Abstract
The goal of the present study was to evaluate the effect of Berberine chloride (BC) on lipid profile, oxidant status and insulin signaling molecules in Streptozotocin (STZ) induced diabetic rat model. Diabetes was induced in rats by a single dose of intraperitoneal administration of STZ (40 mg/kg b.w). Diabetic rats were treated with BC (50 mg/kg b.w) and glibenclamide (6 mg/kg b.w) for 45 days. BC treated diabetic rats showed significant (p <0.05) decrease in the levels of TC, TG, phospholipids, LDL, VLDL and lipid peroxidation markers such as LOOH and TBARS. An increase in enzymatic antioxidant (SOD, CAT and GPx), non-enzymatic antioxidant (GSH, vitamin C and E) and insulin signaling molecules expression, like Insulin receptor substrate-1 (IRS-1), Protein kinase B (PKB or Akt) and glucose transporter-4 (GLUT-4) were found to be significantly raised in BC treated STZ induced diabetic rats. Thus, the results of the current study demonstrated that BC significantly reversed the abnormal levels of lipids, oxidant status and insulin signaling molecules in the diabetic rat model, which may be contributed to its anti-diabetic and antioxidant activities.
Keywords: Berberine chloride, Antioxidant, Insulin receptor substrate and Glucose transporter
Introduction
Diabetes mellitus (DM) is a condition with a complex and multifarious group of disorders associated with high level of blood sugar over an extended period. It results from shortage or lack of insulin secretion or reduced sensitivity of the tissue to insulin [1]. Current reports reveal that Globally 415 million people suffer from diabetes. It is predictable to increase to 642 million people in 2040. In India alone, 69.2 million people are suffering from diabetes, and this will increase to 123.5 million in 2040 [2]. Diabetes mellitus has been associated with an increased risk of mortality and prevalence of cardiovascular disease. Oxidative stress may occur as a consequence of abnormalities in glucose and lipid metabolism, which favor hyperglycemia and dyslipidemia. These phenomena are coupled with developing of atherosclerosis and cardiovascular complications in the diabetic patients [3, 4].
In diabetic patients, hyperglycemia arises due to defects in the body’s ability to control glucose and insulin homoeostasis. Both the regulation of glucose uptake as well as it’s utilization is critical for the normal maintenance of glucose homoeostasis. Insulin plays a significant role in controlling the rates of glucose uptake, glycogen synthesis and glycolysis in the peripheral tissues. It is well known that the glucose uptake is regulated by glucose transporter (Glut-4) in the plasma membrane of cells [5].
An important organ for insulin response is skeletal muscle. Under normal conditions, insulin when bound to its receptor, leads to IR tyrosine kinase activation, that can activate a cascade of various phosphorylation–dephosphorylation reactions, including phosphatidylinositol-3-kinase (PI3K-p85), serine/threonine kinases Akt/PKB (e.g.Ser-473 and Thr-308 for the Akt/PKB isoform), which lead to GLUT-4 translocation and intracellular glucose metabolism [6, 7]. In patients with type 2 diabetes, defects in insulin-stimulated glucose metabolism in skeletal muscle have also been attributed to impaired glucose transport [8], glycogen synthesis [9] and glycogen synthase activation [10]. These defects may result from impaired insulin signal transduction [11]. Ameliorating insulin resistance is an important strategy in the development of new pharmacological treatment for type 2 diabetes. Previously Chandirasegaran et al. [12] reported that BC has ameliorating and anti-diabetic activities in STZ induced diabetic rats. The present work was designed to evaluate the modulatory effects of BC on insulin signaling molecules in skeletal muscles and on the levels of lipid profile and oxidant status in STZ induced diabetic rats.
Materials and Methods
Chemicals
Berberine chloride and Streptozotocin were procured from Sigma–Aldrich (St. Louis, MO, USA). The primary antibodies IRS1, Akt and insulin, were procured from Santa-Cruz Biotechnology, Inc., USA and GLUT-4 was purchased from Abcam. All other chemicals as well as the reagents used were of analytical grade and were purchased from Merck, Himedia, Mumbai and India.
Experimental Animals
About 180–200 g of male albino Wistar rats were obtained from Central Animal House, Faculty of Medicine, Rajah Muthiah Medical College, Annamalai University. All the rats were housed in clean polycarbonate cages under constant 12 h light and dark cycle 25 ± 2 °C room temperature. Throughout the experiment, rats were feed with standard rodent pellet food (Hindustan Lever Ltd, Mumbai, India) and tap water provided with ad libitum. The experiment was accepted by the Animal Ethics Committee of Rajah Muthiah Medical College and Hospital (Reg. No 166/1999/CPCSEA, Proposal No. 1085).
Induction of Diabetes
Diabetic rat model was created by administrating a single dose (in fasting condition) of freshly prepared solution of STZ (40 mg/kg b.w) in a 0.1 M citrate buffer with pH 4.5 [13]. Then STZ treated rats were allowed to drink 5% glucose water for preventing the STZ induce hypoglycemia [13, 14]. After 72 h, blood was collected from STZ injected rats, and the level of blood glucose was measured. Rats with of blood glucose level above 230 mg/dl were considered for further study.
