Abstract
Background
Optimum management of low-grade gliomas remains controversial, and widespread practice variation exists. This evidence-based meta-analysis evaluates the association of extent of resection, radiation, and chemotherapy with mortality and progression-free survival at 2, 5, and 10 years in patients with low-grade glioma.
Methods
A quantitative systematic review was performed. Inclusion criteria included controlled trials of newly diagnosed low-grade (World Health Organization Grades I and II) gliomas in adults. Eligible studies were identified, assigned a level of evidence for every endpoint considered, and analyzed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The relative risk of mortality and of progression at 2, 5, and 10 years was calculated for patients undergoing resection (gross total, subtotal, or biopsy), radiation, or chemotherapy.
Results
Gross total resection was significantly associated with decreased mortality and likelihood of progression at all time points compared to subtotal resection. Early radiation was not associated with decreased mortality; however, progression-free survival was better at 5 years compared to patients receiving delayed or no radiation. Chemotherapy was associated with decreased mortality at 5 and 10 years in the high-quality literature. Progression-free survival was better at 5 and 10 years compared to patients who did not receive chemotherapy. In patients with isocitrate dehydrogenase 1 gene (IDH1) R132H mutations receiving chemotherapy, progression-free survival was better at 2 and 5 years than in patients with IDH1 wild-type gliomas.
Conclusions
Results from this review, the first to quantify differences in outcome associated with surgery, radiation, and chemotherapy in patients with low-grade gliomas, can be used to inform evidence-based management and future clinical trials.
Keywords: chemotherapy, extent of resection, low-grade glioma therapy, radiation, overall survival
Low-grade gliomas (defined as World Health Organization [WHO] Grades I and II) account for 17% to 22% of all primary brain tumors (approximately 20 000 cases per year in the United States) and are characterized by a low proliferation index.1–5 These tumors are known for their diverse pathology and clinical behavior. Recent insights into the molecular biology of low-grade gliomas and discovery of isocitrate dehydrogenase gene (IDH)1 and IDH2 mutations, 1p19q codeletion, and their impact on survival have added clarity to the molecular subgroups in glioma and have led to a recent reclassification of gliomas by the WHO.3,5–8 Median survival for patients with low-grade gliomas ranges from 5.6 to 13.3 years and is dependent on specific histology and molecular characteristics.9 The relative rarity of low-grade gliomas combined with long overall survival and new developments in molecular diagnostics have complicated the conduct and completion of large, high-quality, prospective studies. As a result, optimal treatment remains controversial, and is often based on the selective application of results from a few high-quality studies and extrapolation from studies of patients with higher-grade gliomas.10 Treatment options include surgery, radiotherapy, and chemotherapy. Surgical intervention is generally performed with the goal of maximum safe resection and aids in diagnosis by providing tissue for molecular testing (1p19q codeletion, IDH, ATRX [α-thalassemia/mental-retardation-syndrome-X-linked] mutations). Radiation is typically reserved for patients considered to be at “high risk” (for example, patients over 40 years old or patients with an incomplete resection) or with progressive disease.10 Only a few prospective trials have been performed on the effect of chemotherapy on gliomas.9,11 Recently, a large, prospective, randomized trial evaluating the effect of procarbazine, lomustine, and vincristine (PCV) with radiation showed an overall survival and progression-free survival benefit compared to radiation alone.9 Nevertheless, considerable practice variation and spirited debate persist regarding the roles of all 3 of these treatment modalities. Although a meta-analysis of surgical resection thresholds has recently been completed, a quantitative analysis of all 3 aspects of the management of low-grade glioma has never been performed and a rigorous assessment of the body of literature has never been undertaken.12
In the present study, we estimate the strength of association between reduction in mortality and risk of progression following surgery, radiation, and chemotherapy by performing a systematic review, grading the quality of the evidence, and conducting a study-level meta-analysis of the existing literature.
Methods
Systematic Review
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline-based approach was used to query PubMed from inception until September 1, 2017.13 Medical Subject Headings (MeSH) and non-MeSH terms were applied broadly with filters utilized to reject studies of patients with tumor histologies other than low-grade gliomas (see supplementary materials for specific search terms). The reference lists of previously published reviews were also examined to identify additional potentially relevant studies. Primary outcomes of interest included relative risk (RR) of mortality and progression at 2, 5, and 10 years comparing gross total resection (GTR) to subtotal resection (STR), chemotherapy to no chemotherapy, and radiation at diagnosis to no radiation or delayed radiation. Secondary outcomes of interest included RR of mortality and progression at 2, 5, and 10 years comparing high-dose to low-dose radiation, STR to biopsy (Bx), and any resection to Bx. Prespecified inclusion criteria included all studies of patients with newly diagnosed, histologically confirmed, supratentorial, low-grade glioma (WHO Grade I or II) that included appropriate outcome data in treated and untreated groups. Exclusion criteria included patient age <18 years, recurrent tumors, and studies that did not provide comparative data on outcomes of interest or otherwise did not meet inclusion criteria. For the chemotherapy analysis, all published regimens were included. For the resection analyses, resection thresholds were defined by the authors of the primary studies, but were generally consistent among studies.
