Abstract
Background
Neurocognitive assessments have become integral to comprehensive neuro-oncology care. Existing screening tools may be insensitive to cognitive changes caused by medical treatments. Research supports the clinical value and psychometric properties of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in various medical populations; however, there is minimal evidence for its use in neuro-oncology. The purpose of the current study was to further explore the cognitive profile of patients with primary brain tumor (PBT) using the RBANS and to assess rates of below-expectation performance compared to normative data and estimated intellectual functioning.
Methods
Data were collected on 82 PBT patients (54% male; age range, 19-81 years). All patients were administered the RBANS-Update and the Advanced Clinical Solutions–Test of Premorbid Functioning (TOPF) according to standardized instructions. Cognitive strengths and weaknesses were identified for PBT patients. Descriptive analyses, t tests, and chi-squared tests were utilized to identify and compare cognitive profiles.
Results
Overall, cognitive performance was low average for PBT patients. When compared to standardization data, PBT patients performed significantly worse across all 5 RBANS indexes, with Attention and Memory showing the largest discrepancies. Estimated intelligence analyses reflected greater deficits in cognitive functioning than when compared to a normal distribution.
Conclusions
Preliminary research demonstrates the RBANS is an efficient screening tool to assess cognitive deficits in PBT patients. Data also support the importance of comparison to self, rather than normative distribution in ensuring proper identification and classification of patients.
Keywords: cognitive functioning, neuropsychological functioning, primary brain tumor patients, RBANS, quality of life
The detrimental effects of brain cancer and its related treatments on cognitive capacity have gained considerable attention and recognition.1–4 Cognitive deficits, even when mild, can result in diminished functional independence,5 as well as produce unfavorable psychiatric consequences, especially if left untreated.6,7 Up to 90% of primary brain tumor (PBT) patients demonstrate at least one area of cognitive dysfunction at baseline testing,8 while up to 80% show further deficits following treatment.9,10 Deficits among PBT patients are evident across many cognitive domains, including attention, executive function, processing speed, memory, and language, which can significantly affect daily functioning and quality of life.1,3,11–13
The inclusion of neurocognitive assessments throughout treatment and as end points in clinical trials is increasing, especially when related to CNS tumors.14 In fact, sustained or improved quality of life, including cognitive functioning, is now considered as equally an important outcome for PBT patients as prolonged survival rates and postponed tumor progression.15,16 The field lacks established standards of best practice for neurocognitive assessments. This lack of uniformly accepted and validated instruments prevents cross-study comparison, adequate understanding of cognitive dysfunction, and proper clinical guidance on which neuropsychological tests should be used for identifying impairments.
Given that individuals with PBT are prone to fatigue, they are less likely to withstand the typical 6- to 7-hour comprehensive neuropsychological evaluation that other populations may endure. Furthermore, most cancer centers are housed in academic medical centers and other integrated care settings where brief neuropsychological evaluations are mandatory because of space, time, and billing limitations. However, selection of an appropriate assessment battery should also be comprehensive enough to differentiate cognitive dysfunction in patients.17 Cognitive deficits within neuro-oncology may have been underreported historically, as medical practitioners have traditionally relied on brief mental-status checks. These existing screening tools, such as the Mini-Mental State Examination18 and the Montreal Cognitive Assessment,19 continue to be criticized because they are insensitive to cognitive changes due to medical treatments.20–22 Therefore, to efficiently serve patients and providers in increasingly integrated care settings, neuropsychological evaluations for a variety of clinical populations including PBTs may benefit from not exceeding 2 to 3 hours. Recently, The International Cognition and Cancer Task Force17 provided recommendations for a core set of neuropsychological tests to be utilized within the neuro-oncology population for research purposes. The recommended tests included measures of verbal memory, processing speed, and executive function. Noticeably absent, however, were indexes of right-hemisphere visual memory, working memory, and language-based confrontational naming, all identified cognitive domains subject to impairment following neurosurgery and brain tumor treatment. Additionally, using coordinated norms (the same norms for multiple cognitive domains) helps reduce error variance and the risk for false-positive findings. As such, the question remains whether there is a more comprehensive and appropriate measure available for this population.