Experimental Design
In the present study, a total of 24 rats were randomly divided into four groups (6 control and 18 diabetic rats) and each group having six rats. Based on previous reports Chandirasegaran et al. [15] BC was intragastrically administered at a concentration of 50 mg/kg b.w and glibenclamide at a concentration of 6 mg/kg b.w. Both the drugs were suspended in distilled water. The groups are as follows:
- Group 1
Normal control rats
- Group 2
STZ (40 mg/kg body weight)-induced diabetic control rats
- Group 3
Diabetic rats + BC (50 mg/kg b.w) treated rats
- Group 4
Diabetic rats + Glibenclamide (Reference drug) (6 mg/kg b.w) treated rats
Every day morning BC (50 mg/kg b.w) and glibenclamide (6 mg/kg b.w) were given to diabetic rats by intragastric intubation for 45 days. On the 45th day, animals were sacrificed by cervical dislocation. The blood samples were collected in two tubes, i.e. one with an anticoagulant for separation of plasma and another without anticoagulant for serum separation. After the separation of plasma, the buffy coat enriched in white cells was detached, and the residual erythrocytes were washed three times with physiological saline and made up to a known volume. A specific volume of erythrocyte was lysed with hypotonic phosphate buffer at pH 7.4. The hemolysate was separated by centrifugation at 2500 rpm for 10 min, and the supernatant was used for the estimation of enzymatic antioxidants. Skeletal muscle was harvested for western blot analysis.
Biochemical Analysis
The lipid profile parameters such as total cholesterol [16], HDL cholesterol [17] and triglycerides [18] were estimated respectively. The level of serum LDL cholesterol and VLDL cholesterol were estimated [19]. Phospholipid was determined by the method of Zilversmit and Davis [20].
TBARS and LOOH in the plasma were measured by the method of Ohkawa [21]. SOD was evaluated by the method of Kakkar [22]. CAT was measured by the method given by Sinha [23]. GPx was evaluated by the method given by Rotruck [24]. GSH was assessed by the method of Ellman [25]. Vitamin C and E were evaluated by Omaye et al. [26] and Baker et al. [27] respectively.
Western Blotting Analysis
The total protein from tissues were extracted by homogenizing tissues with 1 ml of a buffer containing 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, and 10 μl protease inhibitor cocktail. The lysate was then centrifuged at 8000×g for 2 min at 4 °C, the supernatant was removed, and aliquots were frozen at −80 °C. The total protein concentration of the supernatant was determined by the method of Lowry et al. [28]. SDS-PAGE was performed using equivalent protein extracts (50 μg) from each sample. The resolved proteins obtained were then electrophoretically transferred to poly vinylidene difluoride membranes. The blots were incubated in 1 × PBS containing 5% non-fat dry milk for 2 h to block nonspecific binding sites. The blots were further incubated with 1:200 dilution of primary antibodies overnight at 4 °C. After washing, the blots were transferred and incubated with 1:1000 dilution of horseradish peroxidase-conjugated secondary antibody for 45 min at room temperature. After continuous washes with high and low salt buffers, the immunoreactive proteins were visualized using enhanced chemiluminescence detection reagents (Sigma-Aldrich). Densitometry was performed on IISP flat bed scanner and quantitated with Total Lab 1.11 software.
Statistical Analysis
All the data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey multiple comparison tests by using a commercially available statistics software package (IBM SPSS Statistics for Windows, version 15). Results were presented as mean ± SD of six rats in each group. The value of p < 0.05 was considered as statistically significant.
Results
Lipid Profile in Control and Treated Rats
The diabetic control rats depicted a rise in the levels of TC, TG, phospholipids, LDL and VLDL, whereas a decline in HDL level was observed when compared with normal control rats. Oral treatments of diabetic animals with BC and glibenclamide significantly restored the abnormal levels of TC, TG, phospholipids, LDL, VLDL and HDL when compared with diabetic control rats. The result of BC was also found to be similar in effectiveness and was comparable with glibenclamide treatment (Table 1).
Table 1.
Lipid profile in control and treated rats: diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. All the data were expressed as the mean ± SD for 6 rats. The results with different superscripts (a, b, c) in each experimental groups are significantly different at p <0.05
| Groups/parameters | TG (mg/dl) | TC (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) | Phospholipids (mg/dl) |
|---|---|---|---|---|---|---|
| Normal control | 52.24 ± 3.98a | 80.14 ± 6.10a | 46.10 ± 3.51a | 23.60 ± 1.80a | 10.44 ± 0.80a | 90.23 ± 6.87a |
| Diabetic control | 126.42 ± 9.68b | 162.24 ± 12.42b | 26.64 ± 2.04b | 110.38 ± 8.45b | 25.29 ± 1.94b | 183.18 ± 14.02b |
| D + BC (50 mg/kg bw) | 79.57 ± 6.06c | 114.44 ± 8.71c | 36.23 ± 2.76c | 62.44 ± 4.75c | 15.80 ± 1.20c | 120.08 ± 9.14c |
| D + GC (6 mg/kg bw) | 66.05 ± 5.06d | 98.67 ± 7.55d | 42.84 ± 3.28a | 41.69 ± 3.19d | 13.22 ± 1.01d | 109.65 ± 8.39a |
D diabetic; BC berberine chloride; GC glibenclamide
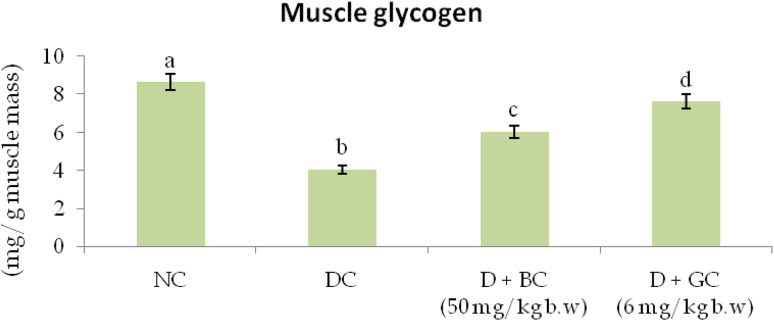
Glycogen Level in Muscle Tissues of Control and Treated Rats
Figure 1 shows the level of muscle glycogen in normal and diabetic animals. The level of muscle glycogen was reduced in diabetic control rats when compared to normal control rats. Treatment of BC significantly improved the muscle glycogen levels in diabetic rats when compared to diabetic control rats.