Grading of Studies
Studies meeting inclusion criteria and accepted for at least 1 comparison were assigned a level of evidence for each outcome for which they were used in accordance with American Academy of Neurology grading criteria.14 Class I represents the highest level of evidence, and Class IV the lowest. Grading of studies was performed by 2 of the authors (TJB and MG) and presented in consensus fashion to the entire study group.
Data Extraction
We compared chemotherapy vs no chemotherapy, early radiation vs late (at the time of disease progression) or no radiation, GTR vs STR, STR vs Bx, and any resection vs Bx. Endpoints of interest for all comparisons were 2-, 5-, and 10-year risk of death and 2-, 5-, and 10-year risk of progression. Secondary analyses were performed to examine the association between outcome of chemotherapy and IDH1 R132H mutational status, and moderate-dose (45-55 Gy) vs high-dose (59-65 Gy) radiation. Data for the time points of interest were extracted from the text of included studies whenever possible. When this was not possible, data were manually extracted from Kaplan-Meier curves using a pixel-coordinate technique.15 When a study met inclusion criteria but required outcome data were not obtainable, the article was excluded from the analysis. Baseline demographic information extracted from all included studies included age, tumor size, and IDH1 R132H mutational status. Subset analyses were performed on high-quality (Class I and II) vs low-quality (Class III and IV) evidence whenever possible. An analysis of prognostic factors was conducted using data extracted from the text or figures of included studies when provided.
Meta-Analysis
All relevant data from eligible trials were included in this study-level meta-analysis. RRs of mortality and progression were calculated using an inverse variance method and random-effects model (Review Manager 5.3, Cochrane Collaborative, London, UK).16 Significance was defined as P ≤ .05; 95% confidence intervals (CIs) were calculated for each endpoint, and numbers needed to treat (NNT) were calculated where appropriate. Summary statistics for variables of potential prognostic importance were calculated and are presented in the same fashion. Heterogeneity was assessed with the I2 statistic, and is reported in the supplementary materials.
Results
Systematic Review
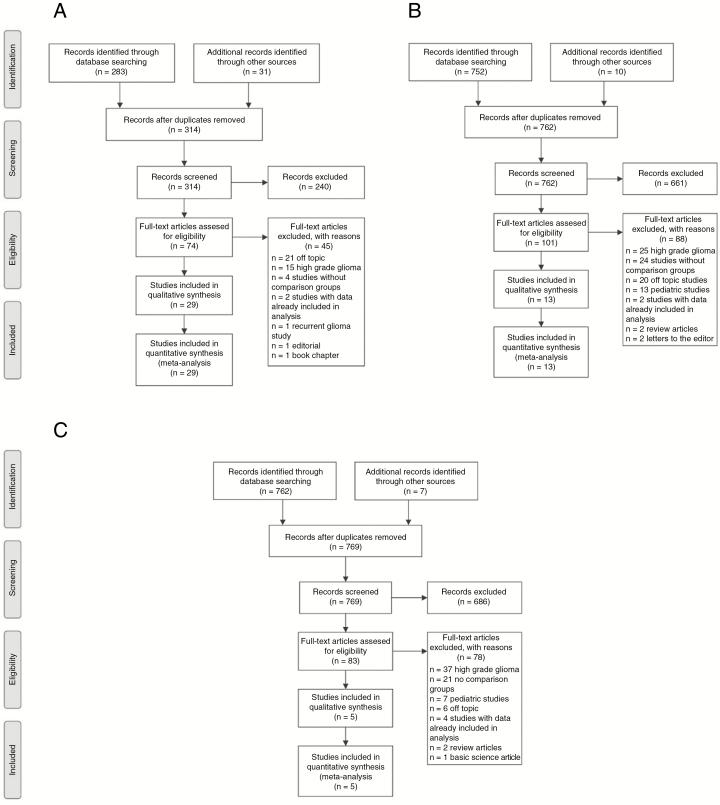
The systematic review regarding extent of resection yielded 283 potentially relevant studies. An additional 31 studies were identified by review of reference lists, review articles, abstract listings, and expert advice. Ultimately 29 studies were included in at least 1 comparison (Fig. 1A).11,17–44 For the radiation questions, the systematic review produced 752 articles. An additional 10 studies were identified outside of the systematic review. Thirteen studies met inclusion criteria for at least 1 comparison (Fig. 1B).27,28,32,33,36,37,39,43–48 The chemotherapy review produced 762 articles. An additional 7 articles were identified using ancillary measures. Five studies on the effect of chemotherapy were ultimately included in at least 1 analysis (Fig. 1C).9,11,33,47,49
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Diagram of Included Studies PRISMA diagram of all studies included in 1 comparison concerning the effect of A, resection, B, radiation, and C, chemotherapy on outcomes in low-grade gliomas.
