Abstract
Thymic epithelial tumors (TETs) belong to orphan oncology. The incidence of TETs is about 1.3–3.2 cases per million worldwide. Following pathology, evolution and prognosis are variable. The World Health Organization classification distinguishes thymomas and thymic carcinomas. TETs are composed of thymic epithelial tumoral cells and normal lymphocytes. The mean age at diagnosis is 50–60 years-old. There are no identified risk factors. TETs are frequently associated with paraneoplastic syndromes as myasthenia gravis. The complete R0 surgical resection is the most significant prognosis factor on survival. In 2010, the French National Institute of Cancer labeled the RYTHMIC network as a specific tumor board including thoracic surgeons, oncologist, and radiation therapist to define standard of care for the management of TETs. The aim of the review was to update knowledge to optimize the standard of care.
Keywords: thymic epithelial tumors, thymomas, thymic carcinomas, surgery, radiation, chemotherapy
Background
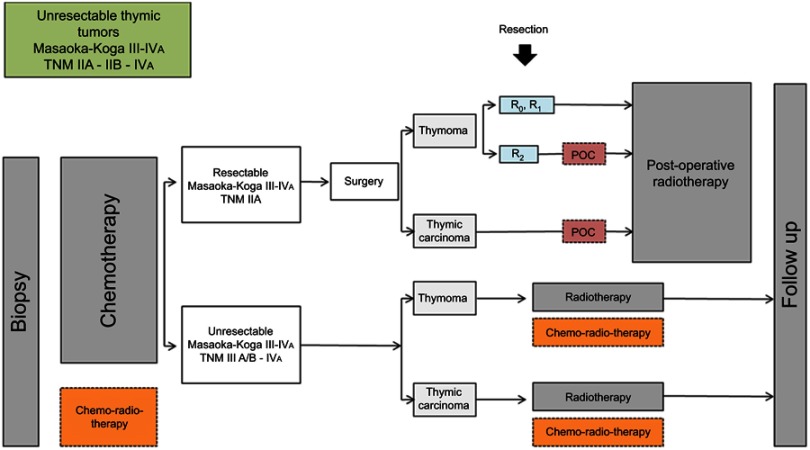
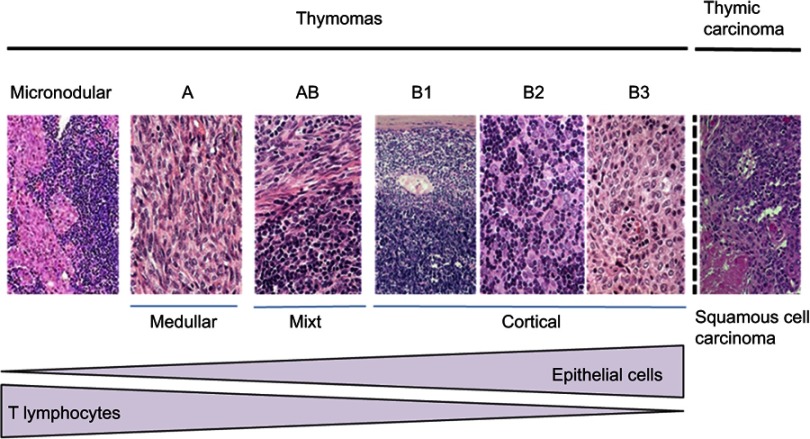
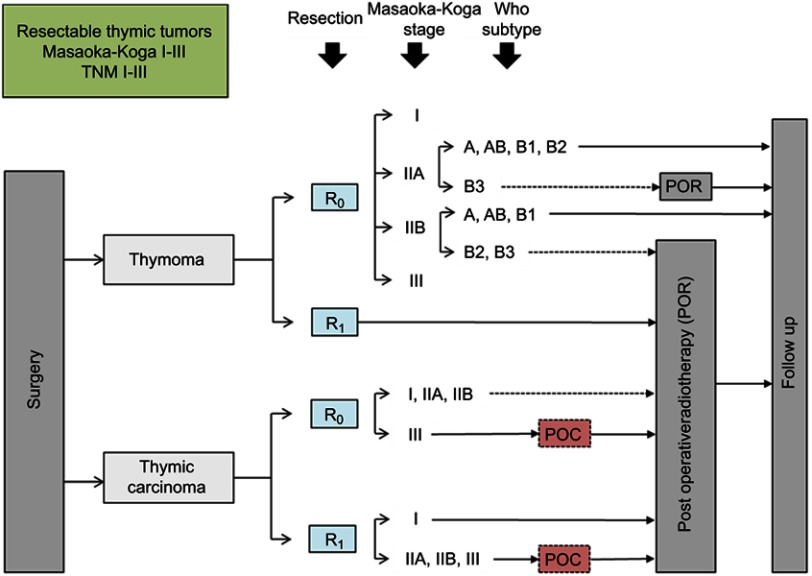
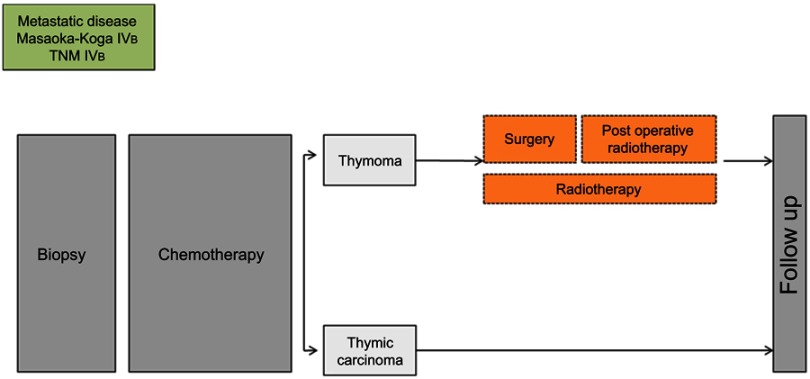
Thymic epithelial tumors (TETs) belong to orphan oncology. The incidence of TETs is about 1.3–3.2 cases per million worldwide.1,2 In France, 250–300 new cases are diagnosed each year.3 The current World Health Organization (WHO) classification distinguishes thymomas and thymic carcinomas (TC)4 (Figure 1). Thymomas are defined as A, AB, B (1–3) subtypes according to the morphology of tumoral thymic epithelial cells (TECs), the proportion of nontumoral lymphocytes (decreasing from B1 to B3) and similarities with the normal thymic architecture. TC are rarer (0.2–0.5 per million5) and defined by the destruction of normal thymic architecture by TECs presenting common characters with carcinomas arising from other organs: high degree of atypia, atypical mitoses, multinucleation, anisocytosis, and anisokaryosis. TC are often associated with severe outcomes and systemic involvement whereas thymomas mostly present with local and regional progression. The presence of these two clinical entities has a major impact on five years overall survival which ranges from less than 20% for stage IV TC to more than 95% for patients with thymomas and early stages.6,7 Complete, radical R0 surgery when feasible is the cornerstone of a multimodal therapy and has been shown as an independent prognosis factor of favorable outcome.8 Secondary to the rarity of the disease, the knowledge of thymic malignancies was primarily made by mean of numerous retrospective studies. However, since a decade, with the creation of the ITMIG (International Thymic Malignancies Interest Group), the JART (Japanese Association for Research on the Thymus), the European society of thoracic surgery (ESTS)9 thymic working group, the CHART (Chinese Alliance for Research in Thymomas), and the RYTHMIC (French thymic tumors and cancer network) substantial efforts have been made to optimize and standardize the care of thymic malignancies. Many classifications are useful to manage thymic malignancies; however, the Masaoka-Koga10 and TNM 8th edition are the most used (Tables 1–3). In France, since 2012; the RYTHMIC networks choose the use the two classifications to manage the care of thymic malignancies with the publication of a national guideline in 2016.3,11 The RYTHMIC network recommendations are summarized (Figures 2–4).
