Significance
Functional loss of gut barrier integrity with subsequent increased antigen trafficking and occurrence of low-grade intestinal inflammation precede the onset of type 1 diabetes (T1D) in patients and preclinical models, thus suggesting that these events are mechanistically linked to the autoimmune pathogenesis of the disease. However, a causal relationship between increased intestinal permeability and autoimmune diabetes was never demonstrated. Our data show that breakage of gut barrier continuity leads to activation of islet-reactive T cells in the intestine, thus triggering autoimmune diabetes. An important implication of our findings is that restoration of a healthy gut barrier through microbiota and diet modulation in diabetes-prone individuals could reduce intestinal activation of islet-reactive T cells and prevent T1D occurrence.
Keywords: autoimmune diabetes, microbiota, gut inflammation
Abstract
Low-grade intestinal inflammation and alterations of gut barrier integrity are found in patients affected by extraintestinal autoimmune diseases such as type 1 diabetes (T1D), but a direct causal link between enteropathy and triggering of autoimmunity is yet to be established. Here, we found that onset of autoimmunity in preclinical models of T1D is associated with alterations of the mucus layer structure and loss of gut barrier integrity. Importantly, we showed that breakage of the gut barrier integrity in BDC2.5XNOD mice carrying a transgenic T cell receptor (TCR) specific for a beta cell autoantigen leads to activation of islet-reactive T cells within the gut mucosa and onset of T1D. The intestinal activation of islet-reactive T cells requires the presence of gut microbiota and is abolished when mice are depleted of endogenous commensal microbiota by antibiotic treatment. Our results indicate that loss of gut barrier continuity can lead to activation of islet-specific T cells within the intestinal mucosa and to autoimmune diabetes and provide a strong rationale to design innovative therapeutic interventions in “at-risk” individuals aimed at restoring gut barrier integrity to prevent T1D occurrence.
Type 1 diabetes (T1D) is an autoimmune disease mediated by self-reactive T cells that destroy insulin-producing beta cells of the pancreatic islets. Although it is known that genetic and environmental factors are involved in T1D pathogenesis, the mechanisms governing the activation of islet-specific autoimmune T cells are still unclear (1). Several environmental risk factors for T1D act at the intestinal level such as enteric infections (i.e., enteroviruses and rotaviruses) (2, 3), reactions to dietary antigens (i.e., cow’s milk and gluten) (4, 5), and modifications of the gut microbiota induced by diet composition, excessive hygiene, antibiotics, and other modulators (6–9). Those factors, specifically proinflammatory diet and alteration of the microbiota composition (dysbiosis), induce intestinal inflammation and modify the metabolic and immunological profile in the intestinal mucosa of T1D patients (10, 11). In line with this idea, the development of clinical diabetes in patients and preclinical models of T1D is often preceded by clinically silent signs of intestinal inflammation such as increased permeability, lymphocyte infiltration, expression of MHC II molecules, and the presence of inflammatory cytokines in the intestinal mucosa (12–18). In humans, signs of intestinal inflammation are detectable before clinical onset of T1D in individuals with beta cell autoimmunity (islet autoantibody positivity) and no hyperglycemia (19). Similarly, augmented gut permeability appears before the development of insulitis in diabetes-prone rats (BB-DP) in comparison with diabetes-resistant rats (BB-DR) (12, 20). Those findings indicate that the breakage of gut barrier integrity with subsequent increased antigen trafficking and occurrence of low-grade intestinal inflammation precede the onset of T1D and are directly related to its pathogenesis rather than secondary to diabetes-induced metabolic alterations, i.e., hyperglycemia. However, although these data suggest a causal relationship between the presence of a leaky gut and the pathogenic process of T1D (21), it is yet to be determined whether functional loss of gut barrier integrity does directly trigger beta cell autoimmunity and, if it does, how this process occurs. It has been proposed that leakage of intestinal barriers could lead to uncontrolled passage into the systemic circulation of bacterial components that directly mediate beta cell damage and/or activate beta cell autoimmunity within pancreatic lymph nodes (PLNs) and tissues (22). Alternatively, autoimmune T cells specific for beta cell antigens could be activated by bacterial products at the intestinal level and subsequently travel to PLNs and islets to mediate beta cell damage (23).
The gastrointestinal barrier is a fundamental gatekeeper to avoid the contact between luminal content and the human body. The barrier is composed of a mucus layer and an intestinal epithelial barrier (IEB), and both are crucial to prevent the passage of commensal bacteria, pathogens, and food antigens from the lumen into the gut tissue and systemic circulation. The IEB is a single layer of epithelial cells held together by a complex junctional system composed of tight junctions, adherent junctions, and desmosomes. Tight junctions are composed of transmembrane proteins such as occludin, claudin, junctional adhesion molecules (JAMs), tricellulin, and angulins whose interaction between themselves and with intracellular scaffolding proteins, i.e., zonula occludens proteins (ZOs) [such as tight junction protein 1 (Tjp1)], is fundamental to maintain tight junction integrity and control paracellular trafficking. In patients and rat models of T1D alterations of the IEB have been reported in association with gut inflammation (12, 24). However, the IEB is not the only intestinal barrier that is important to prevent bacterial translocation. In fact, the first barrier that commensal bacteria encounter before entering in contact with the host is the mucus layer that covers the entire gastrointestinal canal (25). The main function of the mucus barrier is to limit the contact between the gut mucosa and harmful molecules present in the intestinal lumen such as hydrochloric acid, digestive enzymes, and bile salts. However, the mucus layer does not simply act as a diffusion barrier but plays important dynamic functions that regulate the type of commensal bacteria residing in the inner mucus layer, allow the passage of food and bacterial products into the gut tissue and systemic circulation, and has important immune regulatory functions. For example, a healthy mucus architecture is fundamental to allow hosting of beneficial commensal bacteria such as the short-chain fatty acid (SCFA)-producing bacteria (26) that promote immune tolerance in the gut and extraintestinal tissues (27, 28). Moreover, the mucus layer contains antimicrobial peptides (AMPs) and mucins, such as Muc1, Muc2, Muc3, Muc4, Muc12, Muc13, and Muc17, that play key immune-modulatory functions (29, 30). For example, Muc2 is important for the cooperation between goblet cells and CD103+ dendritic cells (DCs) leading to FoxP3+ Treg cell differentiation and immune tolerance in the gut (31). Muc1 and Muc3 have strong antiinflammatory function acting as decoy molecules on the apical cell surface of enterocytes to limit bacterial adherence, translocation, and intestinal inflammation (32).
The integrated response of these combined barrier systems, i.e., the mucus layer and the IEB, is crucial to regulate the interaction between commensal microbiota and immune cells within the gut mucosa and systemically (26). So far, increased gut leakiness present in animal models and T1D patients has been exclusively ascribed to alteration of the IEB, while the mucus layer integrity and mucin composition have been poorly studied except for some mucin alterations found in the BB rat model of T1D (33).
Here, we show in preclinical models of autoimmune T1D that the damage of the intestinal barrier involves primarily the mucus layer with profound modifications of its structure and composition at the level of the small and large intestine. Most importantly, we demonstrated that induction of chronic colitis by low-dose dextran-sulfate sodium (DSS), by altering gut barrier integrity and mucus layer composition, triggers activation of islet-reactive T cells in the gut mucosa [diabetogenic T cells of T cell receptor (TCR)-transgenic (tg) BDC2.5XNOD mice] leading to autoimmune diabetes. In our colitis-induced diabetes model, the presence of the endogenous commensal microbiota is necessary to trigger activation of beta cell autoimmunity, and T1D occurrence is abolished in DSS-colitis BDC2.5XNOD mice treated with antibiotics. Our data establish a causal relationship between breakage of intestinal barriers and autoimmune pathogenesis of T1D in preclinical models and suggest that enteropathy characterized by increased gut permeability and modification of mucus structure is not an epiphenomenon in T1D but directly responsible for triggering beta cell autoimmunity.
Results
Increased Intestinal Permeability and Loss of Mucus Barrier Integrity in Diabetic NOD Mice.
