Significance
Inorganic nitrides are important technological materials, many of which belong to one of two families: hexagonal main-group metal nitride semiconductors and cubic transition-metal nitride superconductors (TMNs). This mixed experimental and theoretical study identifies a class of ternary MgxTM1−xN (TM = Ti, Zr, Hf, Nb) nitrides that bridge these families. While these materials have rocksalt-derived crystal structures similar to binary TMNs, electronically they exhibit semiconducting band structures, large dielectric constants, and disorder-tunable properties. Lattice matching with main-group nitride semiconductors along some crystallographic orientations, and full structural compatibility with superconducting TMNs make these materials uniquely poised for integration with established nitrides.
Keywords: materials discovery, nitride semiconductors, defect-tolerant materials
Abstract
Inorganic nitrides with wurtzite crystal structures are well-known semiconductors used in optical and electronic devices. In contrast, rocksalt-structured nitrides are known for their superconducting and refractory properties. Breaking this dichotomy, here we report ternary nitride semiconductors with rocksalt crystal structures, remarkable electronic properties, and the general chemical formula MgxTM1−xN (TM = Ti, Zr, Hf, Nb). Our experiments show that these materials form over a broad metal composition range, and that Mg-rich compositions are nondegenerate semiconductors with visible-range optical absorption onsets (1.8 to 2.1 eV) and up to 100 cm2 V−1⋅s−1 electron mobility for MgZrN2 grown on MgO substrates. Complementary ab initio calculations reveal that these materials have disorder-tunable optical absorption, large dielectric constants, and electronic bandgaps that are relatively insensitive to disorder. These ternary MgxTM1−xN semiconductors are also structurally compatible both with binary TMN superconductors and main-group nitride semiconductors along certain crystallographic orientations. Overall, these results highlight MgxTM1−xN as a class of materials combining the semiconducting properties of main-group wurtzite nitrides and rocksalt structure of superconducting transition-metal nitrides.
Nitride materials are relevant to several industrial and technological fields, and historically are separated into two families. The first family is main-group metal nitride semiconductors with wurtzite crystal structure, typified by (Al, Ga, In)N, which are known for direct bandgaps and high carrier mobilities (1, 2). Over the last few decades these materials have become particularly important due to proliferation of solid-state lighting, radiofrequency transistors, and high information density optical storage media (1–3). The second family is transition-metal (TM) nitrides with rocksalt structures, such as TiN, VN, and CrN, which are often found as industrial hard coatings and diffusion barriers in semiconductor devices (4). In these compounds, the open d-shell of the TM leads to metallic behavior; many exhibit superconducting transitions approaching 20 K (5). While there are several examples of inorganic nitrides with more structural- and chemical complexity (6–9), and even some cases of semiconducting rocksalt nitrides (e.g., ScN) (10) or metallic wurtzite nitrides (e.g., ZnMoN2) (11), these are exceptions to the general trends observed in the most technologically relevant materials.
In the past decade, increased attention has been devoted to semiconducting II-IV-N2 ternaries, which are structurally similar to III-N wurtzite compounds but with the main-group III3+ metal replaced with group II2+ and group IV4+ metals (for example, Zn2+ and Ge4+ instead of Ga3+) (12). Very recently, we expanded this materials design concept to ternary nitrides with other main-group (e.g., Sb5+ in Zn2SbN3) (13) and TM (Mo6+ in ZnMo3N4) (11) elements in wurtzite-derived crystal structures. However, it is not clear if a similar approach might be used in TM nitrides with rocksalt structures. On one hand, the group IV TMs usually exist in 3+ valence states in nitrides (e.g., TiN) (14). On the other hand, introducing electropositive low-valence alkaline earth (AE) cations, such as Mg2+, could move TMs into higher oxidation states, closing the d-shell and inducing semiconducting behavior (15). Furthermore, TM nitride rocksalts are already known to adopt both cation- and anion-substoichiometric defect phases (16–18), so metal site vacancies could reasonably be accommodated and possibly filled with AE cations. Even though computational studies suggest that some such compounds can be formed (19, 20), few of them have been synthesized (21–24), and reports of thin-film synthesis and functional properties are even rarer (25–28).
Here, we report a family of ternary nitride semiconductors with rocksalt structures and the general formula MgG-3TMNG-2, where G is the group number of TM. We specifically consider the compounds MgTiN2, MgZrN2, MgHfN2, and Mg2NbN3. Ab initio calculations predict rocksalt-derived structures, where multiple cation motifs on the metal sublattice can be close in energy. They also suggest these compounds have disorder-tunable optical absorption but disorder-tolerant electronic bandgaps, low effective masses, and large dielectric constants (30 to 80 ε0). Each of the compounds, synthesized as thin films via sputtering, exhibits diffraction peaks corresponding to a simple rocksalt structure and suggesting mixed cation-site occupancy. Lattice parameters are compatible with epitaxial growth on a variety of substrates and suitable for integration with well-established semiconducting and superconducting nitrides. Mg-rich compositions of MgG-3TMNG-2 exhibit semiconducting electronic properties with visible-range optical absorption onsets, and electron mobilities approaching 100 cm2 V−1⋅s−1 are measured from MgZrN2 grown on MgO. Overall, these results introduce a class of ternary nitrides with semiconducting properties like main-group wurtzite nitrides, but rocksalt-derived crystal structures like superconducting TMNs.