Table 2.
The IASLC/ITMIG thymic epithelial tumors staging
| Category | Definition | ||
|---|---|---|---|
| T | T1 | a | Encapsultated or not with or without extension into mediastinal fat |
| b | Extension into mediastinal pleura | ||
| T2 | Pericardium | ||
| T3 | Lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerve Hilar (extrapericardial) pulmonary vessels | ||
| T4 | Aorta, arch vessels, main pulmonary artery, myocardium, trachea, or oesophagus | ||
| N | N0 | No nodal involvrnent | |
| N1 | Anterior (perithymic nodes | ||
| N2 | Deep intra thoracic or cervical nodes | ||
| M | M0 | No metastatic pleural, pericardial or distant sites | |
| M1 | a | Separate pleural or pericardial nodule(s) | |
| b | Pulmonary intraparenchymal nodule or distant organ metastasis | ||
Figure 3.
RYTHMIC recommendation for initially unresectable thymic tumors from the RYTHMIC network, TNM IIIa–IIIb and IVa, Masaoka-Koga III–IVa.
Figure 1.
HES (Hematoxillin-Eosin-Safran) colorations of different subsets of thymic epithelial tumors, classified according to the World Health Organization classification.
Table 1.
Masaoka-Koga staging
| Masaoka-Koga staging | ||
|---|---|---|
| Stage I | Grossly and microscopiolly anopsulated | |
| Stage II | a | Microscopic transcapsular invasion |
| b | Macroscopic capsular invasion | |
| Stage III | Macroscoping invasion of neighboring organs, incuding pedal rdium, lung, or the main blood vessels | |
| Stage IV | a | Pleural or periardial dissemination |
| b | Hematogenous or lymphatic dssemination | |
Table 3.
Stage grouping according to the IASLC/ITMIG thymic epithelial tumors staging
| Stage | T | N | M |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| IIIa | T3 | N0 | M0 |
| IIIb | T4 | N0 | M0 |
| IVa | T any | N1 | M0 |
| T any | N0, N1 | M1a | |
| IVb | T any | N2 | M0, 1a |
| T any | N any | M1b |
Figure 2.
RYTHMIC recommendation for resectable thymic tumors from the RYTHMIC network, TNM I–II, Masaoka-Koga I–III.
Figure 4.
RYTHMIC recommendation for metastatic disease TNM IVb and Masaoka-Koga IVb.
TETs represent about 20% of the mediastinal tumors and about 50% of the anterior mediastinum tumors (Figure 5). TETs are more commonly diagnosed between the age of 40–60 years without sex-related incidence but can occur at all age including in a pediatric population.8,12,13 There are no identified risk factors for developing TETs. However, a higher incidence of TETs has been reported in multiple endocrine neoplasia type 114 or in the auto-immune Polyendocrinopathy-candidiasis ectodermal dystrophy.15 More than one-third of patients are asymptomatic when diagnosed, superior vena cava obstruction, cough, and hemoptysis are rarely observed and are associated with tumor extension.16 Auto-immune manifestations17 are encountered in 30–60% of the patients with thymomas and myasthenia gravis is the most frequent paraneoplastic-associated disease present in one-third cases.18 A CT-Scan evaluation is essential for the diagnosis establishment and to define the best strategy of care.19–21 The place of 18-FDG positron emission tomography is not essential but helpful in invasive forms, high SUV max is predictable of aggressive patterns of the tumors and could help to detect pleural or systemic metastasis.22–24 Diffusion-weighted-MRI is not indicated for the diagnosis of thymic malignancies but is essential to confirm thymic hyperplasia.25 MRI could also be helpful to establish the diagnosis of benign lesions mimicking solid tumors as a hemorrhagic or inflammatory thymic cyst. There is no place for mediastinal biopsy in first intention removal surgery and should be reserved before induction therapies or in the presence of uncertain diagnosis. Principles of care are principally based on R0 surgery when feasible associated with neo or adjuvant chemo-radio-therapies.
Figure 5.
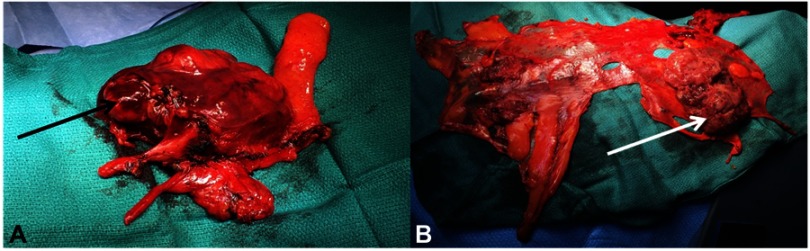
Operating views of thymic surgery: (A) enlarged thymectomy for B2 thymoma, the black arrow indicates the tumor developed in the left side of the thymus with mediastinal pleural involvement. (B) Right pleura after cytoreductive pleural surgery during ITCH procedure. The white arrow indicates pleural node of a A thymoma R0 resected two years before.
Principles of surgery
Operative technic
Multiple surgical options exist for performing extended thymectomy. Each technic carries its own advantages/disadvantages and should be perfectly assimilated by the surgical team. Secondary to the lack of prospective studies in a rare disease, retrospectives studies still advocate the role of complete resection in survival.26 If 100% R0 resection seems easier to perform in early stages (from I to II), the management of stages III or IVa is still challenging.