Fig. 1.
Glycogen level in muscle tissues of control and treated rats: diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. All the data were expressed as the mean ± SD for 6 rats. The results with different superscripts (a, b, c) in each experimental groups are significantly different at p < 0.05 (NC normal control; DC diabetic control; D diabetic; BC berberine chloride; GC Glibenclamide)
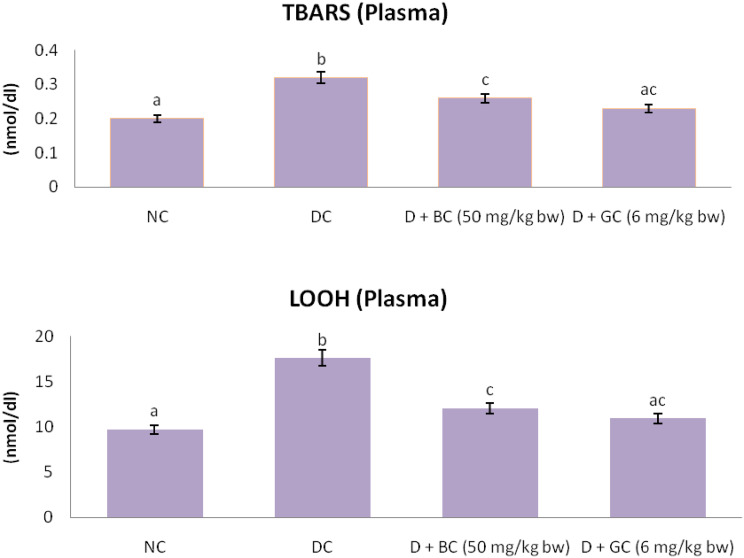
Lipid Peroxidation Markers in Control and Treated Rats
Figure 2 displays the level of TBARS and LOOH in control and experimental rats. There was a significant elevation in the level of TBARS and LOOH in the plasma of diabetic control rats when compared with normal control rats. The oral treatment of BC and glibenclamide were successful in bringing these abnormal levels of TBARS and LOOH back to near normal in diabetic rats.
Fig. 2.
Lipid peroxidation markers in control and treated rats: diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. All the data were expressed as the mean ± SD for 6 rats. The results with different superscripts (a, b, c) in each experimental groups are significantly different at p <0.05 (NC normal control; DC diabetic control; D diabetic; BC berberine chloride; GC glibenclamide)
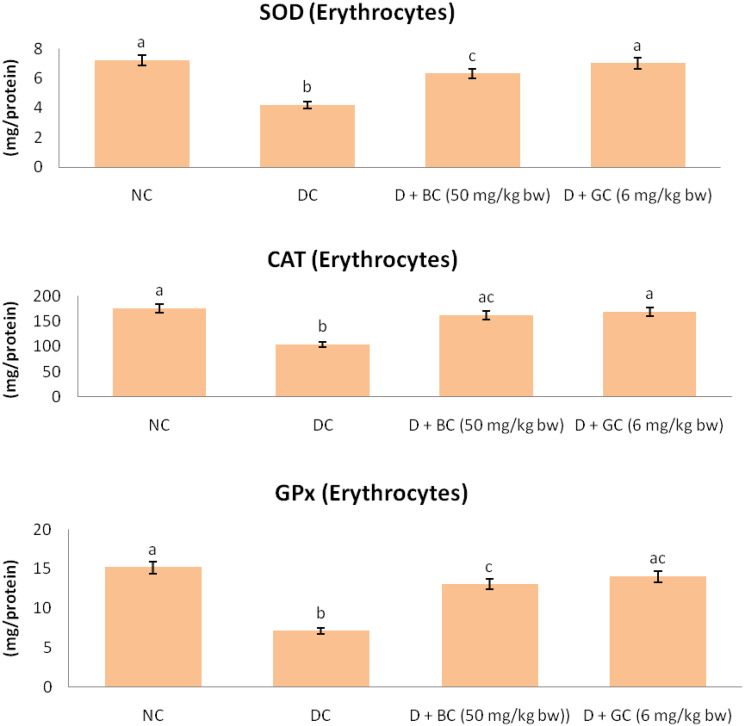
Enzymatic Antioxidants in Control and Treated Rats
The enzymatic antioxidant such as SOD, CAT and GPx activity of control and experimental rats are represented in Fig. 3. There was a significant reduction of SOD, CAT and GPx activity in erythrocytes of diabetic control rats as compared with normal control rats. Continuous treatments of BC or glibenclamide had significantly improved the activities of SOD, CAT and GPx in erythrocytes of diabetic rats.