Results
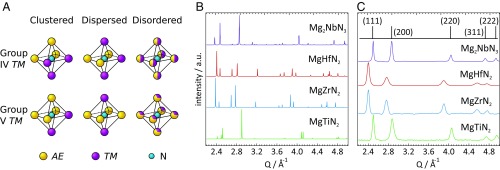
First, we hypothesize about possible structures found in AEG-3TMNG-2 rocksalt-derived nitrides for G = 4 and 5. The basis for any rocksalt-derived structure is octahedrally coordinated cations and anions forming a network of edge-sharing octahedra. In AEG-3TMNG-2 compounds only a few octahedral motifs will satisfy local electroneutrality, just like in the case of oxides (29). The motifs for N-AE32+TM34+ and N-AE42+TM25+ octahedra, corresponding to AE2+TM4+N2 and AE22+TM5+N3 stoichiometries, respectively, are shown in Fig. 1A. Each case has a clustered arrangement, where like cations are grouped together, and a dispersed arrangement, with like cations separated across the octahedron. Alternatively, in a disordered state the motifs can be mixed, and deviations from the ideal AE/TM ratio surrounding the N atoms may occur. However, this becomes energetically more costly the further they deviate from local charge neutrality, as recently shown for tetrahedrally coordinated ternary nitrides (30). Following the observed structural preferences in oxides and similar ternary nitrides, where radius ratio rules have been used to determine the coordination number and adopted motif (19, 21, 29), the smaller TM ions in MgTiN2 are expected to promote clustered octahedral motifs, whereas MgZrN2 and MgHfN2 are expected to have dispersed motifs. Extending the same motif arguments to AE22+TM5+N3 stoichiometries, Mg2NbN3 is expected to have a clustered N-AE42+TM25+ motif.
Fig. 1.
Structural properties of MgG-3TMNG-2 (TM = Ti, Zr, Hf, Nb) materials. (A) The charge-neutral local structural motifs that serve as building blocks for AEG-3TMNG-2 (G = 4, 5) rocksalt-derived structures. An alternative to the clustered and dispersed cation-ordered motifs is a substitutionally disordered cation lattice. (B) Calculated diffraction patterns for the rocksalt-derived ground states of MgG-3TMNG-2 with an ordered cation sublattice. (C) Synchrotron X-ray diffraction data exhibit peaks that can be indexed to simple rocksalt (peak positions shown in black for a = 4.46 Å to match Mg2NbN3), indicating the presence of substitutional disorder on the cation sublattice.
Our recent computational search for new ternary nitrides revealed several AE-TM-N chemistries absent from the inorganic crystal structure database (9). To confirm our hypothetical considerations about the structures in the MgG-3TMNG-2 family, we performed ab initio structure searches using the kinetically limited minimization approach (11, 31). We obtained the layered α-NaFeO2 structure [space group (SG) 166] for MgTiN2 and the γ-LiFeO2 structure (SG 141) for both MgZrN2 and MgHfN2. Each of these are rocksalt-derived structures, showing ABAB cation-site ordering perpendicular to NaCl [111] planes for α-NaFeO2, and parallel to both NaCl [110] and [−110] planes for γ-LiFeO2. For Mg2NbN3, the lowest energy is obtained for a 5-coordinate structure (SG 15). However, a rocksalt-derived structure (SG 12) is only 3.5 meV per atom higher in energy. Fig. 1B shows calculated X-ray diffraction patterns for these rocksalt-derived structures. Crystal structure schematics (SI Appendix, Fig. S1) and .cif files are provided in SI Appendix. For each system the lowest-energy rocksalt-derived structure exhibits local octahedral motifs as expected by the qualitative arguments above. However, our calculations show only a small energy penalty for adopting structures with different motifs (SI Appendix, Table S1), meaning these compounds can likely be disordered under nonequilibrium synthesis conditions.
To validate these predictions, we synthesized both stoichiometric (x = 0) and Mg-rich (x ∼ 0.5) MgG-3+xTM1−xNG-2 (TM = Ti, Zr, Nb, Hf) thin films by combinatorial sputtering on glass substrates, with compositions summarized in Table 1. X-ray diffraction data for stoichiometric samples are shown in Fig. 1C. Diffraction from Mg-rich films (SI Appendix, Fig. S2) are similar, but relative peak intensities suggest different preferential orientation of the grains. Peak widths (SI Appendix, Table S2), indicate crystalline coherence lengths on the order of 10 nm, consistent with observations from transmission electron microscopy (TEM) micrographs from a MgHfN2 lamella (SI Appendix, Fig. S3). TEM energy-dispersive X-ray spectroscopy measurements also support the absence of amorphous Mg3N2 or HfN in the film. For each TM the peaks can be indexed to a simple NaCl-type rocksalt structure (SG 225), with reference positions shown at the top for a = 4.46 Å. The lack of additional reflections, which would be present for the rocksalt-derived ground-state structures (Fig. 1B), suggest substitutional disorder on the cation lattice. This is not surprising given the similar ionic radii of Mg (0.86 Å) and the TMs (0.75 to 0.85 Å) (32), the prevalence of cation disorder in closed-shell oxide rocksalts (33), and the similar formation energy of structures with different local motifs (SI Appendix, Table S1).
