Considerable attention has been given to the well-known growth–longevity trade-off in biology, but mechanistic explanations for this trade-off remain incompletely understood. While a life history trade-off is generally assumed to result from resource allocation conflicts (1), Roskilly et al. (2) provide convincing evidence that a single trait of xylem anatomy governs the growth–longevity trade-off in the conifer species Pinus ponderosa. By applying a dendrochronological approach to uncouple the effects of xylem anatomy on tree age and size, the authors show that the micromorphology of bordered pits may provide a mechanistic explanation for why fast-growing trees of P. ponderosa have a much shorter life span than their slow-growing counterparts.
Bordered pits in conifer xylem have been described in detail for more than 100 y. The unique pit membrane of conifers, composed of a thickened, central torus held in place by a highly porous margo, is believed to have evolved to ensure efficient water transport through narrow, unicellular tracheids, which have a naturally higher resistance to water flow than the broad, multicellular vessels in angiosperms (3). Aspiration of the pit aperture by the torus has a long history of observation (4), with available evidence suggesting that torus displacement to an aspirated position requires a force below 3 µN (5). The key function of pit membrane aspiration is to prevent the spread of drought-induced air embolism into neighboring tracheids (6). Although the actual mechanisms governing embolism resistance in conifers are not fully understood, the torus area that covers the pit border upon aspiration can explain variation in embolism resistance within and between species (7–9). Aspiration of the pit aperture by the torus not only stops air entry into neighboring tracheids but also affects the movement of xylem sap between adjacent cells, as well as the permeability of liquids, preservatives, and gases in timbers, which has noteworthy consequences for the paper and pulp industry.
The most interesting finding of Roskilly et al. (2) is that the amount of torus overlap correlates with the growth rate and longevity of P. ponderosa within and between populations. Large torus overlap values were found in both young and old slow-growing trees, the oldest of which had lived for 450 y, while fast-growing trees showed narrower torus overlap and reached a maximum age of 125 y only. Although Roskilly et al. do not include hydraulic or gas exchange measurements, it is likely that the difference in torus overlap between these 2 cohorts has major consequences for the hydraulic safety and efficiency of the xylem, and consequently photosynthetic capacity and growth rates (Fig. 1). While torus overlap is known to be the main determinant of embolism resistance, it is less clear how torus overlap relates to hydraulic efficiency. Since the margo of P. ponderosa is highly porous and considerably large, the main hydraulic bottleneck is thought to be pit aperture in this species. While computational modeling suggests that pit apertures represent a small proportion (up to 25%) of the total pit resistance to water flow (10–12), other studies suggested that the modeled aperture resistance is considerably higher than margo resistance, especially in embolism-resistant species of the Cupressaceae (13, 14). Assuming that pit density and functional sapwood area between the fast- and slow-growing young trees is fairly similar, minor differences in pit aperture size and therefore torus overlap are likely to affect hydraulic efficiency in P. ponderosa.
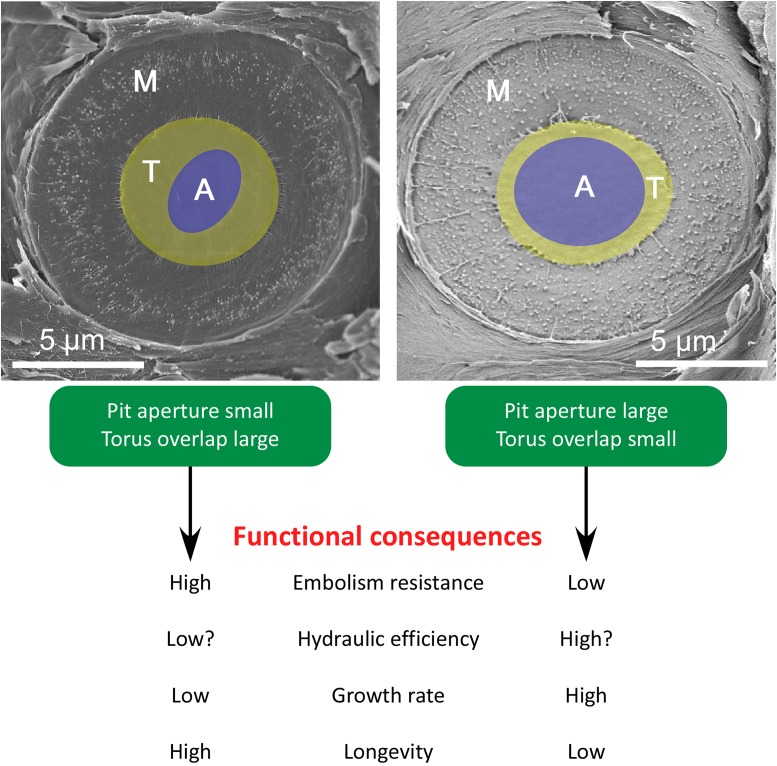
Fig. 1.
