Abstract
The recently described Denisovan hemimandible from Xiahe, China [F. Chen et al., (2019) Nature 569, 409–412], possesses an unusual dental feature: a 3-rooted lower second molar. A survey of the clinical and bioarchaeological literature demonstrates that the 3-rooted lower molar is rare (less than 3.5% occurrence) in non-Asian Homo sapiens. In contrast, its presence in Asian-derived populations can exceed 40% in China and the New World. It has long been thought that the prevalence of 3-rooted lower molars in Asia is a relatively late acquisition occurring well after the origin and dispersal of H. sapiens. However, the presence of a 3-rooted lower second molar in this 160,000-y-old fossil hominin suggests greater antiquity for the trait. Importantly, it also provides morphological evidence of a strong link between archaic and recent Asian H. sapiens populations. This link provides compelling evidence that modern Asian lineages acquired the 3-rooted lower molar via introgression from Denisovans.
Keywords: Denisovan, introgression, dental anthropology, root morphology, Pleistocene Homo
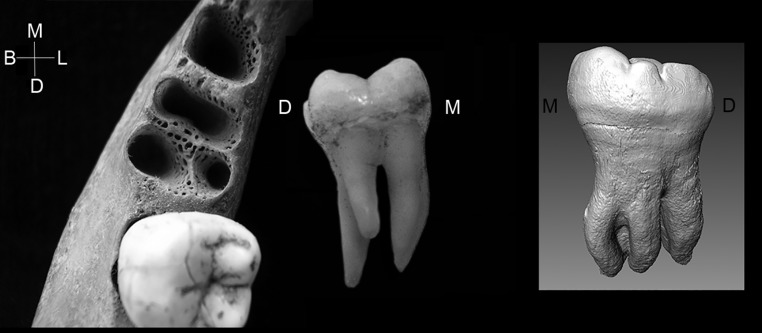
The new Denisovan hemimandible from Xiahe, China (1), exhibits a 3-rooted lower molar, providing a direct morphological link between archaic and recent Asian Homo sapiens populations. Although mandibular molars are most commonly 2-rooted in the genus Homo, root number varies from 1 to 3 (2) or more (3). Three-rooted lower molars maintain both mesial and distal roots, with a third accessory root on either the distolingual aspect or lingually between the mesial and distal roots (Fig. 1). The third root is not a simple bifurcation of either the mesial or distal root tips. While the accessory root can be quite small, it is usually about one-third the size of the normal roots (2). In recent humans, the third root usually occurs on the mandibular first molar (referred to as a 3RM1) but may also occur on the lower second and third molars; we refer to these collectively as 3RM (4–6). The 3RM may appear either unilaterally or bilaterally; the single twin study of which we are aware shows bilateral development in both twins, suggesting a genetic underpinning (7). The 3RM entered the clinical literature in 1844 (8), being called radix entomolaris by Bolk (9); it was codified into the Arizona State University Dental Anthropology System in 1991 (2).
Fig. 1.
The 3-rooted lower molar anomaly. Three-rooted lower first molar alveolar sockets showing distolingual position of accessory root and the 3-rooted lower first molar (lingual view); (Inset) 3-rooted lower second molar of Xiahe Denisovan individual (lingual view). Left and Middle images courtesy of Christine Lee (California State University, Los Angeles, CA). M, mesial; L, lingual; D, distal; B, buccal.
Extensive clinical and bioarchaeological studies confirm the rarity of 3RM outside of Asia and the New World (Dataset S1). In Asian-derived populations, the frequency of the 3RM can exceed 40% (Aleut, Neolithic China), whereas, in non-Asian−derived populations, the frequency ranges from 0 to 3.4%. The rarity of the 3RM in non-Asian H. sapiens is low enough to be explained by mutation alone (2). The high frequencies of 3RM in northeast Asians and Native Americans is a key feature linking Native American origins to Asia (10).
Despite its high frequency in recent Asian-derived populations, the 3RM has not been reported in the earliest H. sapiens from Asia (11), nor have we observed the trait in early H. sapiens from Africa or Homo erectus in Asia.* We note, however, that the lack of radiography of many specimens and the absence of the original Zhoukoudian remains make this conclusion preliminary. Before the recent discoveries, the earliest example of a 3RM came from an H. sapiens mandible from the Philippines (15), perhaps from the site of Tabon, which has fossil-bearing strata dating to 9 ka, 16.5 ka, and as much as 47 ka, and more recent Jar burials (16, 17). The mandible, originally described by Macintosh et al. (18), shows a bilateral 3RM with an accessory root situated lingually between the mesial and distal roots (15). Given a lack of early evidence for 3RM, one explanation for its high frequency in Asia has been a relatively recent acquisition postdating the origin of H. sapiens and occurring well after their dispersal into Eurasia.