Fig. 3.
Enzymatic antioxidants in control and treated rats: diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. All the data were expressed as the mean ± SD for 6 rats. The results with different superscripts (a, b, c) in each experimental groups are significantly different at p < 0.05. (NC normal control; DC diabetic control; D diabetic; BC berberine chloride; GC glibenclamide)
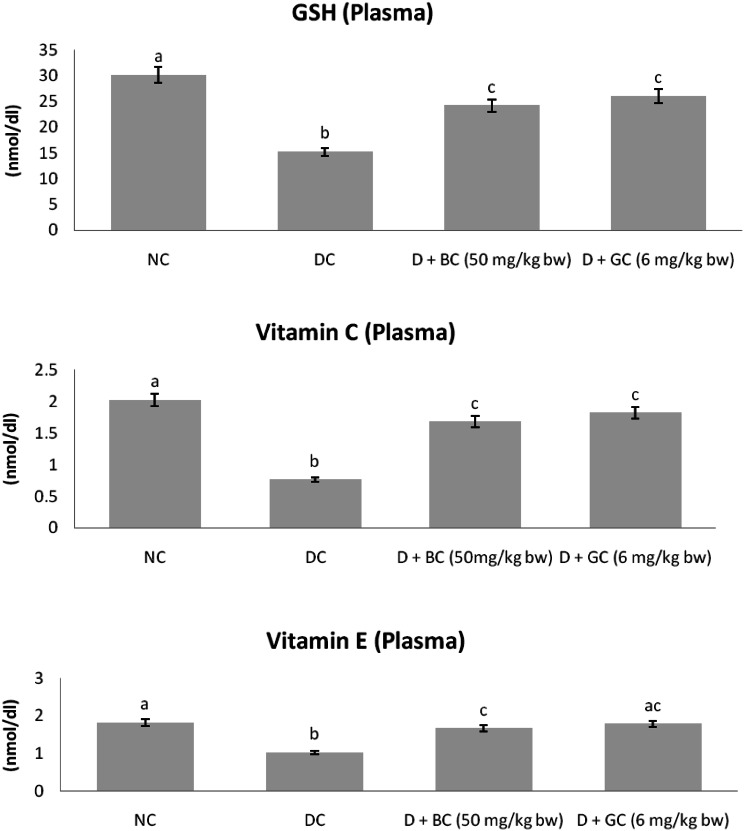
Non-enzymatic Antioxidants in Control and Treated Rats
Figure 4 shows the level of non-enzymatic antioxidants such as GSH, vitamin C and E in plasma of control and experimental rats. In diabetic control rats, the level of GSH, vitamin C and vitamin E were significantly declined as compared with normal control animals. However, BC or glibenclamide treatment had significantly enhanced the level of GSH, vitamin C and E in plasma of diabetic rats as compared with diabetic control rats.
Fig. 4.
Non-enzymatic antioxidants in control and treated rats: diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. All the data were expressed as the mean ± SD for 6 rats. The results with different superscripts (a, b, c) in each experimental groups are significantly different at p <0.05 (NC normal control; DC diabetic control; D diabetic; BC berberine chloride; GC Glibenclamide)
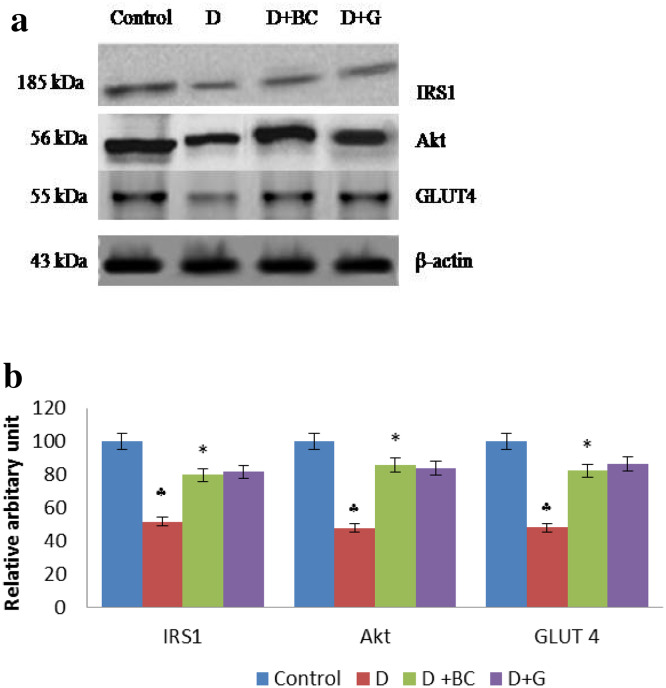
Western Blot Analysis of IRS 1, Akt and GLUT 4 in Skeletal Muscle of Control and Treated Rats
Figure 5 illustrates the protein expression study of IRS1, Akt and GLUT-4 in the skeletal muscle of control and experimental rats. The level of IRS1, Akt and GLUT-4 were significantly declined in diabetic control rats. Oral treatment of BC significantly reverted the diminished levels of IRS1, Akt and GLUT-4 in the skeletal muscle of diabetic rats.