Table 1.
Target and measured compositions of Mg-TM-N thin films on glass substrates
| Cation stoichiometric | Mg-rich | ||
| Target | Measured | Target | Measured |
| MgTiN2 | Mg0.97TiN2.03 | Mg1.5Ti0.5N2 | Mg1.54Ti0.46N1.82 |
| MgZrN2 | Mg0.96ZrN1.87 | Mg1.5Zr0.5N2 | Mg1.54Zr0.46N1.81 |
| MgHfN2 | Mg1.03HfN2.21 | Mg1.5Hf0.5N2 | Mg1.52Hf0.48N1.88 |
| Mg2NbN3 | Mg2.04NbN2.78 | Mg2.5Nb0.5N3 | Mg2.46Nb0.54N2.47 |
Substoichiometric nitrogen measured from Mg-rich compositions is likely partially substituted by oxygen. Oxygen content measured from similar films deposited on C ranged from ∼3 at % (stoichiometric) to ∼7 at % (Mg-rich).
The experimental confirmation of rocksalt-derived materials reported here, along with the already known cation-disordered ternary nitrides in wurtzite-derived crystal structures (11–13), suggests opportunities for future materials design with other “host” crystal structures. By considering additional materials, especially with structures known to accommodate substoichiometric defect phases (e.g., CsCl type, CaF2 type), and elements that have capacity for redox stabilization by the inductive effect (e.g., TMs), it is likely that additional compounds with tunable properties can be realized. This design principle might extend beyond 4-coordinate wurtzites and 6-coordinate rocksalts, since some TMNs can adopt structures with up to 8-coordinate bonding, depending on composition (16, 34).
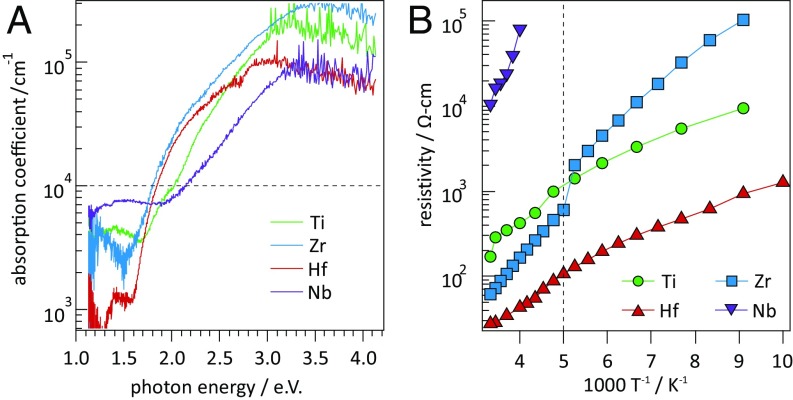
Next, we report measured optical and electronic properties of MgG-3+xTM1−xNG-2 thin films (Fig. 2). The cation-stoichiometric films prepared for this study had conductivities >1 S cm−1 and did not exhibit clearly defined optical absorption onsets, presumably due to degenerate carrier densities. Following the strategy of preparing ZnSnN2 with low carrier densities by making it Zn-rich (35), we prepared Mg-rich compositions of MgG-3+xTM1−xNG-2 (x ∼ 0.5). As presented in Fig. 2A, each Mg-rich composition exhibits an absorption onset (defined here as α = 104 cm−1) in the visible range (1.8 to 2.1 eV) with EZr ∼ EHf < ETi < ENb. Our experimental results agree with recent reports on MgTiN2 and MgZrN2, where a metallic-to-semiconducting transition is observed as Mg is added to TMN (27, 28).
Fig. 2.
Measured optical and electronic properties of Mg-rich MgG-3+xTM1−xNG-2 materials. (A) Experimental optical absorption spectra exhibit absorption onsets in the visible range. The horizontal dashed line indicates absorptivity of 104 cm−1. (B) Semiconducting behavior is supported by an exponential increase in resistivity with decreasing temperature. The vertical dashed line indicates a switch in measurement conditions at ∼200 K.
Variable-temperature resistivity measurements from Mg-rich films are presented as an Arrhenius plot in Fig. 2B. The increase in resistivity with decreasing temperature is slightly weaker than exponential, indicating thermally activated transport is convoluted either with temperature-dependent mobility or other extrinsic factors. The calculated activation energy of the Zr-containing sample is 120 meV, suggesting the presence of a shallow defect. Room-temperature resistivities are significantly higher than at stoichiometric compositions, confirming that extrinsic carriers are partially compensated in Mg-rich films. Negative Seebeck voltages measured from MgZrN2 films on glass substrates show n-type transport, likely from uncompensated oxygen acting as electron donors (∼3–7 at % for witness samples on C substrates). Preliminary Hall effect experiments indicate that MgZrN2 films grown by sputtering on MgO [similar to ScN epitaxy (36–38)] have mobilities approaching 100 cm2 V−1⋅s−1 (Table 2), which is very promising for a sputtered material.
Table 2.
