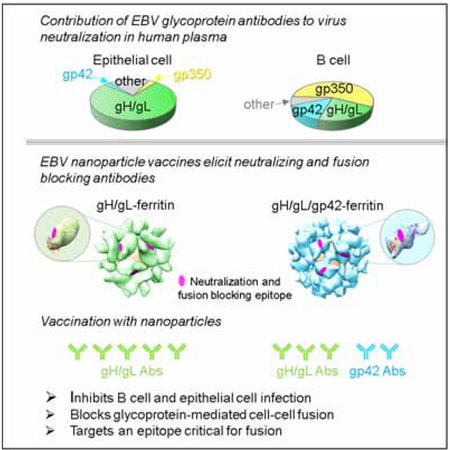
SUMMARY
Epstein-Barr virus (EBV) causes infectious mononucleosis and is associated with epithelial cell cancers and B cell lymphomas. An effective EBV vaccine is not available. We found that antibodies to the EBV glycoprotein gH/gL complex were the principal components in human plasma that neutralized infection of epithelial cells, and that antibodies to gH/gL and gp42 contributed to B cell neutralization. Immunization of mice and nonhuman primates with nanoparticle vaccines that displayed components of the viral fusion machinery, EBV gH/gL or gH/gL/gp42, elicited antibodies that potently neutralized both epithelial cell and B cell infection. Immune serum from nonhuman primates inhibited EBV glycoprotein-mediated fusion of epithelial cells and B cells and targeted an epitope critical for virus-cell fusion. Therefore, unlike the leading EBV gp350 vaccine candidate that only protects B cells from infection, these EBV nanoparticle vaccines elicit antibodies that inhibit the virus fusion apparatus and provide cell-type independent protection from virus infection.
Keywords: Epstein-Barr virus, vaccine, nanoparticle, virus fusion, infectious mononucleosis, B cell lymphoma
Graphical Abstract
In Brief
EBV is associated with B cell and epithelial cell malignancies. Bu et al. showed that an EBV nanoparticle vaccine elicits antibodies to EBV gH/gL and gp42 in mice and non-human primates that inhibit the viral fusion apparatus and block infection of B cells and epithelial cells. This approach may be important for developing an effective EBV vaccine.
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous herpesvirus that infects 95% of adults world-wide. Primary infection in adolescents or young adults often results in infectious mononucleosis (IM). EBV is an oncogenic virus and worldwide there are ~ 200,000 cases of cancer associated with the virus, resulting in ~ 140,000 deaths each year (Cohen et al., 2011). Epithelial cell malignancies are the most common EBV-associated cancers including gastric and nasopharyngeal carcinoma. EBV is also associated with Burkitt and Hodgkin lymphoma.
Primary infection by EBV usually occurs by contact of saliva from infected individuals with epithelial cells in the oropharynx, where virus is amplified through lytic replication and subsequently infects B cells. EBV may directly infect resting B cells in the tonsillar crypts. Infected B cells are usually latently infected and traffic back to the oropharynx, where EBV is amplified by lytic replication in epithelial cells, and shed into saliva (Cohen, 2000). Therefore, both B cells and epithelial cells are important for EBV infection.
Viral entry into B cells is initiated by attachment of EBV glycoprotein gp350 to complement receptor 2 (CD21) on the cell surface (Fingeroth et al., 1984). The viral glycoprotein complex made up of gH, gL and gp42 binds to HLA class II molecules via gp42 (Spriggs et al., 1996). Both gH (Wu and Hutt-Fletcher, 2007) and gp42 (Kirschner et al., 2007) are required for fusion of the virus with B cells. gB is activated and facilitates viral membrane fusion with the B cell membrane (McShane and Longnecker, 2004). Infection of epithelial cells is initiated by attachment of EBV BMRF2 to integrins (Tugizov et al., 2003), followed by binding of gH/gL to integrins and ephrin receptor A2 (Chen et al., 2018; Chesnokova et al., 2009; Zhang et al., 2018), and then activation of gB to facilitate virus-cell membrane fusion. EBV gp350 and gp42 are unique in EBV, while gH/gL and gB comprise the core viral fusion machinery and hence, conserved among all herpesviruses.
