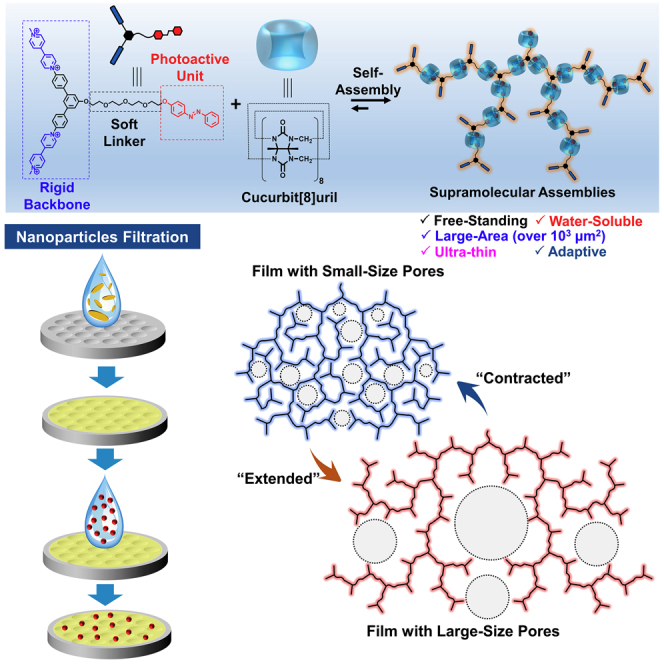
Summary
The construction of synthetic two-dimensional (2D) materials designates a pathway to the versatile chemical functionality by spatial control. However, current 2D materials with intelligence of stimuli-responsibility and adaptiveness have been unfledged. The approach reported here uses a supramolecular strategy to achieve the dynamic non-covalent self-assembly of a rationally designed small molecule monomer, producing large-area, ultra-thin, porous 2D supramolecular assemblies, which are solution-processable in aqueous solution. Importantly, the 2D supramolecular assemblies exhibit distinct adaptive capability to automatically regulate their network density and pore diameters in response to environmental temperature change, which could be developed into an "on-demand" filtration application for nanoparticles. Meanwhile, the 2D supramolecular assemblies can also perform reversible degradation/reformation by photo-irradiation. Our results not only show the simplicity, reliability, and effectiveness of supramolecular strategies in the construction of 2D materials with practical sizes, but also push the dynamic alterability and adaptation features from supramolecular assemblies toward 2D materials.
Subject Areas: Supramolecular Materials, Materials Synthesis, Materials Characterization
Graphical Abstract
Highlights
-
•
2D supramolecular assemblies combine large area, nano-thickness and water solubility
-
•
The 2D assemblies can perform reversible expansion/contraction to tune pore sizes
-
•
The 2D material can be used for on-demand nanoparticles filtration
Supramolecular Materials; Materials Synthesis; Materials Characterization
Introduction
The design and fabrication of supramolecular polymers and materials by precise "bottom-up" self-assembly of building blocks has been an appealing and vital theme for chemists owing to the distinct dynamic properties of supramolecular materials (Lutz et al., 2016, Yang et al., 2015, Yu et al., 2015, Amabilino et al., 2017, Krieg et al., 2016, Chen and Liu, 2015, Qu et al., 2015, Zhang et al., 2018a, Zhang et al., 2018b, Zhang et al., 2018c, Zhang et al., 2018d). Many efforts have been realized in the constructions of sophisticated supramolecular architectures by elaborating the structural design of building blocks and controlling the dimensions, sizes, and manners of the further self-assembly to form diversified supramolecular materials exhibiting the superiority and functionality (Yu et al., 2018, Sun et al., 2018, Zhang et al., 2018a, Zhang et al., 2018b, Zhang et al., 2018c, Zhang et al., 2018d, Xing et al., 2018, Ji et al., 2018, Tian et al., 2014, Tao et al., 2019). Meanwhile, man-made 2D organic materials have also attracted much attention of chemists since the rise of graphene (Colson and Dichtel, 2013, Zhuang et al., 2015). Although amounts of 2D metal/covalent organic frameworks have been built and fabricated by different kinds of synthetic methodologies and libraries (Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Wang et al., 2019, Baek et al., 2013, Matsumoto et al., 2018, Xiao et al., 2018), the design and construction of 2D supramolecular assemblies are still under fledging stage, especially those exhibiting large-area, ultra-thin, free-standing, water-soluble features (Zhang et al., 2013, Pfeffermann et al., 2015, Yue et al., 2016).
A reasonable strategy to fabricate 2D supramolecular assemblies involves the design of rigid and multi-branched monomers, which would define the assemblies growth in a highly ordered direction, resulting in rigid supramolecular frameworks (Dong et al., 2018). However, the strictly rigid structure mostly inhibited the large-area polymer growth because of the vertical packing tendency of rigid structures and the lack of flexibility, which is significant to allow the adaptive interactions among small-molecular-weight 2D assemblies with different edge shapes. Some groups strive to overcome this issue by interfacial self-assembly strategy (Pfeffermann et al., 2015, Dong et al., 2018), however, requiring special processing technique. Flexible hyperbranched monomers are good examples to form large-size supramolecular assemblies (Huang and Gibson, 2004, Fernández et al., 2008, Zhou et al., 2010, Dong et al., 2011a, Dong et al., 2011b, Dong et al., 2014, Tao et al., 2012, Fang et al., 2013, Wang et al., 2014) but remain a challenging issue how to precisely construct the dimensions of the assemblies. In many cases, such flexible hyperbranched building blocks tend to self-assemble into spheres or particles because of the flexibility-induced surface curving (Dong et al., 2011a, Dong et al., 2011b, Groombridge et al., 2017, Tian et al., 2017, Datta et al., 2018, Liu et al., 2018). Hence, it is still a fundamental question of whether the 2D supramolecular assemblies with large area can be achieved by the direct solution-phase growth strategies rather than by the self-assembly on interfaces/surfaces.
Meanwhile, one of the representative features of supramolecular assemblies involves the capability of stimuli-responsive materials owing to the unique dynamic nature. Numerous supramolecular assemblies, such as zero-dimensional vesicle/micelle (Gaitzsch et al., 2016, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Gao et al., 2018, Chen et al., 2018, Hu et al., 2018), one-dimensional fibers/tubes (Hendricks et al., 2017, Cohen et al., 2018, Yagai et al., 2019), and three-dimensional gels (Appel et al., 2012, Jones and Steed, 2016, Voorhaar and Hoogenboom, 2016), have been proved to be talented in many potential applications. However, the alterability and stimuli-responsive behavior of 2D supramolecular assemblies have not been exploited yet. Hence, our motivation in these issues locates on following two original hypotheses: (1) whether we can enable the structural rigidity and flexibility in a single 2D supramolecular assembly; (2) what unprecedented properties and functions can be brought in this rigid and flexible 2D supramolecular assemblies. Herein, we report a rationally designed 2D supramolecular assembly to demonstrate the above-mentioned proposals, achieving the direct aqueous self-assembly to form 2D supramolecular assemblies that integrate large area (up to 1000 μm2), nano-sized thickness, water-solubility, and stimuli-induced alterability.