Scanning electron microscopy images of a bordered pit with a torus–margo pit membrane between adjacent tracheids in wood of C. columellaris (Left) and C. rhomboidea (Right). Clear differences in the torus–aperture ratio can be seen, which result from a difference in aperture dimensions (A, blue area), while the torus (T, yellow) and margo (M) sizes are more or less similar. Functional implications associated with torus overlap are summarized with respect to embolism resistance, hydraulic efficiency, growth rate, and longevity.
Other xylem-related traits such as tracheid diameter and wood density did not show any significant difference between the fast-growing and slow-growing trees of P. ponderosa. This is surprising because both tracheid dimensions, especially tracheid width, and wood density have been given considerable attention with respect to hydraulic safety and efficiency within a wood economics spectrum (15). Measurements of tracheid width and wood density are more straightforward than quantification of torus overlap, which requires pit membrane, torus, and pit aperture measurements based on (electron) microscopy. The finding that torus overlap represents the only xylem characteristic associated with the growth–longevity trade-off illustrates the functional importance of this well-hidden wood anatomical feature in the species studied. To what extent this result can be generalized to other conifer species both at an intraspecific and interspecific level requires further research. Anecdotal evidence from 2 species of Callitris native to Australia suggests that the finding by Roskilly et al. may be general: Torus overlap in Callitris columellaris is considerably larger than torus overlap in Callitris rhomboidea (Fig. 1). This difference in torus overlap corresponds with reported differences in longevity, growth rate, and embolism resistance between these 2 species, with C. columellaris growing slower, living longer, and having considerably more embolism-resistant xylem than C. rhomboidea (16, 17).
Importantly, the torus overlap difference between fast- and slow-growing trees of P. ponderosa is driven by variation in pit aperture area, while dimensions of the pit membrane and torus remain fairly constant, independent of growth rate and the amount of seasonal drought experienced by populations from a wetter and a drier field site. This finding is in line with earlier work that shows how pit aperture variation drives torus overlap in relation to embolism resistance (7–9, 14), and raises the following question: What determines pit aperture size? Since pit membrane, margo, and torus dimensions remain relatively constant, it can be assumed that either secondary wall thickness and/or the slope of the pit chamber roof (i.e., the inner pit border partly overlapping the pit membrane) control pit aperture size, with thicker cell walls and/or a more shallow slope of the pit border resulting in narrow pit apertures. Although direct observations of tracheid wall thickness were not provided, the authors speculate that variation in wall thickness is fairly low due to limited variation in wood density. Secondary wall thickness, which is also associated with the duration of the cell enlargement phase during tracheid ontogeny (18), should be given more attention as a possible explanation for pit aperture variation. It is currently unclear whether pit chamber roofs differ in their slope between fast- and slow-growing trees of P. ponderosa, while future work should also focus on how exactly site-specific secondary cell wall deposition is locally prevented at the pit aperture during bordered pit development and tracheid differentiation (19).
A major unknown from the data of Roskilly et al. is what killed the earlier generations of fast-growing P. ponderosa, given that logging in the remote field sites selected can be excluded. Considering the importance of the torus overlap for preventing embolism spread during drought, it is tempting to speculate that major drought events in the past were the ultimate agents of death for previous generations of fast-growing individuals in a population, with the slow-growing individuals being marginally more resistant to drought-induced embolism and thus surviving. Given the current rate of climate change and the increased severity and frequency of drought events globally, we might experience conditions that will test the hypothesis that fast-growing individuals of P. ponderosa are less likely to survive drought events (20).
The main lesson from Roskilly et al. is that further attention needs to be placed on the torus–aperture ratio when exploring future questions of conifer hydraulic safety and efficiency, growth rates, life span, and the mechanisms driving tree mortality. Although bordered pits are known to present a key evolutionary feature that enabled vascular plants to transport water under negative pressure, our mechanistic understanding of how bordered pit membranes affect water flow in plants remains an important future challenge for both gymnosperms and angiosperms (21–23). Indeed, how exactly plants transport water under negative pressure is not fully understood, even though this topic represents a long-standing question in plant biology (21). Therefore, the integration of descriptive, experimental, and modeling data combining functional plant anatomy with ecophysiological measurements on water transport and gas exchange in plants will undoubtedly remain an important research field over the next decades, with many exciting discoveries to be made.
