Abstract
Diabetic retinopathy (DR) is the most common cause for preventable blindness in India. The onset of micro and macrovascular complications in T2DM is multifactorial and difficult to predict. The status of micronutrients, several inflammatory cytokines, elevated triacylglycerols, oxidative stress etc., are being studied extensively. Hypomagnesemia plays a pivotal role in worsening of insulin resistance. Although, Vascular Endothelial Growth Factor-A (VEGF-A) and Endothelin-1 (ET-1) are known to be elevated in DR, yet few reports cite their role, especially in Indian population. In this study, we included thirty subjects with T2DM in each of the three groups namely, T2DM cases without retinopathy, Non Proliferative DR (NPDR) and Proliferative DR (PDR) retinopathy. The glycemic status, circulating plasma VEGF-A, ET-1 levels, serum magnesium and lipids were estimated and compared among the groups. An ROC was drawn to evaluate VEGF-A, ET-1 and serum magnesium levels as the predictive markers for PDR. On comparison VEGF-A, ET-1 and serum magnesium levels showed a significant difference among the three groups. PDR cases had higher circulating levels of VEGF-A, ET-1 and low serum magnesium levels when compared to others. ROC for VEGF-A and ET-1 showed an optimum cut-off of 1521 ng/ml (AUC 0.975) and 16 pg/ml (AUC 0.96) respectively. A negative ROC was drawn to check the lower cut-off limit for serum magnesium; we documented an optimum cut off of 1.7 mg/dl (AUC 0.837). ET-1, VEGF-A and serum Magnesium levels are significantly altered in PDR and can be used as the predictive markers of PDR.
Keywords: Diabetic retinopathy, VEGF, Endothelin-1, Hypomagnesemia
Introduction
Diabetic retinopathy (DR) is the most common cause for preventable blindness in India [1]. Several factors namely ethnicity, family history, modern dietary habits and sedentary lifestyle contribute to the pathogenesis of T2DM. The onset of micro and macrovascular complications in T2DM is multifactorial in nature and is difficult to predict. Besides poor glycemic control, several inflammatory cytokines, elevated triacylglycerols, oxidative stress etc., are being studied extensively in complications of T2DM [2]. However, it still remains unclear as to why only certain diabetic cases are predisposed to retinopathy ahead of other complications. Poor nutrition combined with an excessive loss of micronutrients in urine attributed to polyuria also contributes to various micro and macrovascular complications in subjects with T2DM [3]. Hypomagnesemia plays a pivotal role in worsening of insulin resistance [4]. It exacerbates the existing insulin resistance and worsens oxidative stress [5]; which in turn is known to accelerate the onset of various micro and macrovascular complications [6, 7]. In recent years, studies have related triacylglycerols (TAGs), magnesium and atherogenic dyslipidaemia in the various complications of T2DM [8]. Although the association of serum magnesium with oxidative stress and insulin resistance is documented, the lower cut-off limit which is associated with the risk of developing these complications was not documented. Several inflammatory cytokines, including Endothelin-1 (ET-1), Vascular Endothelial Growth Factor-A (VEGF-A) are known to be associated with various complications of T2DM [9, 10]. Moreover, very few reports are available from South India linking these parameters to the different grades of retinopathy. With this background, the current study was planned to evaluate the association of VEGF-A, ET-1, serum lipids and magnesium levels with glycemic control. Also we aimed to document the cut-off limits for VEGF-A, ET-1 and serum magnesium to predict DR.
Subjects and Methods
This study was conducted in the Department of Biochemistry in collaboration with Department of Opthalmology, Mahatma Gandhi Medical College and Research Institute, SBV, Pillaiyarkuppam, Puducherry. The project was initiated following the permission from institutional research review board and institute human ethics committee (IHEC). This was a study comprised of three groups of T2DM cases, thirty subjects in each. All the subjects were recruited after informed written consent. Group A-T2DM cases without any evidence of retinopathy, Group B: T2DM cases with non-proliferative diabetic retinopathy (NPDR) and Group C with T2DM cases with proliferative type of diabetic retinopathy (PDR).