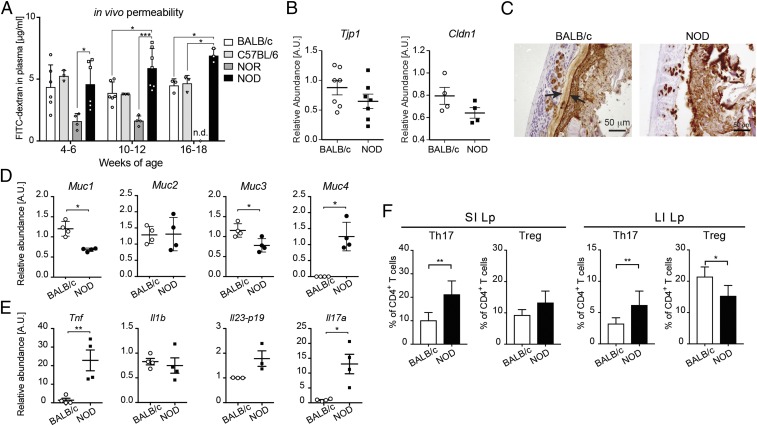
Alterations of the IEB have been reported in T1D patients and diabetic BB rats in association with gut inflammation (12, 24), but the gut barrier integrity has been poorly studied in the NOD mice, the spontaneous murine model of T1D. Moreover, the mucus layer, an important gut barrier containing immunoregulatory molecules such as antimicrobial peptides and mucins, has never been analyzed in T1D. We assessed the integrity of the gut barrier and mucus layer architecture in the intestine of NOD mice. Our fluorescein isothiocyanate (FITC)-dextran test revealed an increased intestinal permeability in female NOD mice in comparison with different control strains (Fig. 1A) that started at 10–12 wk before onset of autoimmune diabetes indicating that breakage of the intestinal barrier integrity is an early event in T1D pathogenesis in NOD mice as previously shown in T1D patients and diabetic BB rats (12, 19). To further assess integrity of the IEB in the intestine of NOD mice, we performed RT-qPCR analysis to measure mRNA expression relative to structural IEB proteins such as the tight junction protein 1 (Tjp1) and claudin-1 (Cldn1). Our analysis revealed a slight but not significant decrease in the relative expression of mRNA encoding for those structural IEB proteins in the large intestine (colon segment) of NOD compared with control mice (Fig. 1B and SI Appendix, Fig. S1A). Next, we asked whether the mucus layer structure and relative expression of mucin proteins are altered in the intestine of NOD mice. Immunohistochemistry of the mucus layer with anti-Muc2 monoclonal antibody showed modifications of the mucus architecture and breakage of the mucus layer continuity in the NOD mice compared with control mice (Fig. 1C and SI Appendix, Fig. S1B). Our quantification of mucin expression highlighted a significant decrease in the relative expression of immune-regulatory mucins Muc1 and Muc3 and increase in inflammatory Muc4 mucin in the large intestine of NOD mice compared with control mice (Fig. 1D and SI Appendix, Fig. S1C). We focused our analysis on the mucus architecture in the large intestine because this is the intestinal segment where the mucus layer is more represented and plays important regulatory functions. In fact, anti-MUC2 immunohistochemistry analysis showed a much thinner and less structured mucus layer in the ileum (small intestine) in comparison with the colon (large intestine) segment in control mice (SI Appendix, Fig. S2). Nevertheless, our comparative analysis revealed differences between NOD and control mice also at the small intestinal level with a reduced thickness and breaks in the mucus layer continuity in the NOD mice in comparison with control mice (SI Appendix, Fig. S2A). At the mucin level, we did not detect alterations in Muc1 and Muc3 expression but increased Muc4 expression in the small intestine of NOD mice compared with control mice in line with what was observed in the large intestine (SI Appendix, Fig. S2B). The mucus layer and mucin composition play important immune-modulatory functions that are fundamental to regulate the cross talk between the gut microbiota and the host immune system. Hence, we asked whether mucus layer modifications are associated with alteration of gut mucosal immunity in the NOD mice. In line with this notion, we found increased levels of several inflammatory cytokines such as TNF-α, IL1-β, IL-23p19, and IL-17 in the large intestinal mucosa of NOD mice compared with control mice (Fig. 1E and SI Appendix, Fig. S1D). We studied modifications of the mucus structure and composition as well as the inflammatory gut environment in the NOD mice early on in T1D progression (4–6 wk of age). Our analysis at a later time point (10–12 wk of age) indicated that most changes such as increased gut permeability, thinning of the mucus layer, and alteration of Muc1 expression (but not of Muc3 and Muc4) are maintained with age (SI Appendix, Fig. S3). Interestingly, we demonstrated that the increased inflammatory gut environment in the NOD mice triggered significant alterations of gut immune homeostasis in the small and large intestine of NOD mice with augmented frequency of effector Th17 cells in both districts and reduction of FoxP3+ Treg cell percentages in the large intestine (Fig. 1F). The immune alterations of Th17/Treg cell frequencies in the small and large intestine of NOD mice compared with control strains were observed at 10–12 wk of age but not earlier (4–6 wk of age), as if the proinflammatory intestinal environment in the first weeks of age requires some time to significantly affect gut mucosal immunity (SI Appendix, Fig. S4).
Fig. 1.
Increased intestinal permeability and loss of mucus barrier integrity in NOD mice. (A) FITC-dextran in vivo permeability assay in female BALB/c and NOD mice at 4, 12, and 18 wk of age. (B) RT-qPCR analysis of tight junction protein 1 (Tjp1) and claudin 1 (Cldn1) on tissue homogenates from the colons of 4-wk-old BALB/c and NOD mice. (C) Immunohistochemistry of Muc2 protein in the colons of 4-wk-old NOD mice and controls. (D) RT-qPCR analysis of Muc1, Muc2, Muc3, and Muc4 mucin genes in the colons of BALB/c and NOD mice. (E) RT-qPCR analysis of cytokine genes encoding TNF-α (Tnf), interleukin-1β (Il1b), subunit p19 of IL-23 (Il23-p19), and IL-17A (Il17a) on tissue homogenates from the colons of 4-wk-old BALB/c and NOD mice. (F) Flow-cytometric analysis of Th17 and Treg cells in the small intestinal (SI) and large intestinal (LI) lamina propria (Lp) of 12-wk-old BALB/c and NOD mice. Data are presented as mean percentages ± SD of IL-17+CD4+ and FoxP3+CD25+CD4+ T cells out of total CD4+ T cells from two independent experiments (n = 4–10 mice per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Chronic Colitis and Breakage of Intestinal Barrier Integrity Trigger Activation of Islet-Reactive T Cells in the Gut Mucosa.
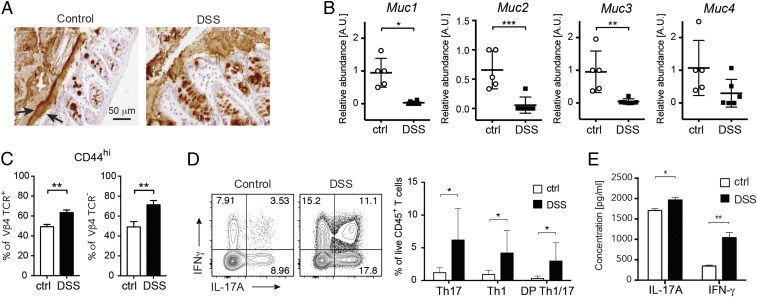
Our data indicate that increased permeability and alterations of immune homeostasis are present in the intestinal mucosa of NOD mice and are associated with important changes in the mucus layer architecture and composition. Next, we wanted to establish a causal link between the presence of a “leaky” gut, breakage of the mucus layer structure, and activation of beta cell autoimmunity. The observation that lymphocytes isolated from mesenteric lymph nodes (MLNs) of young prediabetic NOD mice are capable to transfer autoimmune diabetes suggests that the intestine could be a preferential site for activation and expansion of islet-reactive T cells (34). Hence, we hypothesized that enteropathy and breakage of the intestinal barrier integrity are not epiphenomena but could play a pathogenic role in T1D pathogenesis through triggering intestinal activation of islet-reactive T cells. In the NOD mouse model, due to the low number of circulating islet-reactive T cells, it is not possible to track them and assess their activation state in peripheral lymphoid organs and tissues. To test our hypothesis and monitor the activation state of islet-reactive T cells, we used a TCR-tg murine model (BDC2.5XNOD mice) in which majority of CD4+ T cells (∼90%) express a tg TCR specific for a beta cell antigen (35). Tg BDC2.5XNOD mice bear rearranged genes for the TCR-α (Vα1) and TCR-β (Vβ4) chains from an islet-reactive CD4+ T cell clone (BDC2.5 T cell clone) isolated from a diabetic NOD mouse (36). Those tg mice, despite carrying a large diabetogenic T cell repertoire and having large T cell infiltrates in the pancreatic islets, do not develop autoimmune diabetes unless islet-reactive T cells (BDC2.5 T cells) are activated, for example through islet-antigen presentation in the target tissue (37, 38). In those mice, we altered the gut barrier continuity and, specifically, the mucus architecture by administration of low-dose DSS, according to a protocol that specifically targets the mucus layer integrity (39) (SI Appendix, Fig. S5A), and then analyzed the activation state and cytokine secretion profile of islet-reactive BDC2.5 T cells (Vβ4+CD4+ T cells). BDC2.5XNOD mice undergoing DSS treatment developed chronic colitis, characterized by significant weight loss and colon shortening, as well as increased gut permeability and alterations of tight junction protein expression (SI Appendix, Fig. S5 B–F). Chronic colitis in those mice correlated with substantial modifications of the mucus structure (Fig. 2A) and mucin protein composition (Fig. 2B). Specifically, we detected a mucin RNA expression profile comparable to that observed in the intestine of NOD mice with a significant decrease in mRNA expression of immune-regulatory mucins Muc1 and Muc3 (Fig. 2B). Next, to assess the effect of loss of gut barrier integrity on islet-reactive T cell activation, we analyzed the expression levels of the CD44 activation marker on Vβ4+BDC2.5 T cells in the intestinal mucosa of DSS-colitis BDC2.5XNOD mice (see SI Appendix, Fig. S6, for gating strategy). Our data show that chronic DSS-colitis and breakage of the intestinal barriers increased the percentage of activated CD44+ BDC2.5 (Vβ4+) T cells within the gut mucosa (Fig. 2C). Moreover, we found that the cytokine-secretion phenotype of islet-reactive BDC2.5 T cells in the intestine was skewed toward an effector Th1/Th17 phenotype similarly to that observed in the gut of NOD mice (Fig. 2D). This bias was associated and possibly related to the presence of a highly inflammatory cytokine milieu enriched for IL-23 (a Th17-inducing cytokine) in the gut mucosa of DSS-colitis BDC2.5XNOD mice (SI Appendix, Fig. S5E). The activation state and strong effector phenotype (Th1/Th17 cells) of BDC2.5 T cells was confirmed by their increased secretion of IL-17 and IFN-γ upon in vitro stimulation (Fig. 2E). These data demonstrate that breakage of intestinal barrier integrity and alteration of mucus structure in DSS-colitis BDC2.5XNOD mice are associated with activation of islet-reactive BDC2.5 T cells within the gut mucosa.