Two recently described mandibles suggest a more ancient Asian origin for 3RM, and one that precedes H. sapiens in the region. The newly discovered individual from Xiahe, China—identified as Denisovan through paleoproteomics (1)—possesses a 3-rooted lower second molar (3RM2) (Fig. 1).† This individual is dated to 160 ka. The recently described Penghu 1 mandible from Taiwan (190 to 10 ka) also exhibits a 3RM2 (19). Although the authors suggest the 3RM2 differs from that described by Turner et al. (2), it is clear that the morphology of Penghu 1 falls within the variation described by previous studies (2, 10): The third root appears lingually as an accessory root between the mesial and distal roots. The Penghu mandible retains “archaic” features, including a receding symphysis that lacks a chin, a thick mandibular corpus, and large molar crowns similar in size to Denisovans (20). Like Xiahe, these exceptionally large molars are coupled with agenesis of the third molar (19). For these reasons, Chen et al. (1) suggest that Penghu 1 may also be closely related to Denisovans. Both mandibles show that the 3RM anomaly existed in archaic Asian hominins before H. sapiens in the region.
These 2 recently reported fossils suggest that the 3RM 1) very likely originated in Asia and 2) evolved in a pre-sapiens population. Moreover, until a 3RM is found in more archaic hominins, it should be understood as a morphological trait that was transferred to H. sapiens through gene flow with Denisovans. Gene flow between H. sapiens and Denisovans has been documented, including a mutation (at EPAS1) related to high-altitude adaptation shared by a Siberian Denisovan and modern Tibetans (21). Importantly, Nepal also shows one of the highest occurrences (25%) of 3RM in East Asia (Dataset S1). Like the high-altitude related mutation, which is retained due to positive selection (21), the retention of 3RM at high frequency in Asia may be related to selection for molar retention in populations with heavy masticatory loading (22). Such selection also explains the lower frequency of 3RM in recent populations with higher Denisovan introgression (e.g., Australia/New Guinea) but demonstrably less masticatory robusticity (23). Alternatively, 3RM frequencies may reflect an indirect influence resulting from selection on another trait under high selection, as has been suggested to be the case for incisor crown morphology and EDAR in North and East Asians and in the New World (24). Whatever the cause, we argue that the 3RM anomaly is an example of a morphological character in recent humans that can be clearly traced to this archaic admixture.
The 3RM is an Asian-derived character that we can definitively trace to Denisovans. Thus, we now have very clear evidence that gene flow between archaic groups and H. sapiens resulted in the transfer of identifiable morphological features. There have been a number of Asian H. sapiens fossils described recently that point to admixture with archaic humans as an explanation for the presence of primitive traits [e.g., Dushan (11) and Tianyuan (25)]. If the 3RM was transferred from archaic humans to H. sapiens, other traits may have been as well. Indeed, the presence of “archaic features” in recent Asians that were once used to suggest continuity from Pleistocene Asian H. erectus (26–28) may also have been obtained by introgression from Denisovans.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
*Wu and Xianglong (12) report the presence of a 3RM1 in the 1959 Zhoukoudian mandible (PA 86) based on observations of its left M1 root socket. Published photographs (13, 14), however, show a small septum of the mesial root socket (that may represent a bifurcated mesial root) but no evidence of a lingual accessory root. The 3RM anomaly requires that the accessory root occurs between the mesial and distal root or as a lingual accessory of the distal root.
†The first molar in this individual has 2 roots.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907557116/-/DCSupplemental.