Exclusion Criteria
Patients with other specific types of diabetes mellitus, known alcoholics, Individuals suffering from any other endocrine disorders were excluded. All the relevant details pertaining to clinical, previous drug/treatment and personal history were documented. A detailed ophthalmoscopic examination was undertaken. 3 ml of blood sample (venous) was drawn in the fasting state for analyzing plasma Glucose (GOD-POD method), HbA1c (ion-exchange HPLC), VEGF-A and ET-1 (by ELISA), Serum magnesium (Calmagite indicator method).
Grading of Diabetic Retinopathy
The diabetic cases who met our inclusion criteria were subjected to complete ocular examination. Intraocular pressure was noted and dilated fundus examination was carried out using both direct and indirect ophthalmoscopes (Heine, West Germany). The Early Treatment of Diabetic Retinopathy Study (ETDRS criteria) was used to grade the diabetic retinopathy. The findings of the fundus examination were also confirmed by Fluorescein Angiography.
Statistical Analysis
All data are expressed as mean ± standard deviation. One way ANOVA was used to compare the data among the three groups. STATA software application (version 13.0: StataCorp LP, College Station, TX, U.S.A.) was used for analyzing the data. A p value < 0.05 was considered as statistically significant. Receiver operating curves (ROC) were drawn to elicit the optimum sensitivity, specificity and cut-off values of VEGF-A, ET-1 and magnesium levels for predicting PDR.
Observations and Results
VEGF-A, ET-1 and serum magnesium levels showed a significant difference among the three groups. Group C had higher circulating levels of VEGF-A, ET-1 and low serum magnesium levels when compared to group A and B (Table 1). ROC for VEGF-A showed a showed an optimum cut-off of 1521 ng/ml with 92% sensitivity and 97% specificity (AUC 0.975). The ROC for ET-1 showed an optimum cut-off of 16 pg/ml with 92% sensitivity and 94% specificity (AUC 0.96). A negative ROC was drawn to check the lower cut-off limit for serum magnesium; we documented an optimum cut off of 1.7 mg/dl with sensitivity 92.8% and specificity 77% with a (AUC 0.837) (Table 2).
Table 1.
Comparison of variables among the groups, no retinopathy (group A), non-proliferative (group B) and proliferative (group C) diabetic retinopathy
| Variables | Group A (mean ± SD) | Group B (mean ± SD) | Group C (mean ± SD) | ‘p’ value |
|---|---|---|---|---|
| Age in years | 54 ± 8.5 | 55.2 ± 8.3 | 59 ± 7.2 | 0.2 |
| ET-1 (pg/ml) | 11.5 ± 2.1 | 11.8 ± 3.2 | 21.36 ± 4.6* | 0 |
| VEGF-A (ng/ml) | 1102 ± 67.3 | 1107.9 ± 391.9 | 1973 ± 307.9* | 0 |
| FPG (mg/dl) | 173.5 ± 50 | 174.9 ± 33.8 | 174 ± 28 | 0.9 |
| HbA1c % | 8.8 ± 1.7 | 8.6 ± 1.7 | 8.8 ± 2.1 | 0.8 |
| TC (mg/dl) | 169 ± 25 | 176.9 ± 29 | 185 ± 30 | 0.2 |
| TAG (mg/dl) | 134.2 ± 61.4 | 154.9 ± 67.8 | 167.5 ± 56.1 | 0.32 |
| HDL (mg/dl) | 36 ± 6.8 | 32.2 ± 8.6 | 39.8 ± 7.3* | 0.02 |
| LDL (mg/dl) | 106 ± 22.6 | 125.5 ± 39 | 112.2 ± 28 | 0.17 |
| VLDL (mg/dl) | 26.8 ± 12.3 | 31 ± 13.6 | 33.5 ± 11.1 | 0.3 |
| Magnesium (mg/dl) | 2.01 ± 0.4 | 2 ± 0.4 | 1.4 ± 6.3 | 0* |
The comparison was by one-way ANOVA with TUKEY’s HSD as a POST HOC test. A p value of < 0.05 was considered to statistically significant
NPDR diabetic cases with non-proliferative retinopathy, PDR diabetic cases with proliferative retinopathy, ET-1 endothelien-1, VEGF-A vascular endothelial growth factor, FPG fasting plasma glucose, HbA1c glycated haemoglobin, TC total cholesterol, TAG triacylglycerol, HDL high density lipoprotein, LDL low density lipoprotein, VLDL very low density lipoprotein
*p value < 0.05 considered statistically significant
Table 2.