The present study aims to further address this gap in the literature by investigating the cognitive profile of patients diagnosed with PBT using an alternative, abbreviated, yet comprehensive screening battery of tests (Repeatable Battery for the Assessment of Neuropsychological Status; RBANS). The RBANS was originally developed to screen for dementia and has since been found to be reliable and valid in a variety of disorders including multiple sclerosis, Parkinson disease, and cerebrovascular disorders.23,24 To date, there has been minimal examination into the utility of the RBANS with PBT patients. In 2010, Lageman et al25 revealed that more than 50% of brain tumor patients demonstrated impairment on at least 1 subtest of the RBANS and that when compared to normal distribution, a combination of 4 subtests (Figure Copy, Coding, List Recognition, and Story Recall) captured 90% of the impaired subgroup. Onodera and colleagues22 later determined memory to be the greatest cognitive impairment when utilizing the RBANS at a 4-month follow-up evaluation posttreatment; however, this was conducted with brain metastases patients who undergo treatment regimens different from PBT patients.
The purpose of the current study was to further explore the cognitive profile of PBT patients using the RBANS and to assess rates of below-expectation performance, ie, >1 SD below average compared to a normal distribution or >1 SD below estimated premorbid intellectual functioning. While an ideal within-person comparison would be individuals’ measured intellectual functioning prior to PBT diagnosis and/or treatment, this is often not feasible—especially in a clinical setting. As such, estimated premorbid intellectual functioning has come to be commonly used when these data are not available. Given the current knowledge of cognitive profiling with PBT patients and limited research into the utility of the RBANS in neuro-oncology, we hypothesize that PBT patients will have lower scores on the RBANS when compared to a standardization sample. Also, assuming that the baseline IQ of the population of all brain tumor patients forms a normal curve similar to that of a normal population, it is expected that PBT patients’ prevalence of below-expectation performance will be higher when comparing against their own estimated intelligence rather than a normal distribution.
Materials and Methods
Patients
Eighty-two patients from a National Cancer Institute-designated cancer center were included in the present study. Patients were recruited following neuropsychological evaluations, which are standard of care for all new brain tumor patients at this cancer center. Written informed consent was obtained from all study patients. Inclusion criteria were as follows: 1) confirmed PBT diagnosis via MRI and histopathological diagnosis, 2) a minimum of 2 weeks postsurgical resection or biopsy (if applicable), 3) all neuropsychological assessment measures completed within 1 week to minimize effects of timing, 4) English speaking, and 4) available age-adjusted RBANS normative data (ages 18-89+). Patients with sensory-motor and/or vision limitations were included in this sample.
Procedure
Ethical approval from the institutional review board was obtained. As part of each patient’s standard of care, neuropsychological assessment was performed and written informed consent was obtained prior to inclusion of the participant’s data in the study database. Data from neuropsychological assessments as well as medical chart review were used for the present cross-sectional study.