Partial or total median sternotomy still represents the traditional approach for thymic surgery but has been recently challenging by the introduction of Minimally Invasive Surgical technics as VATS (video-assisted thoracic surgery) or RATS (robotic-assisted thoracic surgery). Indication has to be discussed by the surgical board to manage the optimal decision and depend on several factors such as tumors size, stage, histology, and personal patient anatomical considerations. Guidelines on thymic tumors27 edited by the European Society of Medical Oncology (ESMO) still recommend median sternotomy as a standard of care for Masaoka-Koga I, II, and III disease. ESMO approve the role of MIS for stage I and II. VATS or RATS should be indicated for <5 cm tumors without signs of invasion of intrathoracic vessels, lung, pericardium, or trachea. Recent studies comparing open surgery vs VATS or RATS showed that MIS was performed in early stages (I, II) and in smaller tumors ≈4.09 cm with reduced blood loss, reduced the duration of chest tube and shorter length of stay.28–30 Precautions must be taken in the interpretation of higher rates of R0 surgery in MIS.31 Indeed, open median sternotomy is still a standard in complex tumors invading proximity organs were complete removal is a technical challenge. Otherwise, other retrospective studies showed higher rates of pneumonia in the open surgery group (4.1% vs 1.9%) and higher percent of phrenic nerves injury (6.7% vs 0%) and R+ (0.8% vs 0%) surgery in the VATS group. Finally, recent meta-analyses comparing open surgery – VATS – RATS were unable to find any differences between the 450 patients analyzed in outcomes, intra, and postoperative complications and conversion rate.32–35 Medico-economics studies are needed to evaluate the cost-benefit ratio of open surgery and MIS.
Extended thymectomy or thymomectomy?
Historically, extended thymectomy has been the “gold standard” and is based on the opportunity to decrease the recurrence risk during follow up.27 Recently, non-myasthenic patients have emerged the concept of thymomectomy in stage I–II. Retrospectives studies using the thymic database: JART (Japan),36–38 KART,39 CHART (China),40 and ITMIG41 advocate for a lower rate of complications in thymomectomy and lower surgery time length and blood loss. However, Gu et al,(CHART database) showed a significantly higher recurrence rate in the thymomectomy group (1047 patients; 251 thymomectomy), 5.4% vs 3.1%; p<0.05. However, thymomectomy is enable to define safe anatomic margins and eventually could lead to misdiagnose multifocal TETs42,43 Meanwhile, performing thymomectomy does not allow node removal following the 2015 ITMIG recommendations.44
A new challenge: place of lymphadenectomy in tumoral thymic surgery?
Lymphadenectomy is a standard in most of the tumors and is nowadays relevant to accurately assess TNM staging as a part of the prognosis. However, it has not been demonstrated to have any impact on survival in thymic tumors. The lymphatic involvement in TETs is estimated to be rare. A prevalence of 2% in thymomas and 20% in TC has been historically described45 but might be underestimated. The ITMIG/IASLC working groups proposed a lymph node staging and edited recommendations that led to the implementation of the 8th version of the TNM staging.
The N1 stations are defined as the anterior region including the lower anterior cervical, peri-thymic, pre-vascular, para-aortic, ascending aortic, superior phrenic, supra diaphragmatic, inferior phrenic, and pericardial node groups. The N2 stations are defined as the deep region including deep cervical, supraclavicular, lower (4 R) and upper para-tracheal (2 R), subaortic (4 L), sub-carinal (7), hilar (10 R, 10 L), and internal mammary node. All nodes outside the anterior and the deep regions are considered as metastasis.44,46,47 Lymph node invasion seems to be more elevated in TC. Cheufou et al, identified in a 53 patient retrospective series a rate 30.2% (16; 11 N1 and 5 N2) of lymph node metastases.48 The presence of lymph node invasion was correlated with worst overall survival and advocates the role of lymph node resection associated with enlarged thymectomy.
Surgery for IVa Masaoka-Koga
Surgery for disseminated pleural involvement is not well defined from debulking to extrapleural pneumonectomy (EPP) and intracavitary pleural treatments (HITHOC). Surgery may offer better recurrence free and overall survival when feasible especially in thymomas. Higher rates of recurrences and mortality are encountered in TC.
Debulking has been defined as removing 90% or more of tumor burden.49,50 The indication of debulking still remains controversial but may be purposed in nonresectable TETs with clearly inferior results of R0 surgery51,52 and provides better results in thymomas than in TC.53 Some authors advocate the place of debulking to improve the efficacy of high doses radiotherapy by minimizing fields and showed significantly better survival in stages III and IVa (p<0.05)49 and others showed no benefits.45,54
A multicentric analysis of the ESTS thymic working group55 of 152 patients with pleural involvement highlighted the benefit of surgery (26% EPP, resection of pleural implants 58% and total pleurectomy 15%) on OS. Three- and five years OS were 91% and 87%, respectively, and 3 and 5 years PFS were 58% and 43%. Once again, OS and PFS were worst in TC. A significant prognosis factor was the number of pleural implants.56,57 The presence of more than 10 disseminated pleural lesions is correlated with worse outcomes.58 Finally, a study comparing five and ten years OS between patient surgically managed (110) vs non-surgically (172) was in favor of pleural surgery: 5 years OS 79% vs 52% and 10 years OS 54% vs 36%.59 EPP provides good results on OS in selected patients; 5 years and 10 years OS from 60% to 75% and 30% to 66% with unfortunately major adverse effects (20–41%) as broncho-pleural fistulas, significantly higher in univariate analysis in patients with myasthenia gravis, probably secondary to corticoids impregnation.60–63 In these three series, <30 days mortality was 17.6% and the <90 days mortality rate was 29.4%.
Recently was introduced intrathoracic chemo hyperthermia (ITCH, or HITHOC: hyperthermic intraoperative thoraco-abdominal chemotherapy) based on Cisplatin (from 50 to 100 mg/m2) and subtotal pleural decortication (Figure 2)64 with interesting results on PFS, OS, and postoperative morbidity. One-year and five-year OS were 90–100% and 70–100%, respectively.65–68 Progression-free survival was available in two studies from 42 to 47.2 months. On a total of 77 patients who underwent ITCH, only one postoperative death was numbered (1.3%) and morbid events were encountered in 26 (33%) from postoperative prolonged air leak to re-intervention for wound sepsis or bleeding.
To conclude, surgery for IVa Masaoka-Koga stages must be purposed in selected patients and discussed in thymic tumors board secondary to true benefits on survival and PFS, especially in thymomas with pleural involvement.