ROC optimum cut-off values, sensitivity, specificity, area under the curve for VEGF-A, ET-1 and serum magnesium
| Variables | Optimum cut-off | Sensitivity | Specificity | AUC | SEM | 95% CI |
|---|---|---|---|---|---|---|
| VEGF-A (ng/ml) | 1521 | 92.86 | 97.14 | 0.975 | 0.03 | 0.86–0.99 |
| ET-1 (pg/ml) | 16 | 92.86 | 94.29 | 0.96 | 0.02 | 0.89–0.99 |
| Serum magnesium (mg/dl) | − 1.7 | 92.86 | 77.14 | 0.837 | 0.06 | 0.70–0.92 |
Discussion
The study was planned aiming to compare the levels of FPG, HbA1c, serum magnesium, circulating levels of VEGF-A, ET-1 and serum lipids among T2DM cases with and without retinopathy, also to evaluate these markers to predict DR. Although, we did not find significant difference in the concentration of these parameters between group 1 (no retinopathy) and group 2 (NPDR), we found statistically significant difference among groups 1, 2 with group 3 (PDR).
The pathogenesis of long term complications seen in T2DM (retinopathy, nephropathy, and neuropathy) is attributed to disturbed microvascular endothelial function [10]. ET-1, a potent vasoconstrictor peptide is found associated with both micro and macrovascular complications of T2DM. Studies have shown that ET-1 in circulation occurs well before the onset of insulin resistance (10). Our results show that ET-1 at cut-off value 16 pg/ml was 92.86% sensitive and 94.29% specific in predicting PDR (AUC 0.960).
Retina being the metabolically active and most sensitive tissue to hypoxia, the up regulation of VEGF-A is seen at the early phases of retinopathy [11]. Although there are conflicting reports pertaining to the correlation of circulating VEGF-A with the extent of Micro vascular changes in diabetes mellitus [12, 13], this study shows circulating VEGF is not seen significantly elevated in early DR, but seen elevated in the later stages. The ROC curve showed the cut-off for VEGF-A was 1521 ng/ml with 92.86% sensitivity and 97.14% specificity (AUC 0.975). However, the studies on a larger population could reveal the significance of circulating VEGF-A in early DR. Although conflicts do exist as related to circulating ET-1 as the predictor of DR, ET-1 emerged as the better predictor with VEGF-A in our study. Zhu and Shi [14] documented that area under the curve for ET-1 was 0.857 for predicting PDR much better than VEGF A (0.293). Zanatta et al. [15] showed in a study that ET-1 was associated with urinary albumin excretion after controlling for age, gender, body mass index, blood pressure, HbA1c and total cholesterol. On contrary Vingolo et al. [10] documented vitreous VEGF-A levels were significantly higher in PDR but not ET-1, also quoted its role is limited to induce hypoxic state and promote angiogenesis in the early phases of pathogenesis. Further, studies could focus on comparing vitreous levels with circulating levels of ET-1 with a larger sample size in early stages of DR. Further, the molecular mechanisms by which ET-1 acts needs to be studied in detail (Fig. 1).
Fig. 1.