Level of Evidence Grading
For the endpoint of overall survival, 5 studies provided Class I evidence,9,11,27,37,48 3 studies provided Class II evidence,22,34,47 with the remaining 21 studies providing Class III or IV evidence. For the endpoint of progression-free survival, only 1 study provided Class I evidence,9 with the remainder of included studies providing Class III and IV evidence (see supplementary materials).
Surgery
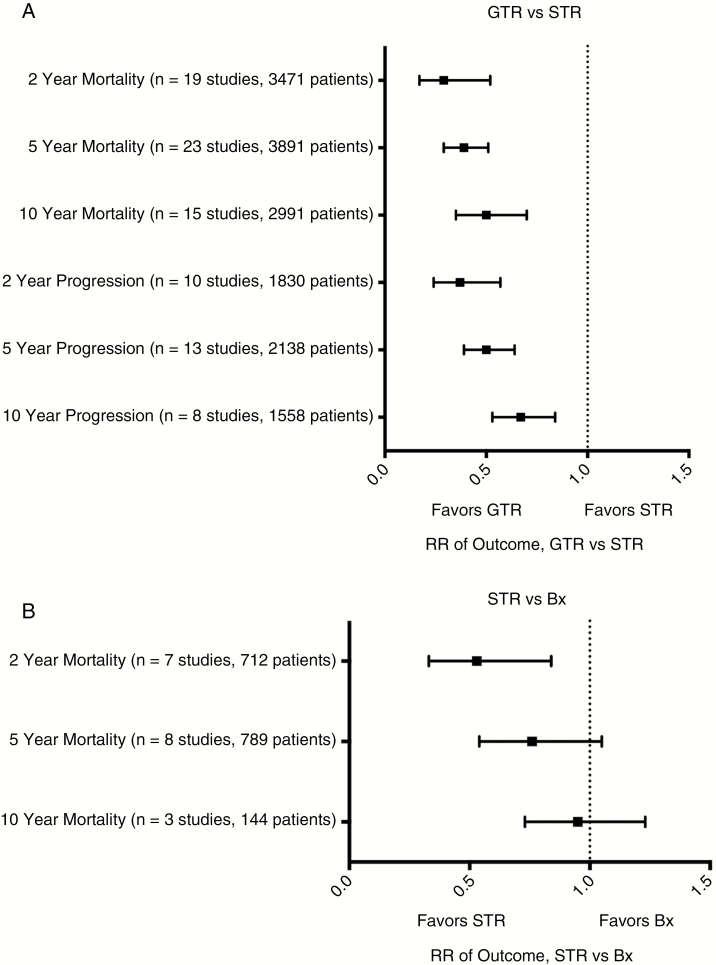
Twenty-nine eligible studies comprising 3891 patients compared GTR vs STR in low-grade gliomas.11,17–44 All included studies were class III or IV. RR and 95% CI of death at 2, 5, and 10 years for GTR vs STR was 0.29 (0.17-0.52, P < .001, NNT = 17),17,18,20–24,26–28,30–33,36,37,39,41,44 0.39 (0.29-0.51, P < .001, NNT = 6),17,18,20–24,26–33,35–37,39,41–44 and 0.50 (0.35-0.70, P < .001, NNT = 4). RR of progression (GTR vs STR) at 2, 5, and 10 years was 0.37 (0.24-0.57, P < .001, NNT = 7),17,22,24,26,27,31,33,38,41,44 0.50 (0.39-0.64, P < .001, NNT = 4), 17,22,24,26,27,29,31,33,35,38,41,42,44 and 0.67 (0.53-0.84, P < .001, NNT = 4)17,22,24,26,29,31,41,44 (see Fig. 2 and supplement for specific comparisons).
Fig. 2.
Meta-Analyses of the Extent of Resection and Outcomes in Low-Grade Gliomas.
A, Forest plot of summary statistics on the 6 comparisons regarding outcomes of patients who received gross total resection (GTR) compared to those who received subtotal resection (STR). Values plotted are relative risks (RR) with 95% confidence intervals. Summary statistics that do not cross X = 1 indicate a benefit favoring GTR over STR. B, Forest plot of summary statistics of STR vs biopsy (Bx).
Comparing STR to Bx, RR of death at 2 (n = 7), 5 (n = 8), and 10 years (n = 3) was 0.53 (0.33-0.84, P = .008, NNT = 10),11,18,22,23,27,30,37 0.76 (0.54-1.05, P = .10),11,18,22,23,27,30, 37,42 and 0.95 (0.73-1.23, P = .70).11,23,30 There was no significant improvement in progression at 2 years, RR 0.49 (0.17-1.38, P = .18),22,27 or at 5 years, RR 0.94 (0.71-1.25, P = .68).22,27,42
The RR of death of any resection vs Bx at 2, 5, and 10 years was 0.46 (0.29-0.75, P = .002, NNT = 10),18,19,22,23,25,27,30,34,37,40 0.60 (0.43-0.84, P = .003, NNT = 6),18,19,22,23,25,27, 30,34,37,40,42 and 0.67 (0.52-0.86, P = .002, NNT = 5).23,25,30,34 Three studies of 511 patients provided data on risk 2-year progression of any resection vs Bx, RR 0.42 (0.09-2.08, P = .29],19,22,27 and 4 studies of 601 patients provided data on 5-year progression of any resection vs Bx, RR 0.83 (0.56-1.23, P = .35).19,22,27,42 Importantly, no Class I or Class II evidence exists to allow a sensitivity analysis of high-quality vs low-quality studies. Histologic subtypes of patients included in these studies can be found in the supplementary material.