Measurements
Medical chart review provided demographic variables (age, gender, ethnicity, and level of education) and medical variables (classification, hemisphere, and grade of brain tumor and treatment regimen). The selected battery for this study included administration of the Advanced Clinical Solutions–Test of Premorbid Functioning (TOPF)26 to assess estimated intelligence and the administration of the RBANS23 to assess cognitive functioning. The TOPF is a well-validated and reliable measure of estimated intelligence based on a reading paradigm. It has been shown to correlate with premorbid intelligence in a variety of clinical populations as well as intelligence testing in nonclinical populations.26–28 Word reading tests, like the TOPF, have long been the standard for determining premorbid functioning because this ability is often preserved following an insult to the brain.29–31 It can be administered in 5 minutes and provides a standard score (SS) based on same-aged peers. The RBANS is a well-validated and reliable cognitive screening battery comprising 12 subtests that can be administered in 20-30 minutes with 4 alternating forms for reevaluation. Further, RBANS index scores have strong convergent validity with other neuropsychological tests on which they were based.23,24 The RBANS provides SS based on same-aged peers for 5 indexes of neuropsychological functioning: Attention, Language, Visuospatial-Construction, Immediate Memory, and Delayed Memory (Table 1). These indexes combine to compute a total scale score of cognitive functioning. Although the RBANS does not have a specific Executive Functioning Index, it should be noted that the subscales Semantic Fluency (part of the Language Index) and Coding (part of the Attention Index) are executive tasks and important components of the RBANS measure.
Table 1.
Description of Repeatable Battery for the Assessment of Neuropsychological Status Indexes (RBANS)23
| RBANS Index | Description | Subtests Included |
|---|---|---|
| Immediate Memory | Indicates the examinee’s ability to remember information immediately after it is presented. | List Learning Story Memory |
| Visuospatial/Construction | Indicates examinee’s ability to perceive spatial relations and to construct a spatially accurate copy of a drawing. | Figure Copy Line Orientation |
| Language | Indicates examinee’s ability to respond verbally to either naming or retrieving learned material. | Picture Naming Semantic Fluencya |
| Attention | Indicates the examinee’s capacity to remember and manipulate both visually and orally presented information in short-term memory storage. | Digit Span Codinga |
| Delayed Memory | Indicates the examinee’s anterograde memory capacity. | List Recall List Recognition Story Recall Figure Recall |
| Total Scale | A total score that is calculated by summing the above 5 index scores. | Five indexes combined |
aExecutive tasks.
Statistical analysis
Data screening and analysis were conducted using SPSS 24. Demographic (age, gender, ethnicity, and level of education) and medical variables (classification, hemisphere, and grade of brain tumor and treatment regimen) were analyzed for sample description. Descriptive analyses (means, SDs, and distribution of scores) were used to identify the cognitive profile of the RBANS in the PBT population. Comparisons (t tests) of RBANS scores were examined by tumor grade and hemisphere. Group comparisons (t tests) were also completed to compare PBT to standardization data. The standardization reference group from the RBANS manual was used for comparison data,32 which included 244 participants selected to proportionally represent the United States population in regard to sex, race/ethnicity, and geographic region. The 244 standardization reference groups included only individuals with more than high school education to adequately match our PBT sample. When investigating performance by reference source, a significant change from the normative distribution and/or estimated intelligence was defined as greater than 1 SD of difference. For normal distribution comparison, scores were considered ‘below expectations’ if participants scored more than 1 SD below average (SS < 85). For estimated intelligence comparison, scores were considered to be ‘below expectations’ if participants scored more than 1 SD below their TOPF score (TOPF – RBANS index score > 15).
Results
Demographic Characteristics
Eighty-two PBT patients (age range, 19-81 years; 54% male; 85% Caucasian; mean education level = 14.8 years, range, 7-20 years) were included in this study. Of the 82, 65% (n = 53) were diagnosed with high-grade tumors and 35% (n = 29) with low-grade tumors. Seventy-four percent (n = 61) of participants underwent surgical resection following diagnosis, 51% (n = 42) radiation therapy, and 45% (n = 37) completed at least one cycle of adjuvant chemotherapy prior to the neuropsychological evaluation. Full demographic data are presented in Table 2.
Table 2.
