Abstract
Heart failure (HF) is the leading cause of morbidity and mortality worldwide and negatively impacts quality of life, healthcare costs, and longevity. Although data on HF in the Arab population are scarce, recently developed regional registries are a step forward to evaluating the quality of current patient care and providing an overview of the clinical picture. Despite the burden of HF in Saudi Arabia, there are currently no standardized protocols or guidelines for the management of patients with acute or chronic heart failure. Therefore, the Heart Failure Expert Committee, comprising 13 local specialists representing both public and private sectors, has developed guidelines to address the needs and challenges for the diagnosis and treatment of HF in Saudi Arabia. The ultimate aim of these guidelines is to assist healthcare professionals in delivering optimal care and standardized clinical practice across Saudi Arabia.
Keywords: Acute heart failure, Chronic heart failure, Heart failure recommendations, Multidisciplinary management, Saudi Arabia
Abbreviations
- ACE-I
angiotensin-converting enzyme inhibitor
- ACS
acute coronary syndrome
- AF
atrial fibrillation
- AHF
acute heart failure
- ARB
angiotensin II receptor blocker
- ARDS
acute respiratory distress syndrome
- ARNI
angiotensin receptor/neprilysin inhibitor
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- AV
atrioventricular
- b.i.d.
twice daily
- BB
beta-blocker
- BiPAP
bilevel positive airway pressure
- BMI
body mass index
- BNP
B-type natriuretic peptide
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CBC
complete blood count
- CHF
chronic heart failure
- CMR
cardiac magnetic resonance
- CPE
cardiogenic pulmonary edema
- CRF
chronic renal failure
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization therapy defibrillator
- DBP
diastolic blood pressure
- ECG
electrocardiogram
- EF
ejection fraction
- EMF
endomyocardial fibrosis
- FCM
ferric carboxymaltose
- GH
growth hormone
- Hb
hemoglobin
- HCM
hypertrophic cardiomyopathy
- HES
hypereosinophilic syndrome
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
heart rate
- ICD
implantable cardioverter defibrillator
- IV
intravenous
- LAD
left anterior descending
- LBBB
left bundle branch block
- LMWH
low-molecular-weight heparin
- LSVD
left ventricular systolic dysfunction
- LV
left ventricle
- LVAD
left ventricular assist device
- LVEF
left ventricular ejection fraction
- LVESV
left ventricle end-systolic volume index
- MI
myocardial infarction
- MR
mitral regurgitation
- MRA
mineralocorticoid antagonist
- NP
natriuretic peptide
- NSAID
non-steroidal anti-inflammatory drug
- NYHA
New York Heart Association
- o.d.
once daily
- OMT
optimal medical therapy
- PAD
peripheral artery disease
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- PMC
percutaneous mitral commissurotomy
- RHD
rheumatic heart disease
- RSVD
right ventricular systolic dysfunction
- RV
right ventricle
- SBP
systolic blood pressure
- SPECT
single-photon emission computed tomography
- TOE
transoesophageal echocardiograph
- TR
tricuspid regurgitation
- TS
tricuspid stenosis
- TSH
thyroid-stimulating hormone
- TTE
transthoracic echocardiography
- VT/VF
ventricular tachycardia/ventricular fibrillation
1. Introduction
1.1. Epidemiology of heart failure in Saudi Arabia
Heart failure (HF) is a leading cause of morbidity and mortality worldwide [1], [2] and negatively impacts quality of life (QoL), healthcare costs, and longevity [2]. The prevalence of HF ranges from 1% to 2% in adults from developed countries and is ≥10% in those aged over 70 years, depending on the definition applied [3]. A myriad of diseases affecting the heart culminate in HF. Although data on HF in the Arab population are scarce, recently developed regional registries are a step forward to evaluating the quality of current patient care and to provide an overview of the clinical picture.
The heart function assessment registry trial in Saudi Arabia (HEARTS) was the first multicenter survey conducted in the Kingdom of Saudi Arabia (KSA) and the Arab population to study the clinical features, management, and short- and long-term outcomes of patients with acute heart failure (AHF) and high-risk chronic heart failure (HCHF; Table 1) [2].
Table 1.
An overview of demographics of patients included in the heart function assessment registry trial in Saudi Arabia (HEARTS) registry [2].
| Variable | Acute heart failure | High-risk chronic heart failure |
|---|---|---|
| Age, mean yr (SD) | 60.6 (15.3) | 56.9 (15.5) |
| n (%) | 772 (66.2) | 368 (33.2) |
| Male, % | 65.2 | 71.7 |
| Body mass index (kg/m2), mean ± SD | 29.3 ± 6.8 | 29.2 ± 5.8 |
| Central obesity, % | 65.0 | 27.2 |
| Medical history, % | ||
| Coronary artery disease | 50.0 | 41.8 |
| Percutaneous coronary intervention | 13.4 | 15.9 |
| Coronary artery bypass graft | 11.1 | 12.5 |
| Rheumatic heart disease | 7.2 | 3.3 |
| Atrial fibrillation | 15.4 | 13.5 |
| Ventricular tachycardia/ventricular fibrillation | 2.2 | 2.6 |
| Implantable cardioverter defibrillator | 10.0 | 28.8 |
| Cardiac resynchronization therapy | 5.3 | 8.0 |
| Stroke | 7.0 | 8.1 |
| Peripheral artery disease | 4.2 | 2.4 |
| Chronic renal failure | 30.7 | 28.1 |
| On dialysis | 6.8 | 1.9 |
| Anemia | 24.5 | 19.8 |
| Major risk factors, % | ||
| History of smoking | 15.5 | 22.8 |
| Current smoker | 18.2 | 21.2 |
| Hypertension | 70.0 | 69.0 |
| Hyperlipidemia | 36.4 | 57.1 |
| Diabetes mellitus | 60.7 | 53.0 |
| Taking insulin | 41.6 | 20.9 |
| Vital signs at presentation | ||
| Systolic blood pressure, median (mmHg) | 125 | 115 |
| Diastolic blood pressure, median (mmHg) | 72 | 69 |
| Heart rate, median (mmHg) | 88 | 77 |
| Major investigations | ||
| Positive serum troponin, % | 30.0 | — |
| Serum sodium (mmol/L) | 135.2 | 137.0 |
| Atrial fibrillation, % | 18.0 | 11.8 |
| QRS ≥120 ms, % | 11.6 | 11.0 |
| Serum NT-proBNP (N-terminal pro B-type natriuretic peptide; pg/mL) | 4616 | 1596 |
| Echocardiography, % | 97.1 | 98.4 |
| Preserved left ventricular function, % | 27.5 | 24.7 |
| Moderate/severe left ventricular systolic dysfunction, % | 72.5 | 75.3 |
| Right ventricular systolic dysfunction, % | 27.2 | 6.6 |
| Pulmonary hypertension, % | 36.4 | 18.1 |
| Coronary angiogram, % | 31.6 | — |
The mean age of patients with AHF and CHF was 57–60 years in the KSA, which is almost 10 years younger than patients from developed countries [2]. Of the patients with AHF, 44.7% had a history of chronic heart failure (CHF), suggesting an early age of onset that may be related to the extremely high prevalence of coronary artery disease (CAD) risk factors [2]. In addition, the prevalence of diabetes mellitus was 60.7% in patients with AHF in the KSA, which is double the rate reported by global AHF registries; however, the rate of hypertension (70%) was similar to global registries despite the population being younger [2]. Similar findings were reported in patients with HCHF; however, the rates of diabetes mellitus (53%) were lower whereas hypertension (69%) was higher compared with patients with AHF. Almost three-quarters of the patients had moderate/severe left ventricle (LV) dysfunction compared with one-half in other studies [2].
A more recent study (HEARTS-chronic) evaluating the clinical features and outcomes of 685 CHF patients enrolled in the registry between 2009 and 2011 reported that CHF patients presented young and commonly suffered from severe LV dysfunction [4]. For instance, the mean age at diagnosis of patients with CHF was 55 years, with CAD (38.8%), dilated cardiomyopathy (DCM; 36.5%), and hypertension (10.5%) being the most common etiologies of HF [4]. Severe LV dysfunction was reported in two-thirds of the patients with median N-terminal-pro B-type natriuretic peptide (NT-proBNP) of 29.34.37 pg/mL [4]. In addition, at 1 year, the all-cause mortality rate was 9% (cardiac related 93.7%), hospitalization rate was 39%, and emergency room visit was 50%, which could be attributed to the relatively high rate of CHF patients with severe LV dysfunction [4].
In general, beta-blockers, angiotensin-converting enzyme inhibitors (ACE-Is)/angiotensin II receptor blockers (ARBs), and diuretics were the most commonly used agents for the management of patients with AHF or CHF (Fig. 1) [2], [4].
Fig. 1.
Overview of medical therapy following diagnosis of patients with (A) acute heart failure (AHF) and (B) chronic heart failure (CHF) in the heart function assessment registry trial in Saudi Arabia (HEARTS) registry [2]. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker. Note.Fig. 1A and B are taken from AlHabib KF, Elasfar AA, AlBackr H, AlFaleh H, Hersi A, AlShaer F, et al. Design and preliminary results of the heart function assessment registry trial in Saudi Arabia (HEARTS) in patients with acute and chronic heart failure. Eur J Heart Fail 2011;13:1178–84. Reproduced with permission from Wiley.
The HEARTS study also described independent predictors of death in patients with de novo AHF and acute CHF (ACHF). Patients with ACHF were older, compared with acute de novo patients (62.2 vs. 60.0 years, respectively); less likely to be men (64% vs. 69%) or smokers (31.6% vs. 36.7%); and more likely to have a history of diabetes mellitus (65.7% vs. 61.3%), hypertension (74% vs. 65%), and severe left ventricular dysfunction (52% vs. 40%). The ACHF group had a higher adjusted 3-year mortality rate [hazard ratio (HR) 1.6, 95% confidence interval (CI) 1.3–2.0; p < 0.001] than the acute de novo group. Overall, patients with ACHF had significantly higher long-term mortality rates than those with de novo AHF [5].
A study assessing the sex-specific differences in clinical features and outcomes of patients with AHF found that, compared with men, women were older (mean 63.6 vs. 60.2 years; p < 0.001) and more likely to have risk factors for atherosclerosis, a history of HF (67.8% vs. 62.3%; p = 0.005), and rheumatic heart disease (11.3% vs. 4.9%; p < 0.001). Ischemic heart disease was the primary cause of HF in men and women, but was less common in women than men (50.6% vs. 54.7%; p = 0.046). Women had higher rates of hypertensive heart disease and primary valve disease (p < 0.001 for both), whereas men were more likely to have severe LV systolic dysfunction. On discharge, men had a higher use of ACE-Is (62.8% vs. 53.4%; p < 0.001), beta-blockers (85.8% vs. 79.3%; p < 0.001), and aldosterone inhibitors (42.1% vs. 30.9%; p < 0.001) compared with women. Apart from higher atrial fibrillation (AF) in women (8.4% vs. 4.7% in men; p < 0.001) and higher ventricular arrhythmias in men (4.8% vs. 3% in women; p = 0.029), no differences were observed in hospital outcomes [6].
Dyslipidemia is a major risk factor for vascular diseases, including coronary heart disease, and is the most common cardiovascular risk factor in the KSA. Dyslipidemia affects both children and adults, and may overwhelm the public health sector in the long run if aggressive interventions are not implemented early. A low level of high-density lipoprotein cholesterol was the most common lipid disorder in patients with HF (82.9%), followed by hypertriglyceridemia (35.2%), atherogenic dyslipidemia (27.8%), and hypercholesterolemia (9.2%). Diabetes mellitus was the single most significant predictor of mortality (p = 0.001) in this population. Of the lipid disorders, only low levels of high-density lipoprotein cholesterol contributed to significant mortality risk [odds ratio (OR) 1.29, 95% CI 1.04–1.59; p < 0.01] adjusted for age, sex, and statin use [7].
HF is a chronic syndrome characterized by significant physical, psychological, and social burden, resulting in poor QoL. Data assessing the QoL of patients with HF revealed that QoL scores were low across all evaluated domains (measured using the Short Form-36 survey). LV ejection fraction (LVEF) was the strongest predictor of both physical and mental summaries [8], [9]. Patients with HF had significant disruptive pain and limitations when performing everyday activities [9].
Given the availability of new data and various global guidelines, a group of local experts came together to develop customized guidelines that best reflect the needs and challenges for the diagnosis and treatment of HF in the KSA. These guidelines also offer an opportunity to address the differences between international guidelines that stem from variations in interpreting the HF literature. Despite the burden of HF in the KSA, there are currently no standardized management protocols or guidelines for the management of patients presenting with AHF or CHF. This paper represents the consensus opinion of 13 experts and two reviewers practicing in the KSA. The aim of these guidelines is to assist healthcare professionals in delivering optimal and standardized clinical practice across the KSA. This paper provides a comprehensive overview of best practices keeping in mind the available local resources and practices. This paper enforces the importance of multidisciplinary care in HF management and discusses steps to measuring and improving quality of care.
2. Methods
2.1. Consensus approach
The Heart Failure Expert Committee, comprising 13 local specialists, representing both public and private sectors and practicing across the KSA, met on October 7–8, 2016, to reach a consensus on the recommendations. This committee included experts practicing in different subspecialties in addition to their HF practice, such as interventional cardiology, cardiothoracic surgery, imaging, electrophysiology, and clinical pharmacology. Each of the expert committee members have a minimum of 10 years’ clinical practice experience in cardiology. In addition, the two external reviewers are senior cardiologists with over 20 years’ clinical practice experience.
To reach consensus, a premeeting survey was conducted prior to drafting these recommendations, to gather opinions on diagnosis, treatment, and follow-up. Recommendations included in the premeeting survey were put together by referencing European and American clinical practice guidance documents. Each expert committee member voted on the key recommendations relating to their subspecialty through the survey, and provided critical feedback on whether they were relevant to practice in the KSA. Dr. Waleed AlHabeeb reviewed all expert committee feedback and worked together with the medical writer on an initial draft for discussion at the expert committee meeting (October 7–8, 2016). During the meeting, all expert committee members discussed recommendations, and outlined clinical care pathways, keeping in mind the available local resources, current unmet needs, and published evidence. Special care was taken to review recent published landmark trials and meta-analysis before drafting treatment recommendations. Postmeeting the writer drafted a recommendation document based on feedback provided at the meeting, which was then critically reviewed by all authors as a validation of the consensus reached during the meeting. The final draft of the manuscript was critically appraised and validated by the two external reviewers.
2.2. Scope
This document is intended for use by local general physicians and cardiac specialists for the management of patients with AHF and CHF. However, physicians are required to manage patients based on the best available evidence and their clinical judgment, and should also take factors such as patient characteristics, drug profile, and available resources into consideration. Given that HF practices are standard globally, there may be inevitable similarities between this paper and other published clinical practice guidance documents.
2.3. Literature review
A literature review was conducted premeeting and postmeeting, primarily using the National Library of Medicine PubMed database (limited to the English language). References were reviewed for relevance based on their title and abstract. References within selected papers were also checked for relevance. The strength of a recommendation for a particular management option was weighed and graded according to the predefined color scale outlined in Table 2.
Table 2.
Definition and class of recommendations.
3. Definition and classification of heart failure
HF can occur in a wide range of patients with different underlying etiologies, demographics, and comorbidities [3]; therefore, measurement of LVEF to define HF is a practical approach that can be used across all patient groups. Table 3 outlines the definition of HF, which includes three types of patients: reduced LVEF of ≤40%, borderline LVEF of 41–49%, and preserved LVEF of ≥50%.
Table 3.
Definition of heart failure.
| Classification | Ejection fraction (%) |
|---|---|
| Heart failure with reduced ejection fraction (HFrEF) | ≤40 |
| Heart failure with borderline ejection fraction (HFbEF) | 41–49 |
| Heart failure with preserved ejection fraction (HFpEF) | ≥50 |
Relevant terminologies related to the time course of HF includes (1) asymptomatic LV systolic dysfunction (a patient who has never exhibited the typical signs and/or symptoms of HF and with a reduced LVEF); (2) stable HF (a treated patient with signs and symptoms that have remained generally unchanged for at least 1 month); and (3) decompensated HF (if chronic stable HF deteriorates, the patient may be described as “decompensated”—this may happen suddenly or slowly) [3]. Staging the increasing severities of HF has been described by the American College of Cardiology Foundation/American Heart Association (AHA), who stratify patients based on the development and progression of disease [10].
4. Diagnosis
4.1. Etiologies
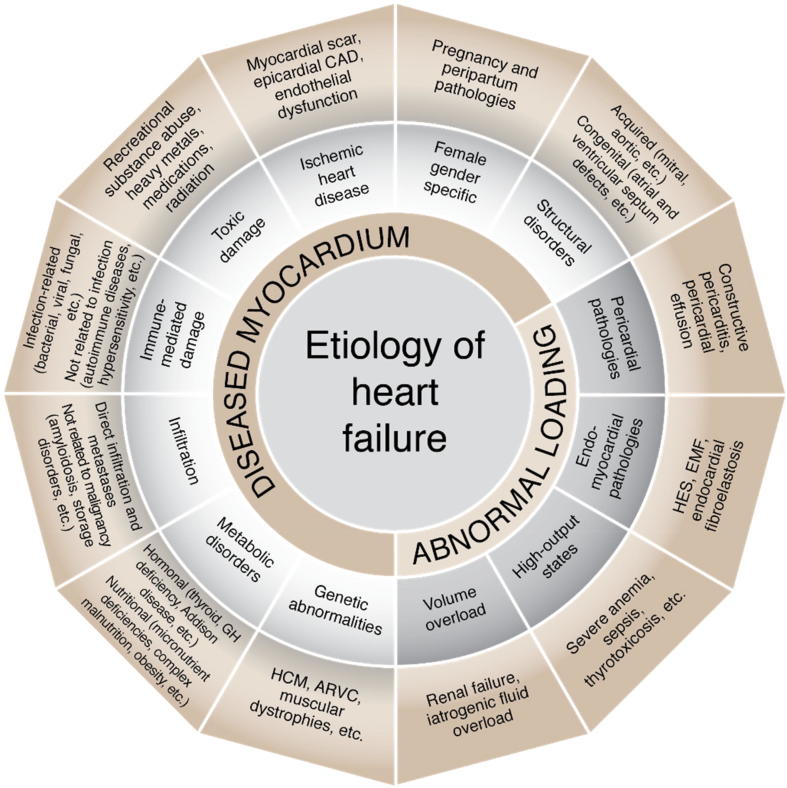
Given the lack of a well-defined classification of the etiologies of HF, the authors have endorsed the 2016 European Society of Cardiology (ESC) guidelines’ scheme for classifying the causes of HF. The scheme divides the etiologies into two broad categories: HF secondary to diseased myocardium and HF secondary to abnormal loading conditions. The causes of HF are highlighted in Fig. 2.
Fig. 2.
Diagram summarizing the etiology of heart failure [3]. ARVC = arrhythmogenic right ventricular cardiomyopathy; CAD = coronary artery disease; EMF = endomyocardial fibrosis; GH = growth hormone; HCM = hypertrophic cardiomyopathy; HES = hypereosinophilic syndrome. Note. Figure is based on content from [3].
4.2. Symptoms and signs
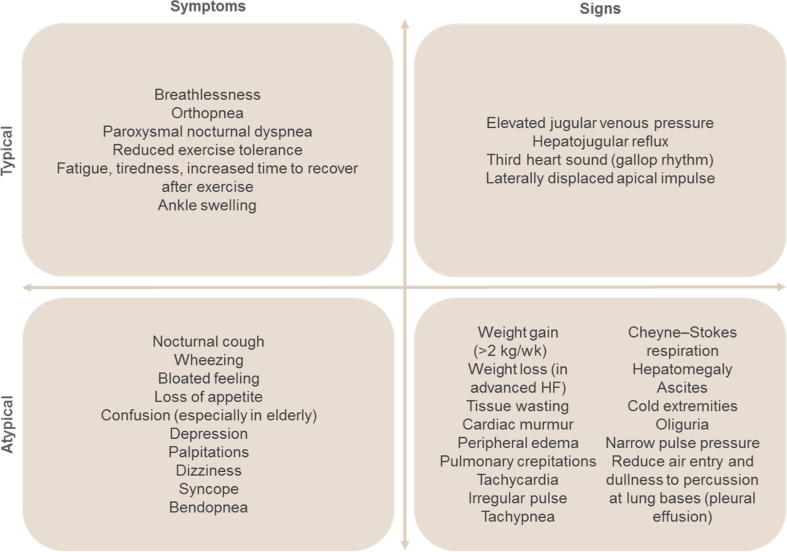
The symptoms of HF can be nonspecific, making it difficult for less experienced practitioners to make a definitive diagnosis (Fig. 3) [11]. Therefore, it is important to document a detailed medical history and to assess the signs and symptoms at each visit, especially for evidence of congestion. A patient’s response to treatment and stability over time is clearly reflected in their signs and symptoms. Persistence of symptoms while on treatment warrants additional therapy. Prompt medical attention is only necessary when symptoms worsen. Although symptoms resolve over time with treatment, the underlying cardiac dysfunction may not necessarily resolve and the patient will continue to be at risk of decompensation. Assessment of patients’ functional ability is also an important predictor of HF, because reduced exercise tolerance over time usually indicates worsening HF and physical deconditioning [11]. The New York Heart Association (NYHA) classification is a useful tool to measure a patient’s physical limitations and for observing a patient’s stability over time [11].