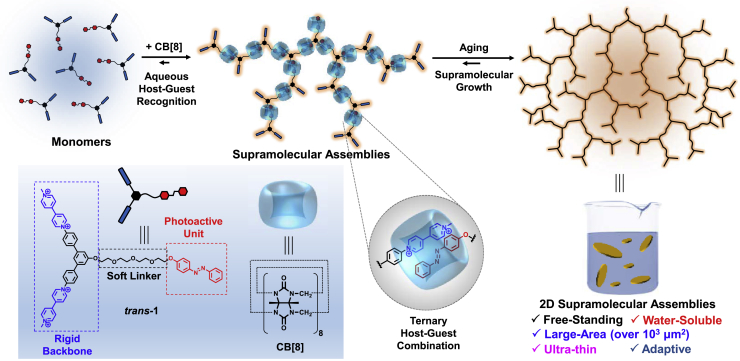
In our design, a semi-rigid tri-branch compound trans-1 was constructed (Figure 1). The unique Y-type structure bears two viologen units and a single azobenzene unit, which can bind together in the cavity of macrocycle cucurbit[8]uril (CB[8]) to form high-affinity ternary host-guest complex (Barrow et al., 2015, Del Barrio et al., 2013, Tian et al., 2012, Pazos et al., 2019). Notably, the viologen-terminated aromatic part bears a rigid backbone with a fixed angle of 120°, whereas the azobenzene-terminated linker is flexible. This semi-rigid design is expected to enable a dendrimer-like supramolecular self-assembly in aqueous solution. We expect that the rigid 120° aromatic backbone could support sufficient space for the tubular macrocycle CB[8] and hence inhibit the steric-hindrance-caused low polymerization degree of the supramolecular assemblies. Meanwhile, the simultaneous presence of soft glycol linker provides flexibility to the resulting supramolecular assemblies. This design is distinct from the previously reported strictly rigid supramolecular frameworks, in which the polymer skeleton is rigid and stable. It is expected that our "semi-rigid" design could generate a unique large-sized supramolecular assembly that simultaneously exhibits the capability of dynamic stimuli responsiveness and adaptiveness.
Figure 1.
Schematic Illustration and Molecular Structure
The molecular structure of trans-1 and the schematic representation of the supramolecular assemblies of trans-1 and CB[8]. The backbone of the final assemblies is simplified for clear presentation.
Results and Discussion
The compound trans-1 was synthesized. Starting with a previously reported compound 2 (Wang et al., 2017), two steps of etherification reactions were performed to yield compound 3 and 4. Then the trifluoroacetic acid (TFA) was used to deprotect the phenylamine groups, and triethylamine was used to neutralize the mixture to expose the amino groups. Finally, the target compound trans-1 was produced by the Zinke reaction with compound 5. The detailed synthesis route of compound trans-1 has been shown in the experimental section of Supplemental Information. The compound structure has been well characterized and confirmed by NMR and HR-MS (see in Supplemental Information).
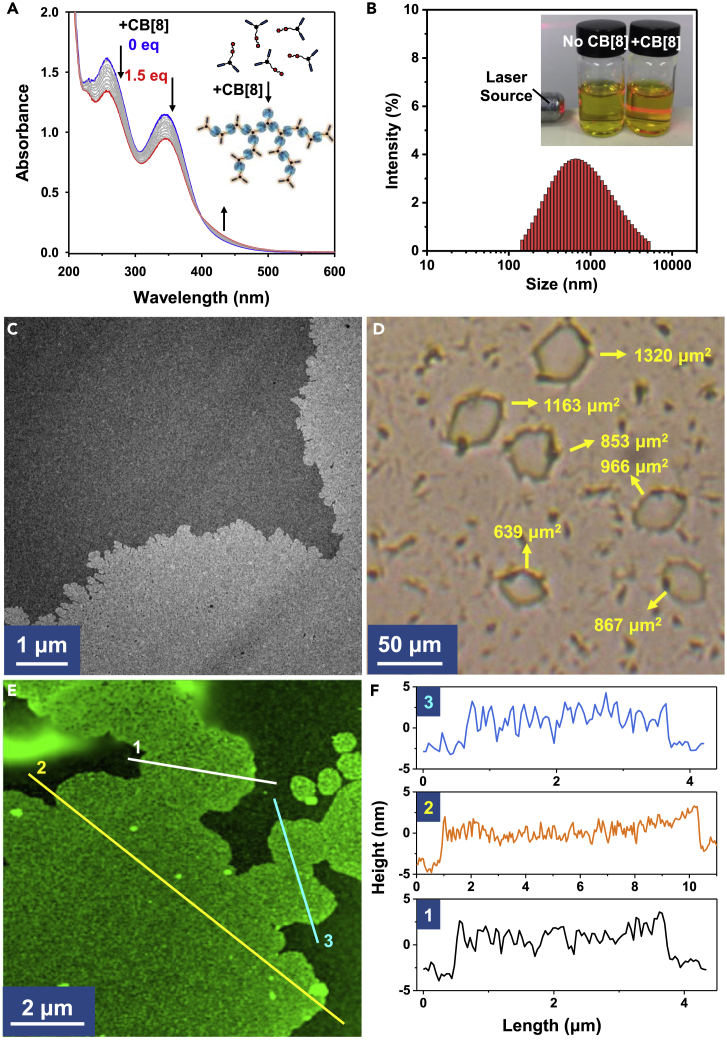
Trans-1 exhibits water solubility owing to the presence of two hydrophilic viologen units, thus producing a homogeneous aqueous solution. The host-guest recognition of trans-1 with macrocycle CB[8] was confirmed by the observed shielded and broadened aromatic peaks of trans-1 after addition of CB[8] in 1H NMR spectra (Figure S1). In the UV-Vis spectra titration experiment (Figure 2A), a remarkable intensity decrease was observed in both the peaks at 260 and 345 nm with the CB[8] solution added, which are attributed to the viologen units and the azobenzene units, respectively. Meanwhile, a shoulder peak after 400 nm rise with the addition of 1.5 equivalents CB[8], and resulted in an isoabsorptive point observed at 400 nm, indicating the formation of the proposed ternary host-guest combination.
Figure 2.