Radiation
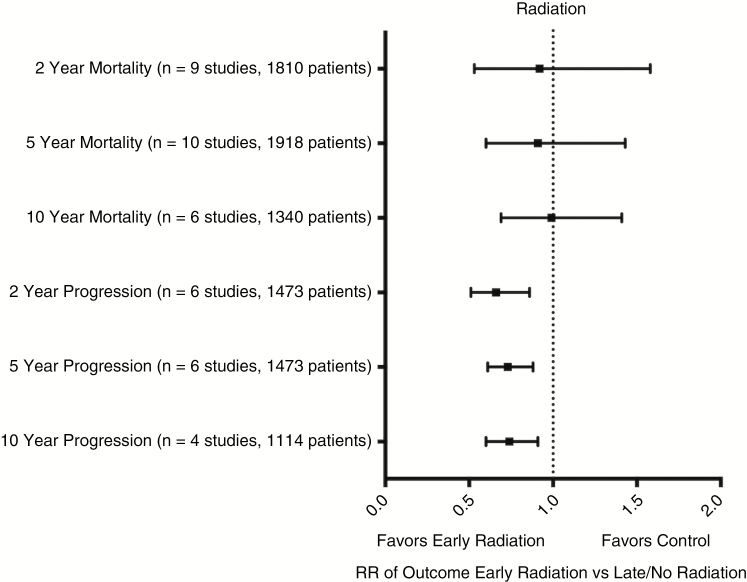
Thirteen eligible studies comprising 1918 patients provided data regarding the effect of postoperative radiation vs delayed or no radiation.28,32,33,36,39,44–48 RR and 95% CI for death at 2, 5, and 10 years was 0.92 (0.53-1.58, P = .76),32,33,36,39,44–48 0.93 [0.60-1.43, P = .73),28,32,33,36,39,44–48 and 0.99 (0.69-1.41, P = .95).28,32,36,39,44,46 RR for progression at 2, 5, and 10 years was 0.66 (0.51-0.86, P = .002, NNT = 10),33,43,44,46–48 0.73 (0.61-0.88, P < .001, NNT = 6),33,43,44,46–48 and 0.74 (0.60-0.91, P = .005, NNT = 5)33,43,44,46 (see Fig. 3 and supplement for specific comparisons). Histologic subtypes can be found in the supplementary material.
Fig. 3.
Meta-Analyses of Early Postoperative Radiation vs Late or No Radiation.
Forest plot of summary statistics on the 6 comparisons regarding outcomes of patients who received early postoperative radiation compared to those who received late or no radiation. Values plotted are relative risks (RR) with 95% confidence intervals. Summary statistics that do not cross X = 1 indicate a benefit favoring early radiation over late or no radiation.
Of the included studies, only 2 provided high-quality data.37,48 The sensitivity analysis of high-quality vs low-quality studies showed an RR of death at 2 and 5 years of 0.91 (0.47-1.76, P = .78) and 0.95 (0.70-1.28, P = .72) for early postoperative radiation compared to delayed or no radiation. Data for overall survival at 10 years or progression-free survival were not available in the high-quality literature.
A preplanned analysis comparing high-dose to moderate-dose radiation included 2 Class I studies.27,37 The RR of death at 2 and 5 years (high dose vs moderate dose) was 1.63 (0.81-3.31, P = .17) and 1.09 (0.88-1.35, P = .45). The RR of progression for high-dose vs moderate-dose radiation at 2 and 5 years was 1.46 (1.06-2.01, P = .02) and 1.00 (0.84-1.18, P = .97). No data were available for a comparison of outcomes at 10 years.