Serum antibodies from EBV-infected persons are able to neutralize virus infection of B cells and epithelial cells (Sashihara et al., 2009; Thorley-Lawson and Poodry, 1982; Tugizov et al., 2003). Since the majority of serum neutralizing antibodies that block B cell infection target gp350 (Thorley-Lawson and Poodry, 1982), nearly all clinical trials of EBV prophylactic vaccines have used gp350 as the sole immunogen (Cohen, 2015). The contribution of antibodies targeting other EBV glycoproteins, such as gH/gL and gp42, on neutralizing viral infection in B cells and epithelial cells has not been investigated. Despite the importance of epithelial cell infection in the EBV life cycle and the finding that EBV-associated epithelial cell malignancies are more common than B cell cancers, vaccines targeting epithelial cell infection have not been reported.
Here we show that anti-gH/gL antibodies are major determinants of EBV neutralization in human plasma and develop nanoparticle-based gH/gL and gH/gL/gp42 vaccines that elicit antibodies in animals that inhibit virus infection of both epithelial cells and B cells by targeting the virus membrane fusion proteins.
RESULTS
Antibodies to EBV gH/gL in human plasma are the principal components that neutralize infection of epithelial cells and contribute to neutralization of B cell infection
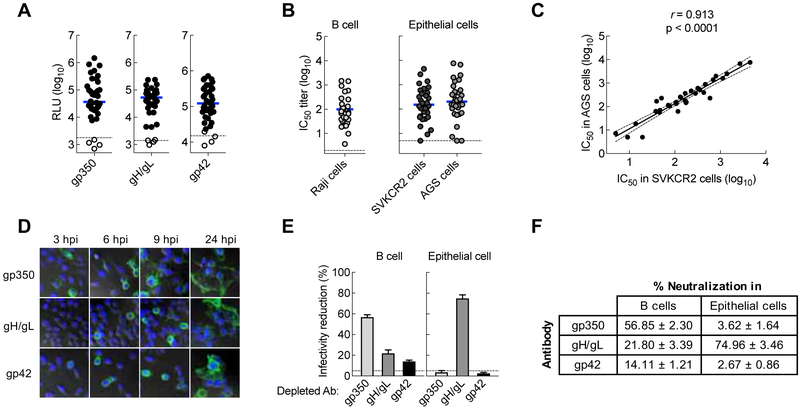
To determine the contribution of neutralizing antibodies in human plasma to EBV viral glycoproteins, we assessed the specificity of neutralizing antibodies in both B cells and epithelial cells. Serum samples from either EBV seronegative or seropositive (viral capsid antigen seropositive) healthy donors were tested for antibodies to EBV gp350, gH/gL, and gp42 using a luciferase immunoprecipitation system (LIPS) assay (Sashihara et al., 2009). Of 38 samples, all 34 seropositive individuals had detectable antibodies to EBV gp350, gH/gL, and gp42 (Figure 1A). No antibodies to gp350 and gH/gL were detected in seronegative subjects, while antibodies to gp42 were detected at a very low level in 2 of 4 seronegative individuals (Figure 1A).
Figure 1.
Contribution of glycoprotein antibodies to B cell and epithelial cell neutralizing titers in human sera and plasma. (A) EBV gp350 antibody, gH/gL antibody, and gp42 antibody titers in human plasma measured by LIPS assay. Antibody titers are expressed as luciferase relative light units (RLU). Solid circles are samples from 34 EBV seropositive subjects (positive for viral capsid antigen [VCA] IgG antibody); open circles are the serum from EBV seronegative subjects (4 subjects). The horizontal blue line represents the median of antibody titers. The horizontal dotted lines are the cut off value defined as twice the value of the buffer control. (B) Neutralizing antibody titers in sera of EBV seropositive subjects were measured using B cell neutralization (Raji cells) and epithelial cells neutralization (SVKCR2 cells and AGS cells) assays. The horizontal dark line is the median of the neutralizing antibody titers. The dotted line represents the detection limit of the assay. (C) Neutralizing antibody titers in the two epithelial cell lines, SVKCR2 and AGS, show a signfiicant correlation (p < 0.0001). (D) Surface of vaccinia virus (VV)-infected cells expressing EBV glycoproteins gp350, gH/gL or gp42 was stained with mAbs 72A1, E1D1, or F-2–1, respectively, followed by Alexa Fluor 488 goat anti-mouse IgG antibody at 3, 6, 9, and 24 hr post infection (hpi). (E) Reduction in EBV infection of B cells (Raji cells) and epithelial cells (SVKCR2 cells) by IVIG in which antibodies to individual EBV glycoproteins were depleted using VV-infected cells expressing EBV glycoproteins. The percentage of infectivity reduction was calculated by (1-IC50-depleted/IC50-control) ×100 in which IC50-depleted is the neutralizing antibody titer of depleted IVIG and IC50-control is the neutralizing antibody titer of IVIG incubated with control VV-infected cells. (F) Contribution of gp350, gH/gL and gp42 in human plasma to B cell and epithelial cell neutralizing antibodies. Data shown are the mean + standard errors of the mean (SEM) based on 3 independent experiments. See also Figure S1.
