Abstract
We report a case of bulky cardiac metastasis of intracranial solitary fibrous tumor/hemangiopericytoma (SFT/HPC). A 72-year-old woman developed a chief complaint of chest pain. Contrast-enhanced computed tomography revealed multiple enhanced masses in the heart, retroperitoneum, and femur. Initially, multiple metastases of cardiac primary angiosarcoma were suspected because the cardiac mass was the largest. However, it was diagnosed as SFT/HPC on the basis of biopsy and immunostaining for the retroperitoneal lesion. She had a history of resected brain tumor surgery for a meningioma 11 years earlier, and pathological reconfirmation revealed this was not a meningioma but rather a SFT/HPC. Thus, we found that the enhanced masses were extracranial metastases of an intracranial primary SFT/HPC. She died approximately 3 years after the onset of chest pain. Autopsy confirmed metastasis in the retroperitoneum, liver, lung, mesentery, skeletal muscle, and bone in addition to the heart. SFT/HPC has been reported to easily recur locally and to show systemic metastasis over the long term. Given that SFT/HPC has been recognized as a subtype of meningioma, the differential diagnosis for patients with a history of intracranial tumors, such as meningioma, should include SFT/HPC.
Keywords: Solitary fibrous tumor, Hemangiopericytoma, Meningioma, STAT6, Cardiac metastasis, Delayed metastasis
Introduction
Solitary fibrous tumor/hemangiopericytoma (SFT/HPC) is a rare tumor arising from various organs, such as the soft tissue, bone, dura, and pleura. Intracranial SFT/HPC recurs locally and has a high rate of metastasis even very long after the initial treatment. Thus, the past history of SFT/HPC may not be recognized. We experienced a case with multiple well-enhanced masses in the trunk with the largest in the heart and initially suspected a primary cardiac angiosarcoma. Eventually, the tumor was biopsy-proven to be an extracranial metastasis of a primary intracranial SFT/HPC that occurred a long time after the previous resection. We report imaging findings of this instructive case.
Case report
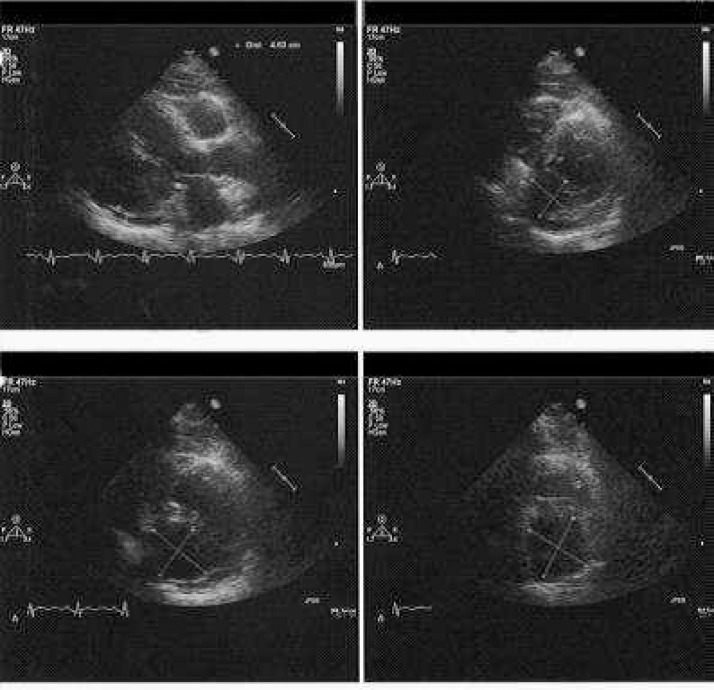
A 72-year-old female presented with sudden chest pain to her primary care physician. Echocardiography revealed pericardial effusion and a cardiac mass. Contrast-enhanced computed tomography (CT) showed a large cardiac mass in the left ventricular wall and multiple tumors in the retroperitoneum. The patient was subsequently referred to the general hospital and close examinations were performed. She had history of multiple intracranial tumor resections. The first was resection of an occipital brain tumor diagnosed as a meningioma 30 years earlier. The second was for local recurrence 11 years earlier. She had received prednisolone for autoimmune hepatitis, and her medical condition with respect to hepatitis was stable. There were no abnormal findings on routine blood and urine tests. Electrocardiogram showed lower wall infarction and complete right bundle block. Echocardiography revealed a 57 × 46-mm tumor with an ill-defined margin in the lower wall of the left ventricle, her ejection fraction was normal at 78%, and no other functional impairment was noted (Fig. 1). Chest pain and pericardial effusion were suspected to be caused by tumor constriction or invasion causing local myocardial infarction.