Chemotherapy
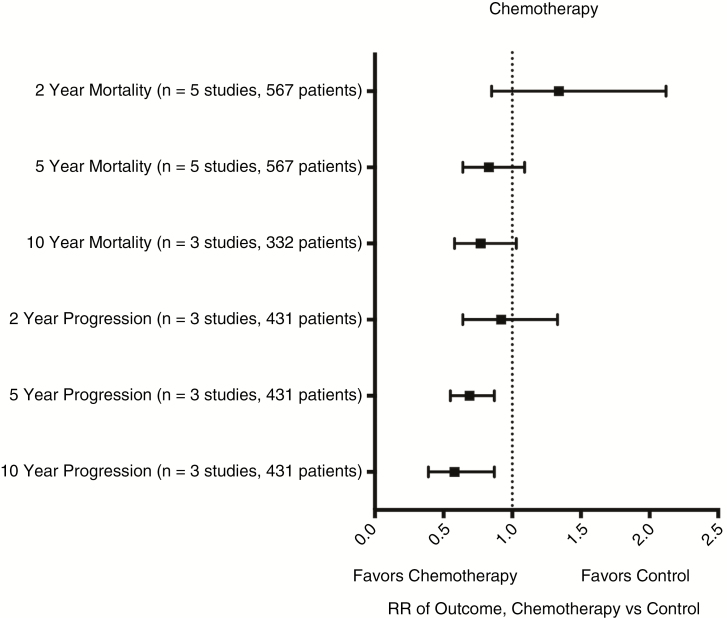
Five eligible studies comprising 567 patients reported on low-grade glioma patients treated with chemotherapy following radiotherapy.9,11,33,47,49 Chemotherapy regimens included PCV with radiation vs radiation,9 nimustine or nimustine/vincristine or nimustine/vincristine/dacarbazine with radiation vs radiation,49 lomustine with radiation vs radiation,11 nimustine/vincristine or procarbazine/nimustine/vincristine with radiation vs radiation,33 or procarbazine/nimustine/vincristine or PCV with radiation vs radiation.47 The RR and 95% CI for death (chemotherapy vs no chemotherapy) at 2, 5, and 10 years were 1.34 (0.85-2.12, P = .21),9,11,33,47,49 0.83 (0.64-1.09, P = .18),9,11,33,47,49 and 0.77 (0.58-1.03, P = .08).9,11,47 RR for progression at 2, 5, and 10 years was 0.92 (0.64-1.33, P = .86),9,33,47 0.69 (0.55-0.87, P = .001, NNT = 6),9,33,47 and 0.58 (0.39-0.87, P = .008, NNT = 3)9,33,47 (see Fig. 4 and supplement for specific comparisons). Histologic subtypes for patients in the included studies can be found in the supplementary material.
Fig. 4.
Meta-Analyses of Chemotherapy vs Control.
Forest plot of summary statistics on the 6 comparisons regarding outcomes of patients who received chemotherapy. Values plotted are relative risks (RR) with 95% confidence intervals. Summary statistics that do not cross X = 1 indicate a benefit favoring chemotherapy. Individual forest plots for all 6 comparisons can be found in the supplementary materials.
A sensitivity analysis of OS including only high-quality (Class I and II) studies showed an RR of death (chemotherapy vs no chemotherapy) at 2, 5, and 10 years of 1.23 (0.72-2.08, P = .45), 0.78 (0.58-1.05, P = .1), and 0.69 (0.56-0.86, P < .001, NNT = 5).9,11 Data from Class III and IV studies produced RRs of death at 2, and 5 years of 1.74 (0.70-4.35, P = .24) and 1.10 (0.60-2.00, P = .76).33,47,49
Two studies provided data on IDH1-R132H mutation (defined by immunohistochemistry) and outcomes.9,33 Among IDH1 R132H-mutated patients, the RR of progression with chemotherapy compared to control at 2, 5, and 10 years was 0.48 (0.06-4.1, P = .5), 0.27 (0.08-0.84, P = .02, NNT = 3), and 0.21 (0.03-1.59, P = .13). A single trial provided overall survival data supporting chemotherapy for IDH1-mutated patients.9
Prognostically Important Features in Low-Grade Glioma
Considering all available evidence from the included trials, the risk of death was greater for patients with tumors >5 cm (hazard ratio [HR] 2.00 [1.53-2.61, P < .001]),22,33,37,44 tumors located in eloquent brain areas (HR 1.98 [1.20-3.26, P = .008]),22,26,41IDH1 R132H-nonmutated (“wild-type”) tumors (HR 2.38 [1.16-5.00, P = .02]),9 and patients with a preoperative KPS ≤80% (HR 3.66 [0.82-16.3, P = .088]).22,26 Including only studies that provided Class I or Class II evidence substantially reduces the number of studies contributing evidence. In this latter analysis, the risk of death was greater for patients with tumors >5 cm (HR 2.27 [1.48-3.50, P < .001]),22,37 tumors located in eloquent brain areas (HR 9.37 [1.82-48.3, P = .007]),22IDH-nonmutated tumors (HR 2.38 [1.16-5.00, P = .02]),9 patients with a postoperative KPS ≤80% (HR 2.47 [1.10-5.55, P = .28]),47 and patients with a preoperative KPS ≤80% (HR 7.35 [2.96-18.2, P < .001]).22 Age ≥40 was not a statistically significant predictor of inferior survival in either the inclusive analysis (HR 0.98 [0.71-1.35, P = .90])22,29,37,44 or in the analysis restricted to high-quality trials (HR 1.33 [0.74-2.40, P = .34]).22,37
Discussion
The management of patients with low-grade glioma remains challenging, and is often based on clinician experience and patient preference. However, the gradual accumulation of data from randomized, controlled trials, supplemented with observational study data where randomized trials are lacking or have not proved feasible, now offers the possibility of a more evidence-based approach to the management of low-grade gliomas.50 Our study-level meta-analysis confirms that extensive resection (GTR) is associated with improved survival and progression-free survival at 2, 5, and 10 years compared to STR. This conclusion fulfills most of Hill’s criteria for a causal association:51 biologic plausibility, consistency, dose-effect (volumetric analyses concerning extents of resection have been mostly consistent with this conclusion as well),24,41,42 temporality, and analogy (to patients with glioblastoma),52 but is based exclusively on Class III and Class IV studies, which convey a substantial risk of bias and confounding (for example by age, performance status, tumor location, molecular diagnostics, and neurosurgeon judgment). The currently available evidence supports attempted GTR if safe and feasible, but the strength of this recommendation must be tempered by the low quality of the existing evidence. A prospective, randomized, controlled trial could overcome these concerns and would certainly be welcome, but multiple previous failed attempts at such studies suggest that this is unlikely to occur.