We next measured neutralizing antibody titers in an EBV-negative B cell line (Sashihara et al., 2009) and two different epithelial cell lines (SVKCR2 and AGS cells) using a GFP-reporter neutralization assay. For the neutralization assays, we utilized a set of monoclonal antibodies (mAbs) as controls, 72A1, E1D1, and F-2–1, which are known to bind gp350 (Hoffman et al., 1980), gL (Sathiyamoorthy et al., 2016) and gp42 (Li et al., 1995), respectively. mAbs 72A1 and F-2–1, but not E1D1, neutralized virus on B cells (Figure S1A). In contrast, E1D1 neutralized virus on SVKCR2 epithelial cells, while 72A1 or F-2–1 had negligible neutralizing activity in epithelial cells (Figure S1B). The geometric mean titer of sera that neutralized virus infection by 50% (IC50) in B cells for EBV seropositive individuals was 87.0 (95% CI, 54.4–139.2) (Figure 1B). Serum samples from EBV seropositive persons neutralized virus infection of SVKCR2 epithelial cells with a geometric mean IC50 of 153.8 (95% CI, 89.8–263.5) (Figure 1B). We also measured neutralization using AGS gastric adenocarcinoma cells that do not express CR2 (Yoshiyama et al., 1997) to rule out potential neutralization through inhibition of a gp350-CR2 interaction. The titer of neutralizing antibodies in AGS cells correlated with those in SVKCR2 cells (r = 0.913), indicating that exogenous expression of CR2 did not affect the measurement of neutralizing activity in epithelial cells (Figure 1C). Binding antibody titers to gH/gL correlated with neutralizing IC50 titers in SVKCR2 (r = 0.721) and AGS epithelial cells (r = 0.748) (Figure S1C and D), and also correlated with neutralizing IC50 titers in B cells (r = 0.794) (Figure S1E).
While antibodies to gp350 have been reported to be the major component in human sera that neutralizes B cell infection (Thorley-Lawson and Poodry, 1982), the relative contributions of antibodies to gH/gL and gp42 in human sera to neutralize EBV have not been studied. To address this, we depleted antibodies to EBV glycoproteins from human plasma and quantified their relative contribution to neutralize virus infection of B cells and epithelial cells. We used HeLa cells infected with recombinant vaccinia viruses (VVs) expressing gp350, gH/gL, or gp42 to deplete antibodies from human plasma. We confirmed gp350, gH/gL and gp42 expression on the cell surface after infection with VVs by staining with mAbs 72A1, E1D1, and F-2–1, respectively (Figure 1D). For antibody depletions, we used human intravenous immunoglobulin (IVIG) which is derived from plasma of >1,000 healthy donors; therefore, the results are more representative than using a small number of blood donors. To ensure that specific EBV glycoprotein antibodies were sufficiently depleted, we performed 4 sequential rounds of depletion using HeLa cells expressing individual EBV glycoproteins and the remaining antibody to EBV glycoproteins in IVIG after each round of depletion were quantified by LIPS assay. After up to 4 rounds of depletion we successfully depleted >95%, >90%, and ~90% of gp350-, gH/gL-, and gp42-specific antibodies from IVIG, respectively (Figure S1F).
