Abstract
Transformation of a normal cell to cancerous one is dependent on the accumulation of several genetic and epigenetic alterations. One of the candidate driver genetic alterations can happen in succinate dehydrogenases (SDHx) coding gene include SDHA, SDHB, SDHC, SDHD, and SDHAF2. The most important SDH mutation is in the SDHD gene, which encodes the smallest subunit of mitochondrial complex II (SDH). It has key function both in familial and non-familial hereditary paraganglioma/phaeochromocytoma syndrome (HPGL/PCC). SDHx genes mutations can have resulted in genetic and epigenetic changes like histone hypermethylation. These properties can lead to succinate-mediated inhibition of α-ketoglutarate-dependent dioxygenases. So hypoxic conditions can generate subsequent neoplastic transformation, and in this review, we are presenting the role of SDHx in several malignancies.
KeyWords: Succinate dehydrogenases, Tumor, Genetic
Introduction
Tumorigenesis is a multistep process depending on a sequential accumulation of genetic and epigenetic alterations within the cells 1,2 . In spite of the fact that during tumorigenesis a large number of mutations are involved, only a relatively small subset of driver mutations is crucial for starting steps of neoplastic development. These mutations consequence in destruction of tissue homeostasis as the transformed cells gain fitness by increasing their proliferation rate, decreasing their death rate, and creating a growth-promoting environment3. Histological studies and Immunohistochemistry (IHC) are applicable for the tissue distribution of targeted antigens in the way of neoplastic and non-neoplastic diagnosis because specific antigens are expressed de novo or up-regulated in certain lesions4-8. One of the remarkable of these driver genetic alterations can happen in succinate dehyrogenase genes (SDHx). SDHx is a multipart enzyme made of subunits encoded by SDHA, SDHB, SDHC and SDHD genes. Succinate dehydrogenase (SDH) heterotetrameric complex catalyzes the oxidation of succinate to fumarate in the tricarboxylic acid (TCA) cycle and in the aerobic respiratory sequences of eukaryotes and bacteria9. Succinate dehydrogenase and fumarate hydratase (other enzyme of Krebs cycle) inactivation result in an obstruction of Krebs cycle, impaired respiration and abnormal accretion of their substrates, succinate and fumarate10. Krebs cycle genes, fumarate hydratase (FH) and SDH are mutated in a compartment of several malignancies, secondary to accretion of their substrates, fumarate and succinate, respectively11. SDH enzyme (also known as succinate-ubiquinone oxydoreductase) is a well-preserved heterotetrameric protein, with SDHA and SDHB as catalytic subunits, which is produced in the mitochondrial matrix and anchored to the inner membrane12. The results of inactivation of SDH and FH have both been associated with abnormalities of cellular metabolism, responsible for the activation of hypoxic gene response pathways and epigenetic alterations (eg, DNA methylation) 13. Loss of the SDH complex is described in extra-adrenal paragangliomas, gastrointestinal stromal tumors, renal cell carcinomas and rare in other epithelial tumors14. Germline mutations in FH gene influence individuals with leiomyomas and renal cell cancer (HLRCC), while mutations in SDH can be the cause of paragangliomas and phaeochromocytomas (endocrine tumors)15. Paragangliomas are neural crest-derived tumors that begin from parasympathetic ganglia of the head and neck areas or from sympathetic ganglia sited in the thorax, abdomen or pelvis. These tumors may grow in the adrenal medulla, in which case, they are called pheochromocytomas16. Gastrointestinal stromal tumors (GISTs) are infrequent mesenchymal tumors of the GI tract. These tumors start in very early forms of special cells in the wall of the GI tract named the interstitial cells of Cajal (ICCs)17,18. Gastrointestinal stromal tumor (GIST) and paraganglioma are caused by germline mutations in SDH subunits B, C or D 19 . Because of their strong syndromic and heritable source and distinctive history, SDH-deficient tumors are essential to be identified20. As a rule, it is suggested that genetic testing for SDHx which could be SDHA, SDHB, SDHC and SDHD be available each time an SDH-deficient tumor is observed21. In this review article, several types of SDH deficient tumors are focused on.