Transport properties of MgZrN2 grown on MgO substrates
| Substrate | ρ, Ω-cm | μ, cm2 V−1⋅s−1 | n, cm−3 |
| (111) MgO | 0.0192 | 90 ± 60 | (−5 ± 3) × 1018 |
| (100) MgO | 0.00118 | 42 ± 9 | (−1.3 ± 0.3) × 1020 |
Error is SD from 3 measurements.
To support these experimental property measurements, we computed electronic structure and resulting properties of MgG-3TMNG-2 materials using many-body perturbation theory in the GW approximation (where G is the Green’s function and W is the screened Coulomb interaction) (39). The calculation results are summarized in Table 3. Electronic bandgaps of the rocksalt-derived structures are between 1 and 2 eV. The optical absorption (shown in Fig. 3B for MgZrN2), exhibits a slow onset, nearly 1 eV above the bandgap, due to the indirect/forbidden nature of the optical transition (compare SI Appendix, Fig. S4 for other MgG-3TMNG-2 compounds and for comparison with experiment). Notably, we obtain very large dielectric constants of 30 to 80 ε0, where ε0 is vacuum permittivity, similar to the case of halide perovskites (40). This is likely because the d0 configuration of the TM cations allows for large cation displacements at minute energy cost, thereby leading to a large ionic contribution to the dielectric constant (33). The electron and hole DOS effective masses (derived from density of states calculations) are between 0.6 and 1.9 me (Table 3) which is comparable to Si (41), and is likely overestimated compared with E(k) effective masses (derived from band structure calculations) due to degeneracy and/or nonparabolicity in the bands.
Table 3.
Calculated properties of MgG-3TMNG-2 materials
| Compound | Disorder state | Gap type | Eg, eV | Egopt, eV | εi/ε0 | εe/ε0 | ||
| MgTiN2 | Ground state | Indirect | 0.91 | 2.1 | 1.4 | 1.4 | 71.4 | 8.4 |
| Moderate-dis | NA | 0.92 | 1.2 | 1.4 | 1.4 | |||
| Strong-dis | NA | 0.81 | 1.0 | 1.5 | 1.5 | |||
| MgZrN2 | Ground state | Direct forbidden | 1.47 | 2.5 | 0.6 | 1.6 | 31.6 | 6.9 |
| Moderate-dis | NA | 1.67 | 2.2 | 1.1 | 2.1 | |||
| Strong-dis | NA | 1.39 | 1.8 | 1.3 | 2.4 | |||
| MgHfN2 | Ground state | Direct forbidden | 1.79 | 2.9 | 0.6 | 1.5 | 25.6 | 6.3 |
| Moderate-dis | NA | 1.92 | 2.6 | 0.8 | 2.0 | |||
| Strong-dis | NA | 1.79 | 2.4 | 1.1 | 2.1 | |||
| Mg2NbN3 | Ground state | Indirect | 1.84 | 2.7 | 0.6 | 1.9 | 61.0 | 7.1 |
| Moderate-dis | NA | 2.14 | 2.3 | 1.5 | 3.6 | |||
| Strong-dis | NA | 1.66 | 1.8 | 1.3 | 3.7 |
Electronic bandgap types, electronic bandgaps, optical absorption onsets (Egopt, defined as energy when α = 103 cm−1), DOS effective masses, and dielectric constants (εi = ionic, εe = electronic). In disordered materials, the gap is not well defined (NA, not applicable).
Fig. 3.
Calculated properties of MgZrN2. (A) Fraction of octet-rule-violating motifs as a function of effective temperature. (B) Optical absorption spectra and bandgap for the rocksalt-derived ground-state structure, and absorption of moderately (moderate-dis) and strongly (strong-dis) disordered structures. (C) IPR data show that carrier localization and bandgap change negligibly with disorder.
To understand the effects of cation-site disorder on material properties, we performed Monte Carlo supercell calculations, using the effective temperature (Teff) concept (42). Note that Teff is used to quantify nonequilibrium disorder due to kinetic limitations during thin-film growth at low and moderate temperatures, and does not directly relate to the actual growth temperature. We considered both a moderate level of disorder (Teff ∼ 2,000 K), common for oxides and nitrides (42, 43), and strong disorder (Teff ∼ 10,000 K). Detailed energetics of the disordered structures are given in SI Appendix, Table S3. The fraction of octet-violating polyhedra has been useful for describing disorder in tetrahedrally coordinated ternary nitrides (30, 44, 45). We take a similar approach and Fig. 3A shows the fraction of motifs found in the disordered structures of MgZrN2. With moderate disorder almost 50% of octahedra have octet-rule-violating motifs, which do not significantly increase at higher Teff. This is possibly because octahedrally coordinated compounds can have both clustered and dispersed forms of charge-neutral motifs, so increased disorder could be reasonably accommodated without additional charged motifs.
Calculated absorption spectra in Fig. 3B suggest that disorder alleviates the selection rules, thereby bringing the absorption onset closer to the bandgap energy. Relative to tetrahedrally coordinated ternary nitrides (30, 43), bandgaps are less affected by moderate and even strong disorder [Table 3, ΔEg = 0.1 to 0.3 eV for MgG-3TMNG-2 vs. ΔEg = 0.6 eV at Teff = 0 K vs. 3,000 K for ZnSnN2 (30)], despite considerable deviations from the ideal octet-rule-conserving motifs in disordered MgG-3TMNG-2. The changes to the bandgaps and effective masses with disorder depend on the interplay between band dispersion and more localized perturbations and can be nonmonotonic (e.g., one would expect Eg to close in the limit Teff → ∞). We also note that disorder stabilizes the rocksalt-like structure of Mg2NbN3 over the distorted hexagonal structure of the ordered ground state (compare SI Appendix, Table S3), consistent with our observation of rocksalt in experiment.