Fig. 1.
Cardiac ultrasound and echocardiogram. Cardiac ultrasound showing a hypoechoic mass (57 × 46 mm in the axial view) in the lower wall of the left ventricle. An unclear margin suggests tumor invasion to the ventricular muscle. The ejection fraction is within normal limits (78%).
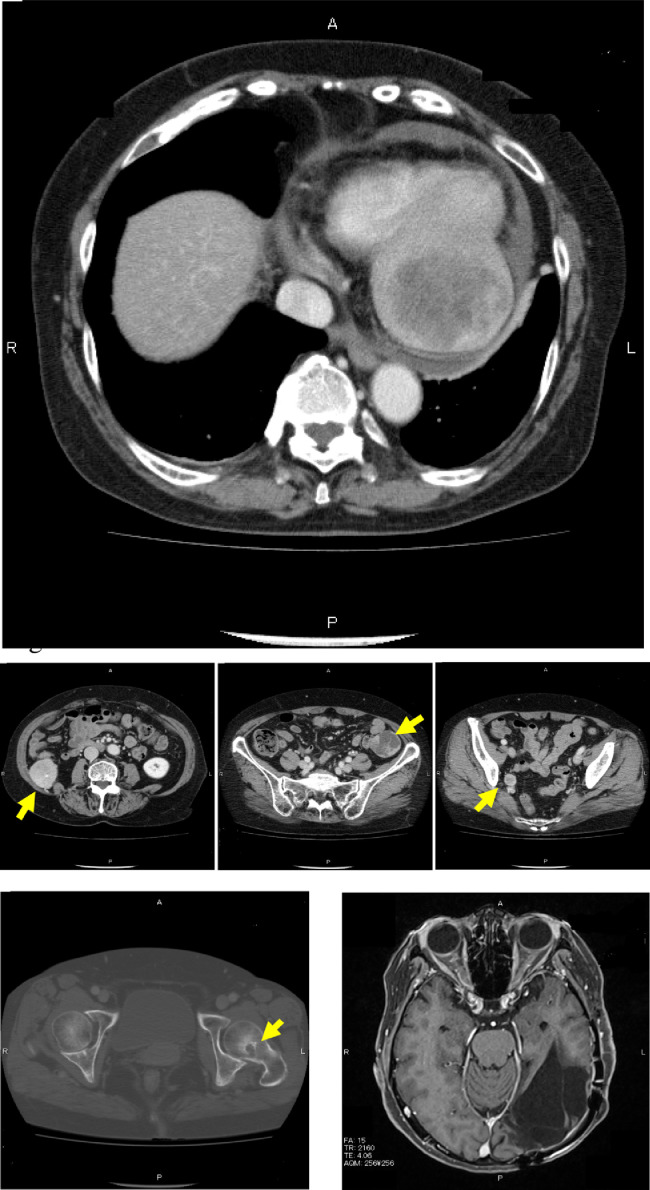
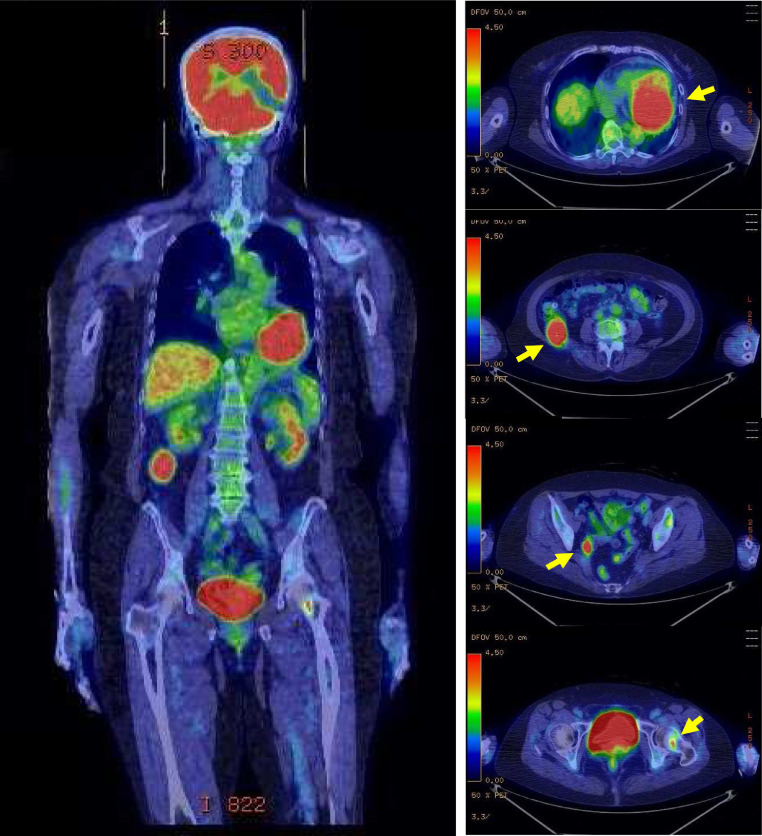
Contrast-enhanced CT showed a well-enhanced 68 × 72-mm mass occupying the left ventricle of the heart and an inhomogeneous density inside of the mass with a well-defined margin. There were multiple tumors up to 38 × 42 mm in diameter in the retroperitoneal space. All tumors were enhanced but had no enhanced area in their centers, which implied necrosis. An osteolytic lesion surrounding an osteoplastic area was observed in the femur neck (Fig. 2). 18F-fluorodeoxyglucose positron emission tomography for whole-body screening showed accumulation of FDG in the left ventricle of the heart and in the retroperitoneal space, and other FDG-avid lesions were noted in the pelvic cavity and femoral neck (Fig. 3)
Fig. 2.
Computed tomography (CT) and brain magnetic resonance imaging (MRI). Contrast-enhanced CT shows a heterogeneously enhanced cardiac mass measuring up to 68 × 72 mm in the left ventricular wall and pericardial effusion. Moreover, there are multiple disseminated lesions in the retroperitoneum and bone of the left femur (arrow). Contrast-enhanced MRI shows a cavity after resecting the brain tumor and no local recurrence.
Fig. 3.
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) shows multiple accumulations including the heart, right retroperitoneum, pelvic wall, and left femoral neck (arrow).
Given that the tumor in the left ventricle of the heart was the largest, it was considered to be the primary lesion. Initially, primary cardiac angiosarcoma with retroperitoneal and bone metastases was suspected. CT-guided biopsy was performed for the retroperitoneal tumor. The provisional diagnosis was a sarcoma of unknown classification. Since there was concern about increasing pericardial effusion and return of chest pain, chemotherapy with paclitaxel against angiosarcoma was started before definitive pathological diagnosis was confirmed. Histopathological evaluation revealed high-density spindle-shaped cells forming an irregular pattern. Immunohistochemical analysis showed tumor cells that were positive for vimentin and CD34 and negative for AE1/3, S-100, desmin, CD31, and EMA. On the basis of these results, a definitive diagnosis of SFT/HPC was made.