Fig. 3.
Clinical signs and symptoms typical of heart failure (HF) [11].
4.3. Diagnosing heart failure
For patients presenting for the first time with signs and symptoms suggestive of HF, it is important to consider the patient’s prior clinical history, physical examination, and resting electrocardiogram. If all results are within the normal range, it is highly unlikely the patient has HF and other diagnoses should be considered.
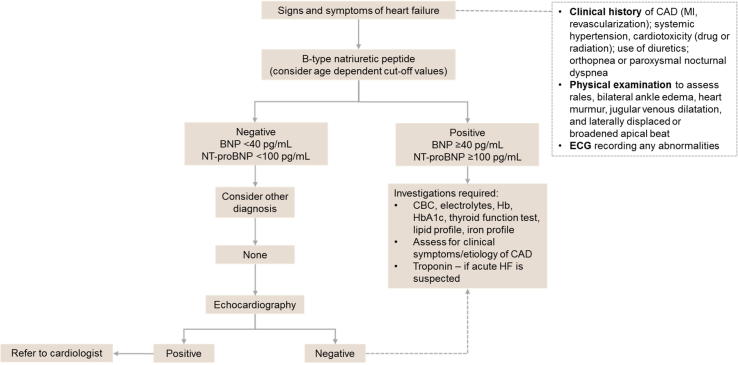
BNP level is a biomarker for the diagnosis and prognosis of HF [12], and a normal NT-proBNP level has a high negative predictive value for HF [13]. A BNP level ≥100 pg/mL [14], [15] and/or an NT-proBNP ≥300 pg/mL [16] (depending on age) would almost certainly confirm the presence of HF. A BNP <40 pg/mL and an NT-proBNP <125 pg/mL excludes HF in a non-acute setting. The BNP cutoff values for “ruling in” and “ruling out” HF are referred to as “gray zone” values, and are seen in approximately 20% of patients with dyspnea in the emergency department [17]. It is important to remember that a gray zone value of NT-proBNP is not a benign finding, and these patients have a higher risk for adverse outcomes than patients with a negative result [17]. A few possible diagnoses to consider in patients with gray zone NT-proBNP levels include cardiac ischemia, AF, and infectious/inflammatory pulmonary disease [17].
A normal natriuretic peptide level would indicate that HF is unlikely, prompting consideration of other diagnoses. If another diagnosis cannot be determined, then the patient should undergo echocardiographic assessment. Echocardiography provides immediate information on chamber volumes, ventricular systolic and diastolic function, wall thickness, valve function, and pulmonary hypertension [3]. Confirmation of HF merits further investigation to determine the etiology and initiate the most appropriate treatment. Therefore, at this point, we recommend referring the patient to a specialist cardiologist to facilitate appropriate patient management. An algorithm for the diagnosis of HF is shown in Fig. 4.
Fig. 4.
Algorithm for the diagnosis of heart failure. BNP = B-type natriuretic peptide; CAD = coronary artery disease; CBC = complete blood count; ECG = electrocardiogram; Hb = hemoglobin; HF = heart failure; MI = myocardial infarction; NT-proBNP = N-terminal pro B-type natriuretic peptide.
4.4. Diagnosis of heart failure with preserved ejection fraction
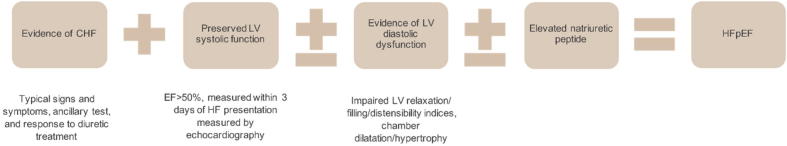
HF with preserved EF (HFpEF) contributes to a substantial societal burden and is becoming a predominant phenotype of HF [18]. Various criteria have been suggested for the diagnosis of HFpEF; however, to make a definitive diagnosis, the presence of three key clinical, echocardiographic, and hemodynamic abnormalities are required (Fig. 5) [18].
Fig. 5.
Criteria for diagnosis of HFpEF [18]. CHF = chronic heart failure; EF = ejection fraction; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; LV = left ventricle.
The diagnosis of HFpEF is challenging because of the nonspecificity of the signs and symptoms, echocardiography, and relative paucity of markers for diastolic dysfunction [18]. Biomarkers are increasingly being used for screening, diagnosis, and risk stratification in HF. Data from the recent Swedish Heart Failure Registry found that decreases in NT-proBNP were associated with improved mortality and morbidity in patients with HF with borderline EF (HFbEF; EF 40–49%) and HFpEF (EF ≥50%) [19]. Although echocardiography plays an important role in the diagnostic work-up of patients with HFpEF, evaluating LV diastolic dysfunction with conventional echocardiography produces variable profiles and has no impact on long-term survival. By contrast, right ventricle (RV) dysfunction, paradoxical septal motion, and higher RV systolic pressure were associated with poor survival [20]. Importantly, data from the RELAX trial showed that, in patients with HFpEF, impaired LV global longitudinal strain was indicative of covert systolic dysfunction despite normal LVEF. Impaired LV global longitudinal strain was associated with lower NT-proBNP and collagen synthesis and diastolic dysfunction, but was not associated with improved QoL or exercise capacity [21]. A recent study assessing biomarkers in 5000 individuals from the population-based Gutenberg Health Study reported that the index of CRP + GDF-15 s + sST2/NT-proBNP may be used to discriminate HFpEF from HF with reduced EF (HFrEF) [22].
Possible clinical parameters to aid diagnosis of HFpEF and minimize the need for invasive testing are listed in Table 4. HFpEF has a unique pathophysiology, characterized by severe dysfunction of the diastolic phase of the cardiac cycle that results in elevated ventricular pressures [18]. In addition, impairment of myocardial relaxation and stiffness lead to reduced LV filling, elevated diastolic pressures, and HF symptoms [18]. Hemodynamic measurements reveal prolonged isovolumic pressure decline and upward–leftward shift in the pressure–volume loop, with aberrant myocardial relaxation coupled with high indices of passive stiffness [18].
Table 4.
Clinical parameters for the diagnosis of heart failure with preserved ejection fraction [18].
| Parameters |
|---|
|
5. Cardiac imaging and diagnostic work-up
Identifying reduced LV function is critical to the diagnosis of HFrEF. It can be detected using multiple modalities (Table 5, Table 6, Table 7, Table 8), including transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), cardiac magnetic resonance (CMR) imaging, left ventriculography during cardiac catheterization, radionuclide ventriculography, and single-photon emission computed tomography (SPECT) [23].
Table 5.
Recommendations for cardiac imaging in patients with suspected or established heart failure.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AHF = acute heart failure; ARVC = arrhythmogenic right ventricular cardiomyopathy; CAD = coronary artery disease; CMR = cardiac magnetic resonance; CRT = chronic heart failure; HF = heart failure; HFbEF = heart failure with borderline ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; PET = positron emission tomography; SPECT = single-photon emission computed tomography; TTE = transthoracic echocardiography.
Table 6.
Recommendations for conducting diagnostic tests in patients with heart failure.
 |
Table 7.
Recommendations for lung ultrasound in patients with heart failure.
Table 8.
Recommendations for genetic testing.
5.1. Chest X-ray
Although the benefits of chest X-rays in the diagnostic work-up of patients with suspected HF are limited, it is the most useful tool in identifying an alternative pulmonary cause [3].
5.2. Transthoracic echocardiography
Echocardiography is an essential tool for establishing diagnosis and etiology, and understanding the pathophysiology of HF. It is recommended that all patients with signs and symptoms of HF, or incidental findings of a low EF on other imaging modalities, be evaluated with TTE as an initial depth analysis because of its well-established accuracy, availability, safety, and low cost [24]. In addition, TTE is useful for assessing LV function. An initial and complete TTE study is pivotal and must include the following [25], [26]:
-
1.
LV chamber and wall assessment
-
2.
LV function quantification
-
3.
Transmitral Doppler patterns
-
4.
Pulmonary venous flow patterns
-
5.
Left atrial volume index
-
6.
Valvular assessment
-
7.
RV chamber size and function assessment
Where advanced echocardiography techniques are available, strain rates and global longitudinal strain imaging should be used, especially in patients who receive cardiotoxic cancer therapies.
5.3. Transesophageal echocardiography
TEE may be valuable for the diagnostic work-up of patients with valve disease, suspected aortic dissection, suspected endocarditis or its complications, or congenital heart disease, and for ruling out intracavitary thrombi in patients with AF requiring cardioversion [3]. It is also recommended that, when the severity of mitral or aortic valve disease is inconsistent with the patient’s symptoms, TEE be used for confirmation [3].
5.4. Stress echocardiography
Stress echocardiography has multiple uses in patients with HF. It may be used to detect diastolic dysfunction related to exercise in patients with exertional dyspnea, preserved LVEF, and inconclusive diastolic parameters at rest. It is also useful for the assessment of inducible ischemia, myocardial viability, and in valve disease [3]. Resting echocardiography often underestimates the severity of HFpEF, therefore, exercise stress echocardiography and cardiopulmonary exercise testing are useful for dynamic assessment of HFpEF [27]. Practitioners are encouraged to use stress echocardiography especially in patients with shortness of breath and no clear resting abnormality. In HF patients with a normal EF, the deterioration of ventricular and peripheral performance is evident during exercise. Furthermore, patients with HF exhibit chronotropic noncompetence during exercise [28].
5.5. Cardiac magnetic resonance
CMR is the gold standard for measurements of volume, mass, and the EF of both the LVs and RVs [3]. CMR is preferred for assessment of myocardial fibrosis and complex congenital heart disease, and may be useful for the assessment of myocardial ischemia and viability in patients with HF and CAD [3]. CMR facilitates the characterization of myocardial tissue of myocarditis, amyloidosis, sarcoidosis, Chagas disease, Fabry disease, noncompaction cardiomyopathy, and hemochromatosis [3]. However, the usefulness of CMR in the Saudi local setting is limited by insufficient local expertise, availability, and cost, compared with echocardiography.
5.6. SPECT, radionuclide ventriculography, and positron emission tomography
SPECT may be useful in assessing myocardial viability or ischemia. Gated SPECT may be used to capture details on ventricular volumes and function; however, it exposes the patient to ionizing radiation [3]. Positron emission tomography (PET), with or without CT, may be used to assess ischemia and viability, but limited availability, radiation exposure, and cost are the main limitations [3].
5.7. Coronary angiography
Coronary angiography is recommended in patients with HF who suffer from angina pectoris recalcitrant to medical therapy, those with a history of symptomatic ventricular arrhythmia or aborted cardiac arrest, and may also be considered in patients with HF and intermediate-to-high pretest probability of CAD as well as the presence of ischemia (assessed by noninvasive stress tests) [3]. Invasive coronary angiography is the gold standard for the anatomical assessment of CAD in patients with HF [29].
5.8. Cardiac computed tomography
Computed tomography angiography (CTA) is an important noninvasive tool for the diagnosis of HF [23]. Compared with invasive coronary angiography, CT coronary angiography (CTCA) has several advantages, including reliability in proving or ruling out the presence of CAD in patients with low-or-intermediate pretest probability of CAD. Cardiac CT at high spatial and temporal resolution is fast, patient friendly, and is associated with declining doses of ionizing radiation [30].
CTA can be used to diagnose reduced LV function by determining EF, which correlates well with echocardiographic assessment [23]. Although CTA and CMR imaging have a strong correlation for EF calculation, CTA has limited temporal resolution compared with CMR imaging, resulting in slight overestimations of end-systolic volume and EF, especially in patients with HFrEF [23]
Although multiple echocardiographic indices (including mitral valve flow velocities and tissue Doppler velocities) are used to diagnose HF with preserved EF, CTA can measure diastolic properties and may have a future role in HFpEF [23].
5.9. Lung ultrasound
In patients with pleural effusion, lung ultrasound can assist in diagnosing the nature of effusion and visualization of internal echoes, either of mobile particles or septa, and is highly suggestive of exudate or hemothorax [31]. It is more accurate than a chest X-ray, particularly for the anterior–posterior view of a supine patient [31].
In patients with HF, lung ultrasound is an alternative tool for monitoring changes in pulmonary congestion during treatment, which are detected by variations in ultrasound patterns [31]. A study assessing the prognostic value of residual pulmonary congestion in HF inpatients reported that residual pulmonary congestion at discharge, assessed by a B-line count of ≥30, was a strong predictor of all-cause death or HF rehospitalization [32]. Similarly, a prospective cohort study of patients with suspected AHF found that the 6-month event-free survival was lowest in patients with B-lines >15; and persistent congestion prior to discharge (B-lines >15) was a strong predictor of rehospitalization for HF at 6 months [33].
5.10. Genetic testing for heart failure
In the presence of adequate expertise, it is recommended that genetic counseling be offered to patients with hypertrophic cardiomyopathy (HCM), DCM, and arrhythmogenic right ventricular cardiomyopathy (ARVC). Combining the CMR findings with genetic testing can contribute greatly to the diagnosis and risk stratification of HCM, and to assessing the need for placement of implantable cardioverter defibrillators (ICDs) for primary prevention of complications [34].
DCM is characterized by genetic heterogeneity, with more than 40 genes implicated in the disease [35], [36]. Idiopathic DCM is familial in 25% of cases and, although it may be difficult to identify asymptomatic relatives due to lack of a molecular marker, screening may result in early treatment, which may improve prognosis in affected individuals [37]. Although genetic testing is challenging, it is a useful tool in the clinical management of DCM. Testing for pathogenic mutations facilitates appropriate treatment and may assist in predicting disease risk for family members before the onset of symptoms [38].
ARVC is an inherited disease of the heart muscle that may lead to life-threatening ventricular arrhythmias, sudden cardiac death, and/or biventricular HF [39]. ARVC is predominantly associated with mutations in desmosomal genes, and has a broad spectrum of phenotypic variation and age-related penetrance [40]. Although the diagnosis of ARVC is challenging due to lack of definitive testing methods, genetic testing and CMR imaging play an important role in the identification of disease [40]. ICD implantation is considered a life-saving therapy for patients with ARVC, and exercise restriction may delay disease progression [40].
In general, the approach to cardiac screening and genetic testing should be family specific and requires expertise in the genetics of cardiomyopathy [41].
6. Preventing heart failure (Table 9)
Table 9.
Recommendations for the prevention of heart failure.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
ACE-I = angiotensin-converting enzyme inhibitor; CAD = coronary artery disease; HF = heart failure; ICD = implantable cardioverter defibrillator; LV = left ventricle; LVEF = left ventricle ejection fraction.
Antihypertensive drugs (diuretics, ACE-Is, ARBs, and beta-blockers) exert a strong protective effect against HF, particularly in older people [42], [43], [44]. The SPRINT study, involving 9361 high-risk, hypertensive, nondiabetic patients, showed that a target systolic blood pressure of <120 mmHg, compared with <140 mmHg, was significantly effective in reducing the rates of myocardial infarction (MI), other acute coronary syndromes, stroke, HF, or death from cardiovascular causes (1.65% vs. 2.19% per year, HR with intensive treatment 0.75, 95% CI 0.64–0.89; p < 0.001) [45].
Smoking has been associated with a significant risk of HF [46]. In a separate study, the incidence of HF was measured at 11.4 per 1000 person-years in nonsmokers, 15.2 in past smokers (HR vs. nonsmokers 1.33, 95% CI 1.01–1.76; p = 0.045), and 21.9 in current smokers (HR vs. nonsmokers 1.93, 95% CI 1.30–2.84; p = 0.001) [47]. Smoking cessation has a significant and swift (within 2 years) effect on reducing morbidity and mortality in patients with LV dysfunction [48]. Abstinence from smoking for more than 15 years reduces the risk of HF and all-cause mortality to that of never-smokers [49].
There is a strong relationship between increased physical activity and a reduced risk of HF. In fact, a substantial risk reduction in HF was observed in individuals who engaged in physical activity two times (HR 0.81, 95% CI 0.77–0.86) and four times (HR 0.65, 95% CI 0.58–0.73) a week above the minimum guideline recommended levels (500 metabolic equivalent-minutes/week; 2008 US federal guidelines) [50].
Statins are considered to be promising candidates for HF treatment because of their role in improving endothelial function, enhancing nitric oxide synthesis, restoring impaired autonomic function, and inhibiting inflammatory cytokine release [51].
A meta-analysis involving 647,388 participants showed a risk ratio of 1.41 for the incidence of HF (95% CI 1.34–1.47), and 1.26 for HF mortality (95% CI 0.85–1.87) with every 5-unit increment in body mass index. Furthermore, every 0.1-unit increase in waist-to-hip ratio was associated with a risk ratio of 1.29 for HF incidence (95% CI 1.13–1.47) [52]. However, the benefit of weight loss in obese patients with HF remains unclear, which represents what is commonly known as the obesity paradox in HF [53].
Prophylactic implantation of a defibrillator improves survival in patients with prior MI and advanced LV dysfunction [54].
7. Pharmacological treatment of HFrEF (Table 10, Table 11)
Table 10.
Recommendations for pharmacological treatments for patients with HFrEF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
aOr an ARB if the ACE-I is not tolerated or contraindicated.
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid antagonist.
Table 11.
Recommendations for treatment combinations that may cause harm in patients with HFrEF.
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; COX = cyclooxygenase; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid antagonist; NSAID = non-steroidal anti-inflammatory drug.
The algorithm for the pharmacological treatment of HFrEF is displayed in Fig. 6. Neurohormonal antagonists (ACE-Is and beta-blockers) have been shown to improve survival in patients with HFrEF and are recommended for the treatment of all patients with HFrEF, unless contraindicated or not tolerated. ARBs are recommended only as an alternative in patients who are intolerant of ACE-Is. A new compound (LCZ696), which combines the moieties of an ARB (valsartan) and a neprilysin inhibitor (sacubitril), has recently been shown to be superior to an ACE-I (enalapril) in reducing the risk of death and of hospitalization for HF in a single trial with strict inclusion/exclusion criteria [55]. Therefore, sacubitril/valsartan is recommended to replace ACE-Is in ambulatory HFrEF patients who remain symptomatic despite optimal therapy and who fit these trial criteria. Digoxin may be considered in patients with AF with symptomatic HFrEF to reduce the risk of hospitalization (both all-cause and HF hospitalizations). A recent meta-analysis concluded, based on non-randomized controlled trials (RCTs), that digoxin has no deleterious effect on mortality in patients with AF and concomitant HF, most of whom had HFrEF [56].
Fig. 6.
Algorithm for pharmacological treatment of HFrEF. ACE-I = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor/neprilysin inhibitor; BB = beta-blocker; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HR = heart rate; MRA = mineralocorticoid antagonist.
7.1. Treatments recommended in all symptomatic patients with HFrEF
7.1.1. Renin–angiotensin–aldosterone system inhibitors and beta-blockers
A recent meta-analysis conducted by Thomsen et al. [57] evaluated RCTs of drugs recommended by the ESC and AHA guidelines for the treatment of patients with HFrEF. The analysis included 47 RCTs that included patients with an average age of 63 years, 22% of whom were women, and looked at outcomes of all-cause mortality and hospitalization due to HF [57].