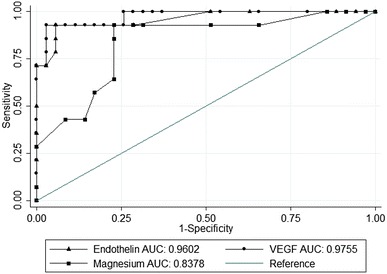
ROC curve for circulating ET-1, VEGF-A levels and serum magnesium status for predicting PDR
Wiwanitkit V used new gene ontology technology to predict the molecular function of ET-1 and Protein kinase C (PKC) in an episode of co-expression, with the use of the GoFigure server, According to this study, different pathways can be derived from ET-1 and PKC; however, ET-1-PKC produces the same pathway as PKC [16]. Elevated ET-1 levels were found to be associated with intercellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1) [4], TNF-alpha, IL-6, vWF, sE-selectin [17], hsCRP levels and oxidative stress [18]. ET-1 was found to be associated with abnormal retinal hemodynamics too [19], but only in the later stages of DR particularly in T2DM patients with a BMI ≥ 30 kg/m. The current study also proved that there was significant elevation of ET-1 and VEGF-A only in the PDR stage but not taking BMI and treatment history into account. Further studies with a higher sample size and also based on waist circumference/waist hip ratio would provide a detailed and more objective insight into the role of central obesity as linked to insulin resistance mediated proliferative diabetic retinopathy. There was no significant difference noted with respect to the glycemic status and lipid profile among NPDR and PDR. The obvious reason would be that, the study did not take the duration of T2DM, treatment, and also the intake of statins into account.
Association of hypomagnesaemia with oxidative stress, hypoxia and poor glycemic index is an established fact already [20–22]. In addition, in this study we have drawn a negative ROC curve to see the minimum cut off level of serum magnesium, below which we can predict.
DR. Serum magnesium below 1.7 mg/dl with (AUC = 0. 837), was proved to be 92.86% sensitive and 77.14% specific. Documentation and correction of hypomagnesaemia and most importantly the strict monitoring of glycemic status could be beneficial in preventing the onset of DR.
Supplementation of magnesium and antioxidants especially in T2DM subjects with co-morbid factors like BMI > 30, hypertension and CAD could be useful. Cardinal points that have emerged from our study
ET-1, VEGF-A and serum Magnesium are predictive markers of PDR.
Since earlier studies have shown that ET-1 levels emerge well before the onset of IR, it is advisable as depicted by our study to include VEGF-A and Magnesium that would enhance objectivity and predictable nature of PDR.
Nutritional supplementation of Magnesium in association with other micronutrients, viz Selenium and Zinc would ameliorate the microvascular complications. Though we did not evaluate the status of selenium and zinc
Limitations of the Study
Small sample size was obvious limitation of this study. The anthropometric parameters and other minerals which are known to help in bringing down the insulin resistance were not considered.
Conclusion
ET-1, VEGF-A and serum Magnesium are significantly altered in PDR and could be used as the predictive markers of PDR.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Khaled AA, Sekaran M, Ikram SI. Type 2 diabetes and vascular complications: a pathophysiologic view. Biomed Res. 2010;21(2):147–155. [Google Scholar]
- 3.Sales CH, Pedrosa LFC. Magnesium and diabetes mellitus: their relation. Clin Nutr. 2006;25:554–562. doi: 10.1016/j.clnu.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Ruy LR, Walter CW, Eric BR, Simin L, Meir JS, Joann EM, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134–140. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 5.Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med. 1996;156(11):1143–1148. doi: 10.1001/archinte.1996.00440100029005. [DOI] [PubMed] [Google Scholar]
- 6.Nasri H, Baradaran HR. Lipids in association with serum magnesium in diabetes mellitus patients. BratislavskeLekarskeListy. 2008;109(7):302–306. [PubMed] [Google Scholar]