To further examine the role of structural disorder on electronic properties of MgG-3TMNG-2 compounds, we performed inverse participation ratio (IPR) calculations (46, 47) on ground state, moderately- and strongly-disordered MgZrN2. Fig. 3C shows that the IPR of the ground-state structure is around 2 near the band edges, indicating about half the atoms contribute to these states, as is the case for many compound semiconductors. In the disordered materials, the magnitude of the IPR in the vicinity of the band edges is hardly affected, and no localized midgap states are observed. Thus, these IPR data indicate that MgZrN2 and related semiconductors are electronically highly tolerant to disorder, even for the “worst-case” scenario of Teff = 10,000 K. The results are striking compared with tetrahedrally coordinated ternary nitrides, where already for Teff = 2,000 K the IPR increased by over 2× at the valence band edge with a concomitant bandgap reduction by one-third (30). We attribute such “defect tolerance” (48, 49) to the exceptionally strong dielectric response in the rocksalt structure, where the potentially adverse effects of breaking the local octet rule are largely screened out.
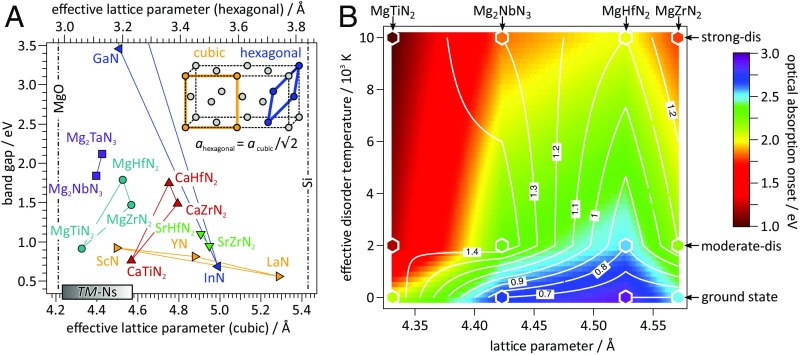
The calculated structural, electronic, and optical properties of the MgG-3TMNG-2 rocksalt materials are summarized in Fig. 4. As inspired by “bandgap engineering” (50) in zincblende and wurtzite compound semiconductors, Fig. 4A shows bandgap vs. effective lattice parameter for the rocksalt-derived crystal structures of MgG-3TMNG-2 in comparison with related nitrides (51, 52). Also included is Mg2TaN3, which was not experimentally investigated in this study, but is predicted to form in the same rocksalt-derived structure as Mg2NbN3 (SI Appendix, Table S1). The group-III TMN rocksalts and heterovalent AEG-3TMNG-2 rocksalts contain several compounds with a bandgap range of ∼1 to 2 eV. These ternary rocksalt nitrides have effective cubic lattice parameters between 4.3 and 5.0 Å and effective hexagonal lattice parameters of 3.0 to 3.5 Å. Since TM-N rocksalts with a similar span of lattice parameters are known to form as ternary alloys and superlattices (36, 53), we postulate that similar materials could be made from AE-TM-N compounds. The hexagonal projection of MgG-3TMNG-2 lattice parameters also falls within the range of InN-GaN-AlN a-lattice parameters.
Fig. 4.
Tunable properties of MgG-3TMNG-2 semiconductors. (A) Bandgap vs. effective lattice parameter for MgG-3TMNG-2, group-III nitrides, other AE-TM-N2 materials, and Si/MgO substrates (51, 52). (B) Interpolation of optical absorption onset (color) and electron effective mass (contour lines) between different MgG-3TMNG-2 lattice parameters and degrees of disorder, as indicated by effective temperature.
Fig. 4B shows a color map of optical absorption onsets (α = 103 cm−1) for MgG-3TMNG-2 with contour lines indicating electron effective masses, both as a function of effective temperature and lattice parameter. The 2D linear interpolation is generated from the calculated values highlighted by white hexagons for ground-state, moderately disordered, and strongly disordered structures. Even though disorder only marginally affects the electronic bandgaps, absorption onsets systematically decrease in energy with disorder. Thus, the degree of disorder could be utilized to tune the absorption strength to the needs of an application without deteriorating charge-transport properties. Since the absorption onset is generally more sensitive to disorder than to lattice parameter, these “disorder-tuned” materials might also be “structurally tuned” to the desired lattice constant via TM alloying.
Finally, we speculate about potential future applications of the MgG-3TMNG-2 materials reported here. The coexistence of multiple useful phenomena, such as superconducting and semiconducting properties, is a key feature of metal nitrides. For example, structural compatibility between (111) NbN and (001) GaN was recently demonstrated in epitaxial heterostructures with a 2D electron gas in GaN and superconductivity in NbN (54). These MgG-3TMNG-2 materials, which are structurally compatible with both III-N semiconductors and TM-N superconductors, will allow for such types of integration. In particular, semiconductor/superconductor heterojunctions with complete structural and chemical compatibility across MgG-3TMNG-2/TMN interfaces might be achievable. Such high-quality interfaces, with defect-tolerant high-k MgG-3TMNG-2 barriers, might enable low-loss Josephson junctions for next-generation quantum computers (55, 56) and other emerging applications.