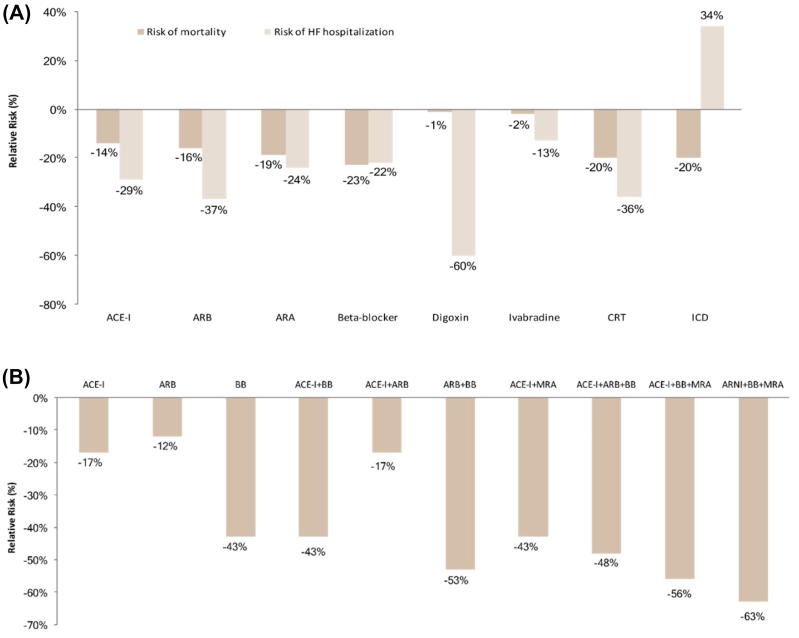
The relative risk (RR) for mortality was similar for drugs targeting the renin–angiotensin–aldosterone system (RAAS), beta-blockers, cardiac resynchronization therapy (CRT), and ICDs (Fig. 7A). Although drugs targeting the RAAS, beta-blockers, digoxin, and CRT substantially reduced the risk of HF hospitalization, ICDs were associated with a significantly increased risk of HF hospitalization (34%) [57]. Overall, ivabradine showed no significant effect on reducing the risk of mortality or HF hospitalization [57]. Although drugs recommended for HFrEF offer significant benefit, studies included in the analysis were from the 1990s or earlier and included a population of men with a different age distribution than the current average for HF [57]. Therefore, the authors advise that extrapolating results to the current population should be done with caution.
Fig. 7.
(A) Relative risk for mortality and HF hospitalization categorized by treatment group [57]. (B) Relative risk for all-cause mortality categorized by treatment group [58]. ACE-I = angiotensin-converting enzyme inhibitor; ARA = aldosterone receptor antagonist; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor/neprilysin inhibitor; BB = beta-blocker; HF = heart failure; CRT = cardiac resynchronization therapy; ICD = implantable cardioverter defibrillator; MRA = mineralocorticoid receptor antagonist.
In a recent network meta-analysis by Burnett et al. [58] including 57 RCTs, the random-effects model suggested that the combination of an ACE-I plus a beta-blocker plus a mineralocorticoid antagonist (MRA) was associated with a 56% reduction in mortality versus placebo [HR 0.44, 95% credible interval (CrI) 0.26–0.66], and an angiotensin receptor/neprilysin inhibitor (ARNI) plus a beta-blocker plus an MRA was associated with a 63% reduction in all-cause mortality versus placebo (HR 0.37, 95% CrI 0.19–0.65; Fig. 7B) [58].
A recent sensitivity analysis of nine RCTs with a high background use of an ACE-I and/or an ARB (>80%) indicated that adding an aldosterone receptor antagonist (ARA) to current standard therapy substantially reduced mortality by 27% (OR 0.73, 95% CrI 0.51–0.95) and hospitalization risk by 33% (OR 0.67, 95% CrI 0.47–0.87), and did not significantly increase the discontinuation risk (OR 1.29, 95% CrI 0.83–2.31) [59].
Beta-blockers have been the backbone of HF treatment because of their ability to reverse the neurohumoral effects of the sympathetic nervous system, with prognostic and symptomatic benefits. A meta-analysis of 21 clinical trials including 23,122 patients treated with beta-blockers (focusing on atenolol, bisoprolol, bucindolol, carvedilol, metoprolol, and nebivolol) reported that beta-blockers reduced the risk of mortality compared with placebo or standard treatment after a median of 12 months of treatment (OR 0.69, 95% CrI 0.56–0.80) [60]. When comparing the different beta-blockers for risk of death, sudden cardiac death, death due to pump failure, or drug discontinuation, no differences were found [60]. Improvements in LVEF were also similar irrespective of the individual study drug, indicative of a class effect [60]. A more recent meta-analysis of 11 trials including 13,833 patients (aged 40–85 years, of whom 24% were women) found that beta-blockers were effective in reducing mortality across all ages: the absolute reduction in mortality was 4.3% over a median follow-up of 1.3 years (number needed to treat 23) [61]. The rate of drug discontinuation was found to be similar irrespective of treatment allocation, age, or sex, with 14.4% discontinuations in patients on beta-blockers and 15.6% in those receiving placebo [61].
7.1.2. Angiotensin receptor/neprilysin inhibitors
In the landmark PARADIGM-HF trials, sacubitril/valsartan, a first-in-class ARNI, was reported to be superior to enalapril in reducing the risk of death from cardiovascular causes or first hospitalization for HF (HR 0.80, 95% CI 0.73–0.87; p < 0.001), risk of death from cardiovascular causes (HR 0.80, 95% CI 0.71–0.89; p < 0.001), and risk of hospitalization for HF (HR 0.79, 95% CI 0.71–0.89; p < 0.001) [55]. Furthermore, sacubitril/valsartan was more beneficial than enalapril across all age categories (<55, 55–64, 65–74, and ≥75 years) in terms of benefit–risk profile. Treatment withdrawal due to intolerance was uncommon, even in elderly individuals [62]. Sacubitril/valsartan also led to significant treatment for outpatient worsening (HR 0.84, 95% CI 0.74–0.94; p = 0.003), emergency department visits for HF (HR 0.66, 95% CI 0.52–0.85; p < 0.001), cardiovascular hospitalization (HR 0.88, 95% CI 0.81–0.95; p < 0.001), all-cause hospitalization (HR 0.88, 95% CI 0.82–0.94; p < 0.001), and intensive care unit (ICU) admission (HR 0.87, 95% CI 0.78–0.98; p = 0.019) [63]. In addition, it is important to note that not all patients in the PARADIGM trial were on MRA.
RAAS blockers are effective therapies for patients with HF and reduced EF or LV dysfunction [59]. A recent meta-analysis of 21 double-blind RCTs including 69,229 patients reported that, compared with placebo, an ARNI had the highest probability of reducing all-cause mortality (OR 0.67, 95% CrI 0.48–0.86), followed by an ARA (OR 0.74, 95% CrI 0.62–0.88) and an ACE-I (OR 0.80, 95% CrI 0.71–0.89) [59]. An ARNI was found to be the most efficacious therapy for preventing HF hospitalization (OR 0.55, 95% CrI 0.40–0.71), followed by an ARB plus an ACE-I (OR 0.61, 95% CrI 0.49–0.75) and an ACE-I alone (OR 0.69, 95% CrI 0.61–0.77) [59]. Therefore, it was concluded that ARNI has the highest probability of being the most efficacious therapy for HFrEF in reducing death and hospitalization for HF.
7.1.3. If channel inhibitor
A high resting heart rate (≥70–75 bpm) is a sign of sympathetic hyperactivity and/or reduced parasympathetic tone, and has several detrimental consequences including the acceleration of coronary atherosclerosis, plaque rupture, subclinical inflammation, reactive oxygen species generation, myocardial ischemia, induction of left ventricular dysfunction, and life-threatening arrhythmias [64].
The BEAUTIFUL study, which assessed the effect of ivabradine in patients with stable CAD and left ventricular systolic dysfunction in 10,917 patients (5479 ivabradine, 5438 placebo), reported that although ivabradine reduced heart rate by 6 bpm at 12 months, it did not improve cardiac outcomes (HR 1.00, 95% CI 0.91–1.1; p = 0.94) [65]. In a subgroup analysis of patients on placebo (2693 had ≥70 bpm, 2745 had <70 bpm), it was found that for every increase of 5 bpm, there were significant increases in cardiovascular death (8%; p = 0.0005), hospital admissions for HF (16%; p < 0.0001), admission to hospital for MI (7%; p = 0.052), and coronary revascularization (8%; p = 0.034) [66].
Contrary to the findings of the BEAUTIFUL study, the SHIFT trial, which randomized 3268 patients to ivabradine and 3290 patients to placebo, reported a significant risk reduction of 18% (HR 0.82, 95% CI 0.75–0.90; p < 0.0001) in the composite primary endpoint (cardiovascular death or hospital admission for worsening HF) in those on ivabradine versus placebo [67]. These effects were mainly due to reduced hospital admissions for worsening HF (21% vs. 16%, HR 0.74, 95% CI 0.66–0.83; p < 00001) and reduced deaths due to HF (5% vs. 3%, HR 0.74, 95% CI 0.58–0.94; p = 0.014) [67]. Analysis of cardiovascular outcomes revealed that the risk of primary composite endpoint events increased by 3% with every beat increase from baseline heart rate, and 16% for every 5 bpm increase [68]. In the ivabradine group, the heart rate achieved at 28 days on treatment was directly associated with cardiac outcome [68]. Patients with heart rates lower than 60 bpm after 28 days on treatment had fewer primary composite endpoint events during the study (n = 1192, event rate 17.4%, 95% CI 15.3–19.6) than patients with higher heart rates [68].
Similar to the SHIFT trial, the INTENSIFY study reported that after 4 months of treatment with ivabradine, heart rate was reduced to 67 ± 8.9 bpm from 85 ± 11.8 bpm at baseline [69]. In addition, the proportion of patients with signs of decompensation reduced from 22.7% to 5.4%, and the proportion of BNP levels >400 pg/mL reduced from 53.9% to 26.7% [69]. These benefits were also accompanied by improved QoL and good general tolerability [69].
7.1.4. Mineralocorticoid antagonists
MRAs have been shown to reduce mortality and morbidity in patients with mild-to-severe HF with reduced LVEF, however, their use is limited as they cause hyperkalemia [70]. An analysis of the EMPHASIS-HF study showed that in patients with chronic HFrEF, in NYHA functional Class II and meeting specific inclusion and exclusion criteria (including an estimated glomerular filtration rate >30 mL/min/1.73 m2 and potassium <5.0 mmol/L), eplerenone was both efficacious and safe when carefully monitored, even in subgroups with a high risk of developing hyperkalemia or worsening renal function [71].
By contrast, it was still unclear whether elevations in potassium reduced the clinical benefit of MRAs in patients with severe HF. Therefore, the RALES study assessed the incidence and predictors of hyperkalemia (potassium ≥5.5 mmol/L) and hypokalemia (potassium <3.5 mmol/L), and hypothesized that hyperkalemia would not modify the efficacy of spironolactone (25 mg) in 1663 patients with severe HF [70].
The RALES study revealed that 1 month after initiating treatment, mean potassium levels increased in the spironolactone group but not in the placebo group (4.54 ± 0.49 vs. 4.28 ± 0.50 mmol/L; p < 0.001), and remained elevated during the trial. Participants randomized to spironolactone had a higher risk of hyperkalemia and a lower risk of hypokalemia compared with those randomized to placebo [70]. Furthermore, those attaining a spironolactone dose of 25 mg had a 13.5% risk of hyperkalemia and those reaching a dose of 50 mg had a 41.4% risk of hyperkalemia, with no difference noted in mortality rates [70]. Compared with placebo, mortality rates were highest in patients with the lowest (<3.5 mmol/L) and highest (>6.0 mmol/L) 4-week potassium values [70]. In general, mortality rates were higher in participants randomized to placebo compared with those taking spironolactone, at all potassium levels (p < 0.0001) [70]. The treatment benefit of spironolactone was maintained when potassium levels exceeded 5.5 mmol/L, although this benefit lost statistical significance as potassium value neared 6.0 mmol/ L [70].
7.1.5. Diuretics
Diuretics are regarded as the first-line treatment for patients with CHF because they provide symptomatic relief. A Cochrane review of 14 trials (7 placebo controlled, 7 active controlled) including 525 patients reported that mortality was lower for patients receiving diuretics compared with placebo (OR 0.24, 95% CI 0.07–0.83; p = 0.02), and admission for worsening HF was reported to be lower in two trials (OR 0.07, 95% CI 0.01–0.52; p = 0.01) [72]. Diuretics were found to improve exercise capacity compared with active comparators [weighted mean difference (MD) 0.72, 95% CI 0.40–1.04; p < 0.0001] in four of the trials [72].
Diuretics are particularly effective in ameliorating clinical signs and symptoms of HF, especially systemic and pulmonary congestion [73]. Although diuretics are the most commonly prescribed drugs for HF management, there is little quality evidence to guide their use [73]. In addition, observation data suggest that diuretics may be harmful and contribute to neurohormonal activation, renal dysfunction, and ultimately, mortality [73]. Despite these concerns, diuretics are the mainstay of HF management: the main classes include loop diuretics, potassium-sparing diuretics, and thiazides [73]. It is important that electrolytes and renal function are carefully monitored during diuretic therapy [73]. Furthermore, fluid overload refractory to loop diuretics can complicate HF patient management, and the CLOROTIC trial, which is the first large-scale trial to evaluate the safety and efficacy of the addition of a thiazide diuretic to a loop diuretic for improving congestive symptoms resulting from HF, may provide important information on treatment strategy in such patients [74].
7.1.6. Combination of hydralazine and isosorbide dinitrate
The V-Heft I trial evaluated the combination of hydralazine and isosorbide dinitrate in 642 men over a 5-year period versus placebo with prazosin [75]. It was found that peak oxygen consumption (VO2) significantly increased at 2 months (p < 0.16) and was sustained up to 1 year (p < 0.04) with hydralazine and isosorbide dinitrate compared with placebo [75]. In the V-Heft II trial, hydralazine and isosorbide dinitrate significantly increased peak VO2 compared with enalapril (p < 0.01 at 3 months; p < 0.02 at 6 months and 2 years) [75]. The authors concluded that long-term data were confounded by mortality and other events, which may have led to the benefits of hydralazine and isosorbide dinitrate over placebo, and enalapril on exercise performance, being underestimated. Overall, the authors concluded that short-term improvement in exercise performance is a suitable therapeutic endpoint. In addition, an RCT conducted in self-identified patients of African descent found that the addition of hydralazine and isosorbide dinitrate reduced mortality and HF hospitalization in patients with HFrEF (NYHA Class III–IV), compared with conventional therapy (ACE-Is, beta-blockers, and MRAs) [76]. It is difficult to translate data from this study to patients of other racial and ethnic origins, so the combination of hydralazine and isosorbide dinitrate should be considered in symptomatic patients with HFrEF who can tolerate neither ACE-Is nor ARBs to reduce mortality [3].
7.1.7. Digoxin
Observational studies report conflicting results on the association of digoxin with mortality in patients with HF. In the ENGAGE AF TIMI 48 trial, in patients with AF and HF (n = 12,124), digoxin use was associated with a 37% increased risk of all-cause death, cardiovascular death, sudden cardiac death, and death caused by HF/cardiogenic shock (p < 0.01 for each) [77]. Given that there is strong evidence suggesting an association of serum concentration of digoxin with its safety and efficacy, it is necessary to achieve low serum digoxin concentrations (0.5–0.9 ng/mL) to optimize therapeutic benefit and avoid harm [78].
Recommended target doses of key agents used for managing patients with HF are outlined in Table 12.
Table 12.
Recommended target doses of disease-modifying agents and diuretics for HF.
| Disease-modifying agents | Target doses (mg) |
|---|---|
| ACE-Is | |
| Captopril | 50 t.i.d. |
| Enalapril | 20 b.i.d. |
| Lisinopril | 20–40 o.d. |
| Ramipril | 10 o.d. |
| Trandolapril | 4 o.d. |
| Beta-blockers | |
| Bisoprolol | 10 o.d. |
| Carvedilol | 25 b.i.d. |
| Metoprolol succinate | 200 o.d. |
| Nebivolol | 10 o.d. |
| ARBs | |
| Candesartan | 32 o.d. |
| Valsartan | 160 b.i.d. |
| Losartan | 150 o.d. |
| Mineralocorticoid antagonist | |
| Eplerenone | 50 o.d. |
| Spironolactone | 50 o.d. |
| Angiotensin receptor/neprilysin inhibitor | |
| Sacubitril/valsartan | 97/103 b.i.d. |
| If channel blocker | |
| Ivabradine | 7.5 b.i.d. |
| Diuretic | Usual daily doses (mg) |
| Loop diuretics | |
| Furosemide | 40–240 |
| Bumetanide | 1–5 |
| Torasemide | 10–20 |
| Thiazides | |
| Hydrochlorothiazide | 12.5–100 |
| Metolazone | 2.5–10 |
| Indapamide | 2.5–5 |
| Potassium-sparing diuretics | |
| +ACE-I/ARB | |
| Spironolactone/eplerenone | 50 |
| Amiloride | 5–10 |
| Triamterene | 100 |
| –ACE-I/ARB | |
| Spironolactone/eplerenone | 100–200 |
| Amiloride | 10–20 |
| Triamterene | 200 |
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; b.i.d. = twice daily; mg = milligrams; o.d. = once daily; t.i.d = thrice daily.
8. Nonsurgical device treatment of HFrEF (Table 13, Table 14)
Table 13.
Recommendations for implantable cardioverter defibrillators in patients with HF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
CRT = cardiac resynchronization therapy; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association; OMT = optimal medical therapy; VT/VF = ventricular tachycardia/ventricular fibrillation.
Table 14.
Recommendations for cardiac resynchronization therapy implantation in patients with HF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AF = atrial fibrillation; CRT = cardiac resynchronization therapy; EF = ejection fraction; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; OMT = optimal medical therapy; RV = right ventricle.
8.1. Implantable cardioverter defibrillator
Patients who survive an out-of-hospital cardiac arrest or symptomatic sustained ventricular tachycardia are at considerable risk of recurrence of these arrhythmias and of death [79], and ICDs play a major role in the prevention of sudden cardiac death [80]. An ICD leads to a 28% reduction in RR of death, which is primarily due to a 50% reduction in arrhythmic death [79]. Patients with an LVEF ≤35% were reported to derive significantly more benefit from ICD therapy than those with a better preserved LV function, as per a meta-analysis of three RCTs (AVID, CASH, and CIDS). Based on a subanalysis of secondary prevention trials of ICDs, patients treated with an ICD in the AVID study had a maximal survival benefit when the EF was 20–34%, compared with amiodarone [81]. Greater survival benefit (50% reduction in risk of mortality) was observed in the higher-risk group, described as those older than 70 years, EF <35%, and NYHA Class III–IV, in the CIDS study [81]. Of note, in the AVID study, the recurrence of arrhythmia was 64% after 3 years in patients with the ICD [81]. Similar results were reported by the Sudden Cardiac Death in Heart Failure Trial, where patients with NYHA Class II or III CHF and an LVEF ≤35% did not experience a favorable effect on survival with amiodarone, but shock-only ICD therapy was found to reduce overall mortality by 23% [82].
A combined analysis of four RCTs found that the unadjusted HR of death for patients with an ICD versus those without was significantly lower, especially in patients with two or more comorbidities (HR 0.71, 95% CI 0.61–0.84) compared with patients with less than two comorbidities (HR 0.59, 95% CI 0.40–0.87) [80]. In addition, after adjustment, increasing comorbidity was associated with decreasing treatment benefit from an ICD (p = 0.004) [80]. A recent pooled analysis of five major ICD trials reported that the survival benefit of an ICD is attenuated with increasing age: HR 0.48 (95% posterior CrI 0.33–0.69) in patients aged <55 years, HR 0.69 (95% posterior CrI 0.53–0.90) in patients aged 55–64 years, HR 0.67 (95% posterior CrI 0.53–0.85) in patients aged 65–74 years, and HR 0.54 (95% posterior CrI 0.37–0.78) in patients aged ≥75 years [83]. This may be due to a higher burden of comorbid illness, competing causes of death, or the limited sample size of patients [83]. Furthermore, no evidence was found that age influenced the likelihood of rehospitalization after ICD placement [83]. In addition, a meta-analysis reported that ICD-only therapy provided survival benefit (arrhythmic mortality RR 0.40, 95% CI 0.27–0.67; and all-cause mortality RR 0.73, 95% CI 0.64–0.82) in patients with ischemic or non-ischemic heart disease, with an LVEF ≤35%, 40 days from MI, and ≤3 months from CRT [84].