Optical Properties and Morphology of the Supramolecular Assemblies
(A) UV-Vis absorption spectra of the titration experiment of trans-1 (50 μM in H2O) upon addition of CB[8].
(B) DLS result of the mixed aqueous solution of trans-1 (1 mM in H2O; 25°C) after addition of 1.5 equivalents CB[8]. The inset image shows the different Tyndall effect of the trans-1 aqueous solutions (1 mM) without (left) and with (right) CB[8].
(C) TEM image of the supramolecular assemblies.
(D) Optical microscopy image of the supramolecular assemblies.
(E) AFM image of the supramolecular assemblies.
(F) Section height analysis of the corresponding linear region in (E).
Considering the hyperbranched molecular structure of trans-1, the CB[8]-mediated host-guest combination should generate supramolecular assembled networks. Dynamic light scattering (DLS) indicated the large-sized assemblies in the aqueous solution of trans-1 after mixing with 1.5 equivalents of CB[8] (Figure 2B). Tyndall effect can be also visible after the addition of CB[8], whereas no visible Tyndall effect can be found in the aqueous solution of tran-1 (Figure 2B inset). Transmission electron microscopy (TEM) images showed the morphology of the formed supramolecular assemblies, which are micron-sized and have a homogeneous thickness distribution (Figure 2C). Notably, some edge regions of the assemblies were found to be branched (Figure S2), suggesting a highly dynamic nature at the edge region of the supramolecular assemblies. The area of these supramolecular assemblies was found to be as large as over 1,000 μm2 measured by optical microscopy image (Figure 2D). The large-area supramolecular assemblies can be processed into a free-standing dry film by simple drop-casting method (Figure S3). The resulting supramolecular assemblies exhibited a reasonable broad scattering peak in small-angle X-ray scattering (SAXS) pattern (Figure S4), indicating the relatively low long-range order in the assembled network, which is consistent with the proposed dendrimer-like supramolecular assembling mode (Figure 1).
The thickness of the supramolecular assemblies was measured as 5.26 ± 0.10 nm by atomic force microscopy (AFM) (Figures 2E and 2F). Considering the 1.75-nm outer diameter of CB[8] macrocycle (Zhang et al., 2013), the film should be of few layers. This observation is also reasonable because of the micron-sized area of this 2D material in aqueous solution, which would lead to a thermodynamics-preferred packing process. The area-to-thickness of the film can be up to 19 cm. To the best of our knowledge, the observed large area (1,320 μm2) and large area-to-thickness ratio have never been achieved previously in 2D supramolecular assemblies formed by direct aqueous self-assembly process. The large-scale and ultrathin supramolecular assemblies should be attributed to the following four factors: (1) the high binding affinity of the CB[8] ternary host-guest combination as the noncovalent interaction in the network; (2) semi-rigid molecular design of the monomer, whose rigidity supports the spatial 2D extension toward large scale, and the flexibility simultaneously allows the solubility by inhibiting rigid-packing-caused crystalline process; (3) the tubular CB[8] macrocycles effectively decrease the interlayer packing, and the intrinsic rigidity also supports the orientated ternary host-guest combinations, which is necessary for large-scale 2D extension; (4) the paralleled 1:1:1 ternary host-guest combination between MV2+ and azobenzene units inside the cavity of CB[8] might also facilitate the further planar growth of the 2D assemblies. However, despite the presence of such large-sized assemblies, the aqueous solution of the supramolecular assemblies is homogeneous, transparent, and stable for months. Therefore, this rational design enables a successful solution-phase growth of micro-area, nano-thickness 2D supramolecular assembly via a simple supramolecular self-assembly strategy.
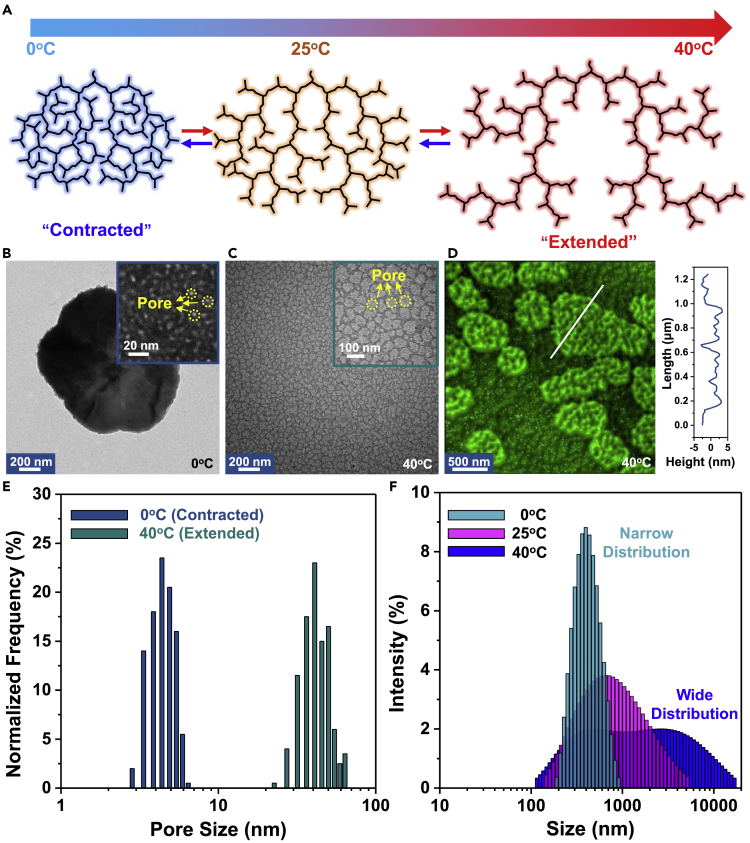
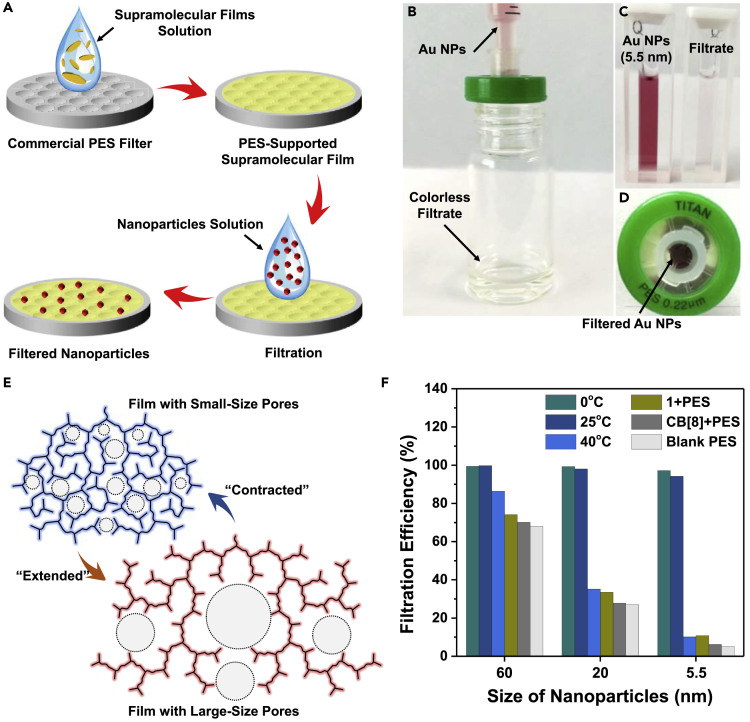