SDH mutations
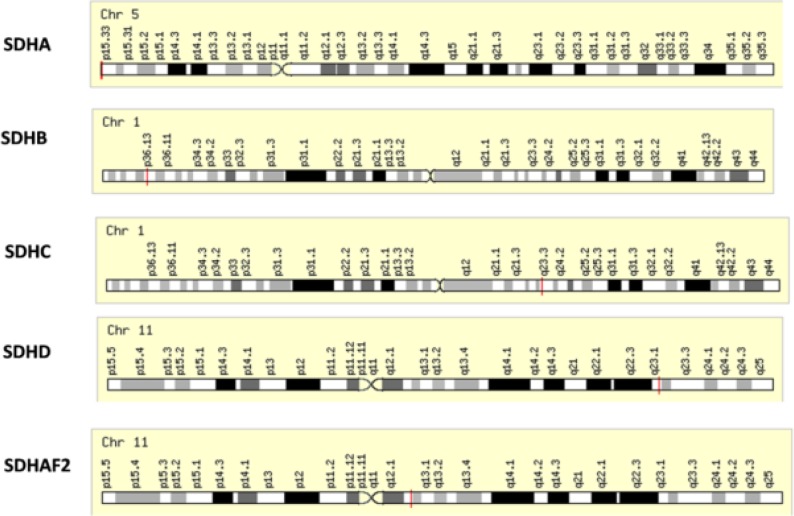
Mitochondrial DNA mutations have been found in dissimilar cancers and seem to change mitochondrial metabolism, increase risk of tumorigenesis and allow cancer cell modification to changing environments22. The nuclear-encoded Krebs cycle enzymes in the mitochondria, fumarate hydratase (FH) and succinate dehydrogenase (SDHB, -C and -D) act as tumor suppressors15. Although mutations in all subunits occur in cancer, tumors containing mutations in the catalytic subunit SDHB are predominantly malignant and associated with enhanced risk of metastasis10. SDHD, SDHB and SDHC mutations are a basis for a series of molecular procedures leading to the abnormal stabilization of hypoxia-inducible factors (HIF) under normoxic or hypoxic conditions or pseudo-hypoxia (via inactivation of SDH, accumulation of succinate, inhibition of prolyl-4-hydroxylases and subsequent HIF hydroxylation), thus, encouraging cell proliferation, angiogenesis and tumor genesis 23. SDH deficiency reduces prolyl hydroxylases and hydroxylation of hypoxia-inducible factor-α, resulting in activation of the hypoxia pathway, angiogenesis, glucose metabolism, cell motility and cancer cells survival 24,25. Although most of the oncogenic activities of SDH mutations have been known to be related to a metabolite, succinate, which accumulates in SDH-deficient cells26, in another way, these cells show high levels of succinate also accessible with elevated caspase 3 and/or caspase 7 levels27. Other subunits of SDH include succinate dehydrogenase complex assembly factor 1 (SDHAF1), which is a novel LYR-motif protein; the first SDH assembly factor recognized in any organism, and is found within the mitochondrial matrix and the succinate dehydrogenase complex assembly factor 2 (SDHAF2) which is shown to be significant for the acceptable flavination of SDHA and function of the SDH complex28. The SDH genes located on chromosomes 1, 5 and 11 (Figure 1) encode subunits of the heterotetrameric succinate dehydrogenase complex, an element of both the mitochondrial-respiratory chain (complex II) and the Krebs cycle in which SDHA (Ch5p15) and SDHB (Ch1p36) encode the two catalytic subunits, the flavoprotein and the iron-sulfur protein, respectively. SDHC (Ch1q21) and SDHD (Ch11q23) encode transmembrane proteins that anchor complex II in the inner mitochondrial membrane, and include a ubiquinone binding site29.
Figure 1.
The genomic location of several Succinate Dehydrogenase Complex Subunits: bands according to Ensemble, locations according to GeneLoc (and/or Entrez Gene and/or Ensembl if different).
Genetic analysis showed a novel frame shift SDHD mutation resulting in premature stop codon at amino acids 133 of the protein30. Finally, the combination of loss-of-function germline mutations in one of the SDH subunit genes and somatic loss-of-function mutations in the tumor cells resulted in inactivation of both alleles. In some cases, the mechanism of SDH inactivation is indistinct and is probably related to epigenetic silencing 31. Negative immunohistochemistry (IHC) staining for SDHB is linked with the SDH mutations (SDHB, SDHC or SDHD) that can weaken the whole enzyme complex activity4,32. SDHB immune staining was extremely concordant with the immunoblot finding33. Totally, lack of staining is more generally found with SDHB mutation, contrary to weak diffuse staining often linked with SDHD mutation34. SDHA and SDHB IHC must be interpreted with caution and potential false-positive or false-negative results should be considered; some supporting results can be provided by molecular testing. For example, in SDHD mutation, weak non-specific cytoplasmic staining usually occurs, and this pattern of staining can be difficult to interpret with confidence35. Also, pseudo-hypoxia, the major phenomenon shown to date to mediate the tumorigenic ending of the loss of mitochondrial tumor suppressors, is a common mechanism for both SDH and FH mutations and in some tumors with SDHD or SDHB mutations, the hypoxia-inducible factor (HIF) pathway, and therefore an angiogenic reaction are activated due to high vascular density36. SDH mutation due to activation of the hypoxia pathway, supports tumor formation by activating angiogenesis, glucose metabolism, cell motility and cell survival24. Activation of the HIF pathway in SDH may be dependable on the stimulation of glycolysis and anaerobic fermentation37. Thus, SDHB and SDHD inactivation is associated with deregulation of the HIF-1 and HIF-2 transcription factors, and a non-HIF-dependent pathway involving JunB, cJun and EglN3/PHD3 in normal developmental apoptosis in sympathetic neuronal progenitor cells38. Also, the loss of SDH mutation causes succinate accretion and reduces α-ketoglutarate-dependent dioxygenase enzymes such as the TET family of DNA hydroxylases. TET proteins catalyze the alteration of 5-methylcytosine to 5-hydroxymethyl cytosine (5-hmC), which is essential for consequent DNA demethylation39. Therefore, SDH and FH mutations can inhibit DNA and histone demethylases, leading to loss of 5hmC and low level 5hmC in SDH-deficient tumors associated notably with nuclear exclusion of TET protein. The results are inhibition of the TET family of DNA hydroxylases, increased succinate and fumarate which negatively affect the enzyme activity of histone demethylases40. Also, SDHD mutation has a distinctive phenotype and recognized increased age-related tumor risks with extremely destabilizing SDHB missense mutations 41. Therefore, the germline mutations could suggest that these patients should be considered for the risk of progression of other cancers 42. A possible advantage in identifying metabolic-enzyme mutations that are pathogenic in specific cancers is that such cancers may be susceptible to pharmacologic administration that are more effective and less toxic than obtainable therapies43. The inhibition of these molecular pathways portrays the widespread vascularization of SDH-related tumors which may be due to metastatic spreading by driving epithelial-to-mesenchymal transition in SDHB-deficient tumors44. Another significant result including SDHA is lost collectively with SDHB in SDHA-mutated tumors, but its expression remains in tumors with other SDH mutations 35 . Thus, genetic testing allows familial consultation and identifies persons at high risk of malignancy (SDHB mutations) or considerable multiorgan disease34.