We next assessed the neutralizing activity of glycoprotein depleted IVIG samples. IVIG depleted with mock-infected cells had no effect on virus neutralization in B cells, while the IC50 titer was substantially reduced when IVIG was depleted with gp350 (56.9% ± 2.30% [mean ± SEM] reduction) (Figure 1E, left panel, Figure S1G, left panel). Depletion of gH/gL and gp42 antibody had less effect on reducing virus neutralization in B cells, with 21.8% ± 3.39% and 14.1% ± 1.21% reduction, respectively (Figure 1E, left panel, Figure S1G, middle and right panels). Together, these results indicate that antibodies to gp350 account for ~50–60% of the total neutralizing activity, while antibodies to gH/gL and gp42 each contribute ~15–20% of the total neutralizing activity against B cell infection. When neutralization of glycoprotein depleted IVIG was evaluated in epithelial cells, we observed virtually no effect in virus infectivity after depletion with gp350 or gp42 compared to mock-depleted control (Figure S1H, left and right panels). In contrast, depletion of gH/gL antibody resulted in a marked reduction in neutralizing activity (Figure S1H, middle panel) in epithelial cells with a reduction in IC50 of 75.0 ± 3.46% (Figure 1E, right panel). Unlike their modest effect on B cell infection, antibodies to gH/gL represent the major component of epithelial cell neutralization in human plasma accounting for ~75% of the total neutralizing activity (Figure 1F). Additional neutralizing activity in B cells may be due to antibodies against gB and in epithelial cells to gB or BMRF2. We also depleted antibodies from an EBV seropositive blood donor and obtained results similar to the depletion observed using IVIG. In the blood donor serum, antibodies to gp350, gH/gL, and gp42 contributed 44.6% ± 4.37%, 46.9% ± 3.29%, and 10.9% ± 1.85% of B cell neutralization, respectively, while gH/gL antibodies contributed to 76.0% ± 0.89% of epithelial cell neutralization. Thus, antibodies to gp350, gH/gL, and gp42 in human plasma comprise the majority of neutralizing activity that prevent B cell infection, while antibodies to gH/gL are the principal components that inhibit epithelial cell infection.
Generation of gH/gL and gH/gL/gp42 Nanoparticle Vaccines
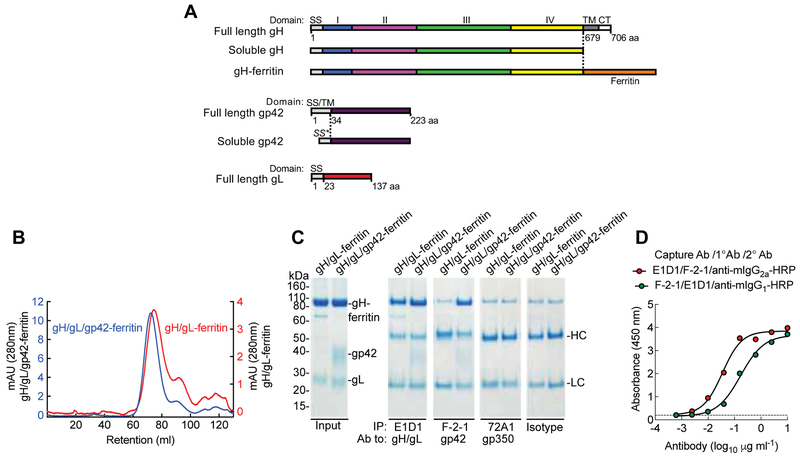
As antibodies to gH/gL, gp42 and gp350 in human plasma contribute to virus neutralization, and since we previously described a potent vaccine for gp350 (Kanekiyo et al., 2015), we sought to design immunogens for gH/gL and gH/gL/gp42 and determine whether they prevent B cell and epithelial cell infection. EBV gH/gL forms a heterodimer with 1:1 stoichiometry and gH/gL/gp42 forms a heterotrimer with 1:1:1 stoichiometry when individual soluble glycoproteins are co-expressed (Kirschner et al., 2006). We generated soluble gH/gL and gH/gL/gp42 proteins by co-transfection of plasmids encoding the ectodomain of each glycoprotein into mammalian cells. To optimize immunogenicity, we prepared a series of truncation variants of the gH ectodomain fused to the N-terminus of ferritin (Kanekiyo et al., 2015) which can give rise to self-assembling nanoparticles that display antigens on their surface (Figure S2A). We found that the full-length gH ectodomain (1 to 679 aa) fused to the ferritin was optimal for producing gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles when gH-ferritin was co-expressed with gL and gL plus gp42, respectively (Figure 2A). After purifying nanoparticles by chromatography, gH/gL-ferritin and gH/gL/gp42-ferritin formed predominantly homogeneous particles of the expected size as judged by size exclusion chromatography (Figure 2B) and dynamic light scattering (Figure S2B). SDS-PAGE confirmed bands of the appropriate size corresponding to gH-ferritin, gL and gp42 in purified nanoparticles (Figure 2C, left panel). While gL from gH/gL/gp42-ferritin nanoparticles migrated slightly faster on SDS-PAGE than gH/gL-ferritin nanoparticles, this difference was no longer apparent after deglycosylation of the nanoparticles, indicating that the difference in migration was due to glycosylation (Figure S2C). Integrity of gH/gL and gH/gL/gp42 proteins displayed on nanoparticles was evaluated by differential scanning fluorometric analysis in which similar thermal transition midpoint temperatures between soluble and nanoparticle forms of gH/gL and gH/gL/gp42 were observed (Figure S2D). These results indicate that the biochemical and biophysical properties of gH/gL and gH/gL/gp42 remain intact when displayed on nanoparticles.