Although ICDs are considered effective in primary and secondary prevention of sudden cardiac death, they are expensive. A systematic review of cost-effectiveness that analyzed data from 34 studies showed that ICDs may be a cost-effective option in patients at high risk of sudden cardiac death in comparison with conventional treatments [85]. Several factors influence the cost-effectiveness of ICDs, including device implantation cost, frequency and cost of battery replacement, and patient demographics and risk profile. These warrant the need for continuous research to ensure the cost-effective use of ICD therapy [85].
8.2. Cardiac resynchronization therapy
An individual patient meta-analysis of five RCTs comparing CRT with no active device or with a defibrillator showed that QRS duration (QRSD) was a powerful predictor of the effects of CRT on morbidity and mortality in patients with symptomatic HF and LV systolic dysfunction who are in sinus rhythm [86]. A QRSD that exceeds 140 ms was determined to lead to substantial survival benefit from CRT. QRS morphology did not provide additional information about clinical response [86].
The REVERSE trial found that, after 24 months of CRT, LV end-systolic volume index (LVESVI) decreased by a mean of 27.5 ± 31.8 mL/m2 versus 2.7 ± 25.8 mL/m2 in patients not on CRT (p < 0.0001) [86]. Time to hospital stay or death was also delayed significantly by CRT (HR 0.38; p = 0.003), suggesting that CRT prevents the progression of disease in patients with asymptomatic or mildly symptomatic LV dysfunction [87]. In a subgroup analysis, patients with an LVEF >30% on CRT showed significant reductions in LVESVI (−6.7 ± 21.1 vs. 2.1 ± 17.6 mL/m2; p = 0.01) and LV mass (−20.6 ± 50.5 vs. 5.0 ± 42.4 g; p = 0.04) after 12 months, in comparison with patients not on CRT, and a trend of improvement in clinical composite response with CRT (p = 0.06) [88]. It was also demonstrated that left bundle branch block (LBBB) and QRS prolongation were markers of reverse remodeling and clinical benefit with CRT in mild HF [89]. In addition, long-term follow-up revealed that CRT was associated with a 68% reduction in mortality in patients with ≥15% decrease in LVESVI [90]. Multivariate analysis showed that a change in LVESVI was a strong independent predictor of mortality (p = 0.0002), with a 14% reduction in mortality for every 10% decrease in LVESVI [90]. Longer QRSD, smaller LVESVI, CRT defibrillator (CRT-D) recipients, and women were associated with better survival [90]. Overall, it can be concluded that CRT in addition to optimal medical therapy produces long-standing clinical benefits in mild HF [91].
In patients at increased risk of arrhythmia-related sudden death and HF, placement of an ICD has been shown to improve survival and reduce risk of sudden death in appropriately selected patients [92]. However, an ICD is associated with an increased risk of first and recurrent HF events. CRT with biventricular pacing may be an effective adjunctive therapy to pharmacologic management in reducing the rates of hospitalizations in patients with NYHA Class III–IV symptoms, an EF ≤35%, and intraventricular conduction delay of ≥120 ms [92].
In the MADIT-CRT trial, during an average follow-up of 2.4 years, the primary endpoint of death from any cause or a nonfatal HF event occurred in 17.2% in the CRT-ICD group and 25.3% in the ICD-only group (HR 0.66, 95% CI 0.52–0.84; p = 0.001 for the CRT-ICD group) [92]. However, no difference in the overall risk of death was observed between treatment groups. In a subgroup of patients with a QRSD of ≥150 ms, CRT use was associated with a 41% reduction in the risk of HF events [92]. A multivariate analysis showed that CRT-D was associated with significant reduction in the risk of the first HF event (HR 0.54, 95% CI 0.44–0.67; p < 0.001) and subsequent events (HR 0.62, 95% CI 0.45–0.85; p = 0.003) [93]. Prevention of HF events was found to be pronounced among patients with LBBB for first (HR 0.38, 95% CI 0.29–0.49; p < 0.001) and subsequent (HR 0.50, 95% CI 0.33–0.76; p < 0.001) events [93]. Overall, sevenfold and 19-fold increases in risk of mortality were reported to be associated with first and second HF events, respectively [93]. Long-term follow-up (7 years) indicated that in patients with mild HF symptoms, LV dysfunction, and LBBB, early intervention with a CRT-D was associated with a significant long-term survival benefit [94]. Of note, the clinical benefit of a CRT-D was not attenuated in patients with LBBB with a history of intermittent atrial tachyarrhythmias or by development of in-trial atrial tachyarrhythmias [95].
Although the MADIT-CRT trial did not show a difference in survival benefit between patients with ischemic or non-ischemic cardiomyopathy [92], a recent meta-analysis of 19 studies including 12,378 patients reported that survival benefit appears to be more pronounced in patients with ischemic cardiomyopathy (HR 0.70, 95% CI 0.59–0.83; p < 0.001, I2 = 0%) compared with non-ischemic cardiomyopathy (HR 0.79, 95% CI 0.61–1.02; p = 0.07, I2 = 36%) [96]. Although the majority of patients treated with CRT had LBBB morphology (QRS <150 ms), its role in non-LBBB morphology (QRSD ≥150 ms) is unclear, with evidence often indicating that CRT should be discouraged in the non-LBBB setting due to lack of benefit [97]. This is clearly shown by a recent study of 973 patients with HF treated with CRT in which LBBB morphology was significantly associated with better survival (HR 0.737, 95% CI 0.584–0.93; p = 0.010), whereas there was no statistically significant association between non-LBBB morphology and survival (HR 0.889, 95% CI 0.726–1.088; p = 0.252) [98]. Therefore, the authors concluded that QRS morphology was independently associated with long-term survival in patients with HF treated with CRT.
In addition, an economic analysis revealed that in patients with an LVEF ≤30%, QRSD of ≥120 ms, and NYHA Class II symptoms, CRT-D is a more cost-effective alternative to ICD alone in terms of survival benefit [99].
A recent study examining outcomes in 24,960 patients reported a greater survival benefit with CRT-D versus standard ICD in patients with LBBB and QRSD ≥180 ms (HR 0.65, 95% CI 0.59–0.72) than in those with QRSD of 120–149 ms (HR 0.85, 95% CI 0.80–0.92) and QRSD of 150–179 ms (HR 0.87, 95% CI 0.81–0.93) [100]. Compared with standard ICD, CRT-D was found to reduce the risk of death by 22% in patients with an LBBB and QRSD ≥180 ms (HR 0.78, 95% CI 0.68–0.91), but not in those with an LBBB and QRSD of 150–179 ms (adjusted HR 1.06, 95% CI 0.95–1.19) [100].
9. Treatment of HFpEF (Table 15)
Table 15.
Recommendations for treatment of HFpEF.
HF with preserved EF is a complex clinical syndrome that comprises approximately half of all patients with HF [101]. The leading cause of death in patients with HFpEF is non-cardiovascular [102]. Guidelines for the treatment and diagnosis of HFpEF are lacking and complicated by the heterogeneous population: multiple comorbidities, race, age, and etiology. Longitudinal studies on this patient population are required for the design of adequate interventional therapies [103]. Health-related quality of life (HRQoL) in symptomatic patients with HF is equally impaired in preserved and low LVEF populations and therefore remains an important treatment target in patients with HFpEF [104]. A meta-analysis of five RCTs involving 245 patients with HFpEF showed that exercise training improved peak exercise oxygen uptake (VO2; weighted MD 2.283, 95% CI 1.318–3.248, mL/min/kg), 6-minute walk distance (30.275 m, 95% CI 4.315–56.234), and Minnesota Living with Heart Failure Questionnaire (MLHFQ) total score (8.974 points, 95% CI 3.321–14.627) compared with usual care [105].
Although several large-scale trials in HFpEF have not met their primary outcome (often mortality), several drugs do improve QoL and exercise capacity, and reduce HF hospitalizations, which are more meaningful outcomes for elderly and debilitated patients with HFpEF compared with reduced mortality [101]. HFpEF, being a heterogeneous syndrome, requires a nuanced, phenotype-specific patient management approach, rather than a one-size-fits-all approach [101].
Evidence supporting the role of diuretics in HF was presented in a meta-analysis of RCTs which showed that treatment with diuretics improved mortality rates, hospitalization rates, and exercise capacity in patients with congestive HF [106]. ACE inhibitors (perindopril 4 mg/day) have been shown to reduce HF hospitalizations and improve symptoms and exercise capacity [101]. Because ACE-Is are indicated for several comorbidities (diabetes, hypertension and chronic kidney disease), they are widely used in patients with HFpEF [101]. The effect of ARBs have been evaluated in two large RCTs, CHARM-Preserved and I-PRESERVE, which have shown that both candesartan and irbesartan reduce overall HF hospitalization and may be useful in less-severe HFpEF and in patients with lower levels of natriuretic peptides [101]. MRAs were shown to consistently improve cardiac structure and function in patients with HFpEF, but not exercise capacity in the ALDO-DHF trial [101]. Although the TOPCAT trial showed similar results, it also found that patients in the lowest tertile of natriuretic peptides were the ones who benefited most from spironolactone [101]. Beta-blockers are commonly used in HFpEF and are the only class of drugs shown to have a potential mortality benefit in patients with HFpEF [107], [108]. A meta-analysis by Bavishi et al. [108] of 15 observational studies and two RCTs including 27,099 patients found that in the observational studies, beta-blocker therapy reduced all-cause mortality by 19% (RR 0.81, 95% CI 0.72–0.90; p < 0.001), but not HF hospitalization (RR 0.79, 95% CI 0.57–1.10; p < 0.001). Based on data from the two RCTs, beta-blockers were not associated with reductions in all-cause mortality or HF hospitalization. It is important to note that beta-blocker survival benefit is limited to studies with a mean age <75 years [108]. A more recent meta-analysis reported that overall, beta-blockers reduced the risk of mortality by 21% (RR 0.79, 95% CI 0.71–0.88). This reduced risk of mortality was reflected through pooled analysis of six observational cohort studies (15,275 patients), and not in a pooled analysis of three RCTs (1046 patients) [107]. However, there is a need for well-designed and powered studies to confirm the survival benefit reported in observational studies.
10. Arrhythmias and conductance disturbances (Table 16, Table 17, Table 18, Table 19, Table 20)
Table 16.
Recommendations for the initial management of a rapid ventricular rate in patients with HF and AF in the acute or chronic setting.
 |
Table 17.
Recommendations for rhythm control management strategy in patients with atrial fibrillation, symptomatic heart failure (NYHA Class II–IV), left ventricular systolic dysfunction, and no evidence of acute decompensation.
Table 18.
Recommendations for the prevention of thromboembolism in patients with symptomatic heart failure (NYHA Class II–IV) and paroxysmal or persistent/permanent atrial fibrillation.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AF = atrial fibrillation; HF = heart failure; LMWH = low-molecular-weight heparin; NOAC = non-vitamin oral anticoagulant; NYHA = New York Heart Association; TOE = transesophageal echocardiograph.
Table 19.
Recommendations for the management of VT in HF.
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy defibrillator; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; MRA = mineralocorticoid antagonist; VT = ventricular tachycardia.
Table 20.
Recommendations for the management of bradyarrhythmias in heart failure.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AF = atrial fibrillation; AV = atrioventricular; CRT = cardiac resynchronization therapy; ECG = electrocardiogram; HFrEF = heart failure with reduced ejection fraction; RV = right ventricle.
10.1. Rate control
Electrical cardioversion can help reduce the risk of stroke, improve cardiovascular hemodynamics, and preclude the need for long-term anticoagulation, by restoring sinus rhythm. A Cochrane-based systematic review evaluated the use of electrical cardioversion versus rate control in 927 participants across four trials (Hot Cafe, RACE, STAF, and J-RHYTHM), and showed that electrical cardioversion helped to significantly improve physical functioning, physical role function, and vitality compared with rate control [109]. An intravenous bolus of amiodarone can immediately control heart rate in patients with AF and a high ventricular rate [110], [111]. RCTs investigating the rates of mortality and morbidity of digoxin in patients with AF are not available. Retrospective analyses of various trials, including AFFIRM, RACE II, and ROCKET-AF, have provided conflicting results regarding the impact of digoxin on mortality in patients with AF [112] and therefore should be interpreted with caution.
If the patient remains symptomatic despite pharmacological treatment, or suffers drug-related adverse effects, then atrioventricular (AV) node catheter ablation may be considered, however, this procedure renders the patient pacemaker dependent [113]. Because of safety concerns, dronedarone is not recommended in patients with HF or AF. A study enrolling 3236 patients with permanent AF and at risk for major vascular events showed that patients receiving dronedarone had increased incidences of mortality (HR 2.11, 95% CI 1.00–4.49; p = 0.046), stroke (HR 2.32, 95% CI 1.11–4.88; p = 0.02), and hospitalization for HF (HR 1.81, 95% CI 1.10–2.99; p = 0.02) compared with the placebo group [114]. A separate study similarly showed increased early mortality related to worsening HF in dronedarone-treated patients with severe HF and left ventricular systolic dysfunction [115].
10.2. Rhythm control
The CHF-STAT study involving 103 congestive patients with HF showed that amiodarone has a significant potential to spontaneously convert patients in AF to sinus rhythm, prevent new-onset AF, and significantly reduce the VR in patients with persistent AF [116]. A meta-analysis of 11 studies with 1481 patients showed superiority of catheter ablation over antiarrhythmic drug therapy in the maintenance of sinus rhythm in drug naïve, resistant, and intolerant patients with AF [117]. A separate meta-analysis comprising 21,305 patients across 59 studies showed that although several Class IA, IC, and III antiarrhythmic drugs were moderately effective in maintaining sinus rhythm after conversion of AF, they were associated with an increase in adverse events and, in a select few, mortality [118].
10.3. Prevention of thromboembolism
According to the nationwide prospective cohort study using Danish registries that included 42,987 patients (21.9% with concomitant AF) not receiving anticoagulation and diagnosed with HF, a high CHA2DS2-VASc score was associated with increased risk of ischemic stroke, thromboembolism, and death. Risks were greater with increasing CHA2DS2-VASc scores as follows: for scores of 1 through 6, respectively: ischemic stroke: 4.5%, 3.7%, 3.2%, 4.3%, 5.6%, and 8.4%; all-cause death: 19.8%, 19.5%, 26.1%, 35.1%, 37.7%, and 45.5%. At high CHA2DS2-VASc scores (≥4), the absolute risk of thromboembolism was high regardless of presence of AF (for a score of 4, 9.7% vs. 8.2% for patients without and with concomitant AF, respectively; overall p < 0.001 for interaction). However, the clinical value of the CHA2DS2-VASc score remains to be determined in patients with HF [119]. A meta-analysis involving 11 studies showed that HAS-BLED had superior performance compared with HEMORR2 HAGES and ATRIA for bleeding scores and with CHADS2 and CHA2DS2-VASc scores for bleeding prediction [120].
A meta-analysis of four RCTs [RELY (dabigatran), ROCKET-AF (rivaroxaban), ARISTOTLE (apixaban), and ENGAGE-TM (edoxaban)] of a total of 19,122 AF patients with HF showed that single-/high-dose non-vitamin oral anticoagulants (NOACs) were significantly better at reducing the risk of stroke/systemic embolic events (OR 0.86, 95% CI 0.76–0.98) and major bleeding (OR 0.76, 95% CI 0.67–0.86) [121]. Dabigatran is the only NOAC investigated in clinical trials in patients with mechanical heart valves [122], but this trial was terminated early due to increased rates of thromboembolic and bleeding complications [123].
10.4. Management of ventricular tachycardia in heart failure
Electrolyte imbalance is a potentially dangerous complication in patients with HF, warranting early recognition and correction [124]. Regarding pharmacological intervention, the COPERNICUS and CAPRICORN trials showed the benefit of carvedilol in reducing ventricular tachycardia and ventricular fibrillation in patients with HF. The MERIT-HF and CIBIS-II trials demonstrated the benefits of metoprolol succinate and bisoprolol, respectively, in preventing arrhythmic deaths in CHF [125]. A prospective study involving 1663 patients assessed the effect of spironolactone in patients with severe HF and reported fewer events of ventricular arrhythmias in the treated group compared with the placebo group [126]. Although still unproven, it has been postulated that sacubitril/valsartan could confer protection against ventricular arrhythmias through reduction in myocardial fibrosis [127].
The AVID, CIDS, and CASH trials studied the treatment of ICD compared with antiarrhythmic drug therapy in patients who had suffered a cardiac arrest or life-threatening VA [128]. A meta-analysis of these three trials showed that ICD therapy was associated with a 50% reduction in arrhythmic mortality (95% CI 0.37–0.67; p = 0.0001) and a 28% reduction in total mortality (95% CI 0.60–0.87; p = 0.006) [79]. A subanalysis of these trials identified that, based on results from the AVID trial, individuals with an EF between 20% and 34% conferred the highest benefit with ICD therapy [81].
10.5. Management of bradyarrhythmias in heart failure
In patients with HFrEF who require pacing and who have high-degree AV block, CRT rather than RV pacing is recommended. A prospective multicenter study involving 186 patients with severely symptomatic permanent AF in whom AV junction ablation and CRT device implantation had been successfully performed showed death from HF, hospitalization due to HF, or worsening HF in 11% of patients in the CRT group compared with 26% of patients in the RV group (CRT vs. RV group: subhazard ratio 0.37, 95% CI 0.18–0.73; p = 0.005) [129].
11. Comorbidities (Table 21, Table 22, Table 23, Table 24, Table 25)
Table 21.
Recommendations for the treatment of stable angina pectoris in patients with symptomatic HFrEF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
ACS = acute coronary syndrome; CAD = coronary artery disease; HF = heart failure; HFrEF = heart failure with reduced ejection fraction.
Table 22.
Recommendations for the treatment of hypertension in patients with symptomatic HFrEF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid antagonist.
Table 23.
Recommendations for the treatment of other comorbidities in patients with HF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; FCM = ferric carboxymaltose; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction.
Table 24.
Treatments not recommended of other comorbidities in patients with HF.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
COX = cyclooxygenase; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; NSAID = non-steroidal anti-inflammatory drug.
Table 25.
Recommendations for the treatment of valvular diseases in patients with HF.
 |
11.1. Treatment of stable angina pectoris in patients with symptomatic HFrEF
Beta-blockers are effective in prolonging and improving symptoms of HF and LVEF in patients with HFrEF [60], and are therefore the preferred first-line treatment for the relief of angina. The vasodilatory effects of nitrates have implicated their use as an effective treatment in angina pectoris and acute coronary syndromes [130], [131], [132], [133]. A recent systematic review, involving 17 RCTs with 9975 participants, assessing the role of ranolazine in patients with stable angina pectoris concluded that there was uncertainty about the effect of ranolazine on all-cause mortality, QoL, and the incidence of nonfatal acute MI [134]. Diltiazem treatment in postinfarction patients with a reduced EF increases the risk for subsequent congestive HF and is therefore not recommended in patients with left ventricular dysfunction [3].
Ivabradine is of symptomatic value in patients with CAD, but has no prognostic value in this population [64], as evidenced by the SIGNIFY trial [135]. This trial, involving 19,102 patients with both stable CAD without clinical HF and a heart rate of ≥70 bpm, showed that although ivabradine treatment, compared with placebo, was able to reduce heart rate (60.7 ± 9.0 vs. 70.6 ± 10.1 bpm, respectively), there was no significant difference in the incidence of a composite of death from cardiovascular causes or nonfatal MI (6.8% and 6.4%, HR, 1.08; 95% CI 0.96–1.20; p = 0.20). Furthermore, ivabradine was associated with an increase in the incidence of a composite of death from cardiovascular causes or nonfatal MI among patients with activity-limiting angina, but not among those without activity-limiting angina (p = 0.02 for interaction) and a higher incidence of bradycardia (18.0% vs. 2.3%; p < 0.001).
11.2. Treatment of hypertension in patients with symptomatic HFrEF
If an ACE-Is (or an ARB), a beta-blocker, an MRA, and a diuretic fail to control blood pressure, then amlodipine or hydralazine can be safely used. The safety and efficacy of amlodipine for the treatment of hypertension in patients with left ventricular dysfunction have been established, with CHF patients reporting fewer cases of hypertension with amlodipine treatment compared with placebo [3].
The ALLHAT study showed that the risk of congestive HF was doubled in patients with hypertension receiving doxazosin compared with the diuretic chlorthalidone (4-year rates, 8.13% vs. 4.45%, RR 2.04, 95% CI 1.79–2.32; p < 0.001) [136]. The induction of neurohormonal activation, fluid retention, and worsening HF associated with alpha-adrenoceptor antagonists make it unsafe in patients with HFrEF. The negative inotropic effects of diltiazem and verapamil make them similarly unsafe in this patient population [3].