Demographics of Primary Brain Tumor Patients
| Demographics | n (%) |
|---|---|
| Mean age | 50.6 years (range, 19-81 years) |
| Gender | |
| Male | 44 (53.7) |
| Female | 38 (46.3) |
| Ethnicity | |
| Caucasian | 70 (85.4) |
| African American | 11 (13.4) |
| Other | 1 (1.2) |
| Mean level of education | 14.8 years (range, 7-20 years) |
| <HS | 10 (12) |
| HS | 12 (15) |
| >HS | 60 (73) |
| Mean estimated intelligencea | 101.7 (range, 68-127) |
| Tumor type | |
| Meningioma | 6 (7.3) |
| Oligodendroglioma | 15 (18.3) |
| Astrocytoma | 16 (19.5) |
| Glioblastoma multiforme | 34 (41.5) |
| CNS lymphoma | 6 (7.3) |
| Mixed glioma | 1 (1.2) |
| Craniopharyngioma | 1 (1.2) |
| Hemangiopericytoma | 1 (1.2%) |
| Hemangioma | 1 (1.2%) |
| Schwannoma | 1 (1.2%) |
| Tumor grade | |
| Low | 28 (34.1%) |
| High | 54 (65.8%) |
| Tumor hemisphere | |
| Left | 34 (41.5%) |
| Right | 33 (40.2%) |
| Bilateral | 15 (18.3%) |
| Surgery | |
| Biopsy | 20 (24.4%) |
| Resection | 61 (74.4%) |
| None | 1 (1.2%) |
| Hx of radiation therapy | 37 (45.1%) |
| Hx of chemotherapy | 42 (51.2%) |
Abbreviations: HS, high school; Hx, history.
aThe Advanced Clinical Solutions–Test of Premorbid Functioning (TOPF)26 was used to assess estimated intelligence. The TOPF is a reading paradigm that has been shown to correlate with premorbid intelligence in a variety of clinical populations as well as intelligence testing in nonclinical populations.26–28 Word reading tests, like the TOPF, have long been the standard for determining premorbid functioning as this ability is often preserved following an insult to the brain.29–31
Cognitive Functioning
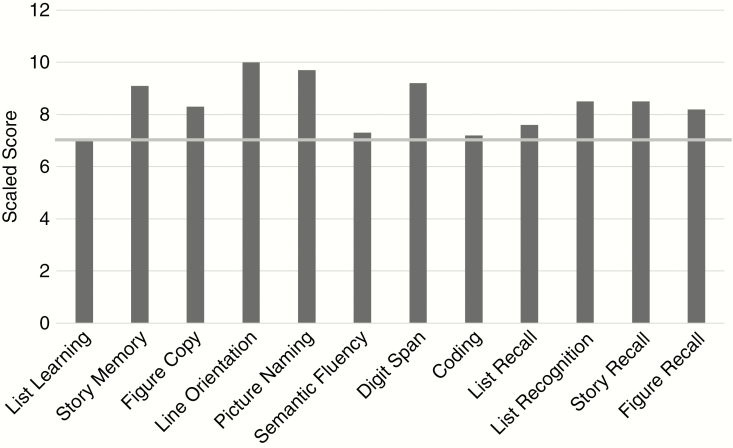
Within the PBT group, the overall total scale score was low average (SS = 88.7). Attention, Immediate Memory, and Delayed Memory indexes were the lowest scores, followed by Language and Visuospatial/Construction (Table 3). Despite relatively intact overall estimated intelligence (TOPF mean = 101), PBT patients performed significantly below the standardization sample on all 5 RBANS index scores (Table 3). Attention and Memory showed the largest discrepancies, with Language following closely behind. PBT subtest SSs are shown in Figure 1. List Learning, Semantic Fluency, Coding, and List Recall were close to 1 SD below the norm. Comparisons of RBANS scores were examined by tumor grade (high vs low) and hemisphere location (left, right, bilateral). There was no significance found between tumor grade groups across RBANS indexes (P > .05). Visuospatial Construction revealed significance between hemisphere groups (P = .013; left > right > bilateral). Though not statistically significant, Language was trending (P = .097; left < right).
Table 3.