Figure 2.
Construction and characterization of gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles. (A) Schematic representation of full-length gH, soluble gH, gH-ferritin, full-length gp42, soluble gp42, and full-length gL. gH-ferritin fusion protein was generated by fusion of gH ectodomain (domains I, II, III, and IV) to the N-terminus of the ferritin. SS is the native signal sequence of the glycoprotein, SS* is the human CD5 signal sequence, TM is the transmembrane domain and CT is the cytoplasmic tail. Amino acid (aa) position of gH, gL, and gp42 is indicated. (B) Chromatograph from size-exclusion chromatography of gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles purified from the supernatants of mammalian cells transfected with gH-ferritin and gL plasmids or gH-ferritin, gL, and gp42 plasmids. Particles were affinity purified using snowdrop lectin prior to size exclusion chromatography. Transient transfection of gH-ferritin/gL and gH-ferritin/gL/gp42 plasmids, resulted in comparable amount of nanoparticle proteins (~ 2mg/L). (C) Characterization of nanoparticles by immunoprecipitation and SDS-PAGE. Bands corresponding to gH-ferritin, gp42, and gL are indicated. Purified nanoparticles were immunoprecipitated with anti-gH/gL mAb (E1D1), anti-gp42 mAb (F-2–1), anti-gp350 mAb (72A1), or isotype control antibody. HC and LC denote antibody heavy and light chains, respectively. Faint bands running slightly slower than gH-ferritin are nonspecific background bands. (D) Sandwich ELISA using mAb E1D1 or F-2–1 to capture purified gH/gL/gp42-ferritin nanoparticles and detection with mAb F-2–1 or E1D1, respectively. The dotted line represents the background. See also Figure S2 and S3.
In addition, we engineered plasmids expressing either a polypeptide with insertion of a furin and 2A protease cleavage site (F2A) (Fang et al., 2005) between EBV gH and gL or a polypeptide with F2A sites between EBV gH, gL, and gp42 that allows individual proteins to be released from the polypeptide by 2A-mediated self-cleavage and activity of the endogenous cellular furin protease (Figure S3A). Nanoparticles made from the polypeptides formed predominantly homogeneous complexes as shown by size exclusion chromatography (Figure S3B, C) and SDS-PAGE (Figure S3D). Compared to nanoparticles made by co-transfection, gH-ferritin derived from the gH/gL-ferritin polypeptide and gH/gL/gp42-ferritin polypeptide was slightly higher in molecular weight (Figure S3D). Thus, self-assembling gH/gL and gH/gL/gp42 nanoparticles were produced in cells either co-transfected with plasmids expressing individual glycoproteins, or tranfected with a single plasmid expressing a polypeptide that underwent self-cleavage to produce individual glycoproteins.