Management of advanced TETs
The therapeutic strategy may be difficult to define, especially when for advanced/recurrent disease. Nearly 30% of the patients present with unresectable locally advanced tumor at diagnosis.69 In such cases, systemic treatments are initiated (Figure 6). Chemotherapy may be delivered in a curative intent approach to reduce the tumor burden and then possibly allowing subsequent carcinologic resection or definitive radiotherapy to achieve prolonged disease control. Unfortunately, about 10% of these patients will ultimately not be eligible for focal treatment after induction chemotherapy. Adjuvant chemotherapy is not recommended in cases of R0 or R1 resections, it can only be discussed in cases of thymic carcinoma from stage II and when no primary chemotherapy has been delivered. Definitive chemotherapy is the standard treatment in advanced, non-resectable, non-irradiable, recurrent, or metastatic disease. The aim is to improve tumor-related symptoms. Along with tumor progression, several lines of treatment can be administered. In this setting, targeted therapies, anti-angiogenic agents, or immunotherapy have been assessed.70
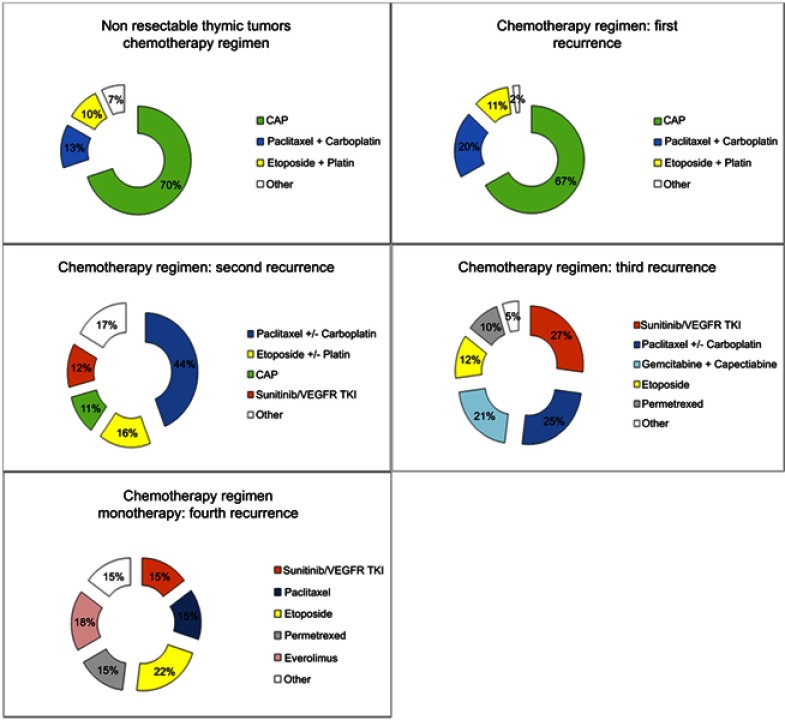
Figure 6.
Chemotherapy and other treatments delivered in patients prospectively included in the French RYTHMIC registry.
Abbreviations: CAP, Cyclophosphamid-Adriblastin-Cisplatin; TKI, Tyrosin Kinase Inhibitors.
Chemotherapy
For patients presenting with locally advanced tumor with no opportunity for upfront complete resection at diagnosis, cisplatin-based combination regimens should be delivered.27 Combinations of cisplatin, doxorubicin, and cyclophosphamide, or cisplatin and etoposide (particularly in thymic carcinoma) are recommended options in the RYTHMIC network, based on previously published retrospective studies. The rate of response to cisplatin-doxorubicin-cyclophosphamide is not different in thymoma (78%) than in thymic carcinoma (74%) and is associated with longer time to progression.71 Two to four cycles should be administered before assessing tumor resectability. If R0 resection is not achievable or if the patient is not in a good enough general condition for surgery, definitive radiotherapy is then recommended.
Exclusive chemotherapy is offered in patients with unresectable, metastatic tumors in a palliative intent approach. Cisplatin-based multi-agent combinations are also recommended. In this setting, cisplatin-doxorubicin-cyclophosphamide is preferred.72 The response rate for this regimen is 31% for thymomas and 37% for TC.71
Recurrences of TETs are not rare and should be managed as if they were newly diagnosed tumors. The question of complete resection of these lesions should be considered and if not deemed possible, re-administration of a previously effective treatment must be discussed.73 For the second line, a combination of carboplatin and paclitaxel is the preferred regimen. For upper lines or in patients not eligible for conventional chemotherapy, single conventional agent can be delivered as pemetrexed,74 oral etoposide,75 or octreotide76 (alone or with prednisone) for octreoscan positive thymomas.
Targeted therapies
Cytotoxic agents, as described earlier, have limited effects in advanced/recurrent thymic tumors. Targeted agents, approved in other solid tumors, have then been evaluated in thymic malignancies.
First of all, mTOR inhibitors (such as Everolimus) showed encouraging results in a Phase II trial77 with a median progression-free survival at 10.1 months and median overall survival at 25.7 months. One of the most severe adverse effects was fatal pneumonitis and caution before instituting this treatment should be considered.
Despite a high rate of c-KIT expression in thymic carcinoma compared to thymoma, less than 10% of TC harbor activating mutations of the c-KIT gene.78 Use of KIT inhibitors is then limited in TC but KIT sequencing may be an option for refractory diseases.79 Phase II trials evaluating the activity of imatinib to unselected TET patients failed to show any benefit.80,81 However, angiogenesis is a relevant pathway in the pathogenesis of thymic tumors. VEGF-A, VEGF-C, VEGF-D, and their receptors VEGFR-1, VEGFR-2, and VEGFR-3 are overexpressed in high-risk thymoma and thymic carcinoma82 and is correlated with tumor aggressiveness.83 Anti-angiogenic agents have then been evaluated in combination with other agents. Sunitinib (oral tyrosine kinase inhibitor of VEGFRs, KIT, and PDGFRs) showed some activity in the treatment of TETs irrespective of histological subtype and presence of KIT mutations with median progression-free survival at 3.7 months and overall survival at 15.4 months.84 Sunitinib is now recommended as an option in the second-line treatment of TC independently from KIT status.27
Somatostatin analogs may be used even in non-neuroendocrine thymic tumors but should be administered in patients with octreoscan positive thymoma, in late lines of treatment and in patients not anymore eligible for conventional chemotherapy.85 The association with prednisone improves the overall response rate.76
Immunotherapy
Recently, immunotherapy has been effective in the treatment of several malignancies and may be promising for refractory thymic neoplasms. PD1 and PDL1 expression were assessed in TETs. PD-1 and/or PD-L1 are expressed in up to 82% of the thymic epithelial neoplasms.86–88 These observations, considering the therapeutic effect of PD-1 inhibitors in other malignant tumors, led to Phase II clinical trials in thymic malignancies. Pembrolizumab has been studied in TC and disease control was achieved in 75% of the patients.89 Median overall survival was 24.9 months. Yet, a significant proportion of patients have had their treatment interrupted due to severe side effects. As expected due to frequent auto-immune disorders paraneoplastic syndrome observed in thymic tumors, 15% of the patients developed severe auto-immune toxicity including elevated liver enzymes, myocarditis, and polymyositis. Likewise, nivolumab is currently studied in patients with thymic carcinoma or type B3 thymoma by the European Organization for Research and Treatment of Cancer – EORTC (https://clinicaltrials.gov ID NCT03134118) and intermediate results have not been yet reported. Immunotherapy with anti-PD1 antibodies showed promising results but further data are needed with special focus on immune-related toxicity.