Interestingly, we found that the resulting 2D supramolecular assemblies exhibited temperature-adaptive deformable ability. After heating the aqueous solution of assemblies at 40°C for 1 h, the 2D supramolecular assemblies transformed from 2D assemblies into highly branched networks (Figures 3A–3D and S5). The observed transformation of the 2D assemblies should be explained as the entropy-driven expansion from high-density dendrimer-like network to disordered low-density branched network. Notably, the porous structure on the assemblies at 0°C can be observed in TEM image with higher magnification (Figure 3B inset). The porous structure can be attributed to the intrinsic space in dendrimer-like network of the resulting supramolecular assemblies. The expansion consequently resulted in the diameter increase of the film pores from about 5 nm (0°C) to about 40 nm (40°C) (Figures 3C–3E). The expansion of the pores might be attributed to the heat-accelerated dynamic exchange process between assemblies and free monomers/oligomers, resulting in the increase in the ratio of the “active” edges, which induced the expansion deformation of the supramolecular film into a highly branched network with more “active” edges. Furthermore, the morphology of the supramolecular assemblies at lower temperature (0°C) were further investigated (Figure S6). The films were found to have disk-like morphology. Notably, the branched edges, also the “active” edges, disappeared at 0°C, further confirming the above proposed mechanism for the thermal expansion deformation. The reversible deformation process by varying temperature can be detected by the observed change of the absorbance intensity at 380 nm (Figure S7). DLS showed the consistent results among the three states (Figure 3F). Therefore, these results confirmed the temperature-adaptive deformation ability of the supramolecular assemblies, which might be potentially applied in smart soft materials (Samanta et al., 2017, Stoffelen et al., 2014, Sankaran et al., 2015).
Figure 3.
Reversible Contraction/Expansion Behavior by Thermally Adaptive Deformation
(A) Schematic representation of the thermal deformation of the supramolecular assemblies between 2D film and hyper-branched network.
(B) TEM images of the supramolecular assemblies at 0°C. Inset image shows the pores with nanometer diameter.
(C) TEM images of the supramolecular assemblies at 40°C. Inset image shows the “extended” pores with larger diameter.
(D) AFM images and section height analysis of the 2D supramolecular assemblies at 40°C.
(E) Normalized frequency distribution of the pore diameter of the 2D assemblies at 0°C and 40°C. The statistical data were collected from 200 randomly selected pores in TEM images.
(F) DLS results of the supramolecular polymers trans-1@CB[8] solution (1 mM, H2O) at varied temperature.
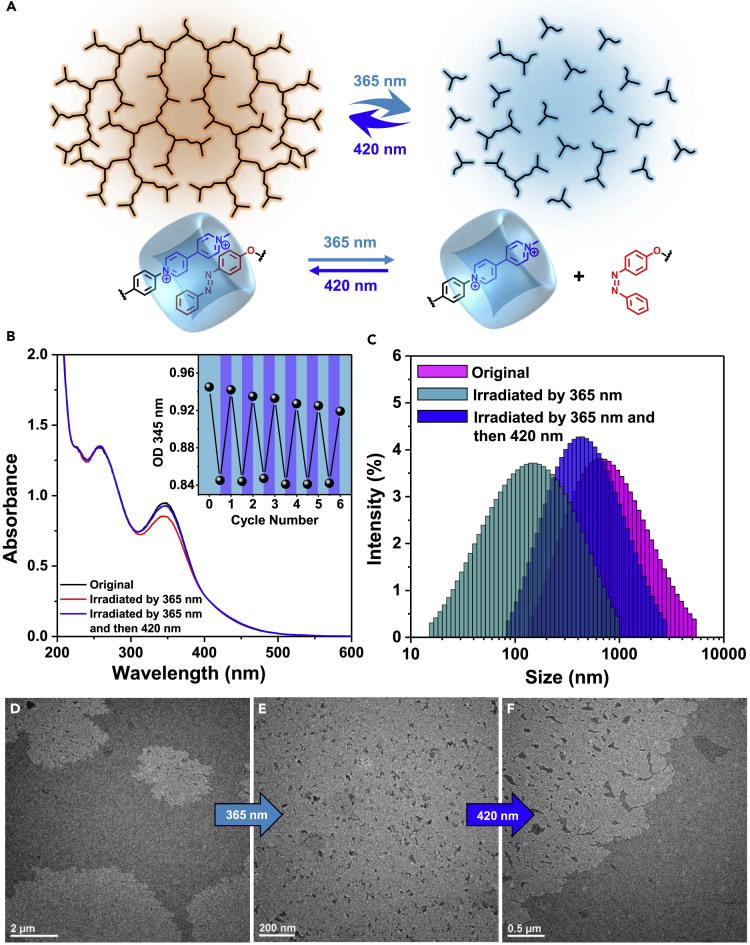
It is also worthwhile to exploit the photo-degradable or recyclable polymers because of the vital importance of rising energy and environment issues. Owing to the presence of photo-responsive azobenzene units, the supramolecular assemblies also exhibited photo-switchability (Figure 4A). Upon irradiation by UV light (λ = 365 nm), the trans-1 performed typical photo-isomerization into cis-form, which had a larger steric hindrance and thus disconnected from the ternary host-guest combination, leading to the light-induced disassembly of the supramolecular assemblies. The light-switching process was detected by UV-Vis spectra (Figures 4B and S8). The azobenzene absorption peak at 345 nm can be switched reversibly in six cycle times by repeating irradiation of UV light (λ = 365 nm, 2 min) and visible light (λ = 420 nm, 5 min). DLS results showed that the size of assemblies decreased remarkably after irradiation of UV light (Figure 4C), suggesting the efficient disassembly process. TEM and AFM images confirmed that the micrometer-sized supramolecular assemblies (Figures 4D, S9, and S10) were disassembled into small nanoparticles (NPs) (Figure 4E), which was consistent with the result measured in DLS (Figure 4C). The visible-light-induced inverse re-assembly process was also confirmed by DLS and TEM images (Figures 4C and 4F). Therefore, these observations indicated the excellent photo-switchability of the resulting supramolecular assemblies, which might provide new strategies and models for the design and construction of photo-switchable supramolecular materials (Del Barrio et al., 2016).