gH/gL- and gH/gL/gp42-Nanoparticles bind to EBV B Cell and Epithelial Cell Neutralizing mAbs
To confirm that neutralizing epitopes for gH/gL and gH/gL/gp42 were displayed on the nanoparticles, we analyzed them with neutralizing mAbs E1D1 and F-2–1 to detect gL and gp42, respectively. Both gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles were immunoprecipitated by mAb E1D1, while only gH/gL/gp42-ferritin nanoparticles interacted with mAb F-2–1, indicating the relevant epitopes were intact and accessible on the nanoparticles (Figure 2C). The presence of Sigma Adjuvant Systems (SAS) adjuvant had minimal effect on binding of mAb E1D1 or F-2–1 to the nanoparticles (Figure S3E, F). gp42 was co-immunoprecipitated when gH/gL/gp42-ferritin was immunoprecipitated with gL-specific mAb E1D1, demonstrating that gp42 formed a complex with gH/gL-ferritin. In addition, we performed a sandwich ELISA with a pair of mAbs E1D1 and F-2–1 for capture and detection, and confirmed that the nanoparticles were heterotrimers of gH/gL and gp42 (Figure 2D).
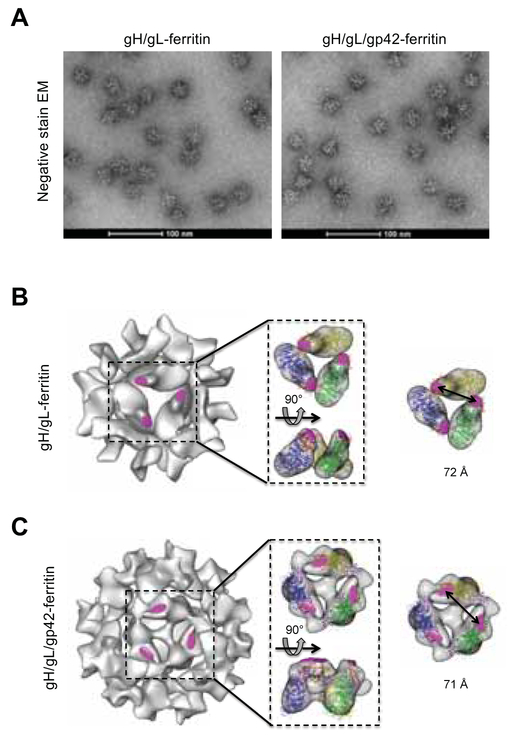
Electron microscopy (EM) analysis showed that gH/gL-ferritin and gH/gL/gp42-ferritin form monodispersed nanoparticles with spikes protruding from their core (Figure 3A). Cryo-electron microscopic reconstruction confirmed the ordered assembly of the gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles (Figure 3B, C). Importantly, the mAb E1D1 epitope was accessible on the surface of both nanoparticles (Figure 3B, C). Comparing the two nanoparticles, gH/gL tilted closer to the surface of the ferritin cage than gH/gL/gp42, presumably due to the absence of gp42. The distance between neighboring E1D1 epitopes in gH/gL molecules at the symmetrical 3-fold axis on both gH/gL-ferritin and gH/gL/gp42-ferrtin was approximately 72 Å, suggesting that the distance is within the range required for B cell receptor cross-linking and microclustering (50 −100 Å) to facilitate B cell activation (Bachmann et al., 1993; Dintzis et al., 1982). Similar distances between neighboring mAb AMMO1 epitopes (Snijder et al., 2018), which is a target for neutralization of EBV in B cells and epithelial cells, were noted for the two nanoparticles (Figure S4). Thus, epitopes recognized by neutralizing antibodies on gH/gL and gH/gL/gp42 nanoparticles were spaced at distances optimal for cross-linking B cell receptors
Figure 3.
Negative-stain transmission electron microscopy images and cryo-EM analysis of nanoparticles. (A) Negative-stain transmission EM of gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles. Cryo EM reconstruction models of gH/gL-ferritin (B) and gH/gL/gp42-ferritin (C) nanoparticles. Coordinates corresponding to gH/gL (PDB ID 3PHF) and gH/gL/gp42 (PDB ID 5T1D) were fitted into cryo-EM density maps. Structures of gH (blue, green, or yellow), gL (red), gp42 (purple), are shown in ribbon representation with the mAb E1D1 binding sites (magenta). The distance between two E1D1 binding sites on particles is indicated. See also Figure S4.