Fig. 2.
Chronic colitis and breakage of intestinal barrier integrity trigger activation of islet-reactive T cells in the gut. (A) Low-dose DSS administration induced alteration of mucus structure as shown by immunohistochemistry analysis with anti-Muc2 mAb in the colon of control and DSS-colitis BDC2.5XNOD mice (DSS). (B) RT-qPCR analysis revealed alteration of mucin genes’ expression in the colon of DSS-colitis BDC2.5XNOD mice with reduced expression of Muc1, Muc2, and Muc3 mRNA. (C) Percentages of activated CD44hi T cells among tg Vβ4+CD4+ BDC2.5 T cells (Left) and non-tg Vβ4−CD4+ T cells (Right) in intestinal lymphocytes isolated from colon of control (ctrl) and DSS-colitis (DSS) BDC2.5XNOD mice. (D) Representative flow cytometry plots (Left) and bar graph with mean percentages ± SD (Right) of cytokine-producing CD4+ T cells (out of CD45+ cells) within the gut mucosa of control (ctrl) and DSS-colitis BDC2.5XNOD mice (two independent experiments; n = 8 mice per group). (E) Cytokine bead array (CBA) assay for IL-17A and IFN-γ production by lymphocytes from the intestine of control (ctrl) and DSS BDC2.5XNOD mice stimulated in vitro with Con A for 72 h. Data are presented as mean percentages ± SEM of IL-17+CD4+ and FoxP3+CD25+CD4+ T cells out of total CD4+ T cells from triplicate wells. Data are from one representative experiment out of two. *P < 0.05; **P < 0.01; ***P < 0.001.
Islet-Reactive T Cells Activated in the Gut Mucosa Migrate to PLNs and Tissues and Induce Autoimmune Diabetes.
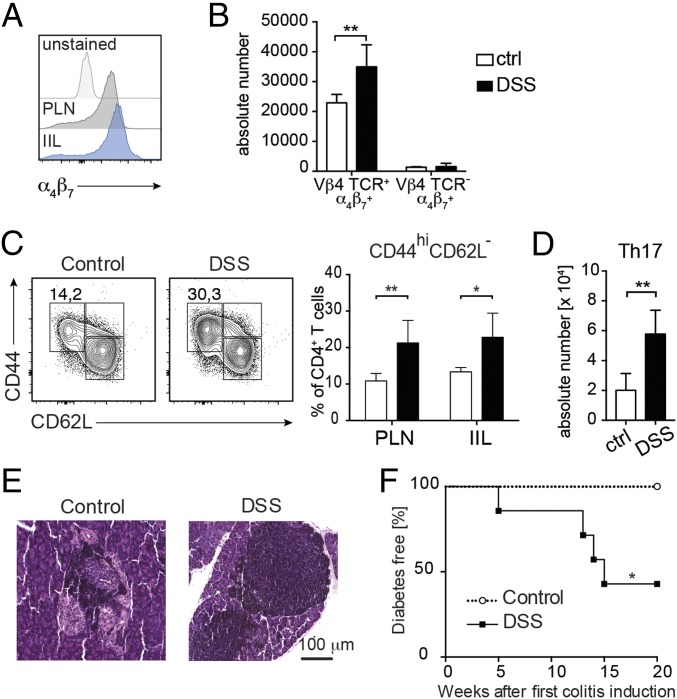
Previous reports indicated that an immunological axis between the gastrointestinal tract and pancreatic tissues exists (23), so that T cells continuously travel from the intestinal mucosa to pancreatic tissues and into the systemic circulation. Hence, we asked whether islet-reactive BDC2.5 T cells, after being activated in the intestinal mucosa, travel to PLN and islets to induce beta cell damage and autoimmune diabetes. As expected, we found that most of the islet-reactive BDC2.5 T cells present in the PLN and intraislet lymphocytes (IILs) of BDC2.5 mice express the gut homing marker α4β7 integrin, a proof of their intestinal origin, even in the absence of DSS-colitis (Fig. 3A). This finding is not surprising since it is known that T cells continuously travel from the intestinal mucosa to pancreatic tissues through the gut–pancreatic axis (23). However, it is important to note that BDC2.5XNOD mice with DSS-colitis showed a higher absolute number of α4β7+ islet-reactive BDC2.5 T cells in the PLN in comparison with their healthy littermates (Fig. 3B). Moreover, BDC2.5 T cells found in PLN and islet infiltrates of DSS-colitis BDC2.5XNOD mice showed an activated CD44highCD62low phenotype (Fig. 3C) and a biased effector Th17 cytokine-secretion phenotype compared with their counterparts from healthy mice (Fig. 3D).
Fig. 3.
Islet-reactive BDC2.5 T cells activated in the gut elicit pancreatic autoimmunity. (A) FACS analysis of expression levels of α4β7 integrin on CD4+ T cells in the pancreatic lymph nodes (PLNs) and intraislet lymphocyte (IIL) compartment of BDC2.5XNOD mice. (B) Absolute numbers of α4β7+ TCR-tg islet-reactive BDC2.5 T cells (Vβ4+CD4+ T cells) and non-tg T cells (Vβ4−CD4+ T cells) in the PLNs of DSS-colitis (DSS) and control (ctrl) BDC2.5XNOD mice. (C) Representative flow cytometry plots (Left) and percentage (Right) of activated CD44hiCD62L− T cells in the PLN and IIL of control and DSS BDC2.5XNOD mice. (D) Absolute numbers of Th17 cells in the PLN of control and DSS BDC2.5XNOD mice. (E) Hematoxylin and eosin staining of pancreatic tissue showing large lymphocyte infiltrates surrounding the pancreatic islets of control BDC2.5XNOD mice (Left) and massive islet infiltration in the DSS BDC2.5XNOD mice (Right). (F) Incidence of autoimmune diabetes in control and DSS-colitis (DSS) BDC2.5XNOD mice (n = 7 mice per group). *P < 0.05; **P < 0.01.
In BDC2.5XNOD mice, large infiltrates of islet-reactive BDC2.5 T cells surround the pancreatic islets, but they do not trigger beta cell damage and autoimmune diabetes unless they are activated by viruses, cytokine milieu, etc. (37, 38). In accordance with previous findings, in our control BDC2.5XNOD mice, we detected large infiltrates around healthy islets (periinsulitis) with no signs of insulitis (Fig. 3E, Left). On the contrary, the pancreatic islets of DSS-colitis BDC2.5XNOD mice were completely invaded by lymphocytes with very limited residual beta cell mass (Fig. 3E, Right). Importantly, in line with the histological signs of islet damage, we observed that 60% of DSS-colitis BDC2.5XNOD mice developed autoimmune diabetes, while 100% of their healthy littermates remained diabetes-free (Fig. 3F). These data indicate that increased activation and acquisition of an effector Th1/Th17 phenotype by islet-reactive BDC2.5 T cells in the intestine of DSS-colitis BDC2.5XNOD mice enhance their aggressiveness, thus enabling them to trigger beta cell destruction and autoimmune diabetes.