Fig. 5.
Western blot analysis of IRS 1, Akt and GLUT 4 in skeletal muscle of control and treated rats: Diabetic control rats were compared with normal control rats; BC treated diabetic rats were compared with diabetic control rats. a Representative immunoblot analysis. Protein samples (50 μg/lane) resolved on SDS-PAGE was probed with corresponding antibodies. β-Actin was used as loading control. b Densitometric analysis. The mean protein expression from control lysates for five determinations was designated as 100% in the graph. Mean ± SD of six determinants is represented in graph for each group. ♣Significantly different from untreated control (p < 0.05) , *Significantly different from diabetic (D) animals (p < 0.05) (D diabetic; BC berberine chloride; GC Glibenclamide)
Discussion
The majority of insulin-mediated glucose disposal is found in skeletal muscles. Insulin resistance and impaired insulin secretion are attributed to impaired insulin signaling pathway [29, 30]. Therefore, in this study, we have investigated whether BC administration improves impaired insulin signaling pathway in muscles of STZ induced diabetic rats. In this study, STZ was selected for induction of diabetes, as the cytotoxic action of STZ mainly destroys β-cells of the pancreas without affecting other cells by producing a high level of ROS and carbonium ion (CH3+), leading to DNA breaks by alkylation DNA bases and oxidative damage [31]. The dose of 40 mg/kg b.w. of STZ can induce incomplete destruction of β-cells of pancreas, which is considered as a type 2 diabetic rat model [13, 14]. In our previous study, we observed that treatment of BC significantly reduced blood glucose and improved the plasma insulin. These observations may be due to enhanced insulin secretion in existing β-cells of the pancreas in BC treated diabetic rats [32].
In diabetes condition, hypertriglyceridemia, as well as hypercholesteremia, are the common factors involved in the development of atherosclerosis and coronary heart disease. These conditions occur because of lack of insulin secretion, which activates the lipase enzyme, hydrolyzes triglyceride (TG) and release the fatty acid and glycerol into the circulating blood. The level of the triglyceride was found to be increased in the STZ induced diabetic rats. This may be due to decreased level of insulin, causing a failure to activate lipoprotein lipase, and thus leading to hypertriglyceridemia [33]. STZ induced diabetes is often associated with abnormal lipid metabolism, which is also a metabolic disorder found in diabetic conditions [34]. We observed augmented levels of TG, and TC in the serum of STZ treated diabetic control. Several previous studies have stated that LDL is the “bad lipid”, and its oxidation can result in increased release of LDL in the arterial walls, resulting in atherosclerotic plaque lesions formation [35, 36]. We observed an increase in serum LDL and VLDL cholesterol fractions along with a decrease in the HDL level in diabetic rats. An increase in the LDL and VLDL cholesterol levels leads to a fall in the HDL level. Treatment with BC and GC helped in restoring the abnormal levels of lipids in diabetic rats. Due to lack of insulin or its action, it leads to inactivation of the lipoprotein lipase in the liver, which is responsible for the change of free fatty acids into phospholipids and cholesterol that is finally released into the blood, resulting in elevated levels of serum phospholipids [37]. The diabetic rats showed a significantly increased level of phospholipids, whereas there was a significant reduction of phospholipids in the serum of BC treated diabetic rats, which may be due to enhanced insulin secretion from the existing β-cells of the pancreas in diabetic rats. Thus, BC could be a potential candidate in preventing the formation of diabetes associated complications like atherosclerosis and coronary heart disease.
Glycogen is the primary form of intracellular glucose storage and is a branched polymer of glucose residues produced by the enzyme glycogen synthase [38]. Its quantity varies in different tissues and is a directly influenced by insulin activity, as insulin supports intracellular glycogen deposition by stimulating glycogen synthase and inhibiting glycogen phosphorylase [39]. Muscle glycogen content was significantly reduced in STZ induced diabetic rats. This may be due to the increased glycogen phosphorylase activity in diabetes. Continuous oral administration of BC to diabetic rats improved the glycogen content in liver and muscles, indicating the possible role of BC in the modulation of the glycogen metabolism.
Prolong period of hyperglycemia generates a huge number of ROS which induces oxidative stress. The increase free radicals produced may react with polyunsaturated fatty acids in cell membranes, leading to lipid peroxidation, and in turn result in elevated free radicals production [40]. LPO is a marker of induced oxidative stress in tissues. TBARS and LOOH are standard lipid peroxidative markers, which are elevated in experimental diabetic rats [41]. In this study, we found that diabetic control rats possess increased levels of TBARS and LOOH in plasma. This result indicated elevated levels of oxidative stress in diabetic control rats. Continuous treatment of BC significantly declined the increased levels of TBARS and LOOH in diabetic rats, which may be attributed to its anti-peroxidative activity.