Group Comparisons Across Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Index Scores
| RBANS Index Scores | PBT Mean (SD) |
>High School Education | |||
|---|---|---|---|---|---|
| Standardization Mean (SD)23a |
Mean Difference | 95% CI | Significance (P) | ||
| Immediate Memory | 88.4 (21.7) | 104.2 (13.4) | 15.8 | 20.5 to 11.1 | <.001 |
| Visuospatial-Construction | 96.6 (15.9) | 103.6 (14.1) | 7.0 | 10.5 to 3.6 | <.001 |
| Language | 90.9 (16.4) | 105.6 (14.7) | 14.7 | 17.1 to 10.0 | <.001 |
| Attention | 88.7 (18.8) | 104.5 (13.7) | 15.8 | 20.9 to 12.8 | <.001 |
| Delayed Memory | 88.9 (17.1) | 103.8 (14.2) | 14.9 | 18.6 to 11.2 | <.001 |
Abbreviation: PBT, primary brain tumor.
aRBANS standardization sample (N = 244; greater than high school level of education).
Fig. 1.
Primary Brain Tumor Group Mean Subtest Scale Profile. Gray line ≥1 SD below expectations.
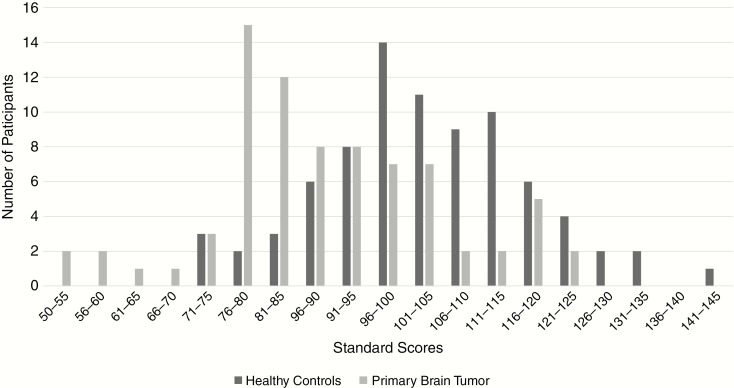
The distribution of PBT RBANS total scale scores was tested for normality. Kolmogorov-Smirnov (P = .178) and Shapiro-Wilk (P = .129) were both nonsignificant, suggesting normal distribution. The distribution of RBANS total scale scores is depicted in Figure 2. The PBT group is presented alongside the Healthy Control RBANS Total Scale score distribution from Karantzoulis et al33 for visual comparison only. No statistics were acquired for comparison.
Fig. 2.
Distribution of Repeatable Battery for the Assessment of Neuropsychological Status Total Scale Scores.a aHealthy Control Repeatable Battery for the Assessment of Neuropsychological Status Total Scale score distribution adapted from Karantzoulis et al.33
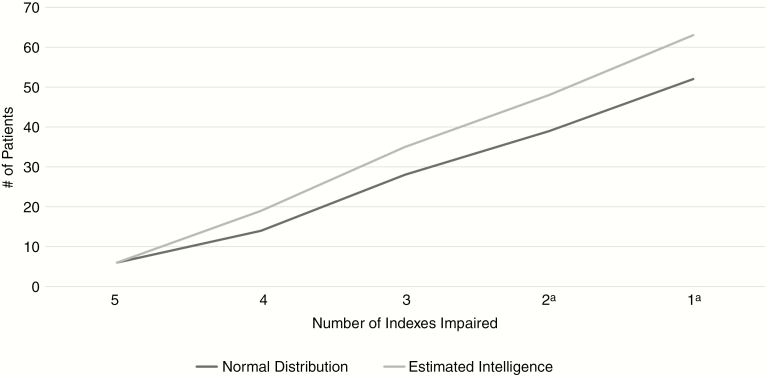
Rates of below-expectation performance differed according to source of reference (ie, estimated functioning vs normal distribution) because the proportion of PBT patients with a performance >1 SD below expectation in total scale score was significantly higher compared to self (48.8%) than when compared to a normal distribution (40.2%), χ2 = 13.715, P < .001. This also held true when looking at a minimum of 1 (76.8% vs 63.4%; χ2 = 14.67, P < .001) and 2 indexes of below-expectation performance (58.5% vs 47.5%; χ2 = 13.45, P < .001; Figure 3).