Induction of Autoimmune Diabetes Requires the Cross Talk Between Islet-Reactive T Cells and the Gut Commensal Microbiota.
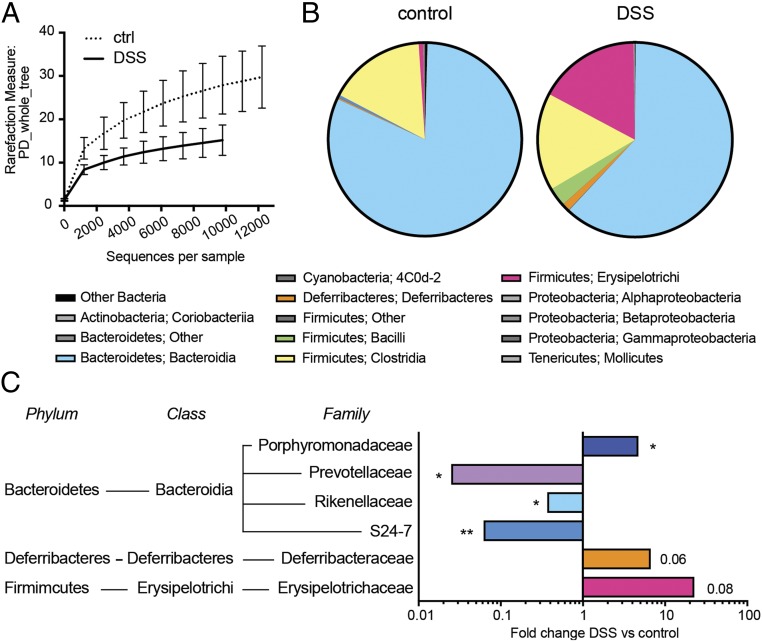
Next, we asked which are the mechanisms that trigger activation of islet-reactive BDC2.5 T cells in the gut mucosa of DSS-colitis BDC2.5XNOD mice. First, we tested whether DSS-colitis affected the composition of gut microbiota favoring overgrowth of proinflammatory bacterial species that could be responsible for activation and acquisition of an effector phenotype by islet-reactive BDC2.5 T cells in DSS BDC2.5XNOD mice. By ultradeep pyrosequencing of barcoded 16S rRNA gene amplicons, we analyzed the gut microbiota community in stool samples of DSS-colitis and healthy BDC2.5XNOD mice at 4 wk after the first DSS administration when the animals showed early signs of colitis (SI Appendix, Fig. S5B) but were still normoglycemic (Fig. 3F). Although we normalized bacterial load between samples based on total DNA and we cannot infer on differences in absolute numbers of live commensal bacteria, we found substantial differences in the alpha-diversity (Fig. 4A) and relative abundance of some bacterial strains in the DSS-colitis mice compared with their healthy littermates. Specifically, we found that, at the phylum level, Firmicutes and Deferribacteres were increased, whereas Bacteroidetes strains were significantly less represented in DSS-colitis BDC2.5XNOD mice compared with their healthy littermates (Fig. 4B). The family-level analysis revealed that the Bacteroidetes strains reduced in DSS-colitis BDC2.5XNOD mice were Prevotellaceae, Rikenellaceae, and S24-7, while others such as Porphyromonadaceae were increased (Fig. 4C). Deferribacteraceae and Erysipelotrichaceae were also augmented in DSS-colitis BDC2.5XNOD mice (Fig. 4C).
Fig. 4.
DSS-colitis alters the composition of the commensal microbiota in BDC2.5XNOD mice. (A) Alpha diversity of gut microbiota from control (ctrl) and DSS-colitis (DSS) BDC2.5XNOD mice as analyzed with QIIME software using a phylogenic diversity (PD) tree. (B) Phylum and class-level phylogenetic classification of 16S rRNA cDNA frequencies in the mucosa and luminal content of the intestine of control and DSS-treated BDC2.5/NOD mice. (C) 16S sequencing comparisons of DSS-colitis vs. control BDC2.5XNOD mice. Bacterial families and genera shown represent those found to be significantly different in the pairwise comparison. *P < 0.05; **P < 0.01.
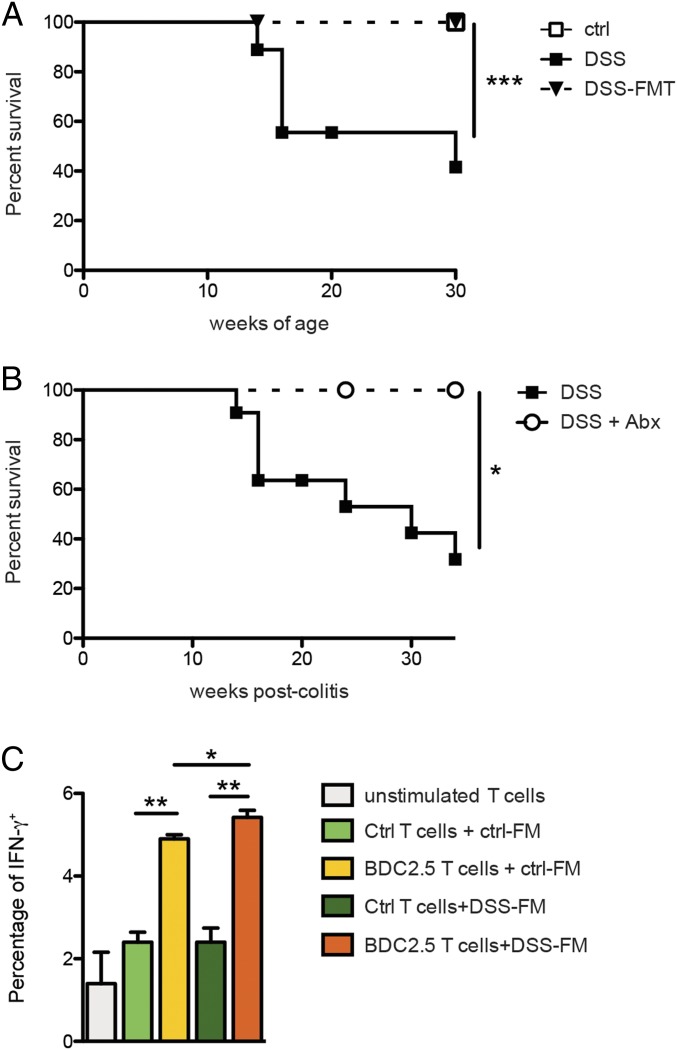
To test whether modifications of the gut microbiota induced by DSS-colitis were directly responsible for intestinal activation of islet-reactive T cells, we performed fecal transfer experiments by orally gavaging stool suspensions from DSS BDC2.5XNOD mice into recipient BDC2.5XNOD mice previously depleted of their endogenous gut microbiota through antibiotic treatment. Our data show that oral transfer of gut microbes from DSS-colitis diabetic donors into healthy BDC2.5XNOD recipients was not sufficient to activate beta cell autoimmunity and induce T1D (Fig. 5A). Although the fecal transfer does not lead to complete colonization of the host with all donor bacterial species, our data suggest that modifications of the gut microbiota induced by DSS-colitis were not sufficient to induce beta cell autoimmunity. Next, we searched the mechanism through which loss of gut barrier integrity could promote autoimmune diabetes. Damage of the gut barrier could lead to bacterial translocation to the gut mucosa and gut-associated lymphoid tissue (GALT) and favor the cross talk between the commensal microbiota and the immune system, thus leading to activation of islet-reactive T cells. In support of bacterial translocation as a consequence of damage of gut barrier integrity, we found commensal bacteria in the MLNs of DSS-treated BDC2.5XNOD mice (SI Appendix, Fig. S7A). To test whether the gut commensal bacteria is required to induce autoimmune diabetes in DSS BDC2.5XNOD mice, we depleted those mice of endogenous commensal microbiota before induction of chronic DSS-colitis. To this aim, we treated BDC2.5XNOD mice with a broad-spectrum antibiotic mixture (ampicillin, metronidazole, neomycin, and vancomycin) that induces complete depletion of endogenous microbiota (SI Appendix, Fig. S7B). Importantly, previous reports showed that the antibiotic treatment depletes the gut microbiota without affecting the DSS-induced damage of the gut barrier integrity in DSS-treated mice (40). Remarkably, none of the DSS BDC2.5XNOD mice depleted of their commensal gut microbiota developed T1D. These results demonstrate that loss of gut barrier integrity and intestinal inflammation are not sufficient to induce T1D and the presence of commensal gut microbiota is required to trigger intestinal activation of islet-reactive BDC2.5 T cells (Fig. 5B).
Fig. 5.
