serology via ELISA is more widely available but has inferior test characteristics; for instance, in use on positive reference samples, the ELISA sensitivity was 41%, compared to 86% for EITB [4,8,9]. For this reason, the 2017 IDSA neurocysticercosis guidelines recommend against use of the ELISA if EITB can be obtained [4]. The sensitivity of neurocysticercosis serology is increased if there are multiple lesions, and if the testing is performed in a high prevalence population. Notably, serum EITB appears superior to CSF EITB [10].
A variety of malignancies can resemble neurocysticercosis. These include metastatic tumors, primary brain cancers, lymphomas, and histiocytosis [4]. When the scolex is definitively identified the diagnosis of neurocysticercosis is solidified [7]. Lesions larger than 20 mm with irregular borders are more likely to be malignancy, as in the patient presented [11].
Conclusions
We present the case of an unfortunate young woman who was found to have multiple cystic brain lesions from metastatic lung neuroendocrine cancer. There was significant anchoring bias [12] toward neurocysticercosis early in her case based on possible exposure due to food sharing with coworkers from endemic areas, and the lack of perilesional mass effect or edema that would have been more suggestive of malignancy. Infectious diseases providers should be aware of the broader differential of cystic CNS lesions, and the ideal imaging and testing modalities in the diagnosis and exclusion of neurocysticercosis.
Notes on contributors
Daniela E. DiMarco, MD, MPH, is an infectious diseases fellow at the University of Vermont Medical Center, Burlington, VT, United States.
Andrew J. Hale, MD is an infectious diseases physician at the University of Vermont Medical Center and Assistant Professor of Medicine at Larner College of Medicine at the University of Vermont, Burlington, VT, United States.
Adam Ulano, MD, is a neuroradiologist at the University of Vermont Medical Center and Assistant Professor of Radiology at Larner College of Medicine at the University of Vermont, Burlington, VT, United States.
Lindsay M. Smith, MD, is an infectious diseases physician at the University of Vermont Medical Center and Assistant Professor of Medicine at Larner College of Medicine at the University of Vermont, Burlington, VT, United States.
Contributor Information
Daniela E. DiMarco, Email: Daniela.DiMarco@uvm.edu.
Andrew J. Hale, Email: Andrew.Hale@UVMhealth.org.
References
- 1.Go K.G., Hew J.M., Kamman R.L., Molenaar W.M., Pruim J., Blaauw E.H. Cystic lesions of the brain. A classification based on pathogenesis, with consideration of histological and radiological features. Eur J Radiol. 1993;17(2):69–84. doi: 10.1016/0720-048x(93)90038-o. [DOI] [PubMed] [Google Scholar]
- 2.Oprisan A., Popescu B.O. Intracranial cysts: an imagery diagnostic challenge. Sci World J. 2013;2013 doi: 10.1155/2013/172154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma V., Prabhash K., Noronha V., Tandon N., Joshi A. A systematic approach to diagnosis of cystic brain lesions. South Asian J Cancer. 2013;2(2):98–101. doi: 10.4103/2278-330X.110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White A.C., Coyle C.M., Rajshekhar V., Singh G., Hauser W.A., Mohanty A. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine And Hygiene (ASTMH) Am J Trop Med Hyg. 2018;98(4):945–966. doi: 10.4269/ajtmh.18-88751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carabin H., Ndimubanzi P.C., Budke C.M., Nguyen H., Qian Y., Cowan L.D. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152. doi: 10.1371/journal.pntd.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dev N., Abbas S.Z. Disseminated cysticercosis. N Engl J Med. 2019;380(13):1267. doi: 10.1056/NEJMicm1810953. [DOI] [PubMed] [Google Scholar]
- 7.Kimura-Hayama E.T., Higuera J.A., Corona-Cedillo R., Chávez-Macías L., Perochena A., Quiroz-Rojas L.Y. Neurocysticercosis: radiologic-pathologic correlation. Radiographics. 2010;30(6):1705–1719. doi: 10.1148/rg.306105522. [DOI] [PubMed] [Google Scholar]
- 8.Carod J.F., Randrianarison M., Razafimahefa J., Ramahefarisoa R.M., Rakotondrazaka M., Debruyne M. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis. 2012;72(1):85–89. doi: 10.1016/j.diagmicrobio.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Proano-Narvaez J.V., Meza-Lucas A., Mata-Ruiz O., Garcia-Jeronimo R.C., Correa D. Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol. 2002;40(6):2115–2118. doi: 10.1128/JCM.40.6.2115-2118.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang V.C., Brand J.A., Boyer A.E. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159(1):50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 11.Rajshekhar V., Chandy M.J. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients presenting with seizures. Acta Neurol Scand. 1997;96(2):76–81. doi: 10.1111/j.1600-0404.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 12.Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775–780. doi: 10.1097/00001888-200308000-00003. [DOI] [PubMed] [Google Scholar]