Figure 4.
Photo-Induced Disassembly/Reassembly
(A) Schematic representation of the photo-switchable disassembly/reassembly process triggered by the photoisomerization of the azobenzene units.
(B) UV-Vis absorption spectra (50 μM in H2O) detecting the photo-isomerization process of the azobenzene units. The inset curve shows the reversible switching cycles driven by repeating UV (365 nm, 2 min) and visible light (420 nm, 5 min) irradiation.
(C) DLS results of the supramolecular films before and after corresponding light irradiation.
(D–F) TEM images show the reversible light-switched disassembly/reassembly morphologies.
The porous structure and micrometer size of the supramolecular assemblies enable this materials potential to be used as a filter for NPs. The 2D supramolecular assemblies can be deposited on a commercial polyethersulfone (PES) filter with an average pore diameter of 0.22 μm by easily filtering the aqueous solution through the PES filter (Figure 5A). The 0.22-μm pores of the PES filter would selectively make the micro-sized supramolecular films left on the PES film to form a PES-supported supramolecular film. The entrapment proportion of the PES filter for the supramolecular assemblies can be evaluated as 8.8% by comparing the relative absorbance at 345 nm before and after filtration (Figure S11). Thus, the immobilized density of the supramolecular film can be evaluated to be 1.42 × 10−9 mol ⋅ cm−2. Such immobilization density is quite low compared with the previous examples in NP filter membranes (Krieg et al., 2011). SEM images also showed the well-dispersed 2D assemblies deposited on the PES substrate (Figure S12). Then the filtration capability of the resulting film was tested for gold NPs with different sizes (Figure S13). The filtration process can be performed by simply filtering the NP solution through the film by a syringe (Figures 5B and S14). In the case of 5.5-nm gold NPs, the filtrate was found to be almost colorless (Figures 5C and S15), and the film after filtration turned into red (Figure 5D), indicating the efficient filtration for the gold NPs with small sizes.
Figure 5.
Application in On-Demand Filtration for Nanoparticles
(A) Schematic representation of the preparation of supramolecular filter based on the trans-1@CB[8] 2D assemblies and its application for separation of NPs.
(B) Photograph shows a supramolecular filter under filtering the 20 nm gold NPs almost quantitatively.
(C) Photograph of the 5.5-nm gold NPs solution before and after filtration.
(D) Top view of a supramolecular filter supported on PES filter after filtering 5.5-nm gold NPs.
(E) Schematic representation of the adaptive capability of the supramolecular film with switchable pore diameters.
(F) Filtration efficiency of the supramolecular film filters prepared under different temperatures (0°C, 25°C, and 40°C) and the three control samples for different sizes of gold NPs. Three control samples include: (1) PES filter pre-filtered with compound trans-1 aqueous solution (1 mM); (2) PES filter pre-filtered with CB[8] aqueous solution (0.25 mM); (3) PES filter without any pre-filtration.
The temperature-adaptive capability of the resulting supramolecular assemblies provides possibilities to enable this filtration material with “on-demand” modulable pore sizes (Figure 5E). As the previous demonstration in Figure 3, the pores of the supramolecular assemblies can be expanded/contracted between about 5 and 40 nm. Hence, we expected that this property could be used for “on-demand” filtration applications, meaning enabling membrane filter materials with “smart” pores, whose diameters could be switched by external stimuli for specific requirements. Quantitative experiments indicated that the deposited supramolecular assemblies at 0°C and 25°C exhibited over 90% filtration efficiency for all the tested gold NPs (Figure 5F), which was much higher than that of series of blank control samples especially for NPs with small sizes. These results indicate the excellent performance of the resulting porous supramolecular assemblies for NP filtration, and the required film amount for high entrapment proportion is as low as 1.42 × 10−9 mol ⋅ cm−2, which represents an advanced membrane filter exhibiting distinct advantages at high entrapment proportion as well as low materials utilization amount. Remarkably, the filter material made by filtering the "expanded" supramolecular assemblies at 40°C through the PES filter showed remarkable decreased filtration efficiency for small NPs of 5.5 and 20 nm, whereas it basically remained high efficiency for large NPs of 60 nm. That indicated the effective regulation of the pore diameters of the supramolecular film filter, showing a distinct adaptiveness of filtration functionality (Geise et al., 2010, Luo et al., 2018, Zhang et al., 2018a, Zhang et al., 2018b, Zhang et al., 2018c, Zhang et al., 2018d, Zou and Zhu, 2018, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Zhu et al., 2018, Liang et al., 2018a, Liang et al., 2018b).
Conclusion
In this research, we have demonstrated synthetic 2D supramolecular assemblies that integrate water-solubility, free-standing ability, micrometer size, nanometer thickness, photo-switchability, and dynamic adaptive pore diameters. The distinct semi-rigid structural design not only significantly supports the large area and solubility features of the 2D assemblies but also makes them dynamically adaptive with the external environmental change. This unique adaptive property also enables the 2D assemblies as an “on-demand” filter material that exhibits the intelligence of modulable pore sizes. We expect this concept as a potential origin toward smart 2D materials, which presents that the combination of dynamic supramolecular assemblies and 2D materials could provide plenty of new possibilities to construct smart soft 2D materials with more complex functions and applications.
Limitations of the Study
This research provides a distinct structural design strategy for 2D supramolecular assemblies based on host-guest combinations. However, the poor water solubility of macrocycle CB[8] limits the water solubility of the resulting assemblies in only 1 mM scale, which is not high enough for further processing and applications. Moreover, the multi-step synthesis of monomers and macrocycles makes the material costly. Hence, functional materials made by easier and cheaper method should be exploited in future research.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by NSFC/China (21790361, 21871084, 21421004, 21672060), the Fundamental Research Funds for the Central Universities (WJ1616011, WJ1213007, 222201717003), the Programme of Introducing Talents of Discipline to Universities (B16017), Program of Shanghai Academic/Technology Research Leader (19XD1421100), and the Shanghai Municipal Science and Technology Major Project (Grant No.2018SHZDZX03). We appreciate Dr. Na Li (BL19U2 beamline of Shanghai Synchrotron Radiation Facility) for her kind help in synchrotron SAXS test. The authors thank the Research Center of Analysis and Test of East China University of Science and Technology for help on the material characterization. Prof. Dr. Li-Hui Zhou is specially thanked for her kind help in TEM tests.
Author Contributions
Q.Z. and D.-H.Q. conceived the project and designed the experiments. R.-J.X. and W.-Z.W. performed the synthetic experiments. Q.Z. and R.X. carried out material characterization. Y.-X.D. assisted in material characterization. Q.Z., D.H.Q., and H.T. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.007.