gH/gL- and gH/gL/gp42-Nanoparticle Vaccines Induce Potent B cell and Epithelial cell Neutralizing Antibodies in Mice
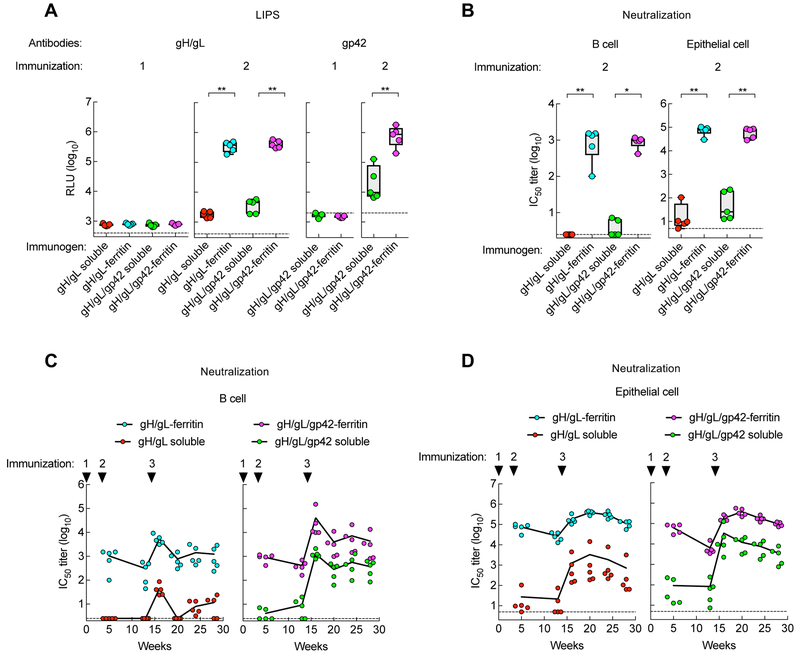
To evaluate the immunogenicity of soluble and nanoparticle vaccines, mice were immunized with 0.5 μg of soluble gH/gL, soluble gH/gL/gp42, gH/gL-ferritin, or gH/gL/gp42-ferritin formulated with SAS adjuvant. Serum antibody titers to gH/gL and gp42 were measured using LIPS assays. Antibodies to gH/gL and gp42 were detected at very low levels in mice immunized with any of the vaccines 2 weeks after the first vaccination (Figure 4A left panel); however, antibody levels increased 2 weeks after the second vaccination (Figure 4A, middle panel). The mean binding antibody titer to gH/gL detected by immunoprecipitation was higher in mice vaccinated with gH/gL-ferritin than soluble gH/gL (105.5 vs. 103.3, respectively, p = 0.0079), and was higher in animals immunized with gH/gL/gp42-ferritin than soluble gH/gL/gp42 (105.6 vs. 103.6, respectively, p = 0.0079). Similarly, antibody titer to gp42 was higher in mice receiving gH/gL/gp42-ferritin compared to soluble gH/gL/gp42 (106.0 vs. 104.6, respectively, p = 0.0079) (Figure 4A, right panel).
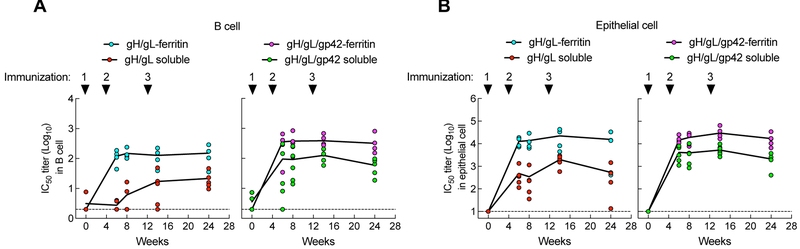
Figure 4.
Immunogenicity of soluble and nanoparticle gH/gL and gH/gL/gp42 immunogens in mice. BALB/c mice (n=5) were immunized intramuscularly with 0.5 μg of the indicated proteins with Sigma Adjuvant System adjuvant at week 0, 3, and 14 (corresponding to injections 1, 2, and 3, respectively). (A) Antibody titers to gH/gL and gp42 in immune sera of 2 weeks after immunizations 1 and 2 were measured by LIPS assay and shown as relative light units (RLU). The horizontal dotted line is the cutoff value defined as twice the value of the buffer control. (B) EBV B cell and epithelial cell neutralizing titers in sera following two immunizations. Data are represented as box-and-whiskers plots (box indicates lower and upper quartiles with horizontal line at median and whiskers at minimum and maximum data points). (C and D) Kinetics of B cell and epithelial cell neutralizing titers in mice. Antibody titers were determined at week 5, 13, 16, 20, 24 and 28. Each dot represents an individual mouse. The dotted lines represent the limit of detection. * p < 0.05; ** p < 0.01. See also Figure S5.