In conclusion, we have introduced a family of MgG-3TMNG-2 (TM = Ti, Zr, Nb, Hf) semiconducting materials with rocksalt-derived crystal structures, and studied their optical and electronic properties using both sputtered thin-film experiments and ab initio calculations. For each studied material, the measured diffraction patterns are consistent with the calculated rocksalt-derived structures, but with significant antisite disorder on the cation sublattice. When the materials are synthesized with Mg-rich stoichiometry, they exhibit semiconducting properties, including temperature-activated electrical conductivity and optical absorption onsets in the visible range. Ab initio calculations suggest that the optical absorption onset can be tuned both by choice of TM and the degree of ordering on the cation sublattice, while the bandgaps and effective masses exhibit a remarkable tolerance to structural cation disorder. These rocksalt semiconductors may be a subset of a larger family of chemically intuitive compounds formed from host materials containing TMs in structures known to accommodate off-stoichiometric defect phases. This suggests opportunities for design of defect-tolerant semiconductors outside of the familiar space of main-group tetrahedrally bonded materials. The potential for integrating these MgG-3TMNG-2 rocksalt nitrides into semiconductor/superconductor heterostructure devices is highlighted by their structural compatibility with several existing nitrides, and by the promising transport properties measured from sputtered epitaxial films.
Methods
Thin films were grown by radiofrequency sputtering from elemental targets onto glass substrates at a deposition temperature of 400 °C. A gas cracker was used to increase nitrogen reactivity. These films were analyzed for structure via X-ray diffraction at the Stanford Synchrotron Radiation Lightsource (SSRL) and for composition using Rutherford backscattering spectrometry. Electronic transport and optical properties were determined by resistivity and Hall measurements in a van der Pauw geometry, and UV-visible spectroscopy, respectively. Experimental data used by this study have been analyzed using the COMBIgor software package (57) and are publicly available in the National Renewable Energy Laboratory (NREL) high-throughput experimental materials database at https://htem.nrel.gov (58).
First-principles density-functional and many-body perturbation theory calculations were performed with the VASP code, employing the generalized gradient and GW approximations, respectively (59, 60). The ground-state structure search was performed using the “kinetically limited minimization” approach, which is unconstrained and does not require prototypical structures from databases (11). Disordered structures were generated through first-principles Monte Carlo sampling in supercells between 64 and 96 atoms (5 random seeds per each case) using the Metropolis criterion for 2,000 and 10,000 K as effective temperature (42) for moderate and strong disorder, respectively. To calculate the electronic structure and optical absorption for these supercells, we used the single-shot-hybrid plus onsite potential approach (30, 51), with parameters fitted to the GW calculations of the ground states (SI Appendix, Table S4). More detailed information regarding the materials and methods are available in the SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Energy (DOE) under Contract DE-AC36-08GO28308 with Alliance for Sustainable Energy, LLC, the Manager and Operator of the NREL. Funding provided by Office of Science (SC), Office of Basic Energy Sciences (BES), as part of the Energy Frontier Research Center “Center for Next Generation of Materials Design: Incorporating Metastability.” High-performance computing resources were sponsored by the DOE, Office of Energy Efficiency and Renewable Energy. Use of the SSRL, SLAC National Accelerator Laboratory, was supported by the DOE, SC, BES under Contract DE-AC02-76SF00515. Work by A.T. and C.M. was supported by the DOE, SC, BES, Materials Sciences and Engineering Division. We thank Dr. Suchismita Sarker and Dr. Apurva Mehta for assistance at SLAC BL1-5.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Computational data available at National Renewable Energy Laboratory (NREL) MatDB: https://materials.nrel.gov/. Experimental data available at NREL HTEM DB: https://htem.nrel.gov/.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904926116/-/DCSupplemental.