Radiation therapy in TETs
Considerable variations in radiotherapy protocols have been described over the 20 past years. The ITMIG published in 2011 recommendations for radiations in thymic malignancies.90 Conformational radiotherapy has been described as a standard in TETs using three-dimensional treatment planning and high-energy photons generated by linear accelerators.90 Conformational radiotherapy needs a previous “dosimetric” CT-scan performing the tumor delineation and defined the gross tumor volume (GTV).91 For postoperative radiotherapy, GTV is defined using pre and postoperative CT-scan and all information collecting via the oriented pathologic finding.92
The major objectives of treatment planning are to deliver a total dose ranging from 45 to 55 Gy in an adjuvant setting, 60 Gy in a definitive treatment setting,16,27,90,93 using a standard fractionation scheme (one 1.8–2 Gy fraction per day), without risking severe toxicities on non-tumoral tissues. The place of intensity-modulated radiotherapy (IMRT) has been recently advocated and allows the administration of beams of variable shapes during a single sequence, possibly proving to be helpful to target tumors that are close to critical tissues.94 However, the proton beam radiotherapy technic assessed in both postoperative or definitive has been described in a 22 patients series radiotherapy after surgical resection of a thymic tumor to significantly reduce doses to the heart, lungs, left ventricle, esophagus, and spinal cord were significantly reduced, as compared to IMRT.95
Postoperative radiotherapy for TETs
Current practices for postoperative mediastinal radiotherapy are highly variable, and there is paucity of prospective, multicenter evidence. The global trend over the past years has been toward a less frequent use of postoperative radiotherapy in thymoma and to keep it in reserve for high-risk cases.16,27
Large database and pooled analyses of retrospective studies revealed that (1) the absence of survival benefit after radiotherapy in stage I thymoma and a debatable survival benefit after R0 resection of stage II–III thymoma,96–98 a similar rate of recurrence whether or not patients received postoperative radiotherapy after complete resection of thymoma,99 and (3) a recurrence-free and overall survival benefit after resection of thymic carcinoma.7,97,100,101 As no randomized or even prospective study has been conducted to assess the effect of postoperative radiotherapy on recurrence rate or survival, available guidelines are of low levels of evidence.16,27 However, the fact that TETs recurrences occur outside the mediastinum in over 60% of the cases98 should be integrated for the discussion of postoperative radiotherapy.
Postoperative radiotherapy in complete resection R0
Thymomas
There is no benefit on survival to use postoperative radiotherapy in stage I Masaoka-Koga.7,45,70 Postoperative radiotherapy is debated in stage II. The Japanese cohort of 257 stage II thymomas treated with postoperative radiotherapy failed to prove any differences in recurrence rate compared to surgery alone.45 Finally, Jackson et al,96 report that postoperative radiotherapy benefit was lower in stage IIA vs IIB. In R0 stage III, the benefit of radiotherapy is better established.96,98
Overall, adjuvant radiotherapy is controversial for invasive thymoma, especially stage II tumors, for which PORT may then be more confidently considered in case of aggressive histology (type B2, B3) or extensive trans capsular invasion (stage IIB). For stage III thymoma, evidence suggests overall survival benefit after complete resection.
Thymic carcinoma
TC are high risk of recurrence tumors, from 20% to 30% in stage I to 80% in advanced disease.7,70,101 Benefits of postoperative radiotherapy on progression-free-survival and overall survival are higher than in thymomas. Recent series of published postoperative radiotherapy advocate an optional role in stage I, should be considered in stage II, and is highly recommended in stage III/IVA tumors.7,97,100,101
Radiotherapy for recurrences
As mentioned, about 60% of the thymic tumors recurrences are extra-thoracic and should be surgically managed when feasible with a true benefit on progression-free-survival and overall survival. In unresectable local recurrences, exclusive radiotherapy may be useful, Urgesi et al,102 showed high response rates with 5 years survival about 70–80% in a small retrospective study. The place of hemi thoracic radiotherapy has to be further evaluated in this setting.
To conclude, radiotherapy is a major therapeutic option for thymic malignancies and is usually considered for stage III thymomas, TC or after incomplete surgical resection. However, the exact role of radiotherapy is still debated, particularly in completely resected stage II tumors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44(1):123–130. doi: 10.1016/j.ejca.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Siesling S, van der Zwan JM, Izarzugaza I, et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer. 2012;48(7):949–960. doi: 10.1016/j.ejca.2012.02.047 [DOI] [PubMed] [Google Scholar]
- 3.Girard N, Merveilleux du Vignaux C, Molina T, Besse B, Rythmic R. Thymic tumors. Rev Prat. 2017;67(4):430–434. [PubMed] [Google Scholar]
- 4.Galateau-Salle F, Churg A, Roggli V, Travis WD, World Health Organization Committee for Tumors of the P. The 2015 World Health Organization Classification of Tumors of the Pleura: advances since the 2004 Classification. J Thorac Oncol. 2016;11(2):142–154. doi: 10.1016/j.jtho.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Marx A, Strobel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9(5):596–611. doi: 10.1097/JTO.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol. 2013;8(12):1467–1473. doi: 10.1097/JTO.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 7.Ruffini E, Detterbeck F, Van Raemdonck D, et al; European Association of Thoracic Surgeons Thymic Working G. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg. 2014;46(3):361–368. doi: 10.1093/ejcts/ezt649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol. 2014;9(7):1018–1022. doi: 10.1097/JTO.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 9.Ruffini E, Van Raemdonck D, Detterbeck F, Rocco G, Thomas P, Venuta F, European Society of Thoracic Surgeons Thymic Questionnaire Working G. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol. 2011;6(3):614–623. doi: 10.1097/JTO.0b013e318207cd74 [DOI] [PubMed] [Google Scholar]
- 10.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48(11):2485–2492. doi: [DOI] [PubMed] [Google Scholar]