- 7.Jonas J, Nangia V, Khare A. Prevalence and associated factors of diabetic retinopathy in rural central India. Diabetes Care. 2013;36:e69. doi: 10.2337/dc12-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan AR, Niranjan G, Velu VK, Parmar P, Anish A. Status of serum magnesium in type 2 diabetes mellitus with particular reference to serum triacylglycerol levels. Diabetes Metab Syndr. 2012;6(4):187–189. doi: 10.1016/j.dsx.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Mcauley AK, Sanfilippo P, Hewitt A, Liang H, Lamoureux EL, Wang J, et al. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Its Compl. 2014;28(3):419–425. doi: 10.1016/j.jdiacomp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Vingolo EM, Fragiotta S, Mafrici M, Cutini A, Marinelli C, Concistrè A, et al. Vitreous and plasma changes of endothelin-1, adrenomedullin and vascular endothelium growth factor in patients with proliferative diabetic retinopathy. Eur Rev Med Pharmacol Sci. 2017;21(4):662–668. [PubMed] [Google Scholar]
- 11.Li T, Hu J, Gao F, Du X, Chen Y, Wu Q. Transcription factors regulate GPR91-mediated expression of VEGF in hypoxia-induced retinopathy. Sci Rep. 2017;7:45807. doi: 10.1038/srep45807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh K, Sandler S, Espes D. The increased circulating plasma levels of vascular endothelial growth factor in patients with type 1 diabetes do not correlate to metabolic control. J Diabetes Res. 2017;2017:6192896. doi: 10.1155/2017/6192896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalini M, Raghavulu BV, Annapurna A, Avinash P, Chandi V, Swathi N, et al. Correlation of various serum biomarkers with the severity of diabetic retinopathy. Diabetes Metab Syndr. 2017;11:451–454. doi: 10.1016/j.dsx.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Shi C. Changes of plasma endothelin-1 and vascular endothelial growth factor in diabetic retinopathy and the clinical application value thereof. Zhonghua Yi Xue Za Zhi. 2007;87(40):2837–2839. [PubMed] [Google Scholar]
- 15.Zanatta CM, Gerchman F, Burttet L, Nabinger G, Jacques-Silva MC, Canani LH, et al. Endothelin-1 levels and albuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;80(2):299–304. doi: 10.1016/j.diabres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Wiwanitkit V. Endothelin-1 and protein kinase C co-expression in the pathogenesis of diabetic retinopathy. J Diabetes Complications. 2007;21(6):359–362. doi: 10.1016/j.jdiacomp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Adamiec-Mroczek J, Oficjalska-Młyńczak J, Misiuk-Hojło M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: analysis of vitreous samples. Cytokine. 2010;49(3):269–274. doi: 10.1016/j.cyto.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Sasongko MB, Wong TY, Jenkins AJ, Nguyen TT, Shaw JE, Wang JJ. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet Med J Br Diabet Assoc. 2015;32(5):686–691. doi: 10.1111/dme.12640. [DOI] [PubMed] [Google Scholar]
- 19.Uğurlu N, Gerceker S, Yülek F, Ugurlu B, Sarı C, Baran P, et al. The levels of the circulating cellular adhesion molecules ICAM-1, VCAM-1 and endothelin-1 and the flow-mediated vasodilatation values in patients with type 1 diabetes mellitus with early-stage diabetic retinopathy. Intern Med Tokyo Jpn. 2013;52(19):2173–2178. doi: 10.2169/internalmedicine.52.8572. [DOI] [PubMed] [Google Scholar]
- 20.Hamdan HZ, Nasser NM, Adam AM, Saleem MA, Elamin MI. Serum magnesium, iron and ferritin levels in patients with diabetic retinopathy attending Makkah Eye Complex, Khartoum, Sudan. Biol Trace Elem Res. 2015;165(1):30–34. doi: 10.1007/s12011-015-0236-4. [DOI] [PubMed] [Google Scholar]
- 21.Niranjan G, Mohanavalli V, Srinivasan AR, Ramesh R. Serum lipid peroxides and magnesium levels following three months of treatment with pioglitazone in patients with type-2 diabetes mellitus. Diabetes Metab Syndr. 2013;7(1):35–37. doi: 10.1016/j.dsx.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Niranjan G, Anitha D, Srinivasan AR, Velu VK, Venkatesh C, Babu MS, et al. Association of inflammatory sialoproteins, lipid peroxides and serum magnesium levels with cardiometabolic risk factors in obese children of South Indian population. Int J Biomed Sci. 2014;10(2):118–123. [PMC free article] [PubMed] [Google Scholar]