Antioxidant enzymes such SOD, CAT and GPx are involved in blocking the free radical process. SOD is the most important scavenging enzyme, which is involved in eliminating free radicals and defends from free oxygen radicals by catalyzing the removal of superoxide radical. This process leads to the prevention of damage to the cell membrane and biological structures [42]. CAT is an antioxidant enzyme which is present in the tissues. It plays a vital role in the decomposition of the hydrogen peroxide molecule, and it prevents the tissues from the reactive hydroxyl radicals [43]. GPx is an enzyme that destroys the peroxides, and it plays a vital role to provide an antioxidant defenses to an organism and also involved in the elimination of hydrogen peroxide [44]. In diabetes, antioxidant enzymes SOD, CAT and GPx are inactivated due to high blood glucose, by glycating of these proteins, consequently leads to the oxidative stress, which in turn causes lipid peroxidation. Previously Zhou et al. [45] demonstrated BC improves the levels of CAT, SOD and GPx in diabetic rats. Thus, the near normal levels of CAT, SOD and GPx activities strongly indicate the efficacy of BC in attenuating the oxidative stress in diabetic rats.
A low level of non-enzymatic antioxidants has been reported in diabetics, which are required to inhibit the peroxidation of lipids in the cell membrane [46]. Among these antioxidants, GSH protects the cell from toxification by scavenging ROS [47]. It has been reported that elevated utilization of GSH in the diabetic cell is one of the reasons for decreased level of GSH in diabetics [48]. According to Sadi et al. [49] and Chen et al. [50] Vitamin C protects all lipids from undergoing oxidation, helps to regenerate vitamin E from its oxidized state and to diminish the count of apoptotic cells. According to Punithavathi et al. [51] vitamin E reduces the chain reactions, which are associated with lipid peroxidation. Several reports have indicated that the reductions in the levels of non-enzymatic antioxidants are associated with STZ induced diabetic rats [52, 53]. In our studies, BC administration expressed near normal levels of GSH, vitamin C and E, indicating BC’s potential to restore the antioxidant reserves in diabetic animals.
Insulin action is a mediated by binding of insulin to the insulin receptor in target tissues. Consequently, IRS gets activated by the insulin receptor and then excited and phosphorylated the signal molecule PI3K and Akt. Both PI3K and Akt can modulate the downstream protein GLUT-4 [54, 55].
The IRS is a member of the ligand-activated receptor of tyrosine kinase family, which is a transmembrane signaling protein with several isoforms. The isoforms IRS 1 and IRS 2 are mainly involved in metabolic regulation. IRS-1 is responsible for glucose metabolism and GLUT-4 translocation [56]. A decreased cellular level of IRS1 is associated with insulin resistance and insufficient insulin secretion [57]. In diabetic control rats showed remarkably declined levels of IRS1 protein, which indicates insulin resistance found in diabetic rats. The levels of IRS 1 notably improved in BC treated groups, which may be due to BC enhanced insulin sensitivity.
PKB or Akt is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes, which is activated by PI3K. The pathway of PKB activation is a process that involves membrane translocation as well as phosphorylation. The activation of this enzyme may stimulate glucose transport by triggering translocation of GLUT-4 from the cytosol towards the plasma membrane of the cell [58]. GLUT-4 present in the skeletal, adipose tissue and cardiac muscles plays a critical role in the regulation of glucose homoeostasis through the translocation and activation triggered by insulin [59]. Intracellular GLUT-4 translocates to the plasma membrane and facilitates glucose uptake stimulated by insulin. Under the diabetic condition, PKB and GLUT-4 expression and translocation are reduced due to the impairment of insulin signaling. These alterations lead to a decrease in the consumption of glucose in adipose tissue and skeletal muscles [59–61]. In this study, Akt and GLUT-4 protein expressions were found to be increased in BC administrated rats compared to diabetic rats. The up-regulation of both Akt and GLUT-4 confirmed increased glucose utilization and thus, help ameliorate the condition of insulin resistance as reported earlier by Nobyuki et al. [62]. Thus, from the above results, it can be concluded that BC enhanced the level of glucose utilization in skeletal muscles by increasing expression of Akt and GLUT-4, improving insulin sensitivity, and thus, also helped ameliorate the abnormal levels of lipids and antioxidant status, and restored near normal insulin levels in diabetic rats.
Conclusion
From this study, it can be concluded that BC can control hyperglycemia, improve the antioxidant status, regulate the abnormal lipid levels and enhances the insulin sensitivity in experimentally induced diabetic rats. Hence, BC can be a potential drug candidate for the treatment of DM. Further studies in BC would prove helpful in better understanding of its antidiabetic properties and long term effects, which would prove beneficial in preparing potent antidiabetic drugs.
Acknowledgement
The authors intend to acknowledge the University Grants Commission, New Delhi, Project File No. 41-178/2012/(SR) for funding this project work and also extend our thanks to Department of Zoology (UGC – SAP Sponsored), Annamalai University for providing infrastructure facility and support.
Compliance with ethical standards
Conflict of interest
All authors declare that there were no conflicts of interest concerning this publication.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s12291-022-01024-0
Change history
1/22/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s12291-022-01024-0
Contributor Information
Govindasami Chandirasegaran, Email: gchandrugobi@gmail.com.
Chakkaravarthy Elanchezhiyan, Email: chezhiyanzooau@gmail.com.
Kavisa Ghosh, Email: kavisa9@gmail.com.