Paraganglioma (PGL) and pheocromocytoma (PCC)
Pheochromocytomas and paragangliomas are rare diseases, but frequently occur with nonspecific symptoms 45-48 . Pheochromocytomas and paragangliomas are tumors beginning from the adrenal medulla and sympathetic/parasympathetic paraganglia, respectively49. The most frequent location of paragangliomas is the carotid body50. Symptoms of this tumors consist of increased blood pressure, headache, sweating and palpitations51. The diagnosis is typically established by calculating the level of catecholamines or their metabolites in urine or plasma, and also single consistent complete sign of malignancy in pheochromocytoma is the presence of metastasis52. Positive staining for chromogranin and synaptophysin is present in the chief cells, whereas the sustentacular cells are positive to S100 protein in immunohistochemistry staining 53,54.
Multifocal tumors, young age and positive family history, known features related to inheritance are not present in all patients, which leads to important study on considerable genetic mutations results55. According to genetic study results, it has been recommended that negative immunostaining of SDHB can be taken as a marker for the presence of a mutation in one of the five SDH genes48,56. Recognition of patients with inherited pheochromocytoma is significant because it can guide medical administration in mutation-positive patients and their families 57 . Pheochromocytomas and paragangliomas are neuroendocrine tumors that occur sporadically and in some heritable tumor syndromes due to germline mutations in SDHB, SDHC or SDHD genes58. Three other important hereditary familial cancer syndromes [von Hippel-Lindau (VHL) disease, multiple endocrine neoplasia (MEN) types 2A and 2B, and neurofibromatosis type 1] are also associated with PCC susceptibility38,59. The benefit of evaluation by IHC and the possible advantages of Ki67 antigen, c-erbB-2 and c-kit proto-oncogenes in the discrimination of benign and malignant pheochromocytomas were reported60. However, there is a widespread genetic description for PGL/PCC61. At least, there is germline mutation in one of the ten recognized susceptibility genes: RET, NF1, VHL, SDHAF2, TMEM127 or MAX, and in genes encoding the four subunits of succinate dehydrogenase (SDHA, SDHB, SDHC or SDHD, referred to as SDHx genes) associated with PGL/PCC. In all SDHD and SDHC cases, but not SDHB tumors, these were found in the head and neck areas62. These syndromes affect mutations in one of the three subunits of the SDH gene. By way of illustration, type 1 is associated with SDHD, type 2 is associated with an unknown gene, type 3 is associated with SDHC and type 4 with SDHB63. Analysis of SDHD can also help to discriminate synchronous primary tumors from abdominal metastases 64. Also, SDHD are more possible to have multifocal disease when compared with patients with SDHB and SDHC mutations 65. SDHB-mutation carriers have higher risk of developing a metastatic disease and shorter survival than patients with a malignant PGL/PCC but without SDHB mutations16. Thus, young age and metastatic disease are both factors for SDHB mutation66. Altogether, extra-adrenal sympathetic tumors are commonly related to SDHB (predominantly solitary, large tumors), less often to SDHD, infrequently to SDHC and SDHA mutations, and because of this connection if SDHD, SDHB and SDHC testing have negative results, then SDHAF2 mutation should be checked48. The SDHAF2 gene encodes an SDH co-factor related to the role of the SDHA subunit and is currently entirely related with head and neck paragangliomas 28. Patients with SDHB mutations are younger, more commonly have extra-adrenal tumors and a shorter metanephrine excretion doubling time-related to shorter survival67. On the whole, SDHB immunohistochemistry on pheochromocytomas and paragangliomas could develop the diagnosis of pheochromocytoma-paraganglioma syndrome68. Also, SDHx-PPGLs overexpress somatostatin receptors (SSTRs) and are consequently targetable with somatostatin analogs (SSAs) labeled with diagnostic radionuclides 69.