Early radiation has not demonstrated a survival benefit, but is associated with improved progression-free survival.48 The inclusion of the European Organisation for Research and Treatment of Cancer (EORTC) 22845 study in this analysis provides particularly robust data; however, it is notable that survival between arms was affected by high rates of crossover at the time of progression. The body of literature suggests delaying radiotherapy until the time of first progression does not compromise overall survival. Additionally, a subset analysis based on 2 high-quality studies favors moderate-dose radiation (45-55 Gy) compared to high-dose radiation (59-65 Gy) for decreased risk of radiation toxicity without loss of efficacy.27,37 A previous pooled analysis of patients in EORTC/Radiation Therapy Oncology Group/North Central Cancer Treatment Group Phase III clinical trials also did not find an association between increasing radiation dose and overall or progression-free survival.53 This synopsis is consistent with current European Federation of Neurological Societies and European Association for Neuro-Oncology recommendations.10,54
The role of chemotherapy only remains less certain. Because no single chemotherapy regimen has yet emerged as the undisputed standard of care for patients with low-grade gliomas, we analyzed all trials that compared any chemotherapy regimen to a control (nonchemotherapy) treatment arm, and accepted the risk of heterogeneity that this approach introduced into our analysis. A trial by Buckner and colleagues in 20169 provides the highest-quality evidence regarding the role of adjuvant chemotherapy in low-grade gliomas. The results of that trial demonstrated a 5.5-year median survival advantage and a progression-free survival benefit from the addition of PCV chemotherapy to radiotherapy. This survival advantage disappeared when studies of lower quality were included in the analysis. We suspect that the biases inherent in lower-quality trials (a consequence of design flaws such as retrospective data collection and analysis, lack of randomization or masked assessment, and nonrandom loss to follow-up) explain these differences in outcome. This is precisely the reason that we give more weight to the results of higher-quality studies. A progression-free survival benefit was demonstrated at 5 and 10 years with the addition of adjuvant chemotherapy and radiation compared to radiation alone. A survival benefit at 10 years for the chemotherapy-treated cohort persists even after our analysis was restricted to high-quality prospective trials. Additionally, an interim analysis of the CATNON trial assessing survival of anaplastic glioma patients receiving concurrent temozolomide and radiotherapy vs radiotherapy alone supports, by analogy, the finding of improved long-term survival with chemotherapy, albeit in higher-grade gliomas.55 The EORTC 22033 study was excluded from our analysis because we could not compare chemotherapy alone vs radiotherapy alone in the meta-analysis.56 Nevertheless, this trial provides important information regarding management of low-grade gliomas, highlights the importance of molecular diagnostics in the characterization of low-grade gliomas, and suggests a role for chemotherapy alone as initial therapy for patients with good-prognosis IDH-mutated and 1p19q-codeleted gliomas.56
Two studies in our analysis including 182 patients with IDH1-mutated tumors by immunohistochemistry showed a progression-free survival benefit at 5 years with the addition of chemotherapy.9,33 Lastly, the Eastern Cooperative Oncology Group E3F05 trial should provide more insight into the use of temozolomide in low-grade gliomas, and early-phase trials of the IDH1 inhibitor AG-120 are ongoing (NCT02073994).
Our meta-analysis corroborated the poor prognosis conveyed by tumors >5 cm in maximum dimension, by tumors located in eloquent brain areas, by tumors without IDH1 mutations, and by a pre- or postoperative KPS ≤80%.53,57 Surprisingly, the widely held view that age ≥40 also conveys a poor prognosis could not be substantiated by studies included in the present analysis, a finding also previously demonstrated in a pooled analysis of the early prospective and randomized clinical trials.53,58
The present analysis has several limitations. First, the quality of evidence of studies included in the analysis was often low, reflecting flaws in study design. High-quality, randomized, controlled trials remain the gold standard for treatment decisions and practice guidelines; however, these have proven difficult to conduct in patients with low-grade gliomas. Data from alternative study designs can, in this case, provide rational guidance for the management of low-grade gliomas.50 When possible, we performed analyses using only high-quality data. Second, some of the trials that contributed patients to this meta-analysis included patients with pilocytic astrocytomas (WHO Grade I tumors). These studies did not provide sufficient detail to permit us to exclude these patients from our analysis, but the number of such patients was small (8.3% of patients for the analysis of survival and extent of resection, and 2.7% for the analysis of progression-free survival; 0.71% for the analysis of survival and chemotherapy; 9.7% for the analysis of survival and radiation, and 0.27% for the analysis of progression-free survival and radiation), therefore the inclusion of these patients likely does not alter the interpretation of the presented data. Third, although no study was excluded because of its language, we did not identify any eligible non-English language trials, which could be an indication of publication bias.59 Fourth, the WHO recently introduced a new classification scheme for CNS tumors.3 Redesigning the analysis to reflect these changes would not be possible without access to individual patient and molecular data. How changes in the classification scheme will affect therapies and outcomes should be an area of investigation in the future. Additionally, many of the studies included in the analysis did not evaluate outcomes based on molecular markers that are now known to have important diagnostic and prognostic implications.8 Finally, although we did apply conventional meta-analytic techniques to attempt to address the risks of publication bias and study heterogeneity, these techniques do not fully mitigate these 2 potential threats to the reliability of meta-analyses.