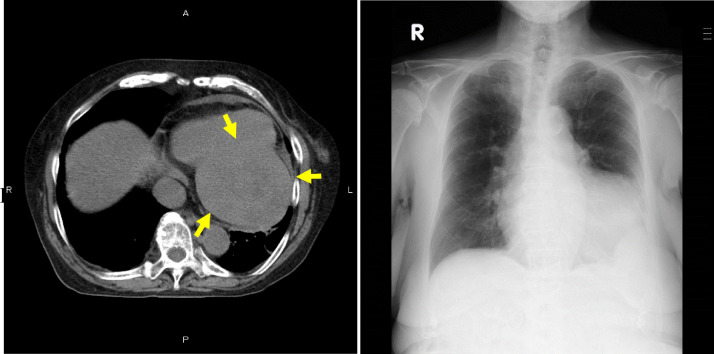
In this case, the patient had past history of meningioma resections. Based on the concern that the resected brain tumor was actually SFT/HPC, the specimen that had been resected 11 years earlier was reviewed. Histopathological and immunohistochemical findings of the previous tumor showed spindle cells positive for vimentin, CD34 (partial), p53 (mild), and SMA (subtle) and negative for EMA, findings that were compatible with SFT/HPC. Therefore, all of the lesions of the trunk were considered to be metastases from a primary intracranial SFT/HPC. Pazopanib was administered, and the lesions regressed temporary. However, the treatment was interrupted because of drug-induced hepatitis, and she opted for supportive care. Although the cardiac lesion gradually grew to >100 mm, she had no complaints related to the heart after the initial presentation (Fig. 4). Two years and 9 months after onset of chest pain, sudden bleeding from one of the retroperitoneal tumors occurred, and the systemic condition worsened. She died 2 months after the bleeding.
Fig. 4.
Final computed tomography (CT) before death. Two years and 6 months after onset of chest pain, noncontrast-enhanced CT shows the mass growing to 100 mm in the left ventricular wall (arrow). No pleural effusion. Chest radiograph demonstrating enlargement of the third heart shadow, but there are no symptoms of cardiac dysfunction, such as pleural effusion or increasing hilum shadow.
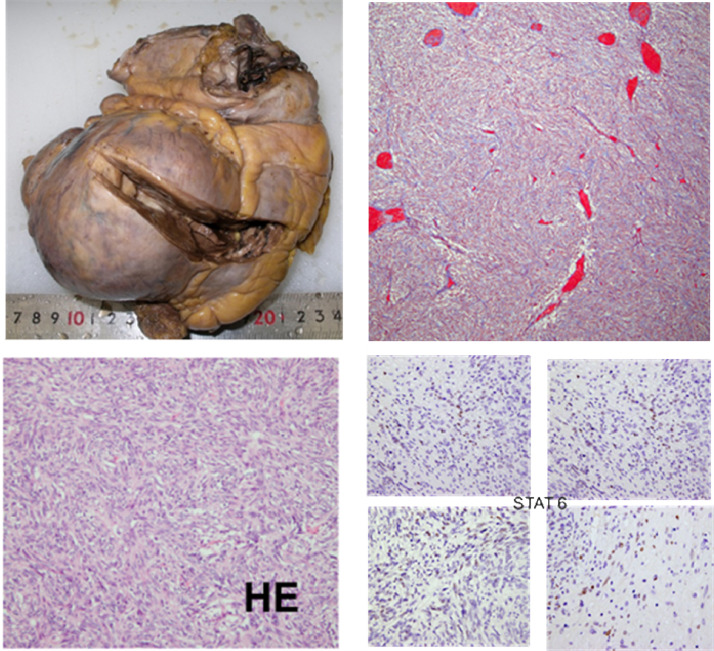
Autopsy was performed under informed consent from her family. There was no recurrence of the intracranial lesion that had been at the primary site. In addition to the 100-mm mass in the left ventricle of the heart, multiple nodules were found in the hepatic duct, iliac artery, omentum, sigmoid colon, femoral head, and skeletal muscle. Disseminated lesions were found in the lungs and mesentery. In specimens from autopsy, hematoxylin-eosin staining showed a spindle cell tumor of curt-wheel pattern, partly accompanied by stag-horn-like vasculature. Additionally, nuclear staining with a STAT6 antibody was confirmed. The diagnosis of SFT/HPC was reconfirmed (Fig. 5).