Fig. 3.
Indexes Impaired in Primary Brain Tumor Group According to Source of Reference. aNumber of impaired indexes between source of reference group reach statistical difference (P < .001).
Discussion
Although the utility of assessing neuropsychological impairment using the RBANS has been well documented across medical populations,24,32–36 this instrument has not been investigated fully within the PBT population. Overall, PBT patients demonstrated low-average functioning when using the RBANS Total Scale as a measure of cognitive performance. However, their performance was not consistent across indexes. Instead, mean performances ranged between low average to average with greater dysfunction in Attention and Memory and relatively spared Language and Visuospatial-Construction. Learning a word list (List Learning), retrieving learned material quickly (Semantic Fluency), manipulating visual information quickly (Coding), and recalling a learned word list (List Recall) accounted for the lowest subtests and largest discrepancies from norms. These results are consistent with previous literature in PBT populations demonstrating deficits in learning and storing information, language fluency, and processing speed.11,13,37,38 In fact, Lageman et al25 found Coding to be an RBANS subtest indicative of impairment in brain tumor patients, along with Line Orientation and Picture Naming, to be the most intact. There was a discrepancy in our findings, however, in regard to Visuospatial-Construction, in which Lageman25 noted significant impairment in her sample and our results revealed a strength. This is surprising given our sample had more right-hemisphere tumor placement (40.2%) as compared to 14.71% in Lageman.25Tumor location, not malignancy grading, demonstrated performance differences consistent with previous reports.39As expected, scores were consistently lower across all indexes in the PBT sample relative to the standardization reference group, reaching significance in all 5 indexes. Consistent with previous research in PBT populations, although this weakness was statistically significant, it was not always clinically drastic (>1 SD). Many patients diagnosed with PBT who subsequently undergo cancer treatment see a decline in functioning from baseline or pretreatment capability, yet for most these declines do not often fall within the severely impaired range. Instead they lower slightly from premorbid functioning. The largest discrepancies in cognitive functioning between groups were evident across measures of Attention and Memory, with Language closely following. These findings are in line with research documenting decreased functioning in attention and memory following treatment for PBTs, including chemotherapy treatment that crosses the blood-brain barrier and radiation treatment targeting the brain.3,11,12
Also as expected, rates of below-expectation performance were more frequently identified when compared to estimated intelligence than a normal distribution. This was consistent when examining both overall cognitive functioning (RBANS total scale score) and when looking at the number of indexes reflecting below-expectation performance. Source of reference appears to play an important role in the identification of performance below expectation and introduces 2 potential classification errors: (1) patients with an estimated intelligence in the low-average range being incorrectly classified as performing “below expectations” and/or (2) patients with an estimated high average intelligence being incorrectly identified as having intact cognitive functioning. The same individual with PBT may or may not be classified as performing “below expectations” depending on the reference source being utilized. In our sample, depending on the number of indexes, up to 13% of patients might have been misidentified or classified differently across scales/practitioners.
There are several important study limitations to mention. First, this study was taken from a convenience sampling approach—patients had diverse tumor characteristics and were at varying time points in their treatment regimens. In our clinical practice, PBT patients receive neuropsychological evaluations as part of standard of care following diagnosis and throughout treatment (time points based on tumor type/grade). As such, this sample mirrors clinical practice. To control for time since diagnosis and treatment, a larger and more homogenous sample would be beneficial. A larger sample would also allow for comparisons based on medical variables; however, these analyses were not feasible in the current sample because of power and data limitations. Second, there were no healthy group data available for comparison. As a result, standardization data retrieved from the RBANS manual were used as a comparison,23 thus limiting the analyses performed. Obtaining raw data from a healthy control group to perform more in-depth statistical comparisons is recommended. In addition, we did not administer a lengthy comprehensive battery or measures of day-to-day functioning in the current study, which limits our ability to draw conclusions regarding how RBANS scores relate to other commonly used markers of functioning. Lastly, there are instances in which the estimated intelligence (TOPF) may not have been an accurate indication but rather an underestimate due to other cognitive deficits including aphasia, visual neglect, or memory impairments. Although not frequent, this is an attribute of this medical population.