- 11.Meurgey A, Girard N, Merveilleux du Vignaux C, et al. Assessment of the ITMIG Statement on the WHO Histological Classification and of the Eighth TNM staging of thymic epithelial tumors of a series of 188 thymic epithelial tumors. J Thorac Oncol. 2017;12(10):1571–1581. doi: 10.1016/j.jtho.2017.06.072 [DOI] [PubMed] [Google Scholar]
- 12.Davis RD Jr., Oldham HN Jr., Sabiston DC Jr.. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg. 1987;44(3):229–237. [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105(4):546–551. doi: 10.1002/ijc.11099 [DOI] [PubMed] [Google Scholar]
- 14.Kojima Y, Ito H, Hasegawa S, Sasaki T, Inui K. Resected invasive thymoma with multiple endocrine neoplasia type 1. Jpn J Thorac Cardiovasc Surg. 2006;54(4):171–173. doi: 10.1007/BF02662474 [DOI] [PubMed] [Google Scholar]
- 15.Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard N, Mornex F, Van Houtte P, Cordier JF, van Schil P. Thymoma: a focus on current therapeutic management. J Thorac Oncol. 2009;4(1):119–126. doi: 10.1097/JTO.0b013e31818e105c [DOI] [PubMed] [Google Scholar]
- 17.Chiche P, Benaim R, Samama M, Maury J. [Thrombolytic agents and acute pulmonary emboli. Study of 28 cases treated by streptokinase or urokinase-heparin]. Coeur Med Interne. 1978;17(1):59–66. [PubMed] [Google Scholar]
- 18.Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev. 2016;15(1):82–92. doi: 10.1016/j.autrev.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Park CM, Park SJ, Bae JS, Lee SM, Goo JM. Value of computerized 3D shape analysis in differentiating encapsulated from invasive thymomas. PLoS One. 2015;10(5):e0126175. doi: 10.1371/journal.pone.0126175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marom EM, Rosado-de-Christenson ML, Bruzzi JF, Hara M, Sonett JR, Ketai L. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. J Thorac Oncol. 2011;6(7 Suppl 3):S1717–S1723. doi: 10.1097/JTO.0b013e31821e8cd6 [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Yanagawa M, Hata A, et al. Volumetric analysis of the thymic epithelial tumors: correlation of tumor volume with the WHO classification and Masaoka staging. J Thorac Dis. 2018;10(10):5822–5832. doi: 10.21037/jtd.2018.09.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;8(4):502–510. doi: 10.1097/JTO.0b013e3182835549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto K, Taniguchi T, Ishikawa Y, et al. The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiothorac Surg. 2012;42(6):e152–e156. doi: 10.1093/ejcts/ezs527 [DOI] [PubMed] [Google Scholar]
- 24.Scagliori E, Evangelista L, Panunzio A, et al. Conflicting or complementary role of computed tomography (CT) and positron emission tomography (PET)/CT in the assessment of thymic cancer and thymoma: our experience and literature review. Thorac Cancer. 2015;6(4):433–442. doi: 10.1111/1759-7714.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priola AM, Priola SM. Imaging of thymus in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin Radiol. 2014;69(5):e230–e245. doi: 10.1016/j.crad.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Detterbeck F, Youssef S, Ruffini E, Okumura M. A review of prognostic factors in thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3)):S1698–S1704. doi: 10.1097/JTO.0b013e31821e7b12 [DOI] [PubMed] [Google Scholar]
- 27.Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S, Committee EG. Thymic epithelial tumours: ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v40–v55. doi: 10.1093/annonc/mdv277 [DOI] [PubMed] [Google Scholar]
- 28.Friedant AJ, Handorf EA, Su S, Scott WJ. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol. 2016;11(1):30–38. doi: 10.1016/j.jtho.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agatsuma H, Yoshida K, Yoshino I, et al. Video-assisted thoracic surgery thymectomy versus sternotomy thymectomy in patients with thymoma. Ann Thorac Surg. 2017;104(3):1047–1053. doi: 10.1016/j.athoracsur.2017.03.054 [DOI] [PubMed] [Google Scholar]
- 30.Hess NR, Sarkaria IS, Pennathur A, Levy RM, Christie NA, Luketich JD. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Thorac Surg. 2016;5(1):1–9. doi: 10.3978/j.issn.2225-319X.2016.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Gu Z, Ding J, et al. Perioperative outcomes and long-term survival in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis. 2016;8(4):673–679. doi: 10.21037/jtd.2016.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buentzel J, Heinz J, Hinterthaner M, et al. Robotic versus thoracoscopic thymectomy: the current evidence. Int J Med Robot. 2017;13(4). [DOI] [PubMed] [Google Scholar]
- 33.Buentzel J, Straube C, Heinz J, et al. Thymectomy via open surgery or robotic video assisted thoracic surgery: can a recommendation already be made? Medicine. 2017;96(24):e7161. doi: 10.1097/MD.0000000000007161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casiraghi M, Galetta D, Borri A, et al. Robotic-assisted thymectomy for early-stage thymoma: a propensity-score matched analysis. J Robot Surg. 2018;12(4):719–724. doi: 10.1007/s11701-018-0816-3 [DOI] [PubMed] [Google Scholar]
- 35.Fok M, Bashir M, Harky A, et al. Video-assisted thoracoscopic versus robotic-assisted thoracoscopic thymectomy: systematic review and meta-analysis. Innovations. 2017;12(4):259–264. doi: 10.1097/IMI.0000000000000382 [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa K, Asamura H, Sakurai H, Watanabe S, Tsuta K. Does the mode of surgical resection affect the prognosis/recurrence in patients with thymoma? J Surg Oncol. 2014;109(3):179–183. doi: 10.1002/jso.23499 [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa K, Yokoi K, Nakajima J, et al. Is thymomectomy alone appropriate for stage I (T1N0M0) thymoma? Results of a propensity-score analysis. Ann Thorac Surg. 2016;101(2):520–526. doi: 10.1016/j.athoracsur.2015.07.084 [DOI] [PubMed] [Google Scholar]
- 38.Yano M, Fujii Y, Yoshida J, et al. A phase II study of partial and subtotal thymectomy for thymoma (JART02). World J Surg. 2017;41(8):2033–2038. doi: 10.1007/s00268-017-3990-y [DOI] [PubMed] [Google Scholar]