Gastrointestinal stromal tumor (GISTs)
The interstitial cells of Cajal are origin site in which GIST arise depending on high-level KIT expression for lineage specification and survival. Majority of the sporadic GISTs harbor activating mutations in KIT and to a lesser extent, in PDGFRA and BRAF70. Genetic mutations affecting KIT, PDGFRA, BRAF and SDH complex functions are thought to be mutually special events71. GIST diagnosis is based on histology changes in tissue sections, but not clinical symptoms. While spindle and/or epithelioid tumor cells in the gastrointestinal tract are positive for KIT or DOG1 in immunostaining, GIST could be considered. DOG1 is more specific for GIST than KIT and is occasionally positive for KIT-negative GIST. Epithelioid tumor cells may be a definite type of GIST, including PDGFRA-mutated GIST or GIST with mutations in the SDH complex, or GIST transformed to highly malignant (typically mixed phenotype)72. Thus, patients accessible to generally epithelioid GISTs were characterized by plump cells containing a centrally located, round nucleus and prominent nucleoli; these changes were approximately distinguishable from those seen in patients with SDH mutated GIST73. Also, among GISTs that begin in children and young adults, insulin-like growth factor 1 receptor (IGF1R) overexpression is usually observed in those with KIT/PDGFRA wild-type but not in those with either mutant kinase 74 . This result which leads to IFG1R-positivity may also be a helpful serology marker to recognize SDH-deficient GISTs75. SDHB and SDHA mutated GIST consist of a subgroup of young adult women patients with a well distinct clinical and biological profile, usually characterized by the gastric primary tumor localization, a principally mixed epithelioid and spindle cell morphology, diffused IHC positivity for KIT and revealed on gastrointestinal stromal tumors 1 (DOG1), recurrent lymph node metastases, and an nonaggressive course of disease even if metastasis is identified. Moreover, they are distinguished by the overexpression of the insulin growth factor 1 receptor (IGF1R). GIST characterized by SDHB, −C or D mutations (most of them germline, and in few cases by SDHA mutations), originate mainly from the stomach, with a lesser female incidence, but histologically like SDHA mutated GIST76. In pediatric patients, mostly GISTs are KIT/PDGFRA wild-type. These findings are principally on girls and usually have a clinically slow progress course777. Also, these tumors did not reveal the KIT or platelet-derived growth factor receptor-α (PDGFRA) gene mutations related to GISTs and correlated lesions that are responsive to Imatinib mesylate and its analogs78. The most common and closest change detected by tumor genetic studies is the deletion of the 1cen-q21 chromosomal region involving the SDHC gene. An additional change was also discovered, together with loss of the 1p region79. Not more than a small subset of SDHB-deficient GISTs carries loss-of-function mutations in SDHB, SDHC or SDHD. Because of the complication of its locus (15 exons) and the presence of three pseudogenes, SDHA is infrequently analyzed. Moreover, immunohistochemistry for SDHA can be used to select patients for SDHA-specific genetic testing80. Carney-Stratakis syndrome can make patients susceptible to GIST and paraganglioma 19. Carney triad (CT) shows the association of paragangliomas (PGLs) with GISTs and pulmonary chondromas in which inactivating mutations of the mitochondrial complex II SDH enzyme subunits SDHB, SDHC and SDHD are found in PGLs, gain-of-function mutations of c-kit (KIT), and platelet-derived growth factor receptor A (PDGFRA) in GISTs81. Also, those with SDHA-negative GISTs had older median age, lower female to male ratio but like mitotic counts and median tumor sizes, with a slow course of disease in most cases, regardless of a vaguely higher rate of liver metastases82. Patients with metastatic KIT/PDGFRA wild-type succinate dehydrogenase–deficient gastrointestinal stromal tumors harboring succinate dehydrogenase subunit A mutations show a remarkable long survival. These patients should be recognized in clinical practice to improve altered treatments and follow-up over time83. Furthermore, patients with SDHX mutations need germline testing to establish whether the mutation is sporadic or germline, and if a germline mutation is shown, genetic consultation is indicated. In contrast, those patients found to have SDHC promoter hypermethylation do not require genetic consultation, as these are not germline alterations. However, these patients still do need screening for paragangliomas as noted, since they are often related to syndromic GIST84.
Renal cell carcinoma (RCC)
Renal cell carcinoma (RCC) originates from the kidney and is frequently present with only some symptoms, but it can have varied paraneoplastic appearances. It shows relative resistance to radiation and chemotherapy and the latest study on the genetic changes related to RCC have shown new targets for therapy 85 . SDH-deficient renal carcinoma has been established as a conditional entity in the 2013 International Society of Urological Pathology Vancouver Classification86. SDH-deficient renal carcinoma is recently accepted under the World Health Organization (WHO) 2016 classification and presents vacuolated eosinophilic cytoplasmic and cytoplasmic inclusions. It is predominantly related to SDHB mutation, although SDHC and SDHA mutations can occur87. Thus, the 2016 World Health Organization (WHO) classification includes new renal tumor classification; recently accepted epithelial renal tumors are hereditary leiomyomatosis and renal cell carcinoma (RCC) syndrome–associated RCC, succinate dehydrogenase–deficient RCC, tubulocystic RCC, acquired cystic disease–associated RCC, and clear cell papillary RCC88. Moreover, germline mutations in Krebs cycle enzyme, SDH, can also cause a hereditary tumor susceptibility syndrome89. Most SDH-deficient tumors contain SDHB mutation, with only a small number of RCC with SDHC or SDHD having been reported to date90. Thus, a monomorphic oncocytic renal tumor with a solid architecture, cytoplasmic inclusions of flocculent material, and intratumoral mast cells should be quickly assessed for SDH status, as it may have indications for screening the patient and families. In this manner, negative IHC for KIT and heterogeneous staining for epithelial antigens are other helpful approaches91. Also, mitochondrial dysfunction may lead to neoplasia through the role of mitochondria in apoptosis. Because RCCs are referred to as “oncocytic” (that is, accumulation with mitochondria), the role of SDH in RCC is reasonable92.
However, renal cell carcinomas (RCCs) with other histologic features have been reported in patients with germline mutations of SDH subunit genes and a few RCCs of other histologic types have been established to be SDH-deficient with the lack of identified germline gene mutation91. The tumor had a mixed histology pattern of high-grade papillary and collecting duct carcinoma and characteristic pale eosinophilic cytoplasmic inclusions like SDHB-deficient RCC; this is the first report that identifies SDHA inactivation in RCC93. Despite the fact that most tumors are low grade, a lot of other tumors can be shown in an aggressive fashion, predominantly if they are high nuclear grade, and have coagulative necrosis or sarcomatoid differentiation 94 . Moreover, these suggested careful observations of patients at risk of SDH mutation related renal cell carcinoma and extensive surgical excision of renal tumors 95 and following the genetic consequences, a succession of tyrosine kinase inhibitors was administered as targeted treatment options and obviously showed how the genetic findings make the accessible motivation for their helpfulness96.