Supplemental Information
References
- Amabilino D.B., Smith D.K., Steed J.W. Supramolecular materials. Chem. Soc. Rev. 2017;46:2404–2420. doi: 10.1039/c7cs00163k. [DOI] [PubMed] [Google Scholar]
- Appel E.A., del Barrio J., Loh X.J., Scherman O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012;41:6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- Baek K., Yun G., Kim Y., Kim D., Hota R., Hwang I., Xu D., Ko Y.H., Gu G.H., Suh J.H. Free–standing, single–monomer–thick two–dimensional polymers through covalent self–assembly in solution. J. Am. Chem. Soc. 2013;135:6523–6528. doi: 10.1021/ja4002019. [DOI] [PubMed] [Google Scholar]
- Del Barrio J., Horton P.N., Lairez D., Lloyd G.O., Toprakcioglu C., Scherman O.A. Photocontrol over Cucurbit[8]uril complexes: stoichiometry and supramolecular polymers. J. Am. Chem. Soc. 2013;135:11760–11763. doi: 10.1021/ja406556h. [DOI] [PubMed] [Google Scholar]
- Del Barrio J., Ryan S.T.J., Jambrina P.G., Rosta E., Scherman O.A. Light–regulated molecular trafficking in a synthetic water–soluble host. J. Am. Chem. Soc. 2016;138:5745–5748. doi: 10.1021/jacs.5b11642. [DOI] [PubMed] [Google Scholar]
- Barrow S.J., Kasera S., Rowland M.J., del Barrio J., Scherman O.A. Cucurbituril–based molecular recognition. Chem. Rev. 2015;115:12320–12406. doi: 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Chen Y., Liu Y. Construction and functions of cyclodextrin–based 1d supramolecular strands and their secondary assemblies. Adv. Mater. 2015;27:5403–5409. doi: 10.1002/adma.201501216. [DOI] [PubMed] [Google Scholar]
- Chen L., Xiang J., Zhao Y., Yan Q. Reversible self–assembly of supramolecular vesicles and nanofibers driven by chalcogen–bonding interactions. J. Am. Chem. Soc. 2018;140:7079–7082. doi: 10.1021/jacs.8b04569. [DOI] [PubMed] [Google Scholar]
- Cohen E., Weissman H., Pinkas I., Shimoni E., Rehak P., Král P., Rybtchinski B. Controlled self–assembly of photofunctional supramolecular nanotubes. ACS Nano. 2018;12:317–326. doi: 10.1021/acsnano.7b06376. [DOI] [PubMed] [Google Scholar]
- Colson J.W., Dichtel W.R. Rationally synthesized two–dimensional polymers. Nat. Chem. 2013;5:453–465. doi: 10.1038/nchem.1628. [DOI] [PubMed] [Google Scholar]
- Datta S., Misra S.K., Saha M.L., Lahiri N., Louie J., Pan D., Stang P.J. Orthogonal self–assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc. Natl. Acad. Sci. U S A. 2018;115:8087–8092. doi: 10.1073/pnas.1803800115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S.-Y., Luo Y., Yan X.-Z., Zheng B., Ding X., Yu Y.-H., Ma Z., Zhao Q.-L., Huang F.-H. A dual–responsive supramolecular polymer gel formed by crown ether based molecular recognition. Angew. Chem. Int. Ed. 2011;50:1905–1909. doi: 10.1002/anie.201006999. [DOI] [PubMed] [Google Scholar]
- Dong R.-J., Liu Y., Zhou Y.-F., Yan D.-Y., Zhu X.-Y. Photo–Reversible supramolecular hyperbranched polymer based on host–guest interactions. Polym. Chem. 2011;2:2771–2774. [Google Scholar]
- Dong R.-J., Zhou Y.-F., Zhu X.-Y. Supramolecular dendritic polymers: from synthesis to applications. Acc. Chem. Res. 2014;47:2006–2016. doi: 10.1021/ar500057e. [DOI] [PubMed] [Google Scholar]
- Dong R.-H., Zhang T., Feng X.-L. Interface-assisted synthesis of 2D materials: trend and challenges. Chem. Rev. 2018;118:6189–6235. doi: 10.1021/acs.chemrev.8b00056. [DOI] [PubMed] [Google Scholar]
- Fang R.-C., Liu Y.-L., Wang Z.-Q., Zhang X. Water–soluble supramolecular hyperbranched polymers based on host–enhanced π–π interaction. Polym. Chem. 2013;4:900–903. [Google Scholar]
- Fernández G., Pérez E.M., Sánchez L., Martín N. An electroactive dynamically polydisperse supramolecular dendrimer. J. Am. Chem. Soc. 2008;130:2410–2411. doi: 10.1021/ja710505h. [DOI] [PubMed] [Google Scholar]
- Gaitzsch J., Huang X., Voit B. Engineering functional polymer capsules toward smart nanoreactors. Chem. Rev. 2016;116:1053–1093. doi: 10.1021/acs.chemrev.5b00241. [DOI] [PubMed] [Google Scholar]
- Gao C., Huang Q.-X., Lan Q.-P., Feng Y., Tang F., Hoi M.P.M., Zhang J.-X., Lee S.M.Y., Wang R.-B. A user–friendly herbicide derived from photo–responsive supramolecular vesicles. Nat. Commun. 2018;9:2967. doi: 10.1038/s41467-018-05437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geise G.M., Lee H.S., Miller D.J., Freeman B.D., McGrath J.E., Paul D.R. Water purification by membranes: the role of polymer science. J. Polym. Sci. Pol. Phys. 2010;48:1685–1718. [Google Scholar]
- Groombridge A.S., Palma A., Parker R.M., Abell C., Scherman O.A. Aqueous interfacial gels assembled from small molecule supramolecular polymers. Chem. Sci. 2017;8:1350–1355. doi: 10.1039/c6sc04103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M.P., Sato K., Palmer L.C., Stupp S.I. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Rev. 2017;50:2440–2448. doi: 10.1021/acs.accounts.7b00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.-H., Ma N.-N., Li F., Fang Y., Liu Y., Zhao L.-L., Qiao S.-P., Li X.-M., Jiang X.-J., Li T.-Z. Cucurbit[8]uril–based giant supramolecular vesicles: highly stable, versatile carriers for photoresponsive and targeted drug delivery. ACS Appl. Mater. Interfaces. 2018;10:4603–4613. doi: 10.1021/acsami.8b00297. [DOI] [PubMed] [Google Scholar]
- Huang F., Gibson H.W. Formation of a supramolecular hyperbranched polymer from self–organization of an AB2 monomer containing a crown ether and two paraquat moieties. J. Am. Chem. Soc. 2004;126:14738–14739. doi: 10.1021/ja044830e. [DOI] [PubMed] [Google Scholar]