Fig. 5.
Pathological specimens from autopsy. A protruded tumor (100-mm diameter) is observed in the left ventricular wall, infiltrating the papillary muscle. Hematoxylin-eosin staining shows spindle cell tumor of curt-wheel pattern with stag-horn vasculature. STAT6 staining was positive for nuclei.
Discussion
The pathological concept of SFT/HPC has changed. HPC was first reported as a rare mesenchymal cell tumor derived from perivascular cells by Stout et al in 1942. In the central nervous system, SFT/HPC and meningioma share imaging findings, such as, dura-attaching, round shape, and avid enhancement, so it is often difficult to clearly distinguish between them by imaging modalities alone. HPC was classified as a subtype of meningioma, a so-called “hemangiopericytic meningioma” in the 1979 WHO classification. Subsequently, HPC was distinguished from meningioma as a “meningeal hemangiomericytoma” in the 1993 WHO classification according to advances in immunopathology [1]. It was reported that both SFT and HPC had a same driver mutation; fusing the NAB2 and STAT6 gene derived from inversions at 12q13 [2], [3], [4]. Therefore, the 2016 CNS WHO classification has created the combined terminology of SFT/HPC for the same disease [5]. Recently, the STAT6 antibody has been used to diagnose SFT/HPC because STAT6 staining in the nucleus is pathognomonic for SFT/HPC [6].
SFT/HPC can recur and metastasize a long time after initial treatment. Surgical resection is the primary treatment. The 5-year recurrence-free rates for patients with complete excision and incomplete excision were 72.7% and 20.8%, respectively [7]. Thus, complete removal is essential to cure, but the complete resection rate is only 46%-85% [1], [7], [8], [9], [10], [11], [12], [13]. According to reports after 2000, the recurrence rate of SFT/HPC in 5 years and 10 years has been relatively high (20%-51% and 54%-72%, respectively). Distant metastasis has also been found to occur in 11.6%-32.6% of patients [7], [9], [10], [11], [12], [15]. The mean time for distant metastases is 8.2-10.2 years [1], [7], [8], [11]. HPC/SFT can metastasize to the bone, liver, lung, lymph node, muscle, kidney, pancreas, skin, subcutaneous tissue, breast, adrenal grand, gallbladder, diaphragm, retroperitoneum, and heart; essentially to all organs of the body [14]. The 5-and 10-year survival rates have been reported to be 79%-96% and 56%-75%, respectively [1], [7], [9], [10], [11], [12], [13]. Even after recurrence, patients with HPC/SFT can survive for a relatively long time (0.7-11.8 years, median 4.2 years) [1].
Local recurrence or metastasis occurs at a relatively high rate, but the overall survival rate is not poor, which suggests that SFT/HPC tends to grow slowly and noninvasively. On the other hand, awareness is needed to the fact that recurrence and metastasis are possible long after initial treatment, even >10 years [1], [9], [12], [13]. For metastasis after a very long time, as in our case, it may be difficult to recognize the history of the initial intracranial tumor. SFT/HPC was previously considered to be a subtype of meningioma. Therefore, some SFT/HPC cases might have been diagnosed and treated as meningioma. Our case was found to be SFT/HPC by re-evaluation of the resected specimen of the head tumor 11 years earlier. Considering the patient's disease history, the tumor resected as a meningioma 30 years earlier might also have been SFT/HPC. In other words, it is possible that she had a very long history (>30 years) of SFT/HPC.