Other epithelial tumors
The succinate dehydrogenase complex catalyzes the oxidation of succinate to fumarate; mutations in its subunits SDHA, SDHB, SDHC and SDHD, and in the assembly factor SDHAF2, result in syndromes with divergent tumor types, including pheochromocytoma/paraganglioma, gastrointestinal stromal tumor, and, less often, renal-cell carcinoma, pituitary adenoma24 ,and breast cancer26. Also, head and neck PGLs, extra-adrenal PGLs, a number of other neuroendocrine or non-neuroendocrine neoplasms have been related to mutations in SDH genes. Thyroid cancer is the most common endocrine tumor in which SDHB and SDHD mutations are linked to epigenetic alterations97. A distinctive case of testicular seminoma has been reported in a carrier of germline SDHD mutations, which presented the loss of the wild type allele in tumor cells. The frequent neural crest embryonal origin of both pheochromocytoma and neuroblastoma with the common loss of the locus 1p35-36 in the last tumors, a section where SDHB gene is located, suggested that genetic alterations in SDHB might be related to the progression or advanced neuroblastoma tumors12. Papillary thyroid cancer from SDHx mutation is also seen in cowden syndrome98. The homozygous/compound heterozygous mutations in SDHA result in rigorous neurological dysfunction and cardiomyopathy, but heterozygous germline mutations in SDHB-D cause a pheochromocytoma-PGL syndrome. Some carriers of SDHB or SDHD mutations have been found to have renal cell carcinoma or papillary thyroid cancer which also appears in Cowden syndrome99. However, mutations in SDHA cause the lethal pediatric neurodegenerative disease and Leigh's syndrome but not paraganglioma100. In Leigh syndrome, a severe neurodegenerative disease, there was a compound heterozygous germline SDHA-mutation (one allele with nonsense and another with missense mutation) 82. Thus, germline SDHA mutations are related to juvenile encephalopathy 101 . Also, several other neoplasms have been reported in SDHx mutation carriers, consisting of pancreatic neuroendocrine tumor, adrenal cortical adenoma, neuroblastoma, ganglioneuroma, adenomatoid tumor of the adrenal gland, melanoma, lung cancer, breast carcinoma, esophageal cancer, rectal and ovarian carcinomas, uterine adenocarcinoma, uterine leiomyoma, testicular seminoma, bladder cancer, meningioma, oligodendroglioma, cecal polyps and hematolymphoid malignancies 102 . Moreover, decreased SDH enzymatic activity supports SDHD's involvement in the pituitary tumor development, testicular seminoma and papillary thyroid carcinoma30. Another finding shows that SDH mutation-related pituitary tumors have an aggressive phenotype103 and can be larger and further expected to produce prolactin than other pituitary adenomas104. A pancreatic neuroendocrine tumor is also within the SDH-related tumor variety105,106.
CONCLUSION
In total, the SDH-germline mutation can be driver mutations of Paraganglioma, Pheochromocytoma, Gastrointestinal stromal tumors, Renal Cell carcinomas and other endocrine-related tumors. The most important gene of SDH enzymatic complex is SDHB. Both genetic alterations and epigenetic changes (methylation) of this gene are considered as an important modification in endocrine malignancies formation. The bi-allelic expression of SDHD and the complete loss of SDH enzymatic activity whenever one of its subunits is mutated can describe the phenotypic variability in some tumors.
ACKNOWLEDGEMENTS
The authors would like to thank the pathology staff of Shariati Hospital and Endocrinology and Metabolism Population Sciences Institute for their kind assistance and cooperation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
Author Contributions
SMT conceived of the presented idea and developed the theory, EN and HS wrote the manuscript, and FKH reviewed and edited the written manuscript and developed it to the final version.
References
- 1.Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21(3):379–85. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi R, Gentry SN, Jackson TL. Pathways to Tumorigenesis—Modeling Mutation Acquisition in Stem Cells and Their Progeny. Neoplasia. 2008;10(11):1170–82. doi: 10.1593/neo.08572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett JC. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ Health Perspect. 1993;100:9–20. doi: 10.1289/ehp.931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarmadi S, Izadi-Mood N, Sotoudeh K, et al. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol. 2009;4:41. doi: 10.1186/1746-1596-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadi-asl J, Larijani B, Khorgami Z, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol. 2011;28(4):1123–8. doi: 10.1007/s12032-010-9587-z. [DOI] [PubMed] [Google Scholar]
- 6.Natanzi MM, Pasalar P, Kamalinejad M, et al. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Med Iran. 2012;50(9):589–96. [PubMed] [Google Scholar]
- 7.Haghpanah V, Shooshtarizadeh P, Heshmat R, et al. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006;14(4):422–5. doi: 10.1097/01.pai.0000213100.88074.b8. [DOI] [PubMed] [Google Scholar]
- 8.Omidfar K, Moinfar Z, Sohi AN, et al. Expression of EGFRvIII in thyroid carcinoma: immunohistochemical study by camel antibodies. Immunol Invest. 2009;38(2):165–80. doi: 10.1080/08820130902735998. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Winge DR. Emerging concepts in the flavinylation of succinate dehydrogenase. Biochim Biophys Acta. 2013;1827(5):627–36. doi: 10.1016/j.bbabio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Pollard PJ. Succinate: a new epigenetic hacker. Cancer Cell. 2013;23(6):709–11. doi: 10.1016/j.ccr.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Xiao M, Yang H, Xu W, et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26(12):1326–38. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011;1807(11):1432–43. doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Clark GR, Sciacovelli M, Gaude E, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99(10):E2046–50. doi: 10.1210/jc.2014-1659. [DOI] [PubMed] [Google Scholar]