Commensal gut microbiota directly triggers activation of islet-reactive T cells and is necessary for diabetes induction in DSS-colitis BDC2.5XNOD mice. (A) Diabetes-free survival of control (ctrl), DSS-colitis (DSS) BDC2.5XNOD mice, and healthy BDC2.5XNOD mice receiving fecal microbiota transplants from DSS mice (DSS-FMT). (B) Diabetes-free survival of DSS-colitis BDC2.5XNOD mice treated (DSS+Abx) or not (DSS) with an antibiotic mixture (0.5 g/L vancomycin, 1 g/L ampicillin, 1 g/L metronidazole, and 1 g/L neomycin) to deplete endogenous commensal microbiota. (C) Fecal material from DSS-colitis (DSS-FM) or healthy (crtl-FM) BDC2.5XNOD mice was added to splenocytes of control non-tg mice (ctrl T cells) or TCR-tg BDC2.5XNOD mice (BDC2.5 T cells), and after 96 h T cell activation was assessed by measuring percentage of IFN-γ+ cells. Data are presented as mean percentages ± SEM of IFN-γ+CD4+ T cells out of total CD4+ T cells. One representative experiment out of two is shown. *P < 0.05; **P < 0.01; ***P < 0.001.
Our results indicate that the gut microbiota is necessary but not sufficient to induce T1D, but how does the commensal bacteria trigger beta cell autoimmunity upon breakage of the intestinal barriers? Loss of intestinal barrier integrity could lead to passage of bacterial products that could stimulate islet-reactive T cells through a TCR-mediated mechanism. In fact, commensal bacterial strains may contain molecules that mimic self-antigens and directly trigger TCR-mediated activation of islet-reactive T cells as recently demonstrated for a diabetogenic CD8+ T cell clone (41). Alternatively, bacterial products could bind pattern recognition receptors on intestinal DCs and increase their capacity to stimulate all T cells in an antigen-independent manner (42). To test whether the commensal gut microbiota can trigger activation of islet-reactive T cells, we performed in vitro experiments in which total splenocytes from BDC2.5XNOD mice or wild-type (wt) mice were challenged with bacterial suspensions isolated from the intestine of DSS-colitis or healthy mice and the acquisition of an activated IFN-γ+ phenotype on islet-reactive BDC2.5 T cells (Vβ4+CD4+) (from tg BDC2.5XNOD mice) or polyclonal CD4+ T cells (from wt mice) was evaluated. Our data show that CD4+ BDC2.5 T cells are activated and acquired an activated IFN-γ+ phenotype when stimulated with commensal microbiota strains from healthy BDC2.5XNOD mice (Fig. 5C). No stimulation was detected on polyclonal CD4+ T cells (splenocytes of wt mice) challenged in vitro with the same bacterial suspensions (Fig. 5C). We cannot exclude an indirect effect of the bacterial lysates on other immune cells contained in total splenocytes (e.g., DCs and macrophages). However, the observation that commensal bacteria triggered IFN-γ secretion on BDC2.5 T cells from TCR-tg mice and not polyclonal CD4+ T cells from wt mice suggests that the commensal bacterial strains contained in the healthy murine intestine are capable to activate autoimmune islet-reactive BDC2.5 T cells with an antigen-specific TCR-mediated mechanism. In support to the TCR dependency of the microbiota-induced stimulation, we found that addition of anti-MHC class II mAb together with fecal material to the BDC2.5 T cell cultures reduced their acquisition of an IFN-γ+ phenotype (SI Appendix, Fig. S8). These data suggest that interaction between components of the endogenous commensal bacteria and T cells that occurs in the intestinal mucosa upon breakage of the gut barrier integrity could potentially lead to activation of islet-reactive T cells through a TCR-mediated mechanism. We also observed that bacterial lysates from commensal gut microbiota collected from DSS BDC2.5XNOD mice were more stimulatory on CD4+ BDC2.5 T cells (Fig. 5C) compared with bacterial strains from healthy BDC2.5XNOD mice. Although the difference was not significant (P = 0.05), the latter observation suggests that dysbiosis induced by intestinal inflammation could lead to selection of bacterial species that are more stimulatory for islet-reactive BDC2.5 T cells.
Discussion
Recent evidence suggests that the intestinal environment and, specifically, modifications of the microbiome profile, regulate the pathogenesis of T1D by inducing intestinal inflammation and increasing gut permeability (43, 44). Although it has been proposed that T1D originates in the intestine (45), it remains to be determined how gut leakiness in T1D patients and animal models promotes beta cell autoimmunity. Here, we demonstrate that loss of gut barrier integrity increases activation of islet-reactive T cells and triggers autoimmune diabetes.
The gut barrier that prevents translocation of bacterial components in the intestinal tissue is composed of a mucus layer and an intestinal epithelial layer (IEB). The IEB regulates intestinal permeability through a well-organized system of intercellular connections between the cells, composed of tight junctions, adherent junctions, and desmosomes. The IEB is fundamental to regulate the trafficking of bacterial products and metabolites and is damaged in patients and rat models of T1D (12). In prediabetic NOD mice, we found that increased gut permeability is associated with damage of the mucus layer and composition and not of the IEB. Although mucus structure and composition were never studied in NOD mice, it was recently reported that, in those mice, gut colonization with Akkermansia muciniphila counterregulates autoimmune diabetes by increasing mucus production and secretion of the antibacterial peptide RegIIIγ in the mucus layer (46), thus suggesting that the mucus barrier plays an important role in T1D prevention. The mucus layer is the first barrier that commensal bacteria encounter before entering in contact with the host and plays important dynamic functions that regulate the type of commensal bacteria that reside in the inner mucus layer, the passage of food and bacterial components from the gut lumen into the mucosal tissue, and the interaction between commensal microbiota and immune cells. For example, commensal bacteria that play important immune regulatory functions such as the SCFA producers reside within the mucus layer (26). In addition, the mucus layer contains mucins and antimicrobial peptides (AMPs) that are released by intestinal cells (enterocytes, goblet cells, and Paneth cells) and play important immune-regulatory functions (30). Expression of AMPs such as α-defensins and cathelicidins and the regenerating islet-derived protein (Reg)IIIγ are altered in T1D. Previous studies have shown that cathelicidins are reduced in the serum of T1D patients (47) and in NOD mice dysbiosis is associated with reduced local expression of cathelicidin-related antimicrobial peptide (CRAMP) by pancreatic endocrine cells (22). Our results demonstrate that also the expression of mucins is altered in NOD mice. In fact, we found that expression of immune-regulatory Muc1 and Muc3 is reduced while expression of proinflammatory Muc4 is increased in the intestine of NOD mice. Those alterations were detected at 4 wk of age, an early time point in T1D progression that is concomitant with the generation of the autoimmune T cell repertoire (48). Later on (10–12 wk of age), changes in mucin expression were partially corrected with decreased Muc1 expression but normalized levels of Muc3 and Muc4, an effect possibly due to compensatory mechanisms. Dysbiosis and reduction of immune-regulatory mucins associated with damage of the mucus layer could ultimately lead to alterations of gut immune homeostasis with increased Teff/Treg cell ratio and chronic inflammation that we observed in prediabetic NOD mice. AMPs maintain gut barrier integrity by regulating expression of mucins and TJ proteins through the WNT pathway (49). For example, REG3γ-deficient mice harbor an altered mucus layer and increased mucosal inflammatory response to the commensal gut microbiota (50). Similarly, CRAMP deficiency in mice leads to a more severe form of DSS-induced colitis (51). Hence, it is possible to speculate that in the NOD mice the defective release of AMPs previously reported (22) is responsible for the damage of gut barrier integrity and the alterations of mucus structure and composition that we observed.