11.2.1. Iron deficiency
The FAIR-HF trial, involving 459 patients, showed that administering intravenous iron to patients with CHF and iron deficiency with or without anemia, improved symptoms, functional capacity, and QoL [137]. These outcomes were sustained over a 1-year period in the CONFIRM-HF trial, which also demonstrated that intravenous iron reduced the risk of hospitalization for worsening HF [138]. Results from a recent meta-analysis of RCTs, involving 551 patients with systolic HF and iron deficiency, are consistent with these findings. Intravenous iron therapy reduced the risk of the combined endpoint of all-cause death or cardiovascular hospitalization (OR 0.44, 95% CI 0.30–0.64; p < 0.0001), and reduced the NYHA class (OR –0.54, 95% CI –0.87 to –0.21; p = 0.001) compared with the control group [139].
11.2.2. Diabetes
A systematic review of observational studies involving 34,000 patients confirmed that the use of metformin in patients with diabetes and HF reduced mortality compared with controls (23% vs. 37%, pooled adjusted risk estimates: 0.80; 0.74–0.87; I2 = 15%; p < 0.001) and was as safe as other glucose-lowering treatments [140]. Metformin is contraindicated in patients with severe renal or hepatic impairment because of the risk of lactic acidosis [3].
Empagliflozin has been shown to reduce HF hospitalizations and cardiovascular-induced death in HF patients with diabetes. Further studies are required to assess the effects of empagliflozin on LV structure and function, NYHA class, and hemodynamics in addition to its long-term safety [141]. Linagliptin is similarly effective for glycemic control in patients with diabetes [142], with data showing no association with cardiovascular risk versus pooled active comparators or placebo [143], [144]. Data are still required to assess the efficacy of linagliptin in patients with diabetes and HF. Thiazolidinediones (glitazones) have been significantly associated with the increased risk of HF-induced mortality and hospitalization, and are therefore not recommended in patients with HF [145], [146].
11.2.3. Depression
Depression often accompanies HF and is associated with increased financial burden, somatic symptoms, hospitalization, poor QoL, poor prognosis, increased mortality, and is a predictor of future cardiac events [147], [148], [149]. Selective serotonin reuptake inhibitors may improve the symptoms of depression and prognosis in patients with HF [150], but only after the consideration of comorbidities and the potential for drug interactions [151]. In another study (MOOD-HF), the use of escitalopram did not significantly reduce mortality and hospitalization or the symptoms of depression; therefore, the study authors did not recommend the routine use of antidepressants in patients with heart failure and depression [152]. Tricyclic antidepressants may cause hypotension, arrhythmias, and worsening HF, and should therefore be avoided [3].
11.2.4. Vaccinations
Vaccinations against influenza are associated with a reduced risk of hospitalization due to heart disease, pneumonia, and influenza [153]. Vaccination against pneumococcal disease and influenza should therefore be considered in patients with HF [154].
11.2.5. Sleep apnea
A study on treated patients with CHF showed that 61% of patients presented with either central or obstructive sleep apnea [155]. The SERVE-HF trial, which studied HFrEF patients with predominant central sleep apnea, showed that use of adaptive servoventilation had no significant effect on the primary endpoints (all-cause death, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF), and was associated with increased all-cause death (HR 1.28, 95% CI 1.06–1.55; p = 0.01) and cardiovascular mortality (HR 1.34, 95% CI 1.09–1.65; p = 0.006) [156].
In patients with central sleep apnea and HF, continuous positive airway pressure significantly improved nocturnal oxygenation (1.6 ± 2.8% vs. 0.4 ± 2.5%; p < 0.001), lowered norepinephrine levels (–1.03 ± 1.84 vs. 0.02 ± 0.99 nmol/L; p = 0.009), increased EFs (2.2 ± 5.4% vs. 0.4 ± 5.3%; p = 0.02), and increased the distance walked in 6 minutes (20.0 ± 55 vs. –0.8 ± 64.8 m; p = 0.016), but failed to affect survival compared with the control group [157].
11.3. Treatment of valvular disease in patients with heart failure
The accurate assessment of the grade of aortic stenosis is vital to selecting the appropriate treatment [158]. Dobutamine stress echocardiography may help to assess the aortic valve area in a different flow status and differentiate between fix severe aortic stenosis and pseudo-severe aortic stenosis [158]. An observational analysis, involving 114,125 patients aged ≥65 years who underwent aortic valve replacement, showed that concomitant HF negatively impacted both operative mortality and long-term survival [159]. Furthermore, longer duration of HF symptoms before aortic valve replacement was associated with worse outcomes. Overall, these data show that careful clinical judgment is required when managing patients with both severe aortic stenosis and HF [159].
11.4. Treatment of heart failure patients with cancer
Myocardial dysfunction and HF are the most concerning cardiovascular complications of cancer treatment. It is important for specialists to work together to prevent and manage cardiotoxicity without compromising cancer care and optimizing patient outcome [160].
Some cancer treatments, such as anthracyclines, may induce progressive cardiac remodeling as a late consequence of earlier myocyte damage, resulting in late cardiomyopathy, whereas others may cause transient cardiac dysfunction without long-term consequences. Data show that anthracycline-associated cardiac dysfunction detected early and treated with HF medications frequently leads to good functional cardiac recovery [160].
Other conventional chemotherapies such as cyclophosphamide, cisplatin, ifosfamide, and taxanes are also known to induce myocardial dysfunction. More recently introduced immunotherapies and targeted therapies, such as HER2 antagonists and tyrosine kinase inhibitors, appear to have similar cardiotoxicity profiles to trastuzumab. Vascular endothelial growth factor inhibitors are known to induce cardiac dysfunction in 3–15% of patients and symptomatic HF in 1–10% of patients. Similarly, other agents such as BCR-ABL kinase inhibitors and proteasome inhibitors, also lead to cardiotoxicity, with carfilzomib (a proteasome inhibitor) being associated with a high risk of HF (up to 25%) [160].
Radiotherapy is associated with an absolute excess risk for mortality ranging from 9.3 to 28 per 10,000 person-years of follow-up, and a 4.9-fold increased risk of HF in survivors. Although the actual incidence of radiation-induced cardiotoxicity is difficult to evaluate, some studies have reported the RR of fatal cardiovascular events to range between 2.2 and 12.7 in survivors of Hodgkin lymphoma and between 1 and 2.2 in patients with breast cancer [160].
Therefore, it is imperative to first identify patients at risk of cardiotoxicity by taking into account the patient’s clinical history, examination, and baseline measurements of cardiac function. Screening and detection of cardiotoxicity may include cardiac imaging (echocardiography, nuclear imaging, CMR) and biomarkers (troponin, natriuretic peptides) based on local expertise and availability. It is important to remember that risk stratification assessment is a guide to ensure that patients at higher risk have an earlier review to avoid missing early signs of toxicity [160].
11.5. Treatment of heart failure patients with infection
Certain antibiotics such as macrolides can cause, or aggravate, LV systolic dysfunction in HF. Macrolides include the antibiotics erythromycin, azithromycin (Zithromax), clarithromycin (Biaxin), and quinolone. A meta-analysis of 33 studies involving 20,779,963 participants revealed that patients taking macrolides experienced an increased risk of developing sudden cardiac death or ventricular tachyarrhythmias (RR 2.42; 95% CI 1.61–3.63), sudden cardiac death (RR 2.52; 95% CI 1.91–3.31), and cardiovascular death (RR 1.31; 95% CI 1.06–1.62), compared with those who did not take macrolides [160]. However, no association was found between macrolide use and all-cause death or any cardiovascular events [161].
12. Acute heart failure
12.1. Definition and classification
AHF is characterized by the sudden worsening of signs and/or symptoms of HF. AHF can be clinically classified into four different hemodynamic profiles, stratified by adequacy of perfusion (warm or cold) and degree of congestion (dry and wet). Perfusion is characterized by cold sweaty extremities, oliguria, mental confusion, dizziness, and narrow pulse pressure [3]. Congestion is characterized by pulmonary congestion, orthopnea/paroxysmal nocturnal dyspnea, peripheral edema, jugular venous dilatation, congested hepatomegaly, gut congestion, ascites, and hepatojugular reflux [3]. Such classification can help guide therapy and predict prognosis [162]. For instance, to achieve a “warm and dry” profile, diuresis may be indicated, or for a “wet and cold” profile vasoactive therapy with diuresis may be indicated [162].
12.2. Management of acute heart failure (Table 26, Table 27, Table 28, Table 29, Table 30, Table 31, Table 32)
Table 26.
Recommendations for applied diagnostic measurements in AHF.
 |
Table 27.
Recommendations for the management of patients with AHF—oxygen therapy and ventilatory support.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AHF = acute heart failure; BiPAP = bilevel positive airway pressure; COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; PaCO2 = partial pressure of carbon dioxide.
Table 28.
Recommendations for the management of patients with AHF—pharmacotherapy.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
AF = atrial fibrillation; AHF = acute heart failure; ECG = electrocardiogram; HF = heart failure; IV = intravenous; LMWH = low-molecular-weight heparin; SBP = systolic blood pressure.
Table 29.
Recommendations for renal replacement therapy in patients with acute heart failure.
Table 30.
Recommendations for oral evidence-based disease-modifying therapies in patients with acute heart failure.
Table 31.
Recommendations for the management of patients with cardiogenic shock.
 |
Table 32.
Recommendations for monitoring clinical status of patients hospitalized due to acute heart failure.
An algorithm for the management of AHF is shown in Fig. 8. Intravenous loop diuretics are recommended for all patients with AHF admitted with signs/symptoms of fluid overload to improve symptoms and a less-than-optimal response following the use of diuretics necessitates further treatment. Intravenous vasodilators should be considered for symptomatic relief in AHF with SBP >90 mmHg and without symptomatic hypotension. Short-term, intravenous infusion of inotropic agents may be considered in patients with hypotension (SBP <90 mmHg); dobutamine if SBP is 70–90 mmHg and dopamine if SBP is <70 mmHg.
Fig. 8.
Algorithm for the management of acute heart failure. IV = intravenous; SBP = systolic blood pressure.
12.3. Diagnostic measurements in acute heart failure
The lower recommended threshold for the diagnosis of AHF for BNP and NT-proBNP is <100 pg/mL and <300 pg/mL, respectively. Use of these thresholds in clinical practice provides an excellent ability for the rapid and accurate exclusion of AHF. A meta-analysis evaluating 15,263 test results showed that using these thresholds for BNP (<100 pg/mL) and NT-proBNP (<300 pg/mL) was associated with a sensitivity of 0.95 (95% CI 0.93–0.96) and 0.99 (95% CI 0.97–1.00) and negative predictive values of 0.94 (95% CI 0.90–0.96) and 0.98 (95% CI 0.89–1.0), respectively, for the diagnosis of AHF. At the lower recommended threshold of 120 pmol/L, MR-pro-atrial natriuretic peptide (MR-proANP) has a sensitivity ranging from 0.95 (0.90–0.98) to 0.97 (0.95–0.98) and a negative predictive value ranging from 0.90 (0.80–0.96) to 0.97 (0.96–0.98) [162]. However, specificity may vary, and thus imaging is required to confirm diagnosis [162]. NT-proBNP and MR-proANP are as useful as BNP in the diagnosis of AHF [163], [164]. Mandating the use of NT-proBNP in the management of patients suspected of AHF significantly enhanced the accuracy of diagnosis and reduced the duration of emergency department visits, hospitalizations, and subsequent outpatient services [165].
12.4. Pharmacotherapy
Diuretics are recommended to relieve the signs and symptoms of fluid overload [166]. Recent data have shown that timely administration of intravenous diuretics in the emergency department is strongly associated with improved outcomes and reduction in the readmission rate. Based on the recent data available, we strongly recommend that intravenous diuretics are administered ideally within 60 minutes of first medical contact, and no later than 120 minutes [167], [168], [169]. The least effective intravenous diuretic dose may be administered to reach euvolemia in the shortest time possible. Studies have shown no difference in the patient’s global assessment of symptoms when administering diuretic therapy as a bolus infusion compared with continuous infusion [170]. Combining diuretic therapy with thiazide-type diuretics can help induce diuresis in patients resistant to high doses of loop diuresis, but increased the risks of hypokalemia, hypotension, hyponatremia, and worsening renal function [171].
Use of intravenous vasodilators is effective in improving hemodynamics and organ perfusion in patients with AHF [172]. Although useful in improving forward flow and organ perfusion, inotropic agents have been associated with increased mortality and therefore require careful patient assessment before use [173].
In a meta-analysis involving 19,958 at-risk hospitalized medical patients, thromboembolism prophylaxis was effective in reducing incidences of deep vein thrombosis (RR 0.47, 95% CI 0.22–1.00) and pulmonary embolism (RR 0.43, 95% CI 0.26–0.71) [174].
12.5. Oxygen therapy and ventilatory support
Noninvasive positive pressure ventilation (NPPV) is widely used to alleviate the signs and symptoms of respiratory distress due to cardiogenic pulmonary edema. Both continuous positive airway press and bilevel positive airway pressure provide a more rapid improvement in respiratory distress and metabolic disturbance compared with standard therapy [175], [176], [177], [178], [179]. Further large-scale trials are needed to evaluate the potential benefit of NPPV in reducing mortality.
Opiates should be cautiously used to relieve dyspnea, as data from 147,362 patients with acute decompensated HF (ADHF) in the ADHERE study showed that patients on morphine had more ICU admissions (38.7% vs. 14.4%), a longer median hospitalization (5.6 vs. 4.2 days), and greater mortality (13.0% vs. 2.4%), and were more likely to require mechanical ventilation (15.4% vs. 2.8%; all p < 0.001) compared with patients who did not receive morphine [180].
12.6. Monitoring clinical status of patients hospitalized due to acute heart failure
Pulmonary edema and low tissue perfusion can lead to changes in the blood acid–base balance and may be related to worse outcomes in patients with AHF. The Korean Heart Failure Registry, which involved 1982 patients, showed that 19% and 44% of AHF patients had acidosis and alkalosis, respectively. In addition, acidosis was associated with higher mortality (acidosis 19.5%, neutral pH 13.7%, alkalosis 14.9%; p = 0.007). Therefore, assessment of pH provides an additional prognostic value in AHF patients and may be used to optimize risk stratification [181].
12.7. Renal replacement therapy
A meta-analysis of 12 RCTs involving 659 patients with ADHF showed that ultrafiltration treatment was effective in reducing fluid retention (MD 1.28, 95% CI 0.43–2.12; p = 0.003) and inducing weight loss (MD 1.23, 95% CI 0.03–2.44; p = 0.04), but had no significant effect on all-cause mortality (OR 1.08, 95% CI 0.63–1.86; p = 0.77) or all-cause hospitalization (OR 0.89, 95% CI 0.39–2.00; p = 0.77) [182]. A separate meta-analysis of 477 patients with AHF showed no significant difference in adverse events between the ultrafiltration and intravenous diuretic treatment groups [183]. Caution is still advised with ultrafiltration treatment, particularly in the setting of worsening renal function [184], [185], [186].
12.8. Cardiogenic shock
The use of norepinephrine for the treatment of cardiogenic shock is associated with fewer numbers of arrhythmic events compared with dopamine (12.4% vs. 24.1%; p < 0.001) [187], and thus is the preferred vasopressor. Supplementing dobutamine with epinephrine in patients with cardiogenic shock has been shown to improve cardiac output (103 ± 8 mL/kg to 125 ± 9 mL/kg) and central venous oxygen saturation (49% ± 3% to 59% ± 4%) compared with dobutamine treatment alone because of an increased heart rate and contractility with minimal change in systemic vascular resistance [188].
Intra-aortic balloon pump is not recommended for routine use in patients with cardiogenic shock because evidence from the IABP-SHOCK II trial (600 patients) failed to show any significant improvement in rates of mortality (RR 1.01, 95% CI 0.86–1.18; p = 0.91), reinfarction (RR 2.60, 95% CI 0.95–7.10; p = 0.05), recurrent revascularization (RR 0.91, 95% CI 0.58–1.41; p = 0.77), or stroke (RR 1.50, 95% CI 0.25–8.84; p = 1.00) [189].
13. Mechanical circulatory support and heart transplantation (Table 33, Table 34, Table 35)
Table 33.
Recommendations for the implantation of mechanical circulatory support in patients with refractory heart failure.
Table 34.
Recommendations on revascularizations in patients with chronic heart failure and systolic LV dysfunction (ejection fraction ≤35%).
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
CABG = coronary artery bypass graft; LAD = left anterior descending; LV = left ventricle; LVESV = left ventricle end-systolic volume index; PCI = percutaneous coronary intervention.
Table 35.
Recommendations for the management of patients with valvular heart disease.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
CABG = coronary artery bypass graft; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; MR = mitral regurgitation; PMC = percutaneous mitral commissurotomy; TR = tricuspid regurgitation; TS = tricuspid stenosis.
13.1. Heart transplantation
In Saudi Arabia, a total of 30 whole heart transplantations were carried out and 17 hearts were recovered as a source of valves in 2015 [190]; however, there is a need for epidemiological studies on the management of patients undergoing heart transplantation. In general, heart transplantation may be considered in patients with end-stage HF with severe symptoms, a poor prognosis, and no remaining alternative treatment options [3]. Patients that are capable of complying with intensive postoperative treatment may also be considered for heart transplantation [3]. Patients with an active infection, severe peripheral arterial or cerebrovascular disease, pharmacologically irreversible pulmonary hypertension, cancer, irreversible renal dysfunction (e.g. creatinine clearance <30 mL/min), multiorgan systemic disease, body mass index over 35 kg/m2, current alcohol or drug abuse, and/or other serious comorbidities with poor prognosis are not eligible for heart transplantation [3]. In addition, heart transplantation is contraindicated in any patient for whom social supports are deemed insufficient to achieve compliant care in the outpatient setting [3]. Relatively, patients above 65 years, and/or patients with the inability to relocate to a city which has a secondary care facility with a trained cardiologist to follow-up the care and refer to an advanced HF center, should ideally not be considered for heart transplantation [3].
13.2. Implantation of mechanical circulatory support in patients with refractory heart failure
A left ventricular assist device (LVAD) is an established treatment for patients with advanced HF, with most patients hospitalized and dependent on intravenous inotropic support [190]. The seventh INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) registry report of >15,000 LVAD-supported patients over a 9-year period found that the actuarial survival rates at Year 1 and Year 2 were 80% and 70%, respectively, for all patients with continuous-flow LVAD [191]. A more recent cohort of the HeartMate II destination therapy postapproval found that survival rates were 82% at Year 1 and 69% for Year 2 for INTERMACS Profiles 4–7, compared with 72% at Year 1 and 60% at Year 2 for INTERMACS Profiles 1–3 [192]. Furthermore, for INTERMACS Level I patients who are known to have a higher risk of mortality after VAD implantation, preoperative optimization using initial venoarterial extracorporeal life support with deferred VAD implantation substantially improves renal, hepatic, and pulmonary function over a period of 8 days [193]. The 30-day and in-hospital mortality rates after VAD implantation were 4.5% and 9.1%, respectively, and overall 1-year survival was 86.4% [193]. During extracorporeal life support, catecholamine dosage may also be reduced [193].
In addition, a prognostic evaluation of ambulatory patients with advanced HF found that patients with systolic HF, a heavy symptom burden, and at least one recent HF hospitalization were at high risk for death or LVAD rescue [194]. Of note, a retrospective, longitudinal, comparative study evaluating the performance of the HeartMate Risk Score reported that patients within each INTERMACS profile group had a wide spectrum of mortality risk and therefore, low INTERMACS profiles should not be considered a contraindication to mechanical support [195].
Despite technological improvements, bleeding, thromboembolism (both of which can cause a stroke), pump thrombosis, driveline infections, and device failure remain significant problems and affect the long-term outcome of patients on mechanical circulatory support [3]. Therefore, newly established advanced centers performing LVAD implantation and/or heart transplant should consult local experts to assess the degree of readiness and to assure that this advanced and highly specialized service is being provided in a way that assures patient safety and produces high-quality outcomes.