References
- 1.Ponce F., Bour D. P., Nitride-based semiconductors for blue and green light emitting devices. Nature 386, 351–359 (1997). [Google Scholar]
- 2.Mishra U. K., Parikh P., Wu Y.-F., AlGaN/GaN HEMTs-an overview of device operation and applications. Proc. IEEE 90, 1022–1031 (2002). [Google Scholar]
- 3.Nakamura S., The roles of structural imperfections in InGaN-based blue light-emitting diodes and laser diodes. Science 281, 955–961 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Wittmer M., Properties and microelectronic applications of thin films of refractory metal nitrides. J. Vac. Sci. Technol. A 3, 1797–1803 (1985). [Google Scholar]
- 5.Chen X.-J., et al. , Hard superconducting nitrides. Proc. Natl. Acad. Sci. U.S.A. 102, 3198–3201 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tareen A. K., Priyanga G. S., Behara S., Thomas T., Yang M., Mixed ternary transition metal nitrides: A comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Prog. Solid State Chem. 53, 1–26 (2019). [Google Scholar]
- 7.Miura A., et al. , Octahedral and trigonal-prismatic coordination preferences in Nb-, Mo-, Ta-, and W-based ABX2 layered oxides, oxynitrides, and nitrides. J. Solid State Chem. 229, 272–277 (2015). [Google Scholar]
- 8.Hinuma Y., et al. , Discovery of earth-abundant nitride semiconductors by computational screening and high-pressure synthesis. Nat. Commun. 7, 11962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun W., et al. , A map of the inorganic ternary metal nitrides. Nat. Mater. 18, 732–739 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Gall D., et al. , Electronic structure of ScN determined using optical spectroscopy, photoemission, and ab initio calculations. Phys. Rev. B 63, 125119 (2001). [Google Scholar]
- 11.Arca E., et al. , Redox-mediated stabilization in zinc molybdenum nitrides. J. Am. Chem. Soc. 140, 4293–4301 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Martinez A. D., Fioretti A. N., Toberer E. S., Tamboli A. C., Synthesis, structure, and optoelectronic properties of II-IV-V2 materials. J. Mater. Chem. A 5, 11418–11435 (2017). [Google Scholar]
- 13.Arca E., et al. , Zn2SbN3: Growth and characterization of a metastable photoactive semiconductor. Mater. Horiz. 10.1039/C9MH00369J (2019). [DOI] [Google Scholar]
- 14.Gregory D. H., Structural families in nitride chemistry. J. Chem. Soc. Dalt. Trans., 1999, 259–270 (1999). [Google Scholar]
- 15.Etourneau J., Portier J., Ménil F., The role of the inductive effect in solid state chemistry: How the chemist can use it to modify both the structural and the physical properties of the materials. J. Alloys Compd. 188, 1–7 (1992). [Google Scholar]
- 16.Toth L., Transition Metal Carbides and Nitrides (Elsevier Science, 1971). [Google Scholar]
- 17.Jackson A. W., Shebanova O., Hector A. L., McMillan P. F., Amorphous and nanocrystalline titanium nitride and carbonitride materials obtained by solution phase ammonolysis of Ti(NMe2)4. J. Solid State Chem. 179, 1383–1393 (2006). [Google Scholar]
- 18.Bhadram V. S., et al. , Semiconducting cubic titanium nitride in the Th3P4 structure. Phys. Rev. Mater. 2, 011602 (2018). [Google Scholar]
- 19.Orisakwe E., Fontaine B., Gregory D. H., Gautier R., Halet J.-F., Theoretical study on the structural, electronic and physical properties of layered alkaline-earth-group-4 transition-metal nitrides AEMN2. RSC Adv. 4, 31981–31987 (2014). [Google Scholar]
- 20.Gharavi M. A., Armiento R., Alling B., Eklund P., Theoretical study of phase stability, crystal and electronic structure of MeMgN2 (Me = Ti, Zr, Hf) compounds. J. Mater. Sci. 53, 4294–4305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory D. H., Barker M. G., Edwards P. P., Siddons D. J., Synthesis and structure of two new layered ternary nitrides, SrZrN2 and SrHfN2. Inorg. Chem. 35, 7608–7613 (1996). [Google Scholar]
- 22.Gregory D. H., Barker M. G., Edwards P. P., Slaski M., Siddons D. J., Synthesis, structure, and magnetic properties of the new ternary nitride BaHfN2 and of the BaHf1-xZrxN2 Solid solution. J. Solid State Chem. 137, 62–70 (1998). [Google Scholar]
- 23.Gregory D. H., Barker M. G., Edwards P. P., Siddons D. J., Synthesis and structure of the new ternary nitride SrTiN2. Inorg. Chem. 37, 3775–3778 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Brokamp T., Jacobs H., Darstellung und Struktur einiger Gemischtvalenter ternärer tantalnitride mit lithium und magnesium. J. Alloys Compd. 183, 325–344 (1992). [Google Scholar]
- 25.Fenker M., Balzer M., Kappl H., Banakh O., Some properties of (Ti,Mg)N thin films deposited by reactive dc magnetron sputtering. Surf. Coat. Technol. 200, 227–231 (2005). [Google Scholar]
- 26.Alling B., Metal to semiconductor transition and phase stability of Ti1-xMgxNy alloys investigated by first-principles calculations. Phys. Rev. B 89, 085112 (2014). [Google Scholar]
- 27.Wang B., et al. , Growth and properties of epitaxial Ti1-x MgxN (001) layers. J. Vac. Sci. Technol. A 36, 061501 (2018). [Google Scholar]
- 28.Bauers S. R., et al. , Composition, structure, and semiconducting properties of Mgx Zr2–x N2 thin films. Jpn. J. Appl. Phys. 58, SC1015 (2019). [Google Scholar]