- Ji X.-F., Wu R.-T., Long L.-L., Ke X.-S., Guo C.-X., Ghang Y.-J., Lynch V.M., Huang F.-H., Sessler J.L. Encoding, reading, and transforming information using multifluorescent supramolecular polymeric hydrogels. Adv. Mater. 2018;30:1705480. doi: 10.1002/adma.201705480. [DOI] [PubMed] [Google Scholar]
- Jones C.D., Steed J.W. Gels with sense: supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016;45:6546–6596. doi: 10.1039/c6cs00435k. [DOI] [PubMed] [Google Scholar]
- Krieg E., Weissman H., Shirman E., Shimoni E., Rybtchinski B. A recyclable supramolecular membrane for size-selective separation of nanoparticles. Nat. Nanotechnol. 2011;6:141–146. doi: 10.1038/nnano.2010.274. [DOI] [PubMed] [Google Scholar]
- Krieg E., Bastings M.M.C., Besenius P., Rybtchinski B. Supramolecular polymers in aqueous media. Chem. Rev. 2016;116:2414–2477. doi: 10.1021/acs.chemrev.5b00369. [DOI] [PubMed] [Google Scholar]
- Liang B., Wang H., Shi X.-H., Shen B.-Y., He X., Ghazi Z.A., Khan N.A., Sin H., Khattak A.M., Li L.-S. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic–solvent nanofiltration. Nat. Chem. 2018;10:961–967. doi: 10.1038/s41557-018-0093-9. [DOI] [PubMed] [Google Scholar]
- Liang B., He X., Hou J.-J., Li L.-S., Tang Z.-Y. Membrane separation in organic liquid: technologies, achievements, and opportunities. Adv. Mater. 2018:e1806090. doi: 10.1002/adma.201806090. [DOI] [PubMed] [Google Scholar]
- Liu T.-T., Wang S.-D., Li Y.-R., Yan H.-X., Tian W. Triple noncovalent–interaction–containing supramolecular polymer vesicle chemosensors with dynamically tunable detection ranges. Chem. Eur. J. 2018;24:4239–4244. doi: 10.1002/chem.201705162. [DOI] [PubMed] [Google Scholar]
- Luo H.-X., Aboki J., Ji Y.-Y., Guo R.-L., Geise G.M. Water and salt transport properties of triptycene–containing sulfonated polysulfone materials for desalination membrane applications. ACS Appl. Mater. Interfaces. 2018;10:4102–4112. doi: 10.1021/acsami.7b17225. [DOI] [PubMed] [Google Scholar]
- Lutz J.F., Lehn J.M., Meijer E.W., Matyjaszewski K. From precision polymers to complex materials and systems. Nat. Rev. Mater. 2016;1:16024. [Google Scholar]
- Matsumoto M., Valentino L., Stiehl G.M., Balch H.B., Corcos A.R., Wang F., Ralph D.C., Marinas B.J., Dichtel W.R. Lewis–acid–catalyzed interfacial polymerization of covalent organic framework films. Chem. 2018;4:308–317. [Google Scholar]
- Pazos E., Novo P., Peinador C., Kaifer A.E., García M.D. Cucurbit[8]uril (CB[8]) –based supramolecular switches. Angew. Chem. Int. Ed. 2019;58:403–416. doi: 10.1002/anie.201806575. [DOI] [PubMed] [Google Scholar]
- Pfeffermann M., Dong R.-H., Graf R., Zajaczkowski W., Gorelik T., Pisula W., Narita A., Müllen K., Feng X.-L. Free–standing monolayer two–dimensional supramolecular organic framework with good internal order. J. Am. Chem. Soc. 2015;137:14525–14532. doi: 10.1021/jacs.5b09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D.-H., Wang Q.-C., Zhang Q.-W., Ma X., Tian H. Photoresponsive host–guest functional systems. Chem. Rev. 2015;115:7543–7588. doi: 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- Samanta S.K., Quigley J., Vinciguerra B., Briken V., Isaacs L. Cucurbit[7]uril enables multi–stimuli–responsive release from the self–assembled hydrophobic phase of a metal organic polyhedron. J. Am. Chem. Soc. 2017;139:9066–9074. doi: 10.1021/jacs.7b05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S., Kiren M.C., Jonkheijm P. Incorporating bacteria as a living component in supramolecular self–assembled monolayers through dynamic nanoscale interactions. ACS Nano. 2015;9:3579–3586. doi: 10.1021/acsnano.5b00694. [DOI] [PubMed] [Google Scholar]
- Stoffelen C., Voskuhl J., Jonkheijm P., Huskens J. Dual stimuli–responsive self–assembled supramolecular nanoparticles. Angew. Chem. Int. Ed. 2014;53:3400–3404. doi: 10.1002/anie.201310829. [DOI] [PubMed] [Google Scholar]
- Sun B., Kim Y., Wang Y.-Q., Wang H.-X., Kim J., Liu X., Lee M. Homochiral porous nanosheets for enantiomer sieving. Nat. Mater. 2018;17:599–604. doi: 10.1038/s41563-018-0107-4. [DOI] [PubMed] [Google Scholar]
- Tao W., Liu Y., Jiang B.-B., Yu S.-R., Huang W., Zhou Y.-F., Yan D.-Y. A linear–hyperbranched supramolecular amphiphile and its self–assembly into vesicles with great ductility. J. Am. Chem. Soc. 2012;134:762–764. doi: 10.1021/ja207924w. [DOI] [PubMed] [Google Scholar]
- Tao R., Zhang Q., Rao S.-J., Zheng X.-L., Li M., Qu D. Supramolecular gelator based on a [c2]daisy chain rotaxane: efficient gel-solution transition by ring-sliding motion. Sci. China Chem. 2019;62:245–250. [Google Scholar]
- Tian F., Jiao D.-Z., Biedermann F., Scherman O.A. Orthogonal switching of a single supramolecular complex. Nat. Commun. 2012;3:1207. doi: 10.1038/ncomms2198. [DOI] [PubMed] [Google Scholar]
- Tian J., Zhou T.-Y., Zhang S.-C., Aloni S., Altoe M.V., Xie S.-H., Wang H., Zhang D.-W., Zhao X., Liu Y. Three–dimensional periodic supramolecular organic framework ion sponge in water and microcrystals. Nat. Commun. 2014;5:5574. doi: 10.1038/ncomms6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Li X.-X., Wang J.-X. Supramolecular hyperbranched polymers. Chem. Commun. 2017;53:2531–2542. doi: 10.1039/c6cc09678f. [DOI] [PubMed] [Google Scholar]