13.3. Myocardial revascularization
The role of coronary artery bypass grafting (CABG) in the treatment of patients with CAD and left ventricular dysfunction (EF ≤35%) was assessed in the Surgical Treatment for Ischemic Heart Failure (STICH) trial [196]. Of the 1212 patients recruited, the primary outcome of death from any cause occurred in 41% of patients on medical therapy and 36% of patients undergoing CABG (p = 0.12) [196]. Although CABG did not significantly reduce the risk of death from any cause compared with medical therapy, the rate from death from any cause or hospitalization for cardiovascular causes was lower within the CABG group (58%) than those on medical therapy (68%; p < 0.001) [196]. Importantly, according to the propensity-matched, risk-adjusted observational cohort of patients with CAD, an LVEF <35%, and no left main disease ≥50%, CABG is associated with a survival advantage over medical therapy, over a 10-year follow-up period [197].
The presence of viable myocardium has been shown to be associated with a greater likelihood of survival in patients with CAD and left ventricular dysfunction [198]. Although the STICH trial did not show improvement in survival with revascularization of viable myocardium, the results were limited by the viability of the imaging techniques used and the lack of inducible ischemia information [199]. During a mean follow-up of 2.8 years, 27.5% of the 648 patients died, and hibernating myocardium (p = 0.0015), ischemic myocardium (p = 0.0038), and scarred myocardium (p = 0.001) were associated with all-cause death [199]. Hibernating myocardium, especially when the extent of the viability exceeded 10% of the myocardium, was associated with improved survival [199].
Left ventricular reconstruction was also shown to provide durable improvements in left ventricular function in patients with a large scarred ventricular wall and who were systematically excluded from the STICH trial [200]. In this study, of the 101 patients with CHF, magnetic resonance imaging revealed that EF improved from 26% preoperatively to 40% at 1 month and 44% at 1 year postoperatively [200]. Simultaneously, the end-diastolic volume index reduced from 130 ± 43 mL/m2 to 81 ± 27 mL/m2 and 82 ± 25 mL/m2, respectively, and end-systolic volume index reduced from 96 ± 45 mL/m2 to 50 ± 21 mL/m2 and 47 ± 20 mL/m2, respectively [200]. Therefore, care should be taken when extrapolating study results and/or restricting treatment to a select group of patients. Furthermore, an analysis of left ventricular volumes at baseline and 4 months after surgery showed that surgical ventricular reconstruction (SVR) resulted in improved survival compared with CABG alone, when the postoperative end-systolic volume index was ≤70 mL/m2 [201]. Subgroup analysis of the STICH trial also suggested that patients with less dilated LV (LVESVI <60 mL/m2) and better LVEF (≥33%) may benefit from SVR compared with patients with larger LV (>90 mL/m2) and poorer LVEF (≤25%) [202].
Meta-analysis data from clinical studies show that percutaneous coronary intervention (PCI) among patients with left ventricular dysfunction yields similar outcomes to CABG, with acceptable in-hospital and long-term mortality. However, both interventions need to be used in tandem with pharmacological therapy for improved outcomes [203]. Of note, according to the APPROACH study in patients with CAD and left ventricular dysfunction, CABG was associated with lower rates of repeat revascularization and improved survival over PCI at 1, 5, 10, and 15 years [204].
13.4. Valvular heart disease
Approximately 30–50% of patients with MI will develop ischemic mitral regurgitation [205]. For patients with moderate regurgitation, the Cardiothoracic Surgical Trials Network found that at 1 year, the addition of mitral valve repair to CABG did not result in a higher degree of LV reverse remodeling [206]. At the 2-year follow-up, mitral valve repair was associated with a significantly higher incidence of moderate or greater recurrent MR, with no difference in the indices of left ventricular reverse remodeling, compared with valve replacement [205].
Although the management of tricuspid regurgitation in patients with mitral regurgitation is controversial, tricuspid repair is indicated in patients undergoing left-sided valve surgery with mild or moderate coexistent tricuspid regurgitation if the annulus is >40 mm or >21 mm/m2 [207].
14. Multidisciplinary team management (Table 36)
Table 36.
Recommendations for exercise, multidisciplinary management, and monitoring of patients with heart failure.
 |
Based on guidance published by the European Society of Cardiology (ESC) [3] and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) [10].
HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction.
It is important that each HF case be managed collaboratively, with a process used to assess, plan, implement, coordinate, monitor, and evaluate the services required to meet a patient’s health and service needs [208]. Several patients suffer from multimorbidity, which has been identified to play a critical role in driving both early and late readmissions in HF, with about 60% of patients requiring readmission within 30 days for a condition other than HF [208].
It is important to remember that patients with HF are often prescribed a complex therapeutic regimen that consists of medication, diet, fluid restriction, and recommendations on activity and rest [209]. Integrating these changes into the patient’s existing regimen related to other comorbidities increases the chances of noncompliance, which is likely to lead to worsening symptoms and/or rehospitalization [209]. Therefore, education and counseling by a multidisciplinary team, each focusing on specific areas, are important to improving compliance and subsequent health-related outcomes [209].
The interdisciplinary approach also plays an important role in HF patient management, such as in primary care or with nursing home residents [208]. Data from three studies show that multidisciplinary care reduces hospital admissions and ER visits, and improves overall QoL in both CHF and AHF settings [210], [211], [212], [213], [214]. Data show that physiotherapy-based treatments not only assist in the prevention of falls, but also reduce functional deficits in those with cardiovascular disease [208]. Aerobic or resistance training has been shown to improve physical performance and HRQoL, and may increase the probability of older patients remaining independent, with home-based exercise programs found to be as effective as supervised exercise programs [208]. A recent meta-analysis of 46 RCTs found that exercise-based cardiac rehabilitation in patients with HF significantly reduces the risk of hospital admission by 35% (RR 0.65, 95% CI 0.50–0.84; p = 0.001) [215]. Besides, another meta-analysis of 55 trials found that increasing exercise intensity was associated with a greater level of postrehabilitation exercise capacity in patients with HF [216]. A Cochrane review of 33 trials including 4740 patients, predominantly with reduced EF (<40%), found that exercise-based cardiac rehabilitation leads to improvements in HRQoL and reductions in hospitalization, regardless of the cardiac rehabilitation program, and may reduce mortality in the long term [217].
Research shows that multimorbid or high-risk patients are most likely to benefit from home-based patient-centered management programs [208]. This was further highlighted by data from the CHAMPION trial reporting that a wireless implantable hemodynamic monitoring system has a long-term benefit in lowering hospital admission rates for HF [218], [219]. Furthermore, automatic, daily, implant-based, multiparameter telemonitoring was shown to significantly improve clinical outcomes in patients with HF [220].
14.1. Establishing a multidisciplinary team
Management of HF is complex, requiring the cooperation of various specialists [221]. A good HF service would typically include an experienced HF cardiology lead supported by primary care physicians, nurse specialists, hospital pharmacists, and community pharmacists with the ability to provide remote care [221].
For patients presenting with AHF, initial stabilization requires cooperation from emergency care providers, interventional cardiologists, heart surgeons, intensive care specialists, nurses, clinical pharmacists, and discharge managers [221]. To facilitate the transition from an inpatient to an outpatient setting, a general practitioner may be key and will likely help improve readmission rates [221].
However, the key to a successful multidisciplinary HF program is coordination of care delivered by various services within the healthcare system and across the spectrum of HF severity [221]. As patients transition from the inpatient to outpatient setting, pharmacists are key to assuring consistency in management [222] and predicting and improving medication adherence in patients with HF [222], [223], [224], making them an invaluable member of the multidisciplinary HF team. In addition, pharmacists are integral for optimizing care for elderly patients with HF [225] to significantly reduce the risk of discrepancies and prescription errors in HF medication postdischarge [222], [225], [226], [227], contribute to lower HF readmission rates and emergency department visits [222], [228], improve overall wellness and perception of self, through education and active involvement [222], [227], and reduce clinically relevant drug–drug interactions [229], [230].
15. Quality metrics (Table 37, Table 38)
Table 37.
Recommendations for performance measures in HF.
Table 38.
Performance indicators for HF.
| Process related |
|
| Outcome related |
|
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNi = angiotensin receptor/neprilysin inhibitor; CRT-D = cardiac resynchronization therapy-defibrillator; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid antagonist.
To achieve optimal outcomes in patients with HF, in addition to establishing a robust multidisciplinary HF team, there is a need for continuous monitoring of healthcare services at a program, provider, and patient level for consistency and quality. Some potentially useful performance indicators are outlined in Table 38 and a patient management pathway to promote organized and efficient patient care is outlined in Fig. 9.
Fig. 9.
Patient management pathway for HF. HF = heart failure; ICD = implantable cardioverter defibrillator.
16. Current limitations
Despite the advances in clinical practice and HF treatment modalities, there is a need for the creation of robust patient care pathways and quality improvement measures to improve overall health and reduce associated costs at both a community and national level. There was general consensus among the Heart Failure Expert Committee on the need for more epidemiological research in patients with HF. Studies looking at average salt intake, effectiveness of multidisciplinary care programs, prognostic markers, preferred imaging techniques, and rate of misdiagnosis in Saudi Arabia are necessary to improve patient management.
In terms of current practice, the Committee pointed out the lack of clarity on the percentage of patients presenting with HF symptoms who did not receive a definitive diagnosis, whether all patients with cardiomyopathy received a complete work-up, the percentage of patients with cardiomyopathy leaving with a presumed diagnosis of HF, and whether all centers assessing HF conduct imaging tests for all patients.
Owing to the lack of a national registry in Saudi Arabia, monitoring HF programs at a national level is difficult. However, the Saudi Health Council is currently working toward building sustainable healthcare registries with a focus on cardiovascular disease, which have the potential to shed light on several aspects of patient management in the country.
Acknowledgments
Acknowledgments
This project is supported by the Saudi Heart Association and funded through a research grant from Novartis, Riyadh, Saudi Arabia (Grant No. SA1610535252). Medical writing support was provided by Leris D’Costa of OPEN Health, and funded through a research grant issued to the Saudi Heart Association by Novartis, Riyadh, Saudi Arabia (Grant No. SA1610535252).
Authors’ contribution
This manuscript has been written by the Saudi Heart Failure Working Group, of the Saudi Heart Association. Dr. Waleed AlHabeeb, President of the Saudi Heart Failure Working Group, of the SHA, and lead author of the manuscript was actively involved in the conception and execution of this project. All authors are board members of the Saudi Heart Failure Working Group, of the Saudi Heart Association, and have actively participated in recommendation generation. The final manuscript was approved by the board of the Saudi Heart Association.
Waleed AlHabeeb, Fakhr Al-Ayoubi, Kamal AlGhalayini, Fahad Al Ghofaili, Yahya Al Hebaishi, Abdulrazaq Al-Jazairi, Mouaz H. Al-Mallah, Ali AlMasood, Maryam Al Qaseer, Shukri Al-Saif, Ammar Chaudhary, Abdelfatah Elasfar, and Adel Tash participated in the premeeting survey and conducted literature review in support of the recommendations; participated in the consensus meeting for generating recommendations for management of HF in Saudi Arabia; and contributed to the development, review, and finalization of the manuscript. Mohamed Arafa and Walid Hassan were responsible for overall critical review of the recommendations and manuscript content. All authors approved the submitted version of the manuscript.
Conflicts of interest
All arrangements for consensus generation such as meeting arrangements, travel, and follow-up were conducted by the SHA. Although the consensus meeting (held on October 7–8, 2016, in Riyadh, Saudi Arabia), and the medical writing was funded by Novartis through a research grant [SA1610535252], Novartis played no direct role in the collection, analysis, and interpretation of data or in the preparation of this manuscript. Dr. Waleed AlHabeeb has received speaker honoraria from Novartis, Pfizer, Merck, MSD, Bayer, and Servier. Yahya Al Hebaishi has received speaker honoraria from Pfizer and Bayer. Mouaz H. Al-Mallah has received speaker honoraria from GE Healthcare. Ali AlMasood has received speaker honoraria from Novartis. Maryam Al Qaseer has received speaker honoraria from Novartis and Bayer. Shukri Al-Saif has received speaker honoraria from Merck and AstraZeneca, and is a clinical investigator for Novartis-sponsored research. Ammar Chaudhary has received speaker honoraria from Novartis. Fakhr Al-Ayoubi, Kamal AlGhalayini, Fahad Al Ghofaili, Abdulrazaq Al-Jazairi, Abdelfatah Elasfar, Adel Tash, Mohamed Arafa, and Walid Hassan have no conflicts of interest to declare.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Al-Shamiri M.Q. Heart failure in the Middle East. Curr Cardiol Rev. 2013;9:174–178. doi: 10.2174/1573403X11309020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlHabib K.F., Elasfar A.A., AlBackr H., AlFaleh H., Hersi A., AlShaer F. Design and preliminary results of the Heart Function Assessment Registry Trial in Saudi Arabia (HEARTS) in patients with acute and chronic heart failure. Eur J Heart Fail. 2011;13:1178–1184. doi: 10.1093/eurjhf/hfr111. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Alhabeeb W., Elasfar A., AlBackr H., AlShaer F., Almasood A., Alfaleh H. Clinical characteristics, management and outcomes of patients with chronic heart failure: results from the heart function assessment registry trial in Saudi Arabia (HEARTS-chronic) Int J Cardiol. 2017;235:94–99. doi: 10.1016/j.ijcard.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 5.AlHabib K.F., Kashour T., Elasfar A.A., Alfaleh H., Hersi A., Alshamiri M. Long-term mortality rates in acute de novo versus acute-on-chronic heart failure: from the Heart Function Assessment Registry Trial in Saudi Arabia. Angiology. 2015;66:837–844. doi: 10.1177/0003319714563138. [DOI] [PubMed] [Google Scholar]
- 6.AlFaleh H.F., Thalib L., Kashour T., Hersi A., Mimish L., Elasfar A.A. Sex differences in patients with acute decompensated heart failure: insights from the Heart Function Assessment Registry Trial in Saudi Arabia. Angiology. 2016;67:647–656. doi: 10.1177/0003319715607298. [DOI] [PubMed] [Google Scholar]
- 7.Al Qahtani M., Al Backer T., Al Anazi T., Al Johani N., Binsalih S., AlGobain M. Impact of lipid disorders on mortality among Saudi patients with heart failure. J Saudi Heart Assoc. 2015;27:91–95. doi: 10.1016/j.jsha.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbuRuz M.E., Alaloul F., Saifan A., Masa’deh R., Abusalem S. Quality of life for Saudi patients with heart failure: a cross-sectional correlational study. Glob J Health Sci. 2015;8:49–58. doi: 10.5539/gjhs.v8n3p49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alaloul F., AbuRuz M.E., Moser D.K., Hall L.A., Al-Sadi A. Factors associated with quality of life in Arab patients with heart failure. Scand J Caring Sci. 2016;31:104–111. doi: 10.1111/scs.12324. [DOI] [PubMed] [Google Scholar]
- 10.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr, Drazner M.H. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 11.Brake R., Jones I.D. Chronic heart failure part 1: pathophysiology, signs and symptoms. Nurs Stand. 2017;31:54–63. doi: 10.7748/ns.2017.e10349. [DOI] [PubMed] [Google Scholar]
- 12.McLellan J., Heneghan C.J., Perera R., Clements A.M., Glasziou P.P., Kearley K.E. B-type natriuretic peptide-guided treatment for heart failure. Cochrane Database Syst Rev. 2016;12:CD008966. doi: 10.1002/14651858.CD008966.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacob D., Butnariu A., Leucuta D.C., Samasca G., Deleanu D., Lupan I. Evaluation of NT-proBNP in children with heart failure younger than 3 years old. Rom J Intern Med. 2017;55:69–74. doi: 10.1515/rjim-2017-0002. [DOI] [PubMed] [Google Scholar]
- 14.Maisel A.S., Krishnaswamy P., Nowak R.M., McCord J., Hollander J.E., Duc P. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 15.Korenstein D., Wisnivesky J.P., Wyer P., Adler R., Ponieman D., McGinn T. The utility of B-type natriuretic peptide in the diagnosis of heart failure in the emergency department: a systematic review. BMC Emerg Med. 2007;7:6. doi: 10.1186/1471-227X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Januzzi J.L., van Kimmenade R., Lainchbury J., Bayes-Genis A., Ordonez-Llanos J., Santalo-Bel M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 17.van Kimmenade R.R., Pinto Y.M., Januzzi J.L., Jr Importance and interpretation of intermediate (gray zone) amino-terminal pro-B-type natriuretic peptide concentrations. Am J Cardiol. 2008;101:39–42. doi: 10.1016/j.amjcard.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Oren O., Goldberg S. Heart failure with preserved ejection fraction – diagnosis and management. Am J Med. 2017;130:510–516. doi: 10.1016/j.amjmed.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Savarese G., Hage C., Orsini N., Dahlstrom U., Perrone-Filardi P., Rosano G.M. Reductions in N-terminal pro-brain natriuretic peptide levels are associated with lower mortality and heart failure hospitalization rates in patients with heart failure with mid-range and preserved ejection fraction. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003105. [DOI] [PubMed] [Google Scholar]
- 20.Raeisi-Giglou P., Lam L., Tamarappoo B.K., Newman J., Dweik R.A., Tonelli A.R. Evaluation of left ventricular diastolic function profile in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. Clin Cardiol. 2016;40:356–363. doi: 10.1002/clc.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVore A.D., McNulty S., Alenezi F., Ersboll M., Vader J.M., Oh J.K. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017;19:893–900. doi: 10.1002/ejhf.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinning C., Kempf T., Schwarzl M., Lanfermann S., Ojeda F., Schnabel R.B. Biomarkers for characterization of heart failure—distinction of heart failure with preserved and reduced ejection fraction. Int J Cardiol. 2017;227:272–277. doi: 10.1016/j.ijcard.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 23.Levine A., Hecht H.S. Cardiac CT angiography in congestive heart failure. J Nucl Med. 2015;56(Suppl 4):46S–51S. doi: 10.2967/jnumed.114.150441. [DOI] [PubMed] [Google Scholar]
- 24.Prastaro M., D’Amore C., Paolillo S., Losi M., Marciano C., Perrino C. Prognostic role of transthoracic echocardiography in patients affected by heart failure and reduced ejection fraction. Heart Fail Rev. 2015;20:305–316. doi: 10.1007/s10741-014-9461-8. [DOI] [PubMed] [Google Scholar]
- 25.Esposito R., Sorrentino R., Galderisi M. The use of transthoracic echocardiography for the assessment of left ventricular systolic and diastolic function in patients with suspected or ascertained chronic heart failure. Expert Rev Cardiovasc Ther. 2016;14:37–50. doi: 10.1586/14779072.2016.1111760. [DOI] [PubMed] [Google Scholar]
- 26.Stevens S.M., Farzaneh-Far R., Na B., Whooley M.A., Schiller N.B. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging. 2009;2:11–20. doi: 10.1016/j.jcmg.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedeljkovic I., Banovic M., Stepanovic J., Giga V., Djordjevic-Dikic A., Trifunovic D. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur J Prev Cardiol. 2016;23:71–77. doi: 10.1177/2047487315604836. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Fang F., Wai-Kwok Yip G., Sanderson J.E., Lee P.W., Feng W. Changes of ventricular and peripheral performance in patients with heart failure and normal ejection fraction: insights from ergometry stress echocardiography. Eur J Heart Fail. 2014;16:888–897. doi: 10.1002/ejhf.124. [DOI] [PubMed] [Google Scholar]