References
- 1.Noor AS, Gunasekaran S, Manickam AS, Vijayalakshmi MA. Antidiabetic activity of Aloe vera and histology of organs in Streptozotocin induced diabetic rats. Curr Sci. 2008;94:254–262. [Google Scholar]
- 2.International Diabetes Federation, Diabetes Atlas. 2015.
- 3.Bray TM. Dietary antioxidants and assessment of oxidative stress. Nutrition. 2000;16:578–580. doi: 10.1016/s0899-9007(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 4.Chertow B, Edwards JC. Advances in diabetes for the milennium: vitamins and oxidant stress in diabetes and its complications. Medscape Gen Med. 2004;6:1–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- 6.Tanti JF, Grillo S, Grémeaux T, Coffer PJ, Van Obberghen E, Le Marchand- Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 7.Tan C, Tan Y, Yan W, Chen Y, Kambadur R, Wahli W. Smad3 deficiency in mice protects against insulin resistance and obesity induced by a high-fat diet. Diabetes. 2011;60:464–476. doi: 10.2337/db10-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryder JW, Yang J, Galuska D, Rincon J, Bjornholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin and hypoxia-stimulated cell surface GLUT 4 content in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:647–654. doi: 10.2337/diabetes.49.4.647. [DOI] [PubMed] [Google Scholar]
- 9.Zierath JR, He L, Guma A, Odegoard-Wahlstrom E, Klip A, Wallberg- Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]
- 10.Damsbo P, Vaag A, Hother-Nielsen O, Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- 11.Bjornholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997;46:524–527. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- 12.Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Determination of antidiabetic compound from Helicteres isora by oral glucose tolerance test. J App Pharm Sci. 2016;6:172–174. [Google Scholar]
- 13.Frode T, Medeiros Y. Animal models to test drugs with potential antidiabetic activity. J Ethnopha. 2008;115(2):173–183. doi: 10.1016/j.jep.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Balamurugan R, Duraipandiyan V, Ignacimuthu S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur J Pharmacol. 2011;667:410–418. doi: 10.1016/j.ejphar.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Efficacy of Berberine chloride on hyperglycemia in Streptozotocin induced diabetic rats. Int Res J Pharm. 2016;7:14–18. [Google Scholar]
- 16.Zlatkis A, Zak B, Boyle GJ. A simple method for determination of serum cholesterol. J Clin Med Res. 1953;41:486–492. [PubMed] [Google Scholar]
- 17.Burnstein M, Scholnic HR, Morfin R. Rapid method of isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–587. [PubMed] [Google Scholar]
- 18.Foster LB, Dunn RT. Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin Chem. 1973;19:338–340. [PubMed] [Google Scholar]
- 19.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of LDL-C in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:449–502. [PubMed] [Google Scholar]
- 20.Zilversmit BB, Davis AK. Micro determination of plasma phospholipids by trichloroacetic acid precipitation. J Lab Clin Med. 1950;35:155–160. [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, YagI K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Ann Biochem. 1979;9:5351–5358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 23.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 24.Rotruck JT, Pope AL, Ganther HE. Selenium biochemical role as a component of glutathione peroxidase purification assay. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluid. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 27.Baker H, Frank O, Angelis B, Feingold S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Rep Int. 1980;21:531–536. [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin’s-Phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Sharma K, Bharti S, Goyal S, Arora S, Nepal S, Kishore K, Joshi S, Kumari S, Arya DS. Upregulation of PPARg by aegle marmelos ameliorates insulin resistance and b-cell dysfunction in high fat diet fed-streptozotocin induced type 2 diabetic rats. Phytother Res. 2011;25:1457–1465. doi: 10.1002/ptr.3442. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CY, Shih HY, Chang YC, Huang ZL, Tsai MJ, Chia YC, Chen C, Lai YK, Weng CF. The beneficial effects of tetracosanol on insulin-resistance by insulin receptor kinase sensibilisation. J Funct Foods. 2015;14:174–182. [Google Scholar]
- 31.Szkudelski T. The mechanism of alloxan and streptozotocin action in beta Cells of the rat pancreas. Physiol Res. 2001;50:536–546. [PubMed] [Google Scholar]
- 32.Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharmacother. 2017;95:175–185. doi: 10.1016/j.biopha.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Shirwaikar A, Rajendran K, Punitha ISR. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin nicotinamide induced type-2 diabetic rats. J Ethnopharmacol. 2005;97:369–374. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Krentz AJ. Lipoprotien abnormalities and their consequences for patients with type 2 diabetes. Diab Obes Metab. 2003;5:19–27. doi: 10.1046/j.1462-8902.2003.0310.x. [DOI] [PubMed] [Google Scholar]
- 35.Marinangeli CP, Varady KA, Jones PJ. Plant sterols combined with exercise for the treatment of hypercholesterolemia: overview of independent and synergistic mechanisms of action. J Nutr Biochem. 2006;17:217–224. doi: 10.1016/j.jnutbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Leiva E, Wehinger S, Guzmán L, Orrego R. 2015. Role of Oxidized LDL in Atherosclerosis, Hypercholesterolemia, Dr. Sekar Ashok Kumar (Ed.). doi:10.5772/59375. http://www.intechopen.com/books/hypercholesterolemia/roleofoxidized-ldl-in-atherosclerosis.