- 39.Narm KS, Lee CY, Do YW, et al. Limited thymectomy as a potential alternative treatment option for early-stage thymoma: a multi-institutional propensity-matched study. Lung Cancer. 2016;101:22–27. doi: 10.1016/j.lungcan.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 40.Gu Z, Fu J, Shen Y, et al. Thymectomy versus tumor resection for early-stage thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. J Thorac Dis. 2016;8(4):680–686. doi: 10.21037/jtd.2016.03.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang W, Yao X, Antonicelli A, et al. Comparison of surgical approach and extent of resection for Masaoka-Koga Stage I and II thymic tumours in Europe, North America and Asia: an International Thymic Malignancy Interest Group retrospective database analysis. Eur J Cardiothorac Surg. 2017;52(1):26–32. doi: 10.1093/ejcts/ezx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori T, Nomori H, Ikeda K, et al. Three cases of multiple thymoma with a review of the literature. Jpn J Clin Oncol. 2007;37(2):146–149. doi: 10.1093/jjco/hyl147 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Yoshida S, Hiroshima K, Nakatani Y, Yoshino I. Synchronous multiple thymoma: report of three cases. Surg Today. 2010;40(5):456–459. doi: 10.1007/s00595-009-4080-z [DOI] [PubMed] [Google Scholar]
- 44.Hwang Y, Park IK, Park S, Kim ER, Kang CH, Kim YT. Lymph node dissection in thymic malignancies: implication of the ITMIG lymph node map, TNM stage classification, and recommendations. J Thorac Oncol. 2016;11(1):108–114. doi: 10.1016/j.jtho.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 45.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76(3):878–884; discussion 884–875. [DOI] [PubMed] [Google Scholar]
- 46.Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC thymic epithelial tumors staging project: a proposed lymph node map for thymic epithelial tumors in the forthcoming 8th edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S88–S96. doi: 10.1097/JTO.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 47.Carter BW, Benveniste MF, Madan R, et al. IASLC/ITMIG staging system and lymph node map for thymic epithelial neoplasms. Radiographics. 2017;37(3):758–776. doi: 10.1148/rg.2017160096 [DOI] [PubMed] [Google Scholar]
- 48.Cheufou DH, Valdivia D, Puhlvers S, et al. Lymph node involvement and the surgical treatment of thymic epithelial and neuroendocrine carcinoma. Ann Thorac Surg. 2019;107:1632–1638. doi: 10.1016/j.athoracsur.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 49.Lin CS, Kuo KT, Hsu WH, et al. Managements of locally advanced unresectable thymic epithelial tumors. J Chin Med Assoc. 2004;67(4):172–178. [PubMed] [Google Scholar]
- 50.Liu HC, Chen YJ, Tzen CY, Huang CJ, Chang CC, Huang WC. Debulking surgery for advanced thymoma. Eur J Surg Oncol. 2006;32(9):1000–1005. doi: 10.1016/j.ejso.2006.03.049 [DOI] [PubMed] [Google Scholar]
- 51.Attaran S, Acharya M, Anderson JR, Punjabi PP. Does surgical debulking for advanced stages of thymoma improve survival? Interact Cardiovasc Thorac Surg. 2012;15(3):494–497. doi: 10.1093/icvts/ivs263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamaji M, Ali SO, Burt BM. A meta-analysis of surgical versus nonsurgical management of recurrent thymoma. Ann Thorac Surg. 2014;98(2):748–755. doi: 10.1016/j.athoracsur.2014.04.028 [DOI] [PubMed] [Google Scholar]
- 53.Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg. 2014;62(8):468–474. doi: 10.1007/s11748-014-0420-z [DOI] [PubMed] [Google Scholar]
- 54.Cohen DJ, Ronnigen LD, Graeber GM, et al. Management of patients with malignant thymoma. J Thorac Cardiovasc Surg. 1984;87(2):301–307. [PubMed] [Google Scholar]
- 55.Moser B, Fadel E, Fabre D, et al. Surgical therapy of thymic tumours with pleural involvement: an ESTS Thymic Working Group Project. Eur J Cardiothorac Surg. 2017;52:346–355. doi: 10.1093/ejcts/ezx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg. 2009;137(5):1185–1189. doi: 10.1016/j.jtcvs.2008.09.033 [DOI] [PubMed] [Google Scholar]
- 57.Yano M, Sasaki H, Yukiue H, et al. Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg. 2009;33(7):1425–1431. doi: 10.1007/s00268-009-0069-4 [DOI] [PubMed] [Google Scholar]
- 58.Okuda K, Yano M, Yoshino I, et al. Thymoma patients with pleural dissemination: nationwide retrospective study of 136 cases in Japan. Ann Thorac Surg. 2014;97(5):1743–1748. doi: 10.1016/j.athoracsur.2014.01.042 [DOI] [PubMed] [Google Scholar]
- 59.Hamaji M, Kojima F, Omasa M, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg. 2015;47(4):602–607. [DOI] [PubMed] [Google Scholar]
- 60.Fabre D, Fadel E, Mussot S, et al. Long-term outcome of pleuropneumonectomy for Masaoka stage IVa thymoma. Eur J Cardiothorac Surg. 2011;39(5):e133–e138. doi: 10.1016/j.ejcts.2010.12.064 [DOI] [PubMed] [Google Scholar]
- 61.Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg. 2009;88(3):952–957. doi: 10.1016/j.athoracsur.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 62.Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg. 2006;82(4):1234–1239. doi: 10.1016/j.athoracsur.2006.05.028 [DOI] [PubMed] [Google Scholar]
- 63.Wright CD. Stage IVA thymoma: patterns of spread and surgical management. Thorac Surg Clin. 2011;21(1):93–97, vii. doi: 10.1016/j.thorsurg.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 64.Maury JM, Drevet G, Collaud S, et al. Cytoreductive pleurectomy and intrathoracic chemohyperthermia for pleural relapse of thymomas. Ann Thorac Surg. 2019;107(2):e157–e160. doi: 10.1016/j.athoracsur.2018.07.058 [DOI] [PubMed] [Google Scholar]
- 65.Ambrogi MC, Korasidis S, Lucchi M, et al. Pleural recurrence of thymoma: surgical resection followed by hyperthermic intrathoracic perfusion chemotherapydagger. Eur J Cardiothorac Surg. 2016;49(1):321–326. doi: 10.1093/ejcts/ezv039 [DOI] [PubMed] [Google Scholar]
- 66.Maury JM, Girard N, Tabutin M, et al. Intra-thoracic chemo-hyperthermia for pleural recurrence of thymoma. Lung Cancer. 2017;108:1–6. doi: 10.1016/j.lungcan.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 67.Refaely Y, Simansky DA, Paley M, Gottfried M, Yellin A. Resection and perfusion thermochemotherapy: a new approach for the treatment of thymic malignancies with pleural spread. Ann Thorac Surg. 2001;72(2):366–370. [DOI] [PubMed] [Google Scholar]
- 68.Ried M, Potzger T, Sziklavari Z, et al. Extended surgical resections of advanced thymoma Masaoka stages III and IVa facilitate outcome. Thorac Cardiovasc Surg. 2014;62(2):161–168. doi: 10.1055/s-0033-1345303 [DOI] [PubMed] [Google Scholar]
- 69.Girard N, Lal R, Wakelee H, Riely GJ, Loehrer PJ. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1749–S1755. doi: 10.1097/JTO.0b013e31821ea5f7 [DOI] [PubMed] [Google Scholar]
- 70.Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG thymic epithelial tumors staging project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S65–S72. doi: 10.1097/JTO.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 71.Merveilleux du Vignaux C, Dansin E, Mhanna L, et al. Systemic therapy in advanced thymic epithelial tumors: insights from the RYTHMIC prospective cohort. J Thorac Oncol. 2018;13(11):1762–1770. doi: 10.1016/j.jtho.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 72.Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol. 2015;141(2):323–331. doi: 10.1007/s00432-014-1800-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lara PN Jr., Bonomi PD, Faber LP. Retreatment of recurrent invasive thymoma with platinum, doxorubicin, and cyclophosphamide. Chest. 1996;110(4):1115–1117. doi: 10.1378/chest.110.4.1115 [DOI] [PubMed] [Google Scholar]