There remains a paucity of high-quality studies addressing the management of low-grade gliomas. Although no Class I studies exist in the surgical literature, consistent results across 3 decades fortify the assertion that GTR is superior to STR, but both selection and outcome assessment bias remain serious concerns. Fewer studies overall address the question of the role of radiation and the role of chemotherapy. In contrast to the extent-of-resection question, however, some high-quality trials do exist, and demonstrate the feasibility of conducting such studies. Additional research incorporating the features of high-quality studies, in particular randomized treatment assignment, allocation concealment, masked assessment, and rational a priori sample size calculations are essential. These studies should also account for the molecular features that form the basis of the new classification scheme for low-grade gliomas.3
Questions regarding the management of low-grade gliomas persist. For clinicians who require guidance in caring for their patients with low-grade gliomas today, the literature reasonably supports the assertion that greater extent of resection is associated with greatest survival benefit. High-quality studies suggest that adjuvant chemotherapy in addition to radiation produces a survival benefit at 10 years, and the overall body of research suggests a progression-free survival benefit at 5 and 10 years with chemotherapy and radiation. Patients with IDH1-mutated tumors may benefit more from chemotherapy than their wild-type counterparts. Radiation is associated with a progression-free survival benefit at 2, 5, and 10 years. Moderate-dose (45-55 Gy) radiation appears to be as effective as high-dose (59-65 Gy) radiation. Maximum tumor diameter >5 cm, eloquent tumor location, lack of an IDH1 mutation, lack of 1p19q codeletion, and a KPS ≤80% either before or after surgery are associated with poorer overall survival, and should be considered when advising patients, deciding on therapy, and designing the next generation of clinical trials.
Funding
None declared.
Tweet: Management of low-grade gliomas: a systematic review and meta-analysis. #btsm
Supplementary Material
Acknowledgments
All authors reviewed and approved the manuscript prior to submission. TJB and MG had full access to all the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Data analysis was performed by TJB and MG (The University of Texas Southwestern Medical Center and Penn State Milton S. Hershey Medical Center).
Portions of this manuscript were presented at the 5th Quadrennial Meeting of the World Federation of Neuro-Oncology Societies, May 5, 2017, Zurich, Switzerland, and the 2017 Annual Meeting of the American Society of Clinical Oncology, June 5, 2017, Chicago, Illinois, USA.
Conflict of interest statement. None declared.
References
- 1. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. [DOI] [PubMed] [Google Scholar]
- 2. Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225; discussion 226–229. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist. 2014;19(4):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cancer Genome Atlas Research Network; Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 11. Eyre HJ, Crowley JJ, Townsend JJ, et al. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a Southwest Oncology Group study. J Neurosurg. 1993;78(6):909–914. [DOI] [PubMed] [Google Scholar]
- 12. Xia L, Fang C, Chen G, Sun C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. French J, Gronseth G. Lost in a jungle of evidence: we need a compass. Neurology. 2008;71(20):1634–1638. [DOI] [PubMed] [Google Scholar]
- 15. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedges LV, Olkin I.. Statistical Methods for Meta-Analysis. Cambridge, MA: Academic Press; 2014. [Google Scholar]
- 17. Ahmadi R, Rezvan A, Dictus C, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien). 2009;151(11):1359–1365. [DOI] [PubMed] [Google Scholar]
- 18. Bahary JP, Villemure JG, Choi S, et al. Low-grade pure and mixed cerebral astrocytomas treated in the CT scan era. J Neurooncol. 1996;27(2):173–177. [DOI] [PubMed] [Google Scholar]
- 19. Brown PD, Buckner JC, O’Fallon JR, et al. ; North Central Cancer Treatment Group; Mayo Clinic Adult patients with supratentorial pilocytic astrocytomas: a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys. 2004;58(4):1153–1160. [DOI] [PubMed] [Google Scholar]
- 20. Capelle L, Fontaine D, Mandonnet E, et al. ; French Réseau d’Étude des Gliomes Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg. 2013;118(6):1157–1168. [DOI] [PubMed] [Google Scholar]
- 21. Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. [DOI] [PubMed] [Google Scholar]
- 22. Gousias K, Schramm J, Simon M. Extent of resection and survival in supratentorial infiltrative low-grade gliomas: analysis of and adjustment for treatment bias. Acta Neurochir (Wien). 2014;156(2):327–337. [DOI] [PubMed] [Google Scholar]