The presence of chronic gut inflammation and loss of gut barrier integrity in patients and preclinical models of T1D has been known for long time but a causal relationship between those intestinal alterations and induction autoimmune diabetes was never established. Our data demonstrate that loss of gut barrier integrity and modifications of the mucus structure and composition, associated with chronic colitis (by low-dose DSS administration), trigger T1D. A previous report showed that disruption of the gut barrier induced by Citrobacterium rodentium colonization in NOD mice increased the percentage of islet-reactive CD8+ T cells in the PLN and accelerated onset of insulitis (52). Our data demonstrate that loss of gut barrier integrity is not only an accelerator in T1D pathogenesis but a causal factor so that chronic DSS-colitis triggers T1D in TCR-tg BDC2.5XNOD mice that otherwise do not develop diabetes. BDC2.5XNOD mice, despite having 90% of the circulating T cells specific for an islet antigen, do not become diabetic and can be considered like genetically susceptible individuals carrying an enlarged islet-reactive T cell repertoire and not developing T1D unless a triggering event activates beta cell autoimmunity. In BDC2.5XNOD mice, different triggering events have been reported such as viral infections (e.g., Coxsackie infection) or any factor that perturbs the islet environment leading to inflammation, tissue damage, and the release of sequestered islet antigen resulting in the stimulation of resting islet-reactive T cells (38). Here, we show that breakage of the gut barrier is one of those triggering events that unleashes beta cell autoimmunity and provokes islet destruction in autoimmune-prone individuals. What is the mechanism that triggers activation of islet-reactive T cells in our DSS-colitis T1D model? One possibility is that bacterial components could have translocated through the intestinal mucosa into the bloodstream and pancreatic islets and mediate tissue damage, exposure of self-antigens, and local activation of islet-reactive T cells (22). Alternatively, islet-reactive T cells could have been activated in the gut mucosa and travel to the PLN and islets to mediate tissue damage and T1D (23). Our data support the latter hypothesis by showing that DSS-colitis increased the percentage of activated islet-reactive T cells already in the intestinal tissue of BDC2.5XNOD mice and large part of activated Vβ4+ T cells found in the PLN and within the islets are of intestinal origin and express the α4β7 marker. Our data are in accordance with a recent report showing that myelin-reactive T cells are activated in the GALT, and from there they travel to peripheral tissue, i.e., the central nervous system, to mediate extraintestinal autoimmune diseases (53). Which are the mechanisms triggering activation of islet-reactive T cells in the gut of DSS-colitis BDC2.5XNOD mice? Gut inflammation could lead to presentation of sequestered self-antigens that are already present in the intestinal tissue. For example, chromogranin A, an islet-antigen that is capable to stimulate the diabetogenic BDC2.5 T cell clone (54), is expressed in the gastro-entero-pancreatic system (55, 56) and, under chronic inflammatory conditions, could be presented to islet-reactive T cells in the intestine. Our data do not support this idea by showing that gut inflammation by itself in DSS-BDC2.5XNOD mice depleted of endogenous microbiota is not capable to activate beta cell autoimmunity. Although in those mice the antibiotic-induced bacterial depletion may have not lasted through the entire duration of the experiment, the observation that microbiota-depleted mice did not develop T1D suggests that the commensal gut microbiota are required for the intestinal activation of diabetogenic T cells. The gut microbiota has a strong impact in T1D pathogenesis as demonstrated both in humans and preclinical models (57, 58), but it is still unclear how commensal bacteria modulate beta cell autoimmunity. In fact, while in other autoimmune diseases such as rheumatoid arthritis in HLA-B27 tg rats and experimental autoimmune encephalomyelitis, the preclinical models of multiple sclerosis, the gut microbiota plays a clear triggering role (53, 59), in the NOD mice, the spontaneous model of T1D, this role is still controversial. In some cases, it plays a beneficial effect, for example in germ-free NOD mice in which the absence of gut commensal bacteria enhances T1D incidence (6). On the contrary, in NOD mice housed in spontaneous monoculture with a Gram-positive aerobic spore-forming rod, T1D has a delayed onset and reduced incidence (60), thus suggesting that commensal strains are important to trigger beta cell autoimmunity. A possible explanation for those controversial findings is that the NOD mice represent a complex and multifactorial animal model of autoimmune diabetes. In fact, in NOD mice the generation of the autoimmune islet-reactive T cell repertoire is strongly affected by exposure to any bacterial antigens early in life. In those mice, the microbial environment in specific-pathogen–free housing facility but also exposure to microbial stimuli, such as injection with mycobacteria (61, 62) or various microbial antigens (63, 64), prevent beta cell autoimmunity by affecting the overall maturation of the immune system and/or with a mechanism of immune deviation. The TCR-tg BDC2.5 murine model differs from NOD mice because it already has a large islet-reactive autoimmune T cell repertoire (35). Hence, in those mice, the confounding effect of the microbial antigens on the generation of the autoimmune T cell repertoire is not present, and we could assess the role of the commensal gut microbiota in activation/expansion of islet-reactive T cells. Importantly, we showed not only that, in DSS-treated BDC2.5XNOD mice, upon damage of the gut barrier, the commensal microbiota was required to trigger activation of islet-reactive T cells, but also demonstrated that microbial antigens contained in bacterial suspensions isolated from the healthy murine intestine are capable to directly stimulate the islet-reactive T cell clone in vitro with a TCR-mediated mechanism. Our observation is aligned with a previous report showing that autoreactive T cells specific for a retinal antigen are directly activated by the commensal gut microbiota through their clonotypic TCR and with a mechanism that is not dependent on an endogenous source of the cognate antigen (65). Recent findings demonstrated that translocation of commensal microbes into intestinal tissue is a common event and gut pathobionts are found in the GALT of healthy, unmanipulated mice (66). Hence, commensal gut microbes can activate autoimmune T cells specific for different extraintestinal self-antigens that circulate through intestinal tissue and GALT even in the absence of gut inflammation and damage of the gut barrier. This notion is supported by our finding that islet-reactive T cells with an activated/memory phenotype are present in the intestine of healthy BDC2.5XNOD mice. We speculate that, under inflammatory conditions, in our DSS-colitis model, this mechanism was enhanced by damage of the gut barrier resulting in an increased percentage of self(islet)-reactive T cells that are activated within the intestinal tissue and migrate to pancreatic islets to provoke T1D. Additionally, the inflammatory gut environment characterized by the presence of proinflammatory cytokines such as IL-23 drove islet-reactive T cells toward an effector Th17 cell phenotype, thus increasing their aggressiveness and capacity to destroy pancreatic beta cells similarly to what was reported in the autoimmune uveitis model (65).
The commensal gut microbiota could activate self(islet)-reactive T cells through different mechanisms. Low-affinity autoreactive T cell clones are highly promiscuous, and their TCRs can be stimulated by microbiome-derived peptides (67) including agonist peptides derived from gut commensal microbiota (65). This hypothesis is supported by the recent finding that three microbial peptides derived from commensal bacteria were found to be able to stimulate the diabetogenic IGRP-specific NY8.3 CD8+ T cell clone and intestinal colonization with Fusobacteria containing those mimicking peptides accelerated diabetes incidence, thus suggesting that commensal microbes could promote T1D through molecular mimicry (41). Alternatively, the commensal gut microbiota could activate islet-reactive T cells through TCR-independent mechanisms. A recent report showed that bacterial products stimulate intestinal DC within the gut mucosa through pattern recognition receptors and induce an innate milieu with an adjuvant-like effect that activates islet-reactive T cells and trigger diabetes (42). Although our results are not conclusive, they support the first hypothesis by showing that the islet-reactive BDC2.5 T cells are directly stimulated by commensal gut microbiota from the intestine of healthy mice through a TCR-mediated mechanism. We also observed that the commensal microbiota from the inflamed intestine of DSS-treated BDC2.5XNOD mice was slightly higher stimulatory; however, additional experiments are necessary to assess whether DSS-colitis promoted overgrowth of proinflammatory bacterial species that were more effective in stimulating islet-reactive T cells in vitro in comparison with microbial suspensions from healthy BDC2.5XNOD mice.
Our study provides direct evidence that increased gut permeability plays an important pathogenic role in T1D possibly by favoring the interaction between islet-reactive T cells and commensal microbes. The murine model that we used, the BDC2.5XNOD mice, have an enlarged islet-reactive T cell repertoire and can be compared with “at-risk” humans with high genetic susceptibility to T1D and beta cell autoimmunity (islet autoantibodies positivity) in which T1D does not occur unless the diabetogenic islet-reactive T cells are activated in the periphery by a triggering event. In diabetes-prone humans, as in BDC2.5XNOD mice, the loss of gut barrier integrity could ultimately lead to intestinal activation of islet-reactive T cells, beta cell destruction, and T1D. An important implication of these findings is that restoration of a healthy gut barrier through microbiota and diet modulation in diabetes-prone individuals could ultimately reduce intestinal activation of islet-reactive T cells and prevent T1D occurrence.
Materials and Methods
Mice.
Female NOD mice were purchased from Charles River Laboratories. TCR-tg BDC2.5XNOD mice were a gift from Dr. Jonathan Katz (Cincinnati Children’s Research Foundation, University of Cincinnati, Cincinnati, OH). All mice were maintained under specific-pathogen–free conditions in the animal facility at San Raffaele Scientific Institute, and all experiments were conducted in accordance with the Institutional Animal Care and Use Committee in accordance with the rules of the Italian Ministry of Health.
Induction of Chronic DSS Colitis.