- Voorhaar L., Hoogenboom R. Supramolecular polymer networks: hydrogels and bulk materials. Chem. Soc. Rev. 2016;45:4013–4031. doi: 10.1039/c6cs00130k. [DOI] [PubMed] [Google Scholar]
- Wang Y.-K., Yang Z.-S., Lv X.-Q., Yao R.-S., Wang F. Construction of supramolecular hyperbranched polymers via the “tweezering directed self–assembly” strategy. Chem. Commun. 2014;50:9477–9480. doi: 10.1039/c4cc03158j. [DOI] [PubMed] [Google Scholar]
- Wang W.-Z., Gao C., Zhang Q., Ye X.-H., Qu D.-H. Supramolecular helical nanofibers formed by achiral monomers and their reversible sol–gel transition. Chem. Asian J. 2017;12:410–414. doi: 10.1002/asia.201601733. [DOI] [PubMed] [Google Scholar]
- Wang X.-Y., Chen L.-J., Chong S.Y., Little M.A., Wu Y.-Z., Zhu W.-H., Clowes R., Yan Y., Zwijnenburg M.A., Sprick R.S. Sulfone–containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018;10:1180–1189. doi: 10.1038/s41557-018-0141-5. [DOI] [PubMed] [Google Scholar]
- Wang Q., Tian L., Xu J.-Z., Xia B., Li J., Lu F., Lu X.-M., Wang W.-J., Huang W., Fan Q.-L. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem. Commun. 2018;54:10328–10331. doi: 10.1039/c8cc05560b. [DOI] [PubMed] [Google Scholar]
- Wang P.-F., Wang M., Liu F., Ding S.-Y., Wang X., Du G.-H., Liu J., Apel P., Kluth P., Trautmann C. Ultrafast ion sieving using nanoporous polymeric membranes. Nat. Commun. 2018;9:569. doi: 10.1038/s41467-018-02941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Zeng Z.-H., Xu P., Li L.-S., Zeng G.-M., Xiao R., Tang Z.-Y., Huang D.-L., Tang L., Lai C. Recent progress in covalent organic framework thin films: fabrications, applications and perspectives. Chem. Soc. Rev. 2019;48:488–516. doi: 10.1039/c8cs00376a. [DOI] [PubMed] [Google Scholar]
- Xiao P., Bu F., Zhao R., Aboud M.F.A., Shakir I., Xu Y. Sub-5 nm ultrasmall metal–organic framework nanocrystals for highly efficient electrochemical energy storage. ACS Nano. 2018;12:3947–3953. doi: 10.1021/acsnano.8b01488. [DOI] [PubMed] [Google Scholar]
- Xing P.-Y., Phua S.Z.F., Wei X., Zhao Y.-L. Programmable multicomponent self–assembly based on aromatic amino acids. Adv. Mater. 2018;30:e1805175. doi: 10.1002/adma.201805175. [DOI] [PubMed] [Google Scholar]
- Yagai S., Kitamoto Y., Datta S., Adhikari B. Supramolecular polymers capable of controlling their topology. Acc. Chem. Res. 2019;52:1325–1335. doi: 10.1021/acs.accounts.8b00660. [DOI] [PubMed] [Google Scholar]
- Yang L.-L., Tan X.-X., Wang Z.-Q., Zhang X. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem. Rev. 2015;115:7196–7239. doi: 10.1021/cr500633b. [DOI] [PubMed] [Google Scholar]
- Yu G.-C., Jie K.-C., Huang F.-H. Supramolecular amphiphiles based on host–guest molecular recognition motifs. Chem. Rev. 2015;115:7240–7303. doi: 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- Yu Q.-L., Zhang Y.-M., Liu Y.-H., Xu X., Liu Y. Magnetism and photo dual–controlled supramolecular assembly for suppression of tumor invasion and metastasis. Sci. Adv. 2018;4:eaat2297. doi: 10.1126/sciadv.aat2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Wang S., Zhou D., Zhang H., Li B., Wu L. Flexible single–layer ionic organic–inorganic frameworks towards precise nano–size separation. Nat. Commun. 2016;7:10742. doi: 10.1038/ncomms10742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.-D., Tian J., Hanifi D., Zhang Y., Sue A.C., Zhou T.-Y., Zhang L., Zhao X., Liu Y., Li Z.-T. Toward a single–layer two–dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 2013;135:17913–17918. doi: 10.1021/ja4086935. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Rao S.-J., Xie T., Li X., Xu T., Li D.-W., Qu D.-H., Long Y.-T., Tian H. Muscle-like artificial molecular actuators for nanoparticles. Chem. 2018;4:2670–2684. [Google Scholar]
- Zhang Y.-M., Zhang N.-Y., Xiao K., Yu Q., Liu Y. Photo–controlled reversible microtubule assembly mediated by cyclodextrin derivative. Angew. Chem. Int. Ed. 2018;57:8649–8653. doi: 10.1002/anie.201804620. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shi C.-Y., Qu D.-H., Long Y.-T., Feringa B.L., Tian H. Exploring a naturally tailored small molecule for stretchable, self–healing, and adhesive supramolecular polymers. Sci. Adv. 2018;4:eaat8192. doi: 10.1126/sciadv.aat8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.-F., Liu N., Zhang Q.-D., Qu R.-X., Liu Y.-N., Li X.-Y., Wei Y., Feng L., Jiang J. Thermo–driven controllable emulsion separation by a polymer–decorated membrane with switchable wettability. Angew. Chem. Int. Ed. 2018;57:5740–5745. doi: 10.1002/anie.201801736. [DOI] [PubMed] [Google Scholar]
- Zhou Y.-F., Huang W., Liu J.-Y., Zhu X.-Y., Yan D.-Y. Self–assembly of hyperbranched polymers and its biomedical applications. Adv. Mater. 2010;22:4567–4590. doi: 10.1002/adma.201000369. [DOI] [PubMed] [Google Scholar]
- Zhu J.-Y., Hou J.-W., Uliana A., Zhang Y.-T., Tian M.-M., van der Bruggen B. The rapid emergence of two–dimensional nanomaterials for high–performance separation membranes. J. Mater. Chem. A. 2018;6:3773–3792. [Google Scholar]
- Zhuang X.-D., Mai Y.-Y., Wu D.-Q., Zhang F., Feng X.-L. Two–Dimensional soft nanomaterials: a fascinating world of materials. Adv. Mater. 2015;27:403–427. doi: 10.1002/adma.201401857. [DOI] [PubMed] [Google Scholar]
- Zou X.-Q., Zhu G.-S. Microporous organic materials for membrane–based gas separation. Adv. Mater. 2018;30:1700750. doi: 10.1002/adma.201700750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.