- 29.Chow B.J., Green R.E., Coyle D., Laine M., Hanninen H., Leskinen H. Computed tomographic coronary angiography for patients with heart failure (CTA-HF): a randomized controlled trial (IMAGE HF Project 1-C) Trials. 2013;14:443. doi: 10.1186/1745-6215-14-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ten Kate G.J., Caliskan K., Dedic A., Meijboom W.B., Neefjes L.A., Manintveld O.C. Computed tomography coronary imaging as a gatekeeper for invasive coronary angiography in patients with newly diagnosed heart failure of unknown aetiology. Eur J Heart Fail. 2013;15:1028–1034. doi: 10.1093/eurjhf/hft090. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D.A., Mathis G., Kirkpatrick A.W. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 32.Coiro S., Rossignol P., Ambrosio G., Carluccio E., Alunni G., Murrone A. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail. 2015;17:1172–1181. doi: 10.1002/ejhf.344. [DOI] [PubMed] [Google Scholar]
- 33.Gargani L., Pang P.S., Frassi F., Miglioranza M.H., Dini F.L., Landi P. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: a lung ultrasound study. Cardiovasc Ultrasound. 2015;13:40. doi: 10.1186/s12947-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer C.M., Appelbaum E., Desai M.Y., Desvigne-Nickens P., DiMarco J.P., Friedrich M.G. Hypertrophic Cardiomyopathy Registry: the rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J. 2015;170:223–230. doi: 10.1016/j.ahj.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakdawala N.K., Funke B.H., Baxter S., Cirino A.L., Roberts A.E., Judge D.P. Genetic testing for dilated cardiomyopathy in clinical practice. J Card Fail. 2012;18:296–303. doi: 10.1016/j.cardfail.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales A., Hershberger R.E. The rationale and timing of molecular genetic testing for dilated cardiomyopathy. Can J Cardiol. 2015;31:1309–1312. doi: 10.1016/j.cjca.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahon N.G., Murphy R.T., MacRae C.A., Caforio A.L., Elliott P.M., McKenna W.J. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Ann Intern Med. 2005;143:108–115. doi: 10.7326/0003-4819-143-2-200507190-00009. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Feng Y., Zhang Y.M., Ding X.X., Song Y.Z., Zhang A.M. Targeted next-generation sequencing of candidate genes reveals novel mutations in patients with dilated cardiomyopathy. Int J Mol Med. 2015;36:1479–1486. doi: 10.3892/ijmm.2015.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etchegary H., Pullman D., Simmonds C., Young T.L., Hodgkinson K. ‘It had to be done’: genetic testing decisions for arrhythmogenic right ventricular cardiomyopathy. Clin Genet. 2015;88:344–351. doi: 10.1111/cge.12513. [DOI] [PubMed] [Google Scholar]
- 40.Philips B., Cheng A. 2015 Update on the diagnosis and management of arrhythmogenic right ventricular cardiomyopathy. Curr Opin Cardiol. 2016;31:46–56. doi: 10.1097/HCO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 41.Miller E.M., Wang Y., Ware S.M. Uptake of cardiac screening and genetic testing among hypertrophic and dilated cardiomyopathy families. J Genet Couns. 2013;22:258–267. doi: 10.1007/s10897-012-9544-4. [DOI] [PubMed] [Google Scholar]
- 42.Kostis J.B., Davis B.R., Cutler J., Grimm R.H., Jr, Berge K.G., Cohen J.D. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 43.Beckett N.S., Peters R., Fletcher A.E., Staessen J.A., Liu L., Dumitrascu D. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 44.Sciarretta S., Palano F., Tocci G., Baldini R., Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171:384–394. doi: 10.1001/archinternmed.2010.427. [DOI] [PubMed] [Google Scholar]
- 45.Wright J.T., Jr, Williamson J.D., Whelton P.K., Snyder J.K., Sink K.M., Rocco M.V. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson S.C., Tektonidis T.G., Gigante B., Akesson A., Wolk A. Healthy lifestyle and risk of heart failure: results from 2 prospective cohort studies. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002855. [DOI] [PubMed] [Google Scholar]
- 47.Gopal D.M., Kalogeropoulos A.P., Georgiopoulou V.V., Smith A.L., Bauer D.C., Newman A.B. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. doi: 10.1016/j.ahj.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suskin N., Sheth T., Negassa A., Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1677–1682. doi: 10.1016/s0735-1097(01)01195-0. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed A.A., Patel K., Nyaku M.A., Kheirbek R.E., Bittner V., Fonarow G.C. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circ Heart Fail. 2015;8:694–701. doi: 10.1161/CIRCHEARTFAILURE.114.001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandey A., Garg S., Khunger M., Darden D., Ayers C., Kumbhani D.J. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132:1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 51.Bonsu K.O., Reidpath D.D., Kadirvelu A. Effects of statin treatment on inflammation and cardiac function in heart failure: an adjusted indirect comparison meta-analysis of randomized trials. Cardiovasc Ther. 2015;33:338–346. doi: 10.1111/1755-5922.12150. [DOI] [PubMed] [Google Scholar]
- 52.Aune D., Sen A., Norat T., Janszky I., Romundstad P., Tonstad S. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose-response meta-analysis of prospective studies. Circulation. 2016;133:639–649. doi: 10.1161/CIRCULATIONAHA.115.016801. [DOI] [PubMed] [Google Scholar]
- 53.Carbone S., Lavie C.J., Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–279. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Moss A.J., Zareba W., Hall W.J., Klein H., Wilber D.J., Cannom D.S. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 55.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 56.Ziff O.J., Lane D.A., Samra M., Griffith M., Kirchhof P., Lip G.Y. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351 doi: 10.1136/bmj.h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomsen M.M., Lewinter C., Kober L. Varying effects of recommended treatments for heart failure with reduced ejection fraction: meta-analysis of randomized controlled trials in the ESC and ACCF/AHA guidelines. ESC Heart Fail. 2016;3:235–244. doi: 10.1002/ehf2.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burnett H., Earley A., Voors A.A., Senni M., McMurray J.J., Deschaseaux C. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie W., Zheng F., Song X., Zhong B., Yan L. Renin-angiotensin-aldosterone system blockers for heart failure with reduced ejection fraction or left ventricular dysfunction: network meta-analysis. Int J Cardiol. 2016;205:65–71. doi: 10.1016/j.ijcard.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Chatterjee S., Biondi-Zoccai G., Abbate A., D’Ascenzo F., Castagno D., Van Tassell B. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346 doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotecha D., Manzano L., Krum H., Rosano G., Holmes J., Altman D.G. Effect of age and sex on efficacy and tolerability of beta blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ. 2016;353 doi: 10.1136/bmj.i1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jhund P.S., Fu M., Bayram E., Chen C.H., Negrusz-Kawecka M., Rosenthal A. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36:2576–2584. doi: 10.1093/eurheartj/ehv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMurray J.J. Neprilysin inhibition to treat heart failure: a tale of science, serendipity, and second chances. Eur J Heart Fail. 2015;17:242–247. doi: 10.1002/ejhf.250. [DOI] [PubMed] [Google Scholar]
- 64.Muller-Werdan U., Stockl G., Werdan K. Advances in the management of heart failure: the role of ivabradine. Vasc Health Risk Manag. 2016;12:453–470. doi: 10.2147/VHRM.S90383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox K., Ford I., Steg P.G., Tendera M., Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 66.Fox K., Ford I., Steg P.G., Tendera M., Robertson M., Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 67.Swedberg K., Komajda M., Bohm M., Borer J.S., Ford I., Dubost-Brama A. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 68.Bohm M., Swedberg K., Komajda M., Borer J.S., Ford I., Dubost-Brama A. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 69.Zugck C., Martinka P., Stockl G. Ivabradine treatment in a chronic heart failure patient cohort: symptom reduction and improvement in quality of life in clinical practice. Adv Ther. 2014;31:961–974. doi: 10.1007/s12325-014-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vardeny O., Claggett B., Anand I., Rossignol P., Desai A.S., Zannad F. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7:573–579. doi: 10.1161/CIRCHEARTFAILURE.114.001104. [DOI] [PubMed] [Google Scholar]
- 71.Eschalier R., McMurray J.J., Swedberg K., van Veldhuisen D.J., Krum H., Pocock S.J. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) J Am Coll Cardiol. 2013;62:1585–1593. doi: 10.1016/j.jacc.2013.04.086. [DOI] [PubMed] [Google Scholar]
- 72.Faris R.F., Flather M., Purcell Poole-Wilson H.P.A., Coats A.J. Diuretics for heart failure. Cochrane Database Syst Rev. 2012:CD003838. doi: 10.1002/14651858.CD003838.pub3. [DOI] [PubMed] [Google Scholar]
- 73.Volz E.M., Felker G.M. How to use diuretics in heart failure. Curr Treat Options Cardiovasc Med. 2009;11:426–432. doi: 10.1007/s11936-009-0045-1. [DOI] [PubMed] [Google Scholar]
- 74.Trullas J.C., Morales-Rull J.L., Casado J., Freitas Ramirez A., Manzano L., Formiga F. Rationale and design of the “Safety and Efficacy of the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure (CLOROTIC) Trial:” a double-blind, randomized, placebo-controlled study to determine the effect of combined diuretic therapy (loop diuretics with thiazide-type diuretics) among patients with decompensated heart failure. J Card Fail. 2016;22:529–536. doi: 10.1016/j.cardfail.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Ziesche S., Cobb F.R., Cohn J.N., Johnson G., Tristani F. Hydralazine and isosorbide dinitrate combination improves exercise tolerance in heart failure. Results from V-HeFT I and V-HeFT II. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI56–V64. [PubMed] [Google Scholar]
- 76.Taylor A.L., Ziesche S., Yancy C., Carson P., D’Agostino R., Jr, Ferdinand K. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 77.Eisen A., Ruff C.T., Braunwald E., Hamershock R.A., Lewis B.S., Hassager C. Digoxin use and subsequent clinical outcomes in patients with atrial fibrillation with or without heart failure in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adams K.F., Jr, Ghali J.K., Herbert Patterson J., Stough W.G., Butler J., Bauman J.L. A perspective on re-evaluating digoxin’s role in the current management of patients with chronic systolic heart failure: targeting serum concentration to reduce hospitalization and improve safety profile. Eur J Heart Fail. 2014;16:483–493. doi: 10.1002/ejhf.64. [DOI] [PubMed] [Google Scholar]
- 79.Connolly S.J., Hallstrom A.P., Cappato R., Schron E.B., Kuck K.H., Zipes D.P. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 80.Steinberg B.A., Al-Khatib S.M., Edwards R., Han J., Bardy G.H., Bigger J.T. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail. 2014;2:623–629. doi: 10.1016/j.jchf.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oseroff O., Retyk E., Bochoeyer A. Subanalyses of secondary prevention implantable cardioverter-defibrillator trials: antiarrhythmics versus implantable defibrillators (AVID), Canadian Implantable Defibrillator Study (CIDS), and Cardiac Arrest Study Hamburg (CASH) Curr Opin Cardiol. 2004;19:26–30. doi: 10.1097/00001573-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Bardy G.H., Lee K.L., Mark D.B., Poole J.E., Packer D.L., Boineau R. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 83.Hess P.L., Al-Khatib S.M., Han J.Y., Edwards R., Bardy G.H., Bigger J.T. Survival benefit of the primary prevention implantable cardioverter-defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–186. doi: 10.1161/CIRCOUTCOMES.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theuns D.A., Smith T., Hunink M.G., Bardy G.H., Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gialama F., Prezerakos P., Maniadakis N. The cost effectiveness of implantable cardioverter defibrillators: a systematic review of economic evaluations. Appl Health Econ Health Policy. 2014;12:41–49. doi: 10.1007/s40258-013-0069-2. [DOI] [PubMed] [Google Scholar]
- 86.Cleland J.G., Abraham W.T., Linde C., Gold M.R., Young J.B., Claude Daubert J. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daubert C., Gold M.R., Abraham W.T., Ghio S., Hassager C., Goode G. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–1846. doi: 10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Linde C., Daubert C., Abraham W.T., St John Sutton M., Ghio S., Hassager C. Impact of ejection fraction on the clinical response to cardiac resynchronization therapy in mild heart failure. Circ Heart Fail. 2013;6:1180–1189. doi: 10.1161/CIRCHEARTFAILURE.113.000326. [DOI] [PubMed] [Google Scholar]
- 89.Gold M.R., Thebault C., Linde C., Abraham W.T., Gerritse B., Ghio S. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 90.Gold M.R., Daubert C., Abraham W.T., Ghio S., St John Sutton M., Hudnall J.H. The effect of reverse remodeling on long-term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: results of the REVERSE study. Heart Rhythm. 2015;12:524–530. doi: 10.1016/j.hrthm.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linde C., Gold M.R., Abraham W.T., St John Sutton M., Ghio S., Cerkvenik J. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J. 2013;34:2592–2599. doi: 10.1093/eurheartj/eht160. [DOI] [PubMed] [Google Scholar]
- 92.Moss A.J., Hall W.J., Cannom D.S., Klein H., Brown M.W., Daubert J.P. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 93.Goldenberg I., Hall W.J., Beck C.A., Moss A.J., Barsheshet A., McNitt S. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2011;58:729–737. doi: 10.1016/j.jacc.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 94.Goldenberg I., Kutyifa V., Klein H.U., Cannom D.S., Brown M.W., Dan A. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 95.Ruwald A.C., Pietrasik G., Goldenberg I., Kutyifa V., Daubert J.P., Ruwald M.H. The effect of intermittent atrial tachyarrhythmia on heart failure or death in cardiac resynchronization therapy with defibrillator versus implantable cardioverter-defibrillator patients: a MADIT-CRT substudy (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) J Am Coll Cardiol. 2014;63:1190–1197. doi: 10.1016/j.jacc.2013.10.074. [DOI] [PubMed] [Google Scholar]
- 96.Barra S., Providencia R., Tang A., Heck P., Virdee M., Agarwal S. Importance of implantable cardioverter-defibrillator back-up in cardiac resynchronization therapy recipients: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Auricchio A., Lumens J., Prinzen F.W. Does cardiac resynchronization therapy benefit patients with right bundle branch block: cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol. 2014;7:532–542. doi: 10.1161/CIRCEP.113.000628. [DOI] [PubMed] [Google Scholar]
- 98.Khidir M.J., Delgado V., Ajmone Marsan N., Schalij M.J., Bax J.J. QRS duration versus morphology and survival after cardiac resynchronization therapy. ESC Heart Fail. 2017;4:23–30. doi: 10.1002/ehf2.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woo C.Y., Strandberg E.J., Schmiegelow M.D., Pitt A.L., Hlatky M.A., Owens D.K. Cost-effectiveness of adding cardiac resynchronization therapy to an implantable cardioverter-defibrillator among patients with mild heart failure. Ann Intern Med. 2015;163:417–426. doi: 10.7326/M14-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sundaram V., Sahadevan J., Waldo A.L., Stukenborg G.J., Reddy Y.N.V., Asirvatham S.J. Implantable cardioverter-defibrillators with versus without resynchronization therapy in patients with a QRS duration >180 ms. J Am Coll Cardiol. 2017;69:2026–2036. doi: 10.1016/j.jacc.2017.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Polsinelli V.B., Shah S.J. Advances in the pharmacotherapy of chronic heart failure with preserved ejection fraction: an ideal opportunity for precision medicine. Expert Opin Pharmacother. 2017;18:399–409. doi: 10.1080/14656566.2017.1288717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Henkel D.M., Redfield M.M., Weston S.A., Gerber Y., Roger V.L. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lekavich C.L., Barksdale D.J., Neelon V., Wu J.R. Heart failure preserved ejection fraction (HFpEF): an integrated and strategic review. Heart Fail Rev. 2015;20:643–653. doi: 10.1007/s10741-015-9506-7. [DOI] [PubMed] [Google Scholar]
- 104.Lewis E.F., Lamas G.A., O’Meara E., Granger C.B., Dunlap M.E., McKelvie R.S. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 105.Fukuta H., Goto T., Wakami K., Ohte N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2016;23:78–85. doi: 10.1177/2047487314564729. [DOI] [PubMed] [Google Scholar]
- 106.Faris R., Flather M., Purcell H., Henein M., Poole-Wilson P., Coats A. Current evidence supporting the role of diuretics in heart failure: a meta analysis of randomised controlled trials. Int J Cardiol. 2002;82:149–158. doi: 10.1016/s0167-5273(01)00600-3. [DOI] [PubMed] [Google Scholar]
- 107.Fukuta H., Goto T., Wakami K., Ohte N. The effect of beta-blockers on mortality in heart failure with preserved ejection fraction: a meta-analysis of observational cohort and randomized controlled studies. Int J Cardiol. 2017;228:4–10. doi: 10.1016/j.ijcard.2016.11.239. [DOI] [PubMed] [Google Scholar]
- 108.Bavishi C., Chatterjee S., Ather S., Patel D., Messerli F.H. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20:193–201. doi: 10.1007/s10741-014-9453-8. [DOI] [PubMed] [Google Scholar]
- 109.Mead G.E., Elder A.T., Flapan A.D., Kelman A. Electrical cardioversion for atrial fibrillation and flutter. Cochrane Database Syst Rev. 2005;Cd002903 doi: 10.1002/14651858.CD002903.pub2. [DOI] [PubMed] [Google Scholar]
- 110.Hofmann R., Steinwender C., Kammler J., Kypta A., Leisch F. Effects of a high dose intravenous bolus amiodarone in patients with atrial fibrillation and a rapid ventricular rate. Int J Cardiol. 2006;110:27–32. doi: 10.1016/j.ijcard.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 111.Hofmann R., Wimmer G., Leisch F. Intravenous amiodarone bolus immediately controls heart rate in patients with atrial fibrillation accompanied by severe congestive heart failure. Heart. 2000;84:635. doi: 10.1136/heart.84.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bavendiek U., Aguirre Davila L., Koch A., Bauersachs J. Assumption versus evidence: the case of digoxin in atrial fibrillation and heart failure. Eur Heart J. 2017;38:2095–2099. doi: 10.1093/eurheartj/ehw577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Van Gelder I.C., Rienstra M., Crijns H.J., Olshansky B. Rate control in atrial fibrillation. Lancet. 2016;388:818–828. doi: 10.1016/S0140-6736(16)31258-2. [DOI] [PubMed] [Google Scholar]
- 114.Connolly S.J., Camm A.J., Halperin J.L., Joyner C., Alings M., Amerena J. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 115.Kober L., Torp-Pedersen C., McMurray J.J., Gotzsche O., Levy S., Crijns H. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 116.Deedwania P.C., Singh B.N., Ellenbogen K., Fisher S., Fletcher R., Singh S.N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98:2574–2579. doi: 10.1161/01.cir.98.23.2574. [DOI] [PubMed] [Google Scholar]
- 117.Khan A.R., Khan S., Sheikh M.A., Khuder S., Grubb B., Moukarbel G.V. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7:853–860. doi: 10.1161/CIRCEP.114.001853. [DOI] [PubMed] [Google Scholar]
- 118.Lafuente-Lafuente C., Valembois L., Bergmann J.F. Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;Cd005049 doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 119.Melgaard L., Gorst-Rasmussen A., Lane D.A., Rasmussen L.H., Larsen T.B., Lip G.Y. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314:1030–1038. doi: 10.1001/jama.2015.10725. [DOI] [PubMed] [Google Scholar]
- 120.Zhu W., He W., Guo L., Wang X., Hong K. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555–561. doi: 10.1002/clc.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong Q., Lau Y.C., Senoo K., Lane D.A., Hong K., Lip G.Y. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: a systemic review and meta-analysis of randomized trials. Eur J Heart Fail. 2015;17:1192–1200. doi: 10.1002/ejhf.343. [DOI] [PubMed] [Google Scholar]
- 122.Trikha R., Kowey P.R. Practical considerations for the nonvitamin K antagonist oral anticoagulants. Cardiology. 2017;136:115–124. doi: 10.1159/000447530. [DOI] [PubMed] [Google Scholar]
- 123.Eikelboom J.W., Connolly S.J., Brueckmann M., Granger C.B., Kappetein A.P., Mack M.J. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 124.Urso C., Canino B., Brucculeri S., Firenze A., Caimi G. Analysis of electrolyte abnormalities and the mechanisms leading to arrhythmias in heart failure. A literature review. Clin Ter. 2016;167:e85–e91. doi: 10.7417/CT.2016.1944. [DOI] [PubMed] [Google Scholar]
- 125.Saltzman H.E. Arrhythmias and heart failure. Cardiol Clin. 2014;32(125–33):ix. doi: 10.1016/j.ccl.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Pitt B., Zannad F., Remme W.J., Cody R., Castaigne A., Perez A. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 127.Desai A.S., McMurray J.J., Packer M., Swedberg K., Rouleau J.L., Chen F. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 128.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Europace. 2015;17:1601–1687. doi: 10.1093/europace/euv319. [DOI] [PubMed] [Google Scholar]