- 74.Liang Y, Padda SK, Riess JW, West RB, Neal JW, Wakelee HA. Pemetrexed in patients with thymic malignancies previously treated with chemotherapy. Lung Cancer. 2015;87(1):34–38. doi: 10.1016/j.lungcan.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 75.Bluthgen MV, Boutros C, Fayard F, Remon J, Planchard D, Besse B. Activity and safety of oral etoposide in pretreated patients with metastatic or recurrent thymic epithelial tumors (TET): a single-institution experience. Lung Cancer. 2016;99:111–116. doi: 10.1016/j.lungcan.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 76.Loehrer PJ Sr., Wang W, Johnson DH, Aisner SC, Ettinger DS, Eastern Cooperative Oncology Group Phase IIT. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol. 2004;22(2):293–299. doi: 10.1200/JCO.2004.02.047 [DOI] [PubMed] [Google Scholar]
- 77.Zucali PA, De Pas T, Palmieri G, et al. Phase II study of everolimus in patients with thymoma and thymic carcinoma previously treated with cisplatin-based chemotherapy. J Clin Oncol. 2018;36(4):342–349. doi: 10.1200/JCO.2017.74.4078 [DOI] [PubMed] [Google Scholar]
- 78.Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res. 2009;15(22):6790–6799. doi: 10.1158/1078-0432.CCR-09-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merveilleux du Vignaux C, Maury JM, Girard N. Novel agents in the treatment of thymic malignancies. Curr Treat Options Oncol. 2017;18(9):52. doi: 10.1007/s11864-017-0495-8 [DOI] [PubMed] [Google Scholar]
- 80.Palmieri G, Marino M, Buonerba C, et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother Pharmacol. 2012;69(2):309–315. doi: 10.1007/s00280-011-1690-0 [DOI] [PubMed] [Google Scholar]
- 81.Giaccone G, Rajan A, Ruijter R, Smit E, van Groeningen C, Hogendoorn PC. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol. 2009;4(10):1270–1273. doi: 10.1097/JTO.0b013e3181b6be57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lattanzio R, La Sorda R, Facciolo F, et al. Thymic epithelial tumors express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy: a tissue micro array-based multicenter study. Lung Cancer. 2014;85(2):191–196. [DOI] [PubMed] [Google Scholar]
- 83.Tomita M, Matsuzaki Y, Edagawa M, et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J Thorac Cardiovasc Surg. 2002;124(3):493–498. [DOI] [PubMed] [Google Scholar]
- 84.Remon J, Girard N, Mazieres J, et al. Sunitinib in patients with advanced thymic malignancies: cohort from the French RYTHMIC network. Lung Cancer. 2016;97:99–104. doi: 10.1016/j.lungcan.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 85.Palmieri G, Montella L, Martignetti A, et al. Somatostatin analogs and prednisone in advanced refractory thymic tumors. Cancer. 2002;94(5):1414–1420. doi: 10.1002/cncr.10374 [DOI] [PubMed] [Google Scholar]
- 86.Arbour KC, Naidoo J, Steele KE, et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS One. 2017;12(8):e0182665. doi: 10.1371/journal.pone.0182665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. doi: 10.1016/j.lungcan.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 88.Weissferdt A, Fujimoto J, Kalhor N, et al. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod Pathol. 2017;30(6):826–833. doi: 10.1038/modpathol.2017.6 [DOI] [PubMed] [Google Scholar]
- 89.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347–355. doi: 10.1016/S1470-2045(18)30062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez D, Komaki R, Yu J, Ikushima H, Bezjak A. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol. 2011;6(7 Suppl 3):S1743–S1748. doi: 10.1097/JTO.0b013e31821ea60c [DOI] [PubMed] [Google Scholar]
- 91.Treglia G, Sadeghi R, Giovanella L, Cafarotti S, Filosso P, Lococo F. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? A meta-analysis. Lung Cancer. 2014;86(1):5–13. doi: 10.1016/j.lungcan.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 92.Huang J, Detterbeck FC, Wang Z, Loehrer PJ Sr.:. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5(12):2017–2023. doi: 10.1097/JTO.0b013e3181f13682 [DOI] [PubMed] [Google Scholar]
- 93.Mornex F, Resbeut M, Richaud P, et al. Radiotherapy and chemotherapy for invasive thymomas: a multicentric retrospective review of 90 cases. The FNCLCC trialists. Federation Nationale des Centres de Lutte Contre le Cancer. Int J Radiat Oncol Biol Phys. 1995;32(3):651–659. [DOI] [PubMed] [Google Scholar]
- 94.Basse C, Thureau S, Bota S, et al. Multidisciplinary tumor board decision making for postoperative radiotherapy in thymic epithelial tumors: insights from the RYTHMIC prospective cohort. J Thorac Oncol. 2017;12(11):1715–1722. doi: 10.1016/j.jtho.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 95.Vogel J, Lin L, Simone CB 2nd, Berman AT. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol. 2017;56(8):1060–1064. doi: 10.1080/0284186X.2017.1302097 [DOI] [PubMed] [Google Scholar]
- 96.Jackson MW, Palma DA, Camidge DR, et al. The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol. 2017;12(4):734–744. doi: 10.1016/j.jtho.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 97.Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer. 2015;121(7):1008–1016. doi: 10.1002/cncr.29166 [DOI] [PubMed] [Google Scholar]
- 98.Rimner A, Yao X, Huang J, et al. Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III thymoma – an analysis of the international thymic malignancies interest group retrospective database. J Thorac Oncol. 2016;11(10):1785–1792. doi: 10.1016/j.jtho.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korst RJ, Kansler AL, Christos PJ, Mandal S. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87(5):1641–1647. doi: 10.1016/j.athoracsur.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 100.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149(1):95–100, 101 e101–e102. doi: 10.1016/j.jtcvs.2014.09.124 [DOI] [PubMed] [Google Scholar]
- 101.Fu H, Gu ZT, Fang WT, et al. Long-term survival after surgical treatment of thymic carcinoma: a retrospective analysis from the Chinese Alliance for Research of Thymoma Database. Ann Surg Oncol. 2016;23(2):619–625. doi: 10.1245/s10434-015-4825-4 [DOI] [PubMed] [Google Scholar]
- 102.Urgesi A, Monetti U, Rossi G, Ricardi U, Maggi G, Sannazzari GL. Aggressive treatment of intrathoracic recurrences of thymoma. Radiother Oncol. 1992;24(4):221–225. [DOI] [PubMed] [Google Scholar]