Overall, the results of this study provide supportive evidence for RBANS’ utility as a brief but comprehensive assessment of cognitive functioning in PBT patients. Furthermore, our results support the use of estimated intelligence as a reference source when assessing cognitive functioning. These findings have important clinical applications that would inform treatment recommendations and planning. More research is warranted investigating the use of RBANS in larger samples of PBT relative to nonclinical controls.
Funding
None declared.
Conflict of interest statement. None declared.
References
- 1. Noll KR, Ziu M, Weinberg JS Wefel JS. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol. 2016;128(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith-Cohn M, Chen Z, Peereboom D, Stevens G. Maximizing function and quality of life of patients with glioblastoma after surgical resection: a review of current literature. J Cancer Ther. 2016;7(12):857–888. [Google Scholar]
- 3. McAleer MF, Brown PD. Neurocognitive function following therapy for low-grade gliomas. Semin Radiat Oncol. 2015;25(3):210–218. [DOI] [PubMed] [Google Scholar]
- 4. Meskal I, Gehring K, Rutten GJ Sitskoorn MM. Cognitive functioning in meningioma patients: a systematic review. J Neurooncol. 2016;128(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sands LP, Yaffe K, Covinsky K et al. Cognitive screening predicts magnitude of functional recovery from admission to 3 months after discharge in hospitalized elders. J Gerontol A Biol Sci Med Sci. 2003;58(1):M37–M45. [DOI] [PubMed] [Google Scholar]
- 6. Wellisch DK, Kaleita TA, Freeman D Cloughesy T Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230–238. [DOI] [PubMed] [Google Scholar]
- 7. Pelletier G, Verhoef MJ, Khatri N Hagen N. Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. [DOI] [PubMed] [Google Scholar]
- 8. Tucha O, Smely C, Preier M Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333; discussion 333. [DOI] [PubMed] [Google Scholar]
- 9. Meyers CA, Geara F, Wong PF Morrison WH. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46(1):51–55. [DOI] [PubMed] [Google Scholar]
- 10. Weiss B. Chemobrain: a translational challenge for neurotoxicology. Neurotoxicology. 2008;29(5):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrey LE. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J Neurooncol. 2012;108(2):285–290. [DOI] [PubMed] [Google Scholar]
- 12. Postma TJ, Klein M, Verstappen CC et al. Radiotherapy-induced cerebral abnormalities in patients with low-grade glioma. Neurology. 2002;59(1):121–123. [DOI] [PubMed] [Google Scholar]
- 13. Bosma I, Vos MJ, Heimans JJ et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. [DOI] [PubMed] [Google Scholar]
- 15. Schagen SB, Klein M, Reijneveld JC et al. Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. EJC Suppl. 2014;12(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyers CA, Hess KR, Yung WK Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 2000;18(3):646–650. [DOI] [PubMed] [Google Scholar]
- 17. Wefel JS, Vardy J, Ahles T Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 18. Patterson J. Multilingual aphasia examination. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. New York:Springer;2011:1674–1676. [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 20. Kashiwazaki D, Takaiwa A, Nagai S et al. Reversal of cognitive dysfunction by total removal of a large lateral ventricle meningioma: a case report with neuropsychological assessments. Case Rep Neurol. 2014;6(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noggle CA, Dean RS, eds. Neuropsychology and cancer: an emerging focus. In: The Neuropsychology of Cancer and Oncology. New York: Springer;2013:3–39. [Google Scholar]