- 129.Brignole M., Botto G., Mont L., Iacopino S., De Marchi G., Oddone D. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur Heart J. 2011;32:2420–2429. doi: 10.1093/eurheartj/ehr162. [DOI] [PubMed] [Google Scholar]
- 130.Vizzardi E., Bonadei I., Rovetta R., D’Aloia A., Quinzani F., Curnis A. When should we use nitrates in congestive heart failure? Cardiovasc Ther. 2013;31:27–31. doi: 10.1111/j.1755-5922.2012.00311.x. [DOI] [PubMed] [Google Scholar]
- 131.Kosmicki M.A. Long-term use of short- and long-acting nitrates in stable angina pectoris. Curr Clin Pharmacol. 2009;4:132–141. doi: 10.2174/157488409788185016. [DOI] [PubMed] [Google Scholar]
- 132.Boden W.E., Finn A.V., Patel D., Peacock W.F., Thadani U., Zimmerman F.H. Nitrates as an integral part of optimal medical therapy and cardiac rehabilitation for stable angina: review of current concepts and therapeutics. Clin Cardiol. 2012;35:263–271. doi: 10.1002/clc.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siama K., Tousoulis D., Papageorgiou N., Siasos G., Tsiamis E., Bakogiannis C. Stable angina pectoris: current medical treatment. Curr Pharm Des. 2013;19:1569–1580. [PubMed] [Google Scholar]
- 134.Salazar C.A., Basilio Flores J.E., Veramendi Espinoza L.E., Mejia Dolores J.W., Rey Rodriguez D.E. Loza Munarriz C. Ranolazine for stable angina pectoris. Cochrane Database Syst Rev. 2017;2:Cd011747. doi: 10.1002/14651858.CD011747.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fox K., Ford I., Steg P.G., Tardif J.C., Tendera M., Ferrari R. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 136.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA 2000;283:1967–75. [PubMed]
- 137.Anker S.D., Comin Colet J., Filippatos G., Willenheimer R., Dickstein K., Drexler H. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 138.Ponikowski P., van Veldhuisen D.J., Comin-Colet J., Ertl G., Komajda M., Mareev V. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jankowska E.A., Tkaczyszyn M., Suchocki T., Drozd M., von Haehling S., Doehner W. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail. 2016;18:786–795. doi: 10.1002/ejhf.473. [DOI] [PubMed] [Google Scholar]
- 140.Eurich D.T., Weir D.L., Majumdar S.R., Tsuyuki R.T., Johnson J.A., Tjosvold L. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 141.Pham D., Albuquerque Rocha N., McGuire D.K., Neeland I.J. Impact of empagliflozin in patients with diabetes and heart failure. Trends Cardiovasc Med. 2017;27:144–151. doi: 10.1016/j.tcm.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Deeks E.D. Linagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2012;72:1793–1824. doi: 10.2165/11209570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 143.Rosenstock J., Marx N., Neubacher D., Seck T., Patel S., Woerle H.J. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol. 2015;14:57. doi: 10.1186/s12933-015-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lehrke M., Leiter L.A., Hehnke U., Thiemann S., Bhandari A., Meinicke T. Safety and efficacy of linagliptin in patients with type 2 diabetes mellitus and coronary artery disease: analysis of pooled events from 19 clinical trials. J Diabetes Complications. 2016;30:1378–1384. doi: 10.1016/j.jdiacomp.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 145.Komajda M., McMurray J.J., Beck-Nielsen H., Gomis R., Hanefeld M., Pocock S.J. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hernandez A.V., Usmani A., Rajamanickam A., Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011;11:115–128. doi: 10.2165/11587580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 147.Newhouse A., Jiang W. Heart failure and depression. Heart Fail Clin. 2014;10:295–304. doi: 10.1016/j.hfc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 148.Fan H., Yu W., Zhang Q., Cao H., Li J., Wang J. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Prev Med. 2014;63:36–42. doi: 10.1016/j.ypmed.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 149.Diez-Quevedo C., Lupon J., Gonzalez B., Urrutia A., Cano L., Cabanes R. Depression, antidepressants, and long-term mortality in heart failure. Int J Cardiol. 2013;167:1217–1225. doi: 10.1016/j.ijcard.2012.03.143. [DOI] [PubMed] [Google Scholar]
- 150.Paraskevaidis I., Palios J., Parissis J., Filippatos G., Anastasiou-Nana M. Treating depression in coronary artery disease and chronic heart failure: what's new in using selective serotonin re-uptake inhibitors? Cardiovasc Hematol Agents Med Chem. 2012;10:109–115. doi: 10.2174/187152512800388894. [DOI] [PubMed] [Google Scholar]
- 151.Watson K., Summers K.M. Depression in patients with heart failure: clinical implications and management. Pharmacotherapy. 2009;29:49–63. doi: 10.1592/phco.29.1.49. [DOI] [PubMed] [Google Scholar]
- 152.Angermann C.E., Gelbrich G., Störk S., Gunold H., Edelmann F., Wachter R. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: the MOOD-HF randomized clinical trial. JAMA. 2016;315:2683–2693. doi: 10.1001/jama.2016.7635. [DOI] [PubMed] [Google Scholar]
- 153.Nichol K.L., Nordin J., Mullooly J., Lask R., Fillbrandt K., Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 154.Dickstein K., Cohen-Solal A., Filippatos G., McMurray J.J., Ponikowski P., Poole-Wilson P.A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 155.MacDonald M., Fang J., Pittman S.D., White D.P., Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 156.Cowie M.R., Woehrle H., Wegscheider K., Angermann C., d’Ortho M.P., Erdmann E. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bradley T.D., Logan A.G., Kimoff R.J., Series F., Morrison D., Ferguson K. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 158.Kamperidis V., Delgado V., van Mieghem N.M., Kappetein A.P., Leon M.B., Bax J.J. Diagnosis and management of aortic valve stenosis in patients with heart failure. Eur J Heart Fail. 2016;18:469–481. doi: 10.1002/ejhf.466. [DOI] [PubMed] [Google Scholar]
- 159.Vassileva C.M., Telila T., Markwell S., Hazelrigg S. Magnitude of negative impact of preoperative heart failure on mortality during aortic valve replacement in the medicare population. Ann Thorac Surg. 2015;99:1503–1509. doi: 10.1016/j.athoracsur.2014.12.106. [discussion 1509–10] [DOI] [PubMed] [Google Scholar]
- 160.Zamorano J.L., Lancellotti P., Rodriguez Munoz D., Aboyans V., Asteggiano R., Galderisi M. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 161.Cheng Y.J., Nie X.Y., Chen X.M., Lin X.X., Tang K., Zeng W.T. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol. 2015;66:2173–2184. doi: 10.1016/j.jacc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 162.Thomas S.S., Nohria A. Hemodynamic classifications of acute heart failure and their clinical application: an update. Circ J. 2012;76:278–286. doi: 10.1253/circj.cj-11-1441. [DOI] [PubMed] [Google Scholar]
- 163.Roberts E., Ludman A.J., Dworzynski K., Al-Mohammad A., Cowie M.R., McMurray J.J. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350 doi: 10.1136/bmj.h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maisel A., Mueller C., Nowak R., Peacock W.F., Landsberg J.W., Ponikowski P. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 165.Moe G.W., Howlett J., Januzzi J.L., Zowall H. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115:3103–3110. doi: 10.1161/CIRCULATIONAHA.106.666255. [DOI] [PubMed] [Google Scholar]
- 166.Hsiao R., Greenberg B. Contemporary treatment of acute heart failure. Prog Cardiovasc Dis. 2016;58:367–378. doi: 10.1016/j.pcad.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 167.Matsue Y., Damman K., Voors A.A., Kagiyama N., Yamaguchi T., Kuroda S. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:3042–3051. doi: 10.1016/j.jacc.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 168.Mebazaa A., Yilmaz M.B., Levy P., Ponikowski P., Peacock W.F., Laribi S. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–558. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 169.Neuhold S., Huelsmann M., Strunk G., Stoiser B., Struck J., Morgenthaler N.G. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52:266–272. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 170.Felker G.M., Lee K.L., Bull D.A., Redfield M.M., Stevenson L.W., Goldsmith S.R. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jentzer J.C., DeWald T.A., Hernandez A.F. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–1534. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 172.Piper S., McDonagh T. The role of intravenous vasodilators in acute heart failure management. Eur J Heart Fail. 2014;16:827–834. doi: 10.1002/ejhf.123. [DOI] [PubMed] [Google Scholar]
- 173.Campia U., Nodari S., Gheorghiade M. Acute heart failure with low cardiac output: can we develop a short-term inotropic agent that does not increase adverse events? Curr Heart Fail Rep. 2010;7:100–109. doi: 10.1007/s11897-010-0021-9. [DOI] [PubMed] [Google Scholar]
- 174.Dentali F., Douketis J.D., Gianni M., Lim W., Crowther M.A. Meta-analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. doi: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 175.Weng C.L., Zhao Y.T., Liu Q.H., Fu C.J., Sun F., Ma Y.L. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590–600. doi: 10.7326/0003-4819-152-9-201005040-00009. [DOI] [PubMed] [Google Scholar]
- 176.Gray A.J., Goodacre S., Newby D.E., Masson M.A., Sampson F., Dixon S. A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial. Health Technol Assess. 2009;13:1–106. doi: 10.3310/hta13330. [DOI] [PubMed] [Google Scholar]
- 177.Vital F.M., Ladeira M.T., Atallah A.N. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;Cd005351 doi: 10.1002/14651858.CD005351.pub3. [DOI] [PubMed] [Google Scholar]
- 178.Park M., Sangean M.C., Volpe Mde S., Feltrim M.I., Nozawa E., Leite P.F. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med. 2004;32:2407–2415. doi: 10.1097/01.ccm.0000147770.20400.10. [DOI] [PubMed] [Google Scholar]
- 179.Gray A., Goodacre S., Newby D.E., Masson M., Sampson F., Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 180.Peacock W.F., Hollander J.E., Diercks D.B., Lopatin M., Fonarow G., Emerman C.L. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25:205–209. doi: 10.1136/emj.2007.050419. [DOI] [PubMed] [Google Scholar]
- 181.Park J.J., Choi D.J., Yoon C.H., Oh I.Y., Lee J.H., Ahn S. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail. 2015;17:601–611. doi: 10.1002/ejhf.276. [DOI] [PubMed] [Google Scholar]
- 182.Kwong J.S., Yu C.M. Ultrafiltration for acute decompensated heart failure: a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2014;172:395–402. doi: 10.1016/j.ijcard.2014.01.069. [DOI] [PubMed] [Google Scholar]
- 183.Wen H., Zhang Y., Zhu J., Lan Y., Yang H. Ultrafiltration versus intravenous diuretic therapy to treat acute heart failure: a systematic review. Am J Cardiovasc Drugs. 2013;13:365–373. doi: 10.1007/s40256-013-0034-3. [DOI] [PubMed] [Google Scholar]
- 184.Mentz R.J., Kjeldsen K., Rossi G.P., Voors A.A., Cleland J.G., Anker S.D. Decongestion in acute heart failure. Eur J Heart Fail. 2014;16:471–482. doi: 10.1002/ejhf.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bart B.A., Goldsmith S.R., Lee K.L., Givertz M.M., O’Connor C.M., Bull D.A. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Raichlin E., Haglund N.A., Dumitru I., Lyden E.R., Johnston M.D., Mack J.M. Worsening renal function in patients with acute decompensated heart failure treated with ultrafiltration: predictors and outcomes. J Card Fail. 2014;20 376.e325–e32. [PubMed] [Google Scholar]
- 187.De Backer D., Biston P., Devriendt J., Madl C., Chochrad D., Aldecoa C. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 188.How O.J., Rosner A., Kildal A.B., Stenberg T.A., Gjessing P.F., Hermansen S.E. Dobutamine-norepinephrine, but not vasopressin, restores the ventriculoarterial matching in experimental cardiogenic shock. Transl Res. 2010;156:273–281. doi: 10.1016/j.trsl.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 189.Thiele H., Zeymer U., Neumann F.J., Ferenc M., Olbrich H.G., Hausleiter J. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 190.Saudi Center for Organ Transplantation. 2015 annual report 2015 [28/02/2017]. Available from: http://www.scot.gov.sa/uploads/Annual_Report_2015_En_(Last_Edit_160516).pdf.
- 191.Estep J.D., Starling R.C., Horstmanshof D.A., Milano C.A., Selzman C.H., Shah K.B. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66:1747–1761. doi: 10.1016/j.jacc.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 192.Kirklin J.K., Naftel D.C., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 193.Jorde U.P., Kushwaha S.S., Tatooles A.J., Naka Y., Bhat G., Long J.W. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2014;63:1751–1757. doi: 10.1016/j.jacc.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 194.Riebandt J., Haberl T., Mahr S., Laufer G., Rajek A., Steinlechner B. Preoperative patient optimization using extracorporeal life support improves outcomes of INTERMACS level I patients receiving a permanent ventricular assist device. Eur J Cardiothorac Surg. 2014;46:486–492. doi: 10.1093/ejcts/ezu093. [discussion 492] [DOI] [PubMed] [Google Scholar]
- 195.Stewart G.C., Kittleson M.M., Patel P.C., Cowger J.A., Patel C.B., Mountis M.M. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) profiling identifies ambulatory patients at high risk on medical therapy after hospitalizations for heart failure. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003032. [DOI] [PubMed] [Google Scholar]
- 196.Adamo L., Tang Y., Nassif M.E., Novak E., Jones P.G., LaRue S. The HeartMate risk score identifies patients with similar mortality risk across all INTERMACS profiles in a large multicenter analysis. JACC Heart Fail. 2016;4:950–958. doi: 10.1016/j.jchf.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 197.Velazquez E.J., Lee K.L., Deja M.A., Jain A., Sopko G., Marchenko A. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Velazquez E.J., Williams J.B., Yow E., Shaw L.K., Lee K.L., Phillips H.R. Long-term survival of patients with ischemic cardiomyopathy treated by coronary artery bypass grafting versus medical therapy. Ann Thorac Surg. 2012;93:523–530. doi: 10.1016/j.athoracsur.2011.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bonow R.O., Maurer G., Lee K.L., Holly T.A., Binkley P.F., Desvigne-Nickens P. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ling L.F., Marwick T.H., Flores D.R., Jaber W.A., Brunken R.C., Cerqueira M.D. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. doi: 10.1161/CIRCIMAGING.112.000138. [DOI] [PubMed] [Google Scholar]
- 201.Dor V., Civaia F., Alexandrescu C., Sabatier M., Montiglio F. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2011;141:905–916. doi: 10.1016/j.jtcvs.2010.10.026. 916.e901–e4. [DOI] [PubMed] [Google Scholar]
- 202.Michler R.E., Rouleau J.L., Al-Khalidi H.R., Bonow R.O., Pellikka P.A., Pohost G.M. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146:1139–1145. doi: 10.1016/j.jtcvs.2012.09.007. e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oh J.K., Velazquez E.J., Menicanti L., Pohost G.M., Bonow R.O., Lin G. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J. 2013;34:39–47. doi: 10.1093/eurheartj/ehs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kunadian V., Pugh A., Zaman A.G., Qiu W. Percutaneous coronary intervention among patients with left ventricular systolic dysfunction: a review and meta-analysis of 19 clinical studies. Coron Artery Dis. 2012;23:469–479. doi: 10.1097/MCA.0b013e3283587804. [DOI] [PubMed] [Google Scholar]
- 205.Nagendran J., Norris C.M., Graham M.M., Ross D.B., Macarthur R.G., Kieser T.M. Coronary revascularization for patients with severe left ventricular dysfunction. Ann Thorac Surg. 2013;96:2038–2044. doi: 10.1016/j.athoracsur.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 206.Mihos C.G., Santana O. Mitral valve repair for ischemic mitral regurgitation: lessons from the Cardiothoracic Surgical Trials Network randomized study. J Thorac Dis. 2016;8:E94–E99. doi: 10.3978/j.issn.2072-1439.2016.01.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Smith P.K., Puskas J.D., Ascheim D.D., Voisine P., Gelijns A.C., Moskowitz A.J. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goldstone A.B., Howard J.L., Cohen J.E., MacArthur J.W., Jr, Atluri P., Kirkpatrick J.N. Natural history of coexistent tricuspid regurgitation in patients with degenerative mitral valve disease: implications for future guidelines. J Thorac Cardiovasc Surg. 2014;148:2802–2809. doi: 10.1016/j.jtcvs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 209.Stewart S., Riegel B., Boyd C., Ahamed Y., Thompson D.R., Burrell L.M. Establishing a pragmatic framework to optimise health outcomes in heart failure and multimorbidity (ARISE-HF): a multidisciplinary position statement. Int J Cardiol. 2016;212:1–10. doi: 10.1016/j.ijcard.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jaarsma T. Inter-professional team approach to patients with heart failure. Heart. 2005;91:832–838. doi: 10.1136/hrt.2003.025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ducharme A., Doyon O., White M., Rouleau J.L., Brophy J.M. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ. 2005;173:40–45. doi: 10.1503/cmaj.1041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Martineau P., Frenette M., Blais L., Sauve C. Multidisciplinary outpatient congestive heart failure clinic: impact on hospital admissions and emergency room visits. Can J Cardiol. 2004;20:1205–1211. [PubMed] [Google Scholar]
- 213.Kasper E.K., Gerstenblith G., Hefter G., Van Anden E., Brinker J.A., Thiemann D.R. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 214.Creaser J.W., DePasquale E.C., Vandenbogaart E., Rourke D., Chaker T., Fonarow G.C. Team-based care for outpatients with heart failure. Heart Fail Clin. 2015;11:379–405. doi: 10.1016/j.hfc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 215.Larsen P.M., Teerlink J.R. Team-based care for patients hospitalized with heart failure. Heart Fail Clin. 2015;11:359–370. doi: 10.1016/j.hfc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 216.Lewinter C., Doherty P., Gale C.P., Crouch S., Stirk L., Lewin R.J. Exercise-based cardiac rehabilitation in patients with heart failure: a meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol. 2015;22:1504–1512. doi: 10.1177/2047487314559853. [DOI] [PubMed] [Google Scholar]
- 217.Uddin J., Zwisler A.D., Lewinter C., Moniruzzaman M., Lund K., Tang L.H. Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur J Prev Cardiol. 2016;23:683–693. doi: 10.1177/2047487315604311. [DOI] [PubMed] [Google Scholar]
- 218.Sagar V.A., Davies E.J., Briscoe S., Coats A.J., Dalal H.M., Lough F. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart. 2015;2 doi: 10.1136/openhrt-2014-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Abraham W.T., Stevenson L.W., Bourge R.C., Lindenfeld J.A., Bauman J.G., Adamson P.B. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 220.Abraham W.T., Adamson P.B., Bourge R.C., Aaron M.F., Costanzo M.R., Stevenson L.W. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 221.Hindricks G., Taborsky M., Glikson M., Heinrich U., Schumacher B., Katz A. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 222.Frankenstein L., Frohlich H., Cleland J.G. Multidisciplinary approach for patients hospitalized with heart failure. Rev Esp Cardiol (Engl Ed) 2015;68:885–891. doi: 10.1016/j.rec.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 223.Milfred-Laforest S.K., Chow S.L., Didomenico R.J., Dracup K., Ensor C.R., Gattis-Stough W. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. J Card Fail. 2013;19:354–369. doi: 10.1016/j.cardfail.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 224.Gattis W.A., Hasselblad V., Whellan D.J., O’Connor C.M. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Arch Intern Med. 1999;159:1939–1945. doi: 10.1001/archinte.159.16.1939. [DOI] [PubMed] [Google Scholar]
- 225.Davis E.M., Packard K.A., Jackevicius C.A. The pharmacist role in predicting and improving medication adherence in heart failure patients. J Manag Care Spec Pharm. 2014;20:741–755. doi: 10.18553/jmcp.2014.20.7.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kitts N.K., Reeve A.R., Tsu L. Care transitions in elderly heart failure patients: current practices and the pharmacist’s role. Consult Pharm. 2014;29:179–190. doi: 10.4140/TCP.n.2014.179. [DOI] [PubMed] [Google Scholar]
- 227.Eggink R.N., Lenderink A.W., Widdershoven J.W., van den Bemt P.M. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci. 2010;32:759–766. doi: 10.1007/s11096-010-9433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cheng J.W., Cooke-Ariel H. Pharmacists’ role in the care of patients with heart failure: review and future evolution. J Manag Care Pharm. 2014;20:206–213. doi: 10.18553/jmcp.2014.20.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Truong J.T., Backes A.C. The impact of a continuum of care resident pharmacist on heart failure readmissions and discharge instructions at a community hospital. SAGE Open Med. 2015;3 doi: 10.1177/2050312115577986. 2050312115577986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roblek T., Deticek A., Leskovar B., Suskovic S., Horvat M., Belic A. Clinical-pharmacist intervention reduces clinically relevant drug-drug interactions in patients with heart failure: a randomized, double-blind, controlled trial. Int J Cardiol. 2016;203:647–652. doi: 10.1016/j.ijcard.2015.10.206. [DOI] [PubMed] [Google Scholar]