References
- 1.Chen F., et al. , A late Middle Pleistocene Denisovan mandible from the Tibetan Plateau. Nature 569, 409–412 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Turner C. G. II, Nichol C. R., Scott G. R., “Scoring procedures for key morphological traits of the permanent dentition: The Arizona State University Dental Anthropology System” in Advances in Dental Anthropology, Kelley M., Larsen C., Eds. (Wiley Liss, New York, NY, 1991), pp. 13–31. [Google Scholar]
- 3.Sidow S. J., West L. A., Liewehr F. R., Loushine R. J., Root canal morphology of human maxillary and mandibular third molars. J. Endod. 26, 675–678 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Ferraz J. A. B., Pécora J. D., Three-rooted mandibular molars in patients of Mongolian, Caucasian and Negro origin. Braz. Dent. J. 3, 113–117 (1993). [PubMed] [Google Scholar]
- 5.Carlsen O., Alexandersen V., Radix entomolaris: Identification and morphology. Scand. J. Dent. Res. 98, 363–373 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Erkman A., Kaya F., Morphological variations in three-rooted mandibular molars in Ancient Anatolian populations (Dilkaya Mound, Van, Turkey): A literature review on world populations. Mediterr. Archaeol. Archaeom. Int. J. 14, 1–11 (2012). [Google Scholar]
- 7.Gabriel A. C., “Genetic types in teeth” in Essays in Biology, Burkitt A. N., Ed. (Australian Medical Publishing, Sydney, NSW, Australia, 1948), pp 7–61. [Google Scholar]
- 8.Carabelli G., Systematisches Handbuch der Zahnheilkunde (Braumüller and Seidel, Vienna, Austria, 1844). [Google Scholar]
- 9.Bolk L., Bemerkungen über Wurzelvariationen am menschlichen unteren Molaren. Z. Morphol. Anthropol. 17, 605–610 (1915). [Google Scholar]
- 10.Turner C. G., II, Three-rooted mandibular first permanent molars and the question of American Indian origins. Am. J. Phys. Anthropol. 34, 229–241 (1971). [DOI] [PubMed] [Google Scholar]
- 11.Liao W., et al. , Mosaic dental morphology in a terminal Pleistocene hominin from Dushan Cave in southern China. Sci. Rep. 9, 2347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L., Xianglong Z., Preliminary impression of current dental anthropology research in China. Dental Anthropology Newsletter 9, 1–5 (1995). [Google Scholar]
- 13.Wu X., Poirier F. E., Human Evolution in China. A Metric Description of the Fossils and a Review of the Sites (Oxford University Press, Oxford, UK, 1995). [Google Scholar]
- 14.Wu J., Chao T., New discovery of a Sinanthropus mandible in Choukoutien. Paleovertebrata et Paleoanthropologia 1, 155–158 (1959). [Google Scholar]
- 15.Barker B., Dental features of the Tabon Mandible: An appendix. Archaeol. Phys. Anthropol. Ocean. 13, 160–166 (1978). [Google Scholar]
- 16.Dizon E., New direct dating of the human fossils from Tabon Cave, Palawan, Phillipines. Proc. Soc. Philipp. Archaeologists 1, 63–67 (2003). [Google Scholar]
- 17.Corny J., “Les restes humains de la grotte de Tabon (Palawan, Philippines): Répartition spatiale et étude d’une collection d’ossements inédite,” MS thesis, Muséum national d’Histoire naturelle, Paris, France (2008).
- 18.MacIntosh N., Barker B., Larnach S., The Tabon Cave mandible. Archaeol. Phys. Anthropol. Ocean. 13, 143–159 (1978). [Google Scholar]
- 19.Chang C.-H., et al. , The first archaic Homo from Taiwan. Nat. Commun. 6, 6037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reich D., et al. , Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468, 1053–1060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huerta-Sánchez E., et al. , Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512, 194–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner C. G., II, Late Pleistocene and Holocene population history of East Asia based on dental variation. Am. J. Phys. Anthropol. 73, 305–321 (1987). [DOI] [PubMed] [Google Scholar]
- 23.Antón S. C., Carter-Menn H., DeLeon V. B., Modern human origins: Continuity, replacement, and masticatory robusticity in Australasia. J. Hum. Evol. 60, 70–82 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Hlusko L. J., et al. , Environmental selection during the last ice age on the mother-to-infant transmission of vitamin D and fatty acids through breast milk. Proc. Natl. Acad. Sci. U.S.A. 115, E4426–E4432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shang H., Tong H., Zhang S., Chen F., Trinkaus E., An early modern human from Tianyuan Cave, Zhoukoudian, China. Proc. Natl. Acad. Sci. U.S.A. 104, 6573–6578 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frayer D., Wolpoff M., Thorne A., Smith F., Pope G., Theories of modern human origins: The paleontological test. Am. Anthropol. 95, 14–50 (1993). [Google Scholar]
- 27.Liu W., “The dental continuity of humans in China from Pleistocene to Holocene, and the origins of Mongoloids” in Proceedings of the 30th Annual Geological Congress (VSP Publishing, Wakefield, UK, 1997), vol. 21, pp. 24–32. [Google Scholar]
- 28.Wolpoff M., Thorne A., Smith F., Frayer D., Pope G., “Multiregional evolution: A world-wide source for modern human populations” in Origins of Anatomically Modern Humans, Interdisciplinary Contibutions to Archaeology, Nitecki M., Nitecki D., Eds. (Plenum Press, New York, NY, 1994), pp. 176–199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.