Acknowledgments
S.M. was supported by a postdoctoral fellowship from the Alexander von Humboldt Foundation.
Footnotes
The authors declare no conflict of interest.
See companion article on page 15282.
References
- 1.Agrawal A. A., Conner J. K., Rasmann S., “Trade-offs and negative correlations in evolutionary ecology” in Evolution after Darwin: The First 150 Years, Bell M. A., Eanes W. F., Futuyma D. J., Levington J. S., Eds. (Sinauer Associates, Sunderland, MA, 2010), pp. 243–268. [Google Scholar]
- 2.Roskilly B., Keeling E., Hood S., Giuggiola A., Sala A., Conflicting functional effects of xylem pit structure relate to the growth-longevity trade-off in a conifer species. Proc. Natl. Acad. Sci. U.S.A. 116, 15282–15287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittermann J., Sperry J. S., Hacke U. G., Wheeler J. K., Sikkema E. H., Torus-margo pits help conifers compete with angiosperms. Science 310, 1924 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Bailey I. W., The preservative treatment of wood. II. The structure of the pit membranes in the tracheids of conifers and their relation to the penetration of gases, liquids, and finely divided solids into green and seasoned wood. Forestry Quart. 11, 12–20 (1913). [Google Scholar]
- 5.Zelinka S. L., et al. , Force-displacement measurements of earlywood bordered pits using a mesomechanical tester. Plant Cell Environ. 38, 2088–2097 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Delzon S., Douthe C., Sala A., Cochard H., Mechanism of water-stress induced cavitation in conifers: Bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant Cell Environ. 33, 2101–2111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domec J.-C., et al. , Maximum height in a conifer is associated with conflicting requirements for xylem design. Proc. Natl. Acad. Sci. U.S.A. 105, 12069–12074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hacke U. G., Jansen S., Embolism resistance of three boreal conifer species varies with pit structure. New Phytol. 182, 675–686 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Bouche P. S., et al. , A broad survey of hydraulic and mechanical safety in the xylem of conifers. J. Exp. Bot. 65, 4419–4431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valli A., Koponen A., Vesala T., Timonen S., Simulations of water flow through bordered pits of conifer xylem. J. Stat. Phys. 107, 121–142 (2002). [Google Scholar]
- 11.Schulte P. J., Computational fluid dynamics models of conifer bordered pits show how pit structure affects flow. New Phytol. 193, 721–729 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Schulte P. J., Hacke U. G., Schoonmaker A. L., Pit membrane structure is highly variable and accounts for a major resistance to water flow through tracheid pits in stems and roots of two boreal conifer species. New Phytol. 208, 102–113 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Hacke U. G., Sperry J. S., Pittermann J., Analysis of circular bordered pit function II. Gymnosperm tracheids with torus-margo pit membranes. Am. J. Bot. 91, 386–400 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Pittermann J., et al. , The relationships between xylem safety and hydraulic efficiency in the Cupressaceae: The evolution of pit membrane form and function. Plant Physiol. 153, 1919–1931 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chave J., et al. , Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Brodribb T. J., Bowman D. J., Nichols S., Delzon S., Burlett R., Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 188, 533–542 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Pearson S., Hua Q., Allen K., Bowman D. M. J. S., Validating putatively cross-dated Callitris tree-ring chronologies using bomb-pulse radiocarbon analysis. Aust. J. Bot. 59, 7–17 (2011). [Google Scholar]
- 18.Cuny H. E., Rathgeber C. B. K., Frank D., Fonti P., Fournier M., Kinetics of tracheid development explain conifer tree-ring structure. New Phytol. 203, 1231–1241 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Savidge R. A., Cell biology of bordered-pit formation in balsam-fir trees. Botany 92, 495–511 (2014). [Google Scholar]
- 20.Hartmann H., et al. , Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 218, 15–28 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Jansen S., Schenk H. J., On the ascent of sap in the presence of bubbles. Am. J. Bot. 102, 1561–1563 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Schenk H. J., et al. , Xylem surfactants introduce a new element to the cohesion-tension theory. Plant Physiol. 173, 1177–1196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen S., et al. , Challenges in understanding air-seeding in angiosperm xylem. Acta Hortic. 1222, 13–20 (2018). [Google Scholar]