- 29.Mather G. C., Dussarrat C., Etourneau J., West A. R., A review of cation-ordered rock salt superstructure oxides. J. Mater. Chem. 10, 2219–2230 (2000). [Google Scholar]
- 30.Lany S., et al. , Monte Carlo simulations of disorder in ZnSnN2 and the effects on the electronic structure. Phys. Rev. Mater. 1, 035401 (2017). [Google Scholar]
- 31.Zawadzki P. P., Perkins J., Lany S., Modeling amorphous thin films: Kinetically limited minimization. Phys. Rev. B 90, 94203 (2014). [Google Scholar]
- 32.Shannon R. D., Cr I. U., Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976). [Google Scholar]
- 33.Urban A., Abdellahi A., Dacek S., Artrith N., Ceder G., Electronic-structure origin of cation disorder in transition-metal oxides. Phys. Rev. Lett. 119, 176402 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Salamat A., Kroll P., McMillan P. F., Nitrogen-rich transition metal nitrides. Coord. Chem. Rev. 257, 2063–2072 (2013). [Google Scholar]
- 35.Fioretti A. N., et al. , Combinatorial insights into doping control and transport properties of zinc tin nitride. J. Mater. Chem. C 3, 11017–11028 (2015). [Google Scholar]
- 36.Saha B., Shakouri A., Sands T. D., Rocksalt nitride metal/semiconductor superlattices: A new class of artificially structured materials. Appl. Phys. Rev. 5, 021101 (2018). [Google Scholar]
- 37.Moram M. A., Zhang Y., Kappers M. J., Barber Z. H., Humphreys C. J., Dislocation reduction in gallium nitride films using scandium nitride interlayers. Appl. Phys. Lett. 91, 152101 (2007). [Google Scholar]
- 38.Moram M. A., et al. , Growth of epitaxial thin films of scandium nitride on 100-oriented silicon. J. Cryst. Growth 310, 2746–2750 (2008). [Google Scholar]
- 39.Hedin L., New method for calculating the one-particle green’s function with application to the electron-gas problem. Phys. Rev. 139, A796–A823 (1965). [Google Scholar]
- 40.Anusca I., et al. , Dielectric response: Answer to many questions in the methylammonium lead halide solar cell absorbers. Adv. Energy Mater. 7, 1700600 (2017). [Google Scholar]
- 41.Green M. A., Intrinsic concentration, effective densities of states, and effective mass in silicon. J. Appl. Phys. 67, 2944–2954 (1990). [Google Scholar]
- 42.Ndione P. F., et al. , Control of the electrical properties in spinel oxides by manipulating the cation disorder. Adv. Funct. Mater. 24, 610–618 (2014). [Google Scholar]
- 43.Fioretti A. N., et al. , Exciton photoluminescence and benign defect complex formation in zinc tin nitride. Mater. Horiz. 5, 823–830 (2018). [Google Scholar]
- 44.Quayle P. C., et al. , Charge-neutral disorder and polytypes in heterovalent wurtzite-based ternary semiconductors: The importance of the octet rule. Phys. Rev. B 91, 205207 (2015). [Google Scholar]
- 45.Skachkov D., Quayle P. C., Kash K., Lambrecht W. R. L., Disorder effects on the band structure of ZnGeN2: Role of exchange defects. Phys. Rev. B 94, 205201 (2016). [Google Scholar]
- 46.Baranowski L. L., et al. , Effects of disorder on carrier transport in Cu2SnS3. Phys. Rev. Appl. 4, 044017 (2015). [Google Scholar]
- 47.Zawadzki P., Zakutayev A., Lany S., Entropy-driven clustering in tetrahedrally bonded multinary materials. Phys. Rev. Appl. 3, 034007 (2015). [Google Scholar]
- 48.Brandt R. E., Stevanović V., Ginley D. S., Buonassisi T., Identifying defect-tolerant semiconductors with high minority-carrier lifetimes: Beyond hybrid lead halide perovskites. MRS Commun. 5, 265–275 (2015). [Google Scholar]
- 49.Zakutayev A., et al. , Defect tolerant semiconductors for solar energy conversion. J. Phys. Chem. Lett. 5, 1117–1125 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Vurgaftman I., Meyer J. R., Ram-Mohan L. R., Band parameters for III–V compound semiconductors and their alloys. J. Appl. Phys. 89, 5815–5875 (2001). [Google Scholar]
- 51.Lany S., Band-structure calculations for the 3d transition metal oxides in GW. Phys. Rev. B 87, 085112 (2013). [Google Scholar]
- 52.Wu J., et al. , Temperature dependence of the fundamental band gap of InN. J. Appl. Phys. 94, 4457–4460 (2003). [Google Scholar]
- 53.Matenoglou G. M., et al. , Structure, stability and bonding of ternary transition metal nitrides. Surf. Coat. Tech. 204, 911–914 (2009). [Google Scholar]
- 54.Yan R., et al. , GaN/NbN epitaxial semiconductor/superconductor heterostructures. Nature 555, 183–189 (2018).29516996 [Google Scholar]
- 55.Mourik V., et al. , Signatures of Majorana fermions in hybrid superconductor-semiconductor nanowire devices. Science 336, 1003–1007 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Oliver W. D., Welander P. B., Materials in superconducting quantum bits. MRS Bull. 38, 816–825 (2013). [Google Scholar]
- 57.Talley K. R., et al. , COMBIgor: Data analysis package for combinatorial materials science. ACS Comb. Sci. 10.1021/acscombsci.9b00077 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Zakutayev A., et al. , An open experimental database for exploring inorganic materials. Sci. Data 5, 180053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kresse G., Joubert D., From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- 60.Shishkin M., Kresse G., Implementation and performance of the frequency-dependent GW method within the PAW framework. Phys. Rev. B 74, 035101 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.