- 14.Miettinen M, Sarlomo-Rikala M, McCue P, et al. Mapping of succinate dehydrogenase losses in 2258 epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2014;22(1):31–6. doi: 10.1097/PAI.0b013e31828bfdd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard PJ, Brière JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 16.Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–52. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973–83. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 18.Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol. 2014;27(Suppl 1):S1–16. doi: 10.1038/modpathol.2013.173. [DOI] [PubMed] [Google Scholar]
- 19.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108(1):314–8. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill AJ, Hes O, Papathomas T, et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 2014;38(12):1588–602. doi: 10.1097/PAS.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwight T, Benn DE, Clarkson A, et al. Loss of SDHA expression identifies SDHA mutations in succinate dehydrogenase–deficient gastrointestinal stromal tumors. Am J Surg Pathol. 2013;37(2):226–33. doi: 10.1097/PAS.0b013e3182671155. [DOI] [PubMed] [Google Scholar]
- 22.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnichon N, Brière JJ, Libé R, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19(15):3011–20. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evenepoel L, Papathomas TG, Krol N, et al. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet Med. 2015;17(8):610–20. doi: 10.1038/gim.2014.162. [DOI] [PubMed] [Google Scholar]
- 25.Alimoghaddam K, Shariftabrizi A, Tavangar M, et al. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemia. Leuk Lymphoma. 2006;47(1):81–8. doi: 10.1080/10428190500300373. [DOI] [PubMed] [Google Scholar]
- 26.Gaude E, Frezza C. Defects in mitochondrial metabolism and cancer. Cancer Metab. 2014;2:10. doi: 10.1186/2049-3002-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentzel JF, Lewies A, Bronkhorst AJ, et al. Exposure to high levels of fumarate and succinate leads to apoptotic cytotoxicity and altered global DNA methylation profiles in vitro. Biochimie. 2017;135:28–34. doi: 10.1016/j.biochi.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Hensen EF, Bayley JP. Recent advances in the genetics of SDH-related paraganglioma and pheochromocytoma. Fam Cancer. 2011;10(2):355–63. doi: 10.1007/s10689-010-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayley JP, Devilee P, Taschner PE. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med Genet. 2005;6:39. doi: 10.1186/1471-2350-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xekouki P, Pacak K, Almeida M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97(3):E357–66. doi: 10.1210/jc.2011-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Eid JE, Paz AC, et al. Metabolic Enzymes in Sarcomagenesis: Progress Toward Biology and Therapy. BioDrugs. 2017;31(5):379–392. doi: 10.1007/s40259-017-0237-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123(9):3652–8. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahia PLM, Ross KN, Wright ME, et al. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill AJ, Benn DE, Chou A, et al. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2010;41(6):805–14. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Santi R, Rapizzi E, Canu L, et al. Potential Pitfalls of SDH Immunohistochemical Detection in Paragangliomas and Phaeochromocytomas Harbouring Germline SDHx Gene Mutation. Anticancer Res. 2017;37(2):805–812. doi: 10.21873/anticanres.11381. [DOI] [PubMed] [Google Scholar]
- 36.King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25(34):4675–82. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 37.Favier J, Brière JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard PJ, El-Bahrawy M, Poulsom R, et al. Expression of HIF-1α, HIF-2α (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91(11):4593–8. doi: 10.1210/jc.2006-0920. [DOI] [PubMed] [Google Scholar]
- 39.Mason EF, Hornick JL. Succinate dehydrogenase deficiency is associated with decreased 5-hydroxymethylcytosine production in gastrointestinal stromal tumors: implications for mechanisms of tumorigenesis. Mod Pathol. 2013;26(11):1492–7. doi: 10.1038/modpathol.2013.86. [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra AS, de Graaff MA, Briaire-de Bruijn IH, et al. Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma/pheochromocytoma and smooth muscle tumors. Oncotarget. 2015;6(36):38777–88. doi: 10.18632/oncotarget.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews KA, Ascher DB, Pires DEV, et al. Tumour risks and genotype–phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J Med Genet. 2018;55(6):384–394. doi: 10.1136/jmedgenet-2017-105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantaleo MA, Astolfi A, Urbini M, et al. Analysis of all subunits, SDHA, SDHB, SDHC, SDHD, of the succinate dehydrogenase complex in KIT/PDGFRA wild-type GIST. Eur J Hum Genet. 2014;22(1):32–9. doi: 10.1038/ejhg.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson CB. Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med. 2009;360(8):813–5. doi: 10.1056/NEJMe0810213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440–6. doi: 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- 45.Emery RT, Brown HL, Emery KQ, et al. Pheochromocytoma: A Rare Presentation. J Ark Med Soc. 2017;113(8):188–190. [PubMed] [Google Scholar]
- 46.Haghpanah V, Soliemanpour B, Heshmat R, et al. Endocrine cancer in Iran: based on cancer registry system. Indian J Cancer. 2006;43(2):80–5. doi: 10.4103/0019-509x.25889. [DOI] [PubMed] [Google Scholar]