- 37.Pushparaj PN, Low HK, Manikandan J, Tan BKH, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Pederson BA, Schroeder JM, Parker G. Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes. 2005;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- 39.Chandramohan G, Ignacimuthu S, Pugalendi KV. A novel compound from Casearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin diabetic rats. Eur J Pharmacol. 2008;590:437–443. doi: 10.1016/j.ejphar.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 40.Lery V, Zaltzber H, Ben-Amotz A, Kanter Y, Aviram M. b-Carotene affects antioxidant status in non-insulin dependent diabetes mellitus. Pathophysiology. 1999;6:157–162. [Google Scholar]
- 41.Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 42.Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of emblica officinalis gaertn leaves extract in Streptozotocin induced type 2 diabetes mellitus (T2M) rats. J Ethnopharmacol. 2012;142:65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Chance B, Greenstein DS. The mechanism of catalase actions–steady state analysis. Arch Biochem Biophys. 1992;37:301–339. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Schopfer P. Hydroxyl radical production in physiological reaction. A novel function of peroxidise. Eur J Biochem. 1999;260:726–735. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou JY, Zhou SW. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b express in diabetic rat liver. Fitoterapia. 2011;82:184–189. doi: 10.1016/j.fitote.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Opara EC. Oxidative stress, micronutrients, diabetes mellitus, and its complications. J R Soc Promot Health. 2002;122:28–34. doi: 10.1177/146642400212200112. [DOI] [PubMed] [Google Scholar]
- 47.Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Furfaro AL, Nitti M, Marengo B, Domenicotti C, Cottalasso D, Marinari UM. Impaired synthesis contributes to diabetes-induced decrease in liver glutathione. Int J Mol Med. 2012;29:899–905. doi: 10.3892/ijmm.2012.915. [DOI] [PubMed] [Google Scholar]
- 49.Sadi G, Yılmaz O, Güray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu-Zn SOD and catalase. Mol Cell Biochem. 2008;309:109–126. doi: 10.1007/s11010-007-9648-6. [DOI] [PubMed] [Google Scholar]
- 50.Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr. 2005;135:1366–1373. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- 51.Punithavathi VR, Anuthama R, Prince PS. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male wistar rats. J Appl Toxicol. 2008;28:806–813. doi: 10.1002/jat.1343. [DOI] [PubMed] [Google Scholar]
- 52.Pavana P, Sethupathy S, Manoharan S. Antihyperglycemic and anti-lipidperoxidative effects of tephrosia purpurea seed extract in streptozotocin induced diabetic rats. Indian J Clin Biochem. 2007;22:77–83. doi: 10.1007/BF02912886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vishnukumar S, Stephan R. Effect of morin on lipidperoxides and antioxidants in streptozotocin-induced diabetic rats. Int J Pharm Bio. 2012;3:770–780. [Google Scholar]
- 54.Gabriel NV, Hector TG, Sergio HF, Juan JRE, Samuel ES, Jose LMF, Ismael LR, Francisco AA, Julio CAP. Synthesis in vitro and in silico study of a PPAR and GLUT-4 modulator with hypoglycemic effect. Bioorg Med Chem Lett. 2014;24:4575–4579. doi: 10.1016/j.bmcl.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 55.Ren ZQ, Zhang PB, Zhang XZ, Chen SK, Zhang H, Lv DT, Zhuang YQW, Ding WC, Zhang C. Duodenal-jejunal exclusion improves insulin resistance in type 2 diabetic rats by upregulating the hepatic insulin signaling pathway. Nutrition. 2015;31:733–739. doi: 10.1016/j.nut.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Krentz Krook A, Bjornholm M, Galuska D, Jiang XJ, Fahlman R, Myers MG, Wallberg-Henriksson H, Zierath JR. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:284–292. doi: 10.2337/diabetes.49.2.284. [DOI] [PubMed] [Google Scholar]
- 57.Leng S, Zhang W, Zheng Y, Liberman Z, Rhodes CJ, Eldar-Finkelman H. Glycogen synthase kinase 3 beta mediates high glucose-induced ubiquitination and proteasome degradation of insulin receptor substrate 1. J Endocrinol. 2010;206:171–181. doi: 10.1677/JOE-09-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zdychova J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]
- 59.Charron MJ, Katz EB, Olson AL. GLUT4 gene regulation and manipulation. J Biol Chem. 1999;6:3253–3256. doi: 10.1074/jbc.274.6.3253. [DOI] [PubMed] [Google Scholar]
- 60.Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M. Proteintyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- 61.Gandhi GR, Stalin A, Balakrishna K, Ignacimuthu S, Paulraj MG, Vishal R. Insulin sensitization via partial agonism of PPARand glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim Biophys Acta. 2013;1830:2243–2255. doi: 10.1016/j.bbagen.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 62.Nobyuki T, Naoto Y, Yuma N, Tetsuya H, Tetsuya N, Astu A, Takaya S. Role of the guanine nucleotide exchange factor in Akt2-mediated plasma membrane translocation of GLUT-4 in insulin-stimulated skeletal muscle. Cell Signal. 2014;26:2460–2469. doi: 10.1016/j.cellsig.2014.07.002. [DOI] [PubMed] [Google Scholar]