In the recent examinations, she was found to have a large mass in the cardiac wall, but cardiac function was normal. Moreover, although multiple metastatic tumors grew gradually, her performance status was good for >2 years. Each lesion was well-defined on CT even after the advanced stage, and the lesions were round and easily resectable on autopsy. Those findings were compatible with SFT/HPC, which has aggressive properties for metastasis, but each tumor showed an indolent tumor appearance. This case showed nonspecific findings, such as a large heterogeneously enhancing mass in the heart and multiple other tumors at first, but the findings were actually consistent with the typical natural history of SFT/HPC.
In conclusion, we experienced a case of bulky cardiac metastasis of an intracranial primary SFT/HPC. SFT/HPC can recur and metastasize even long after initial treatment, so the past history of the initial intracranial tumor may not be recognized. It should also be noted that SFT/HPC was previously regarded as a subtype of meningioma. Therefore, it is important to include SFT/HPC in the differential diagnosis, especially when multiple enhancing tumors with a round shape are detected in patients with a previous history of intracranial tumor.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Schiariti M., Goetz P., El-Maghraby H., Tailor J., Kitchen N. Hemangiopericytoma: long-term outcome revisited. J Neurosurg. 2011;114:747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D.R., Wu Y.M., Kalyana-Sundaram S., Cao X., Lonigro R.J., Sung Y.S. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmielecki J., Crago A.M., Rosenberg M., OʼConnor R., Walker S.R., Ambrogio L. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakada S., Minato H., Takegami T., Kurose N., Ikeda H., Kobayashi M. NAB2–STAT6 fusion gene analysis in two cases of meningeal solitary fibrous tumor/hemangiopericytoma with late distant metastases. Brain Tumor Pathol. 2015;32:268–274. doi: 10.1007/s10014-015-0220-x. [DOI] [PubMed] [Google Scholar]
- 5.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 6.Schweizer L., Koelsche C., Sahm F., Piro R.M., Capper D., Reuss D.E. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.H., Jung H.W., Kim Y.S., Kim C.J., Hwang S.K., Paek S.H. Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol. 2003;59:47–53. doi: 10.1016/s0090-3019(02)00917-5. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie B.L., Ebersold M.J., Scheithauer B.W., Shaw E.G. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. [PubMed] [Google Scholar]
- 9.Rutkowski M.J., Jian B.J., Bloch O., Chen C., Sughrue M.E., Tihan T. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118:1628–1636. doi: 10.1002/cncr.26411. [DOI] [PubMed] [Google Scholar]
- 10.Melone A.G., D'Elia A., Santoro F., Salvati M., Delfini R., Cantore G. Intracranial hemangiopericytoma–our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg. 2014;81:556–562. doi: 10.1016/j.wneu.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Patel A.R., Flores B.C., Ban V.S., Hatanpaa K.J., Mickey B.E., Barnett S.L. Intracranial hemangiopericytomas: recurrence, metastasis, and radiotherapy. J Neurol Surg B Skull Base. 2017;78:324–330. doi: 10.1055/s-0037-1599073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damodaran O., Robbins P., Knuckey N., Bynevelt M., Wong G., Lee G. Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. 2014;21:1310–1314. doi: 10.1016/j.jocn.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Rutkowski M.J., Sughrue M.E., Kane A.J., Aranda D., Mills S.A., Barani I.J. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010;113:333–339. doi: 10.3171/2010.3.JNS091882. [DOI] [PubMed] [Google Scholar]
- 14.Fountas K.N., Kapsalaki E., Kassam M., Feltes C.H., Dimopoulos V.G., Robinson J.S. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29:145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 15.Ghose A., Guha G., Kundu R., Tew J., Chaudhary R. CNS hemangiopericytoma: a systematic review of 523 patients. Am J Clin Oncol. 2017;40:223–227. doi: 10.1097/COC.0000000000000146. [DOI] [PubMed] [Google Scholar]