- 47.Larijani B, Shirzad M, Mohagheghi M, et al. Epidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR) Asian Pac J Cancer Prev. 2004;5(1):36–9. [PubMed] [Google Scholar]
- 48.Khatami F, Tavangar SM. Current diagnostic status of pheochromocytomaand future perspective: A mini review. Iran J Pathol. 2017;12(3):313–322. [PMC free article] [PubMed] [Google Scholar]
- 49.Khatami F, Mohammadamoli M, Tavangar SM. Genetic and epigenetic differences of benign and malignant pheochromocytomas and paragangliomas (PPGLs) Endocr Regul. 2018;52(1):41–54. doi: 10.2478/enr-2018-0006. [DOI] [PubMed] [Google Scholar]
- 50.Wieneke JA, Smith A. Paraganglioma: carotid body tumor. Head Neck Pathol. 2009;3(4):303–306. doi: 10.1007/s12105-009-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacak K, Wimalawansa SJ. Pheochromocytoma and paraganglioma. Endocr Pract. 2015;21(4):406–12. doi: 10.4158/EP14481.RA. [DOI] [PubMed] [Google Scholar]
- 52.Saffar H, Sanii S, Heshmat R, et al. Expression of galectin-3, nm-23, and cyclooxygenase-2 could potentially discriminate between benign and malignant pheochromocytoma. Am J Clin Pathol. 2011;135(3):454–60. doi: 10.1309/AJCPI8AJLUZ3CZLN. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd RV. Adrenal cortical tumors, pheochromocytomas and paragangliomas. Mod Pathol. 2011;24(Suppl 2):S58–65. doi: 10.1038/modpathol.2010.126. [DOI] [PubMed] [Google Scholar]
- 54.Amousha M, Kish NS, Heshmat R, et al. Corrigendum: Expression of the Pituitary Tumor Transforming Gene(PTTG1) in Pheochromocytoma as a Potential Marker for Distinguishing Benign Versus Malignant Tumors. Acta Med Iran. 2015;53(6):392. [PubMed] [Google Scholar]
- 55.Neumann HP, Cybulla M, Shibata H, et al. New genetic causes of pheochromocytoma: current concepts and the clinical relevance. Keio J Med. 2005;54(1):15–21. doi: 10.2302/kjm.54.15. [DOI] [PubMed] [Google Scholar]
- 56.Castelblanco E, Santacana M, Valls J, et al. Usefulness of negative and weak-diffuse pattern of SDHB immunostaining in assessment of SDH mutations in paragangliomas and pheochromocytomas. Endocr Pathol. 2013;24(4):199–205. doi: 10.1007/s12022-013-9269-4. [DOI] [PubMed] [Google Scholar]
- 57.Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95(16):1196–204. doi: 10.1093/jnci/djg024. [DOI] [PubMed] [Google Scholar]
- 58.van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10(8):764–71. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khatami F, Tavangar SM. Multiple Endocrine Neoplasia Syndromes from Genetic and Epigenetic Perspectives. Biomark Insights. 2018;13:1177271918785129. doi: 10.1177/1177271918785129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tavangar SM, Shojaee A, Moradi Tabriz H, et al. Immunohistochemical expression of Ki67, c-erbB-2, and c-kit antigens in benign and malignant pheochromocytoma. Pathol Res Pract. 2010;206(5):305–9. doi: 10.1016/j.prp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Turchini J, Cheung VK, Tischler AS, et al. Pathology and genetics of phaeochromocytoma and paraganglioma. Histopathology. 2018;72(1):97–105. doi: 10.1111/his.13402. [DOI] [PubMed] [Google Scholar]
- 62.López-Jiménez E, Gómez-López G, Leandro-García LJ, et al. Research resource: transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24(12):2382–91. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chetty R. Familial paraganglioma syndromes. J Clin Pathol. 2010;63(6):488–91. doi: 10.1136/jcp.2010.076257. [DOI] [PubMed] [Google Scholar]
- 64.Dannenberg H, van Nederveen FH, Abbou M, et al. Clinical characteristics of pheochromocytoma patients with germline mutations in SDHD. J Clin Oncol. 2005;23(9):1894–901. doi: 10.1200/JCO.2005.07.198. [DOI] [PubMed] [Google Scholar]
- 65.Barletta JA, Hornick JL. Succinate dehydrogenase-deficient tumors: diagnostic advances and clinical implications. Adv Anat Pathol. 2012;19(4):193–203. doi: 10.1097/PAP.0b013e31825c6bc6. [DOI] [PubMed] [Google Scholar]
- 66.Mannelli M, Castellano M, Schiavi F, et al. Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94(5):1541–7. doi: 10.1210/jc.2008-2419. [DOI] [PubMed] [Google Scholar]
- 67.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822–8. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Wang LA, Xie Q, et al. Germline SDHB and SDHD mutations in pheochromocytoma and paraganglioma patients. Endocr Connect. 2018;7(12):1217–1225. doi: 10.1530/EC-18-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taïeb D, Timmers H, Pacak K. Diagnostic Investigation of Lesions Associated with Succinate Dehydrogenase Defects. Horm Metab Res. 2018 doi: 10.1055/a-0586-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ran L, Sirota I, Cao Z, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5(3):304–15. doi: 10.1158/2159-8290.CD-14-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lasota J, Xi L, Coates T, et al. No KRAS mutations found in gastrointestinal stromal tumors (GISTs): molecular genetic study of 514 cases. Mod Pathol. 2013;26(11):1488–91. doi: 10.1038/modpathol.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25(5):479–89. doi: 10.1111/den.12149. [DOI] [PubMed] [Google Scholar]
- 73.Szarek E, Ball ER, Imperiale A, et al. Carney triad, SDH-deficient tumors, and Sdhb+/- mice share abnormal mitochondria. Endocr Relat Cancer. 2015;22(3):345–52. doi: 10.1530/ERC-15-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pantaleo MA, Astolfi A, Indio V, et al. SDHA Loss-of-Function Mutations in KIT–PDGFRA Wild-Type Gastrointestinal Stromal Tumors Identified by Massively Parallel Sequencing. J Natl Cancer Inst. 2011;103(12):983–7. doi: 10.1093/jnci/djr130. [DOI] [PubMed] [Google Scholar]
- 75.Lasota J, Wang Z, Kim SY, et al. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37(1):114–9. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nannini M, Astolfi A, Urbini M, et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST) BMC Cancer. 2014;14:685. doi: 10.1186/1471-2407-14-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oudijk L, Gaal J, Korpershoek E, et al. SDHA mutations in adult and pediatric wild-type gastrointestinal stromal tumors. Mod Pathol. 2013;26(3):456–63. doi: 10.1038/modpathol.2012.186. [DOI] [PubMed] [Google Scholar]