- 22. Onodera S, Aoyama H, Tha KK et al. The value of 4-month neurocognitive function as an endpoint in brain metastases trials. J Neurooncol. 2014;120(2):311–319. [DOI] [PubMed] [Google Scholar]
- 23. Randolph C. RBANS Update: Repeatable Battery for the Assessment of Neuropsychological Status: Manual. Bloomington, MN:Pearson; 2009. [Google Scholar]
- 24. McKay C, Casey JE, Wertheimer J Fichtenberg NL. Reliability and validity of the RBANS in a traumatic brain injured sample. Arch Clin Neuropsychol. 2007;22(1):91–98. [DOI] [PubMed] [Google Scholar]
- 25. Lageman SK, Cerhan JH, Locke DE Anderson SK Wu W Brown PD. Comparing neuropsychological tasks to optimize brief cognitive batteries for brain tumor clinical trials. J Neurooncol. 2010;96(2):271–276. [DOI] [PubMed] [Google Scholar]
- 26. Wechsler D. Advanced Clinical Solutions for WAIS-IV and WMS-IV. San Antonio, TX:Pearson; 2009. [Google Scholar]
- 27. Franzen MD, Burgess EJ, Smith-Seemiller L. Methods of estimating premorbid functioning. Arch Clin Neuropsychol. 1997;12(8):711–738. [PubMed] [Google Scholar]
- 28. Smith-Seemiller L, Franzen MD, Burgess EJ Prieto LR. Neuropsychologists’ practice patterns in assessing premorbid intelligence. Arch Clin Neuropsychol. 1997;12(8):739–744. [PubMed] [Google Scholar]
- 29. Donders J, Stout J. The influence of cognitive reserve on recovery from traumatic brain injury [published online ahead of print April 12, 2018]. Arch Clin Neuropsychol. doi: 10.1093/arclin/acy035. [DOI] [PubMed] [Google Scholar]
- 30. Willshire D, Kinsella G, Prior M. Estimating WAIS-R IQ from the National Adult Reading Test: a cross-validation. J Clin Exp Neuropsychol. 1991;13(2):204–216. [DOI] [PubMed] [Google Scholar]
- 31. Bright P, van der Linde I. Comparison of methods for estimating premorbid intelligence [published online ahead of print March 12, 2018]. Neuropsychol Rehabil. doi: 10.1080/09602011.2018.1445650 [DOI] [PubMed] [Google Scholar]
- 32. Randolph C, Tierney MC, Mohr E Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 33. Karantzoulis S, Novitski J, Gold M Randolph C. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): utility in detection and characterization of mild cognitive impairment due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28(8):837–844. [DOI] [PubMed] [Google Scholar]
- 34. Mooney S, Hasssanein TI, Hilsabeck RC et al. ; UCSD Hepatology Neurobehavioral Research Program Utility of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in patients with end-stage liver disease awaiting liver transplant. Arch Clin Neuropsychol. 2007;22(2):175–186. [DOI] [PubMed] [Google Scholar]
- 35. Duff K, Humphreys Clark JD, O’Bryant SE Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aupperle RL, Beatty WW, Shelton Fde N Gontkovsky ST. Three screening batteries to detect cognitive impairment in multiple sclerosis. Mult Scler. 2002;8(5):382–389. [DOI] [PubMed] [Google Scholar]
- 37. Lageman SK, Brown PD, Anderson SK et al. Exploring primary brain tumor patient and caregiver needs and preferences in brief educational and support opportunities. Support Care Cancer. 2015;23(3):851–859. [DOI] [PubMed] [Google Scholar]
- 38. Wefel JS, Lenzi R, Theriault RL Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. [DOI] [PubMed] [Google Scholar]
- 39. Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30(1):61–69. [DOI] [PubMed] [Google Scholar]