The 2% (wt/vol) DSS (molecular weight: 36,000–50,000 Da; MP Biomedicals) was administered to BDC2.5XNOD mice according with the chronic colitis protocol described by Wirtz et al. (68). Briefly, 4-wk-old BDC2.5XNOD mice received the DSS orally in drinking water for three cycles of 7 d with intervals of 2 wk. In some experiments, BDC2.5XNOD mice were pretreated with antibiotics (0.5 g/L vancomycin, 1 g/L ampicillin, 1 g/L metronidazole, and 1 g/L neomycin; all from Sigma-Aldrich) for 2 wk before each cycle of DSS administration and during the DSS treatment to deplete endogenous commensal microbiota according to a well-established protocol (69). Bacterial depletion was verified by qPCR with universal eubacteria primers as previously reported (70). Mice were either monitored for diabetes occurrence or killed at 8–10 wk of age for immunological and RT-qPCR analyses. Body weight was monitored daily during induction phases and every other day during clinical remission.
In Vivo Permeability Assay.
Intestinal permeability was determined by FITC-dextran assay as previously described (52). Briefly, 20 mL/kg of body weight of PBS containing 25 mg/mL FITC-conjugated dextran (FITC-dextran; molecular mass, 4.4 kDa; FD4, Sigma-Aldrich) was administered to each mouse by oral gavage. After 4 h, blood was collected and added immediately to a final concentration of 3% citrate-phosphate-dextrose solution (Sigma-Aldrich). The blood samples were centrifuged (10,000 rcf at 4 °C) for 10 min, and plasma was collected. Fifty microliters of plasma were mixed with an equal volume of PBS (pH 7.4) and added to a 96-well microplate. The concentration of fluorescein was determined by spectrophotofluorometry (Wallac Victor; Perkin-Elmer Life Sciences) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm using serially diluted samples of the FITC-dextran marker as standard.
Cell Isolation and in Vitro Stimulation Experiments.
Intestinal mononuclear cells were isolated from the small and large intestinal lamina propria as previously described (71, 72). Briefly, after the removal of Peyer’s patches, small and large intestines were flushed with PBS, opened longitudinally, and incubated twice with 5 mM EDTA and 1 mM DTT for 20 min at 37 °C to remove epithelial cells and adipose tissue. Then, the intestines were cut into small pieces and digested in HBSS containing 0.5 mg/mL Collagenase D (Roche Diagnostics), 1 mg/mL Dispase II (Roche Diagnostics), and 5 U/mL DNase I (Sigma-Aldrich) for 20 min at 37 °C in a shaking incubator. The digested tissues were washed, resuspended in 5 mL of 40% Percoll (Sigma-Aldrich), and overlaid on 2.5 mL of 80% Percoll in a 15-mL Falcon tube. Percoll gradient separation was performed by centrifugation at 1,000 × g for 20 min at 20 °C. The interface cells were collected and used as intestinal lymphocytes for fluorescence-activated cell sorting (FACS) analysis. Splenocytes and lymph node cells were isolated by mechanical disruption of the tissues. Pancreatic IILs were isolated by a three-step digestion with 1 mg/mL Collagenase IV (Gibco) followed by extensive washing with HBSS containing 5% FBS.
For bacteria stimulation assays, fecal pellets from DSS-colitis or healthy BDC2.5XNOD mice were lysed through freeze-thaw method and resuspended in RPMI-10, normalized for their protein content (Pierce BCA Protein Assay kit; Thermo Fisher), and added to total splenocytes of wt NOD mice or TCR-tg BDC2.5XNOD mice in a 96-well plate (105 cells/well) in RPMI-10 without antibiotics. After 96 h, activation of CD4+ T cells was estimated by measuring percentage of IFN-γ+ cells by intracellular staining.
Flow Cytometry.
Single-cell suspensions from different organs were resuspended in staining buffer containing PBS, 1% FBS, and 0.09% NaN3, and stained with monoclonal antibodies against surface markers. For intracellular cytokine staining, single-cell suspensions isolated from different organs were stimulated for 2.5 h with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 1 μg/mL ionomycin (both from Sigma-Aldrich) for 4 h in the presence of 10 μg/mL Brefeldin A (Sandoz). Cells were first stained for surface markers, then fixed and permeabilized using the BD Cytofix/Cytoperm kit, and finally stained for intracellular cytokines. The following antibodies were used: PerCP-Cy5.5 anti-mouse CD4 (RM4-5; BD Biosciences), APC-eFluor 780 anti-mouse CD45 (30-F11; eBioscience), APC anti-mouse CD25 (PC61; BioLegend), PE anti-mouse LPAM-1 (DATK32; BioLegend), FITC anti-mouse Vβ4 TCR (KT4; BD Biosciences), APC-Cy7 anti-mouse CD44 (IM7; BD Biosciences), Alexa Fluor 647 anti-mouse CD62L (MEL-14; BioLegend), eFluor 450 anti-mouse Foxp3 (FJK-16s; eBioscience), V450 or FITC anti-mouse IFN-γ (XMG-1.2; BD Biosciences), and Alexa Fluor 647 or 488 anti-mouse IL-17A (TC11-18H10; BD Biosciences). Dead cells were stained with Fixable Viability Dye eFluor 506 (eBioscience) and excluded from the analysis. Flow cytometry was performed using FACSCanto II (BD Biosciences), and data were analyzed with FCS Express V4 software (De Novo Software).
Fecal Transplant Experiments.
Fecal microbiota transplantation (FMT) experiments were performed as previously described (73). The 4-wk-old BDC2.5XNOD mice were depleted of their endogenous microbiota with antibiotics (0.5 g/L vancomycin, 1 g/L ampicillin, 1 g/L metronidazole, and 1 g/L neomycin; all from Sigma-Aldrich) added to drinking water for 2 wk before the FMT experiment. Three to four pellets (100 mg) of fresh stool from adult donor female BDC2.5XNOD mice with/out DSS-colitis were collected and pooled in 1.2 mL of sterile PBS; 200 μL of the mixture was administered to recipient BDC2.5XNOD mice by oral gavage for 3 consecutive days.
Diabetes Incidence.
Diabetes was monitored by weekly measurements of blood glucose levels with a GB35 Ascensia Breeze 2 glucometer (Bayer). The animals were considered diabetic after two consecutive blood glucose measurements ≥250 mg/dL.
Histology and Immunohistochemistry.
For analysis of the mucus layer structure segments of the distal colon were fixed in methanol-Carnoy’s fixative (60% dry methanol, 30% chloroform, and 10% acetic acid), washed in absolute methanol, ethanol, and xylene, embedded in paraffin wax, and sectioned at 6 μm. The sections were stained with primary anti-Mucin-2 antibody (clone Sc-15334; Santa Cruz Biotechnology) and secondary donkey anti-rabbit IgG antibody.
To assess histopathological signs of diabetes, pancreata were fixed in 10% formalin, embedded in paraffin, and sectioned into 4-μm slices. The pancreas sections were then stained with hematoxylin–eosin and scored for insulitis.
Real-Time qPCR.
Immediately after the mice were killed, colons were flushed with PBS, opened longitudinally, and placed in 500 μL of TRIzol reagent (Life Technologies). After homogenization with TissueRuptor (QIAGEN), RNA was extracted by adding 100 μL of chloroform, precipitating the aqueous phase with 300 μL of 70% ethanol, and purifying RNA with RNeasy Mini Kit (QIAGEN). RNA was retrotranscribed with SuperScript III First-Strand Synthesis System following manufacturer’s instructions (Life Technologies). Real-time qPCR assay was performed with SYBR Select Master Mix (Life Technologies) using primers specific for different tight junction proteins, mucins, and cytokines (see SI Appendix, Table S1, for list of primers) on a ViiA 7 Real-Time PCR System (Life Technologies). Transcript expression was normalized against Rpl32.
16S rRNA Microbiota Analysis.
Total DNA was isolated from the mucosa and luminal content of the large intestine of DSS-colitis and control BDC2.5XNOD mice using PowerFecal DNA Isolation kit (MoBio) following the manufacturers’ instructions with only one minor modification in lyses time, 15 min instead of 5 min, to try to retrieve all difficult-to-lyse bacteria. Microbiome characterization was performed by amplification of 200 ng of extracted DNA and amplification of 16S V3-5 regions using barcoded sample-specific primers: 16S-F331 ACT CCT ACG GGA GGC AGC and 16S-R920 CCG TCA ATT CMT TTG AGT TT. The AccuPrime Taq DNA Polymerase (Invitrogen) and the following cycling conditions were employed: 94 °C for 2 min, 35 cycles of (94 °C for 30 s, 56 °C for 30 s, and 68 °C for 1 min), and the samples were stored at 4 °C until usage. Amplicons were loaded on 1% agarose gel and purified with the QiaQuick Gel Extraction Kit (Qiagen). Extracted amplicons were purified with AMPure XP beads (Beckman Coulter, Brea, CA) and used for emulsion-PCR and ultradeep pyrosequencing following the 454 GS Junior manufacturer’s instructions (Roche Diagnostics). Sequences with a high-quality score and length of >250 bp were used for the taxonomic analysis with Quantitative Insights Into Microbial Ecology (QIIME), version 1.6.