- 78.Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney–Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16(1):79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 79.Stratakis C, Carney J. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney–Stratakis syndrome): molecular genetics and clinical implications. J Intern Med. 2009 Jul;266(1):43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner AJ, Remillard SP, Zhang Y-X, et al. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. 2013;26(2):289–94. doi: 10.1038/modpathol.2012.153. [DOI] [PubMed] [Google Scholar]
- 81.Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab. 2007;92(8):2938–43. doi: 10.1210/jc.2007-0797. [DOI] [PubMed] [Google Scholar]
- 82.Miettinen M, Killian JK, Wang ZF, et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37(2):234–40. doi: 10.1097/PAS.0b013e3182671178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pantaleo MA, Lolli C, Nannini M, et al. Good survival outcome of metastatic SDH-deficient gastrointestinal stromal tumors harboring SDHA mutations. Genet Med. 2015;17(5):391–5. doi: 10.1038/gim.2014.115. [DOI] [PubMed] [Google Scholar]
- 84.Boikos SA, Pappo AS, Killian JK, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2(7):922–8. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curti BD. Renal cell carcinoma. JAMA. 2004;292(1):97–100. doi: 10.1001/jama.292.1.97. [DOI] [PubMed] [Google Scholar]
- 86.Gill AJ, Hes O, Papathomas T, et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 2014;38(12):1588–602. doi: 10.1097/PAS.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology. 2018;72(1):106–116. doi: 10.1111/his.13277. [DOI] [PubMed] [Google Scholar]
- 88.Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 89.Ricketts C, Woodward ER, Killick P, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100(17):1260–2. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 90.Ozluk Y, Taheri D, Matoso A, et al. Renal carcinoma associated with a novel succinate dehydrogenase A mutation: a case report and review of literature of a rare subtype of renal carcinoma. Hum Pathol. 2015;46(12):1951–5. doi: 10.1016/j.humpath.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 91.Williamson SR, Eble JN, Amin MB, et al. Succinate dehydrogenase-deficient renal cell carcinoma: detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod Pathol. 2015;28(1):80–94. doi: 10.1038/modpathol.2014.86. [DOI] [PubMed] [Google Scholar]
- 92.Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74(1):153–9. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yakirevich E, Ali SM, Mega A, et al. A Novel SDHA-deficient Renal Cell Carcinoma Revealed by Comprehensive Genomic Profiling. Am J Surg Pathol. 2015;39(6):858–63. doi: 10.1097/PAS.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 94.Kuroda N, Yorita K, Nagasaki M, et al. Review of succinate dehydrogenase-deficient renal cell carcinoma with focus on clinical and pathobiological aspects. Pol J Pathol. 2016;67(1):3–7. doi: 10.5114/pjp.2016.59227. [DOI] [PubMed] [Google Scholar]
- 95.Ricketts CJ, Shuch B, Vocke CD, et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J Urol. 2012;188(6):2063–71. doi: 10.1016/j.juro.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McEvoy CR, Koe L, Choong DY, et al. SDH-deficient renal cell carcinoma associated with biallelic mutation in succinate dehydrogenase A: comprehensive genetic profiling and its relation to therapy response. NPJ Precis Oncol. 2018;2:9. doi: 10.1038/s41698-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larijani B, Mohagheghi MA, Bastanhagh MH, et al. Primary thyroid malignancies in Tehran, Iran. Med Princ Pract. 2005;14(6):396–400. doi: 10.1159/000088112. [DOI] [PubMed] [Google Scholar]
- 98.Ngeow J, Mester J, Rybicki LA, et al. Incidence and clinical characteristics of thyroid cancer in prospective series of individuals with Cowden and Cowden-like syndrome characterized by germline PTEN, SDH, or KLLN alterations. J Clin Endocrinol Metab. 2011;96(12):E2063–71. doi: 10.1210/jc.2011-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ni Y, Zbuk KM, Sadler T, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83(2):261–8. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandon M, Baldi P, Wallace D. Mitochondrial mutations in cancer. Oncogene. 2006;25(34):4647–62. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 101.Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69(1):49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papathomas TG, Gaal J, Corssmit EP, et al. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC–PGL syndromes: a clinicopathological and molecular analysis. Eur J Endocrinol. 2013;170(1):1–12. doi: 10.1530/EJE-13-0623. [DOI] [PubMed] [Google Scholar]
- 103.Tufton N, Roncaroli F, Hadjidemetriou I, et al. Pituitary Carcinoma in a Patient with an SDHB Mutation. Endocr Pathol. 2017;28(4):320–325. doi: 10.1007/s12022-017-9474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gill AJ, Toon CW, Clarkson A, et al. Succinate dehydrogenase deficiency is rare in pituitary adenomas. Am J Surg Pathol. 2014;38(4):560–6. doi: 10.1097/PAS.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niemeijer ND, Papathomas TG, Korpershoek E, et al. Succinate dehydrogenase (SDH)-deficient pancreatic neuroendocrine tumor expands the SDH-related tumor spectrum. J Clin Endocrinol Metab. 2015;100(10):E1386–93. doi: 10.1210/jc.2015-2689. [DOI] [PubMed] [Google Scholar]
- 106.Agaimy A. [Succinate dehydrogenase (SDH)-deficient renal cell carcinoma] Pathologe. 2016;37(2):144–52. doi: 10.1007/s00292-016-0158-8. [DOI] [PubMed] [Google Scholar]