- 23. Hosoda T, Takeuchi H, Hashimoto N, et al. Usefulness of intraoperative computed tomography in surgery for low-grade gliomas: a comparative study between two series without and with intraoperative computed tomography. Neurol Med Chir (Tokyo). 2011;51(7):490–495. [DOI] [PubMed] [Google Scholar]
- 24. Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117(6):1039–1052. [DOI] [PubMed] [Google Scholar]
- 25. Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 26. Jung TY, Jung S, Moon JH, Kim IY Moon KS, Jang WY. Early prognostic factors related to progression and malignant transformation of low-grade gliomas. Clin Neurol Neurosurg. 2011;113(9):752–757. [DOI] [PubMed] [Google Scholar]
- 27. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. [DOI] [PubMed] [Google Scholar]
- 28. Leibel SA, Sheline GE, Wara WM, Boldrey EB Nielsen SL. The role of radiation therapy in the treatment of astrocytomas. Cancer. 1975;35(6):1551–1557. [DOI] [PubMed] [Google Scholar]
- 29. Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15(4):1294–1301. [DOI] [PubMed] [Google Scholar]
- 30. Lo SS, Cho KH, Hall WA, et al. Does the extent of surgery have an impact on the survival of patients who receive postoperative radiation therapy for supratentorial low-grade gliomas?Int J Cancer. 2001;96(Suppl):71–78. [DOI] [PubMed] [Google Scholar]
- 31. McGirt MJ, Goldstein IM, Chaichana KL, Tobias ME, Kothbauer KF, Jallo GI. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63(1):55–60; discussion 60-61. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura M, Konishi N, Tsunoda S, et al. Analysis of prognostic and survival factors related to treatment of low-grade astrocytomas in adults. Oncology. 2000;58(2):108–116. [DOI] [PubMed] [Google Scholar]
- 33. Nitta M, Muragaki Y, Maruyama T, et al. Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus. 2015;38(1):E7. [DOI] [PubMed] [Google Scholar]
- 34. Roelz R, Strohmaier D, Jabbarli R, et al. Residual tumor volume as best outcome predictor in low grade glioma—a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep. 2016;6:32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112(1):1–9. [DOI] [PubMed] [Google Scholar]
- 36. Scerrati M, Roselli R, Iacoangeli M, Pompucci A Rossi GF. Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgery. J Neurol Neurosurg Psychiatry. 1996;61(3):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20(9):2267–2276. [DOI] [PubMed] [Google Scholar]
- 38. Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109(5):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaw EG, Daumas-Duport C, Scheithauer BW, et al. Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg. 1989;70(6):853–861. [DOI] [PubMed] [Google Scholar]
- 40. Singer JM. Supratentorial low grade gliomas in adults. A retrospective analysis of 43 cases treated with surgery and radiotherapy. Eur J Surg Oncol. 1995;21(2):198–200. [DOI] [PubMed] [Google Scholar]
- 41. Smith KA, Ashby LS, Gonzalez LF, et al. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;109(Suppl):106–117. [DOI] [PubMed] [Google Scholar]
- 42. van Veelen ML, Avezaat CJ, Kros JM, van Putten W Vecht C. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatry. 1998;64(5):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeh SA, Ho JT, Lui CC, Huang YJ Hsiung CY Huang EY. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005;78(927):230–235. [DOI] [PubMed] [Google Scholar]
- 44. Youland RS, Brown PD, Giannini C, Parney IF Uhm JH Laack NN. Adult low-grade glioma: 19-year experience at a single institution. Am J Clin Oncol. 2013;36(6):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reedy DP, Bay JW, Hahn JF. Role of radiation therapy in the treatment of cerebral oligodendroglioma: an analysis of 57 cases and a literature review. Neurosurgery. 1983;13(5):499–503. [DOI] [PubMed] [Google Scholar]
- 46. Schomas DA, Laack NN, Rao RD, et al. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009;11(4):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sunyach MP, Jouvet A, Perol D, et al. Role of exclusive chemotherapy as first line treatment in oligodendroglioma. J Neurooncol. 2007;85(3):319–328. [DOI] [PubMed] [Google Scholar]
- 48. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 49. Sakata K, Hareyama M, Komae T, et al. Supratentorial astrocytomas and oligodendrogliomas treated in the MRI era. Jpn J Clin Oncol. 2001;31(6):240–245. [DOI] [PubMed] [Google Scholar]
- 50. Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med. 2017;377(5):465–475. [DOI] [PubMed] [Google Scholar]
- 51. Hill AB. The environment and disease: association or causation?Proc R Soc Med. 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gorlia T, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15(11):1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soffietti R, Baumert BG, Bello L, et al. ; European Federation of Neurological Societies Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. [DOI] [PubMed] [Google Scholar]
- 55. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 58. Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jüni P, Holenstein F, Sterne J, Bartlett C Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31(1):115–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.