Statistical Analysis.
Statistical significance of the differences between two or more samples was calculated by unpaired two-tailed Student’s t test or ANOVA, respectively. Cumulative diabetes incidence was calculated using the Kaplan–Meier estimation, whereas statistical significance was evaluated by the log-rank test. Statistical analysis of the microbiota profiling data was performed on the proportional representation of the taxa (summarized to phyla, class, order, family, and genus levels) using one-way ANOVA test with Bonferroni’s correction. GraphPad Prism, version 6.0 (GraphPad Software, San Diego, CA), was used for statistical analysis, and values of P ≤ 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Prof. Emanuele Bosi (Ospedale San Raffaele, Milan, Italy) for critical reading of the manuscript and Prof. Cecile King (The Garvan Institute of Medical Research, Sydney, NSW, Australia) for helpful suggestions. This work was supported by a research grant from the Juvenile Diabetes Foundation (Grant 2-SRA-2014-28-Q-R) to M.F.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14788.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814558116/-/DCSupplemental.
References
- 1.Todd J. A., Etiology of type 1 diabetes. Immunity 32, 457–467 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Dotta F., et al. , Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. U.S.A. 104, 5115–5120 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honeyman M. C., et al. , Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49, 1319–1324 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Vaarala O., et al. , Cow’s milk formula feeding induces primary immunization to insulin in infants at genetic risk for type 1 diabetes. Diabetes 48, 1389–1394 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Norris J. M., et al. , Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290, 1713–1720 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Wen L., et al. , Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455, 1109–1113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giongo A., et al. , Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 5, 82–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown C. T., et al. , Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 6, e25792 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vatanen T., et al. ; DIABIMMUNE Study Group , Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badami E., et al. , Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes 60, 2120–2124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatanen T., et al. , The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meddings J. B., Jarand J., Urbanski S. J., Hardin J., Gall D. G., Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am. J. Physiol. 276, G951–G957 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Graham S., et al. , Enteropathy precedes type 1 diabetes in the BB rat. Gut 53, 1437–1444 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurano F., et al. , Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia 48, 931–937 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Mooradian A. D., Morley J. E., Levine A. S., Prigge W. F., Gebhard R. L., Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 29, 221–224 (1986). [DOI] [PubMed] [Google Scholar]
- 16.Carratù R., et al. , Altered intestinal permeability to mannitol in diabetes mellitus type I. J. Pediatr. Gastroenterol. Nutr. 28, 264–269 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Westerholm-Ormio M., Vaarala O., Pihkala P., Ilonen J., Savilahti E., Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes 52, 2287–2295 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Secondulfo M., et al. , Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 36, 35–45 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Bosi E., et al. , Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 49, 2824–2827 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Neu J., et al. , Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J. Pediatr. Gastroenterol. Nutr. 40, 589–595 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Vaarala O., Atkinson M. A., Neu J., The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 57, 2555–2562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J., et al. , Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43, 304–317 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Turley S. J., Lee J. W., Dutton-Swain N., Mathis D., Benoist C., Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc. Natl. Acad. Sci. U.S.A. 102, 17729–17733 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapone A., et al. , Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55, 1443–1449 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Pelaseyed T., et al. , The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson M. E., Sjövall H., Hansson G. C., The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith P. M., et al. , The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arpaia N., et al. , Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo R. L., Hooper L. V., Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12, 503–516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chairatana P., Nolan E. M., Defensins, lectins, mucins, and secretory immunoglobulin A: Microbe-binding biomolecules that contribute to mucosal immunity in the human gut. Crit. Rev. Biochem. Mol. Biol. 52, 45–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan M., et al. , Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng Y. H., et al. , MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6, 557–568 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Courtois P., Jurysta C., Sener A., Scott F. W., Malaisse W. J., Quantitative and qualitative alterations of intestinal mucins in BioBreeding rats. Int. J. Mol. Med. 15, 105–108 (2005). [PubMed] [Google Scholar]
- 34.Jaakkola I., Jalkanen S., Hänninen A., Diabetogenic T cells are primed both in pancreatic and gut-associated lymph nodes in NOD mice. Eur. J. Immunol. 33, 3255–3264 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Katz J. D., Wang B., Haskins K., Benoist C., Mathis D., Following a diabetogenic T cell from genesis through pathogenesis. Cell 74, 1089–1100 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Haskins K., Portas M., Bradley B., Wegmann D., Lafferty K., T-lymphocyte clone specific for pancreatic islet antigen. Diabetes 37, 1444–1448 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Mueller R., Bradley L. M., Krahl T., Sarvetnick N., Mechanism underlying counterregulation of autoimmune diabetes by IL-4. Immunity 7, 411–418 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Horwitz M. S., et al. , Diabetes induced by Coxsackie virus: Initiation by bystander damage and not molecular mimicry. Nat. Med. 4, 781–785 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Johansson M. E., et al. , Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 5, e12238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández-Chirlaque C., et al. , Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J. Crohns Colitis 10, 1324–1335 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Tai N., et al. , Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J. Exp. Med. 213, 2129–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa F. R., et al. , Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J. Exp. Med. 213, 1223–1239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Atkinson M. A., The role for gut permeability in the pathogenesis of type 1 diabetes—a solid or leaky concept? Pediatr. Diabetes 16, 485–492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaarala O., Leaking gut in type 1 diabetes. Curr. Opin. Gastroenterol. 24, 701–706 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Vaarala O., Is the origin of type 1 diabetes in the gut? Immunol. Cell Biol. 90, 271–276 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Hänninen A., et al. , Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67, 1445–1453 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Brauner H., et al. , Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin. Exp. Immunol. 177, 478–482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufman D. L., et al. , Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366, 69–72 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson K., Deng Z., Hou Y., Zhang G., Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2, 57 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loonen L. M., et al. , REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 7, 939–947 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Koon H. W., et al. , Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology 141, 1852–1863.e1-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee A. S., et al. , Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia 53, 741–748 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Berer K., et al. , Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Stadinski B. D., et al. , Chromogranin A is an autoantigen in type 1 diabetes. Nat. Immunol. 11, 225–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gleeson C. M., Curry W. J., Johnston C. F., Buchanan K. D., Occurrence of WE-14 and chromogranin A-derived peptides in tissues of the human and bovine gastro-entero-pancreatic system and in human neuroendocrine neoplasia. J. Endocrinol. 151, 409–420 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Curry W. J., et al. , Chromogranin A and its derived peptides in the rat and porcine gastro-entero-pancreatic system. Expression, localization, and characterization. Adv. Exp. Med. Biol. 482, 205–213 (2000). [PubMed] [Google Scholar]
- 57.Sorini C., Falcone M., Shaping the (auto)immune response in the gut: The role of intestinal immune regulation in the prevention of type 1 diabetes. Am. J. Clin. Exp. Immunol. 2, 156–171 (2013). [PMC free article] [PubMed] [Google Scholar]
- 58.Knip M., Siljander H., The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 12, 154–167 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Taurog J. D., et al. , The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King C., Sarvetnick N., The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS One 6, e17049 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadelain M. W., Qin H. Y., Lauzon J., Singh B., Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39, 583–589 (1990). [DOI] [PubMed] [Google Scholar]
- 62.Martins T. C., Aguas A. P., Changes in B and T lymphocytes associated with mycobacteria-induced protection of NOD mice from diabetes. J. Autoimmun. 9, 501–507 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R., Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc. Natl. Acad. Sci. U.S.A. 87, 1576–1580 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saï P., Rivereau A. S., Prevention of diabetes in the nonobese diabetic mouse by oral immunological treatments. Comparative efficiency of human insulin and two bacterial antigens, lipopolysacharide from Escherichia coli and glycoprotein extract from Klebsiella pneumoniae. Diabetes Metab. 22, 341–348 (1996). [PubMed] [Google Scholar]
- 65.Horai R., et al. , Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity 43, 343–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fung T. C., et al. , Lymphoid-tissue-resident commensal bacteria promote members of the IL-10 cytokine family to establish mutualism. Immunity 44, 634–646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cole D. K., et al. , Hotspot autoimmune T cell receptor binding underlies pathogen and insulin peptide cross-reactivity. J. Clin. Invest. 126, 3626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wirtz S., Neufert C., Weigmann B., Neurath M. F., Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Brown R. L., Sequeira R. P., Clarke T. B., The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 8, 1512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atarashi K., et al. , Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weigmann B., et al. , Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Lefrancois L., Lycke N., Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Curr. Protoc. Immunol. Chapter 3, Unit 3.19 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Ghosh S., et al. , Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G39–G49 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.