We next measured neutralizing IC50 titers using both B and epithelial cell infection assays. Virus neutralizing titers in B cells were ~ 400-fold higher in mice immunized twice with gH/gL-ferritin than in animals that received soluble gH/gL protein (103.0 vs. 100.4, respectively, p = 0.0073). Similarly, neutralization in B cells was >200-fold higher in mice that received gH/gL/42-ferritin compared to soluble gH/gL/gp42 (103.0 vs. 100.6, respectively, p = 0.0112) (Figure 4B, left panel). Virus neutralization in epithelial cells was ~3,000-fold higher in mice immunized with gH/gL-ferritin than with soluble gH/gL (104.9 vs. 101.4, p = 0.0079). Immunization with gH/gL/gp42-ferritin elicited a > 600-fold increase in neutralizing titers in epithelial cells compared to soluble gH/gL/gp42 (104.8 vs. 102.0, p = 0.0079) (Figure 4B, right panel). In addition, we compared neutralizing titers in mice immunized with nanoparticles produced by co-transfection of cells with 2 or 3 plasmids with those produced by transfection with a single plasmid expressing a single self-cleaving polypeptide. While a two-fold difference in B or epithelial cell neutralizing titers in mice immunized with nanoparticles made by the two different methods was observed, the differences were not significant (p > 0.1508) (Figure S5A). Consistent with the results shown in Figure 4B, the nanoparticle glycoprotein vaccines from the self-cleaving polypeptide, elicited higher EBV B cell and epithelial cell neutralizing titers than the soluble glycoprotein vaccines (Figure S5A).
To evaluate the time course of immune responses, mice were immunized with a third dose of the same vaccine (2 months after the second dose) and their serum antibody titers were monitored for 3.5 months. Antibody titers to gH/gL were boosted in animals receiving a third dose of soluble gH/gL, but not in mice receiving gH/gL-ferritin (Figure S5B, left panel). In contrast, antibody titers to both gH/gL and gp42 were markedly boosted in animals receiving a third dose of soluble gH/gL/gp42 to levels comparable to those that received gH/gL/gp42-ferritin nanoparticles (Figure S5B, middle and right panels). Although no change was detected in binding antibody titers, gH/gL-ferritin boosted neutralizing titers in both B and epithelial cells and the IC50 titers were higher than those induced by soluble gH/gL (103.7 vs. 101.6, respectively, p = 0.0119 in B cells; 105.3 vs. 103.1, respectively, p = 0.0079 in epithelial cells). Similar findings were observed in mice receiving gH/gL/gp42-ferritin compared to soluble gH/gL/gp42 (104.6 vs. 103.1, respectively, p = 0.0019 in B cells; 105.3 vs. 104.5, respectively, p = 0.0159 in epithelial cells) (Figure 4C, D). Moreover, the neutralizing titers in mice immunized with nanoparticles remained elevated for more than 3 months without further boosting (Figure 4C, D). After 3 doses of each vaccine, gH/gL/gp42-ferritin elicited ~ 4 to 8-fold higher EBV B cell neutralizing titers than gH/gL-ferritin, while at the same time EBV epithelial cell neutralization titers were similar for gH/gL- and gH/gL/gp42-nanoparticles, indicating that the gp42 component was additive for inducing B cell neutralizing antibodies, but not epithelial cell neutralizing antibodies.
As antibody to gp350, along with antibody to gH/gL and gp42, in human plasma is important for neutralizing EBV infection in B cells, we postulated that gp350 might be combined with either gH/gL or gH/gL/gp42 in a prophylactic EBV vaccine. To compare immune responses to these different vaccines, mice were immunized with 0.5 μg of single nanoparticle vaccines (gp350-ferritin, gH/gL-ferritin, or gH/gL/gp42-ferritin) or a combination of 0.5 μg of each of two different nanoparticles (gp350-ferritin plus gH/gL-ferritin or gp350-ferritin plus gH/gL/gp42-ferritin). EBV B cell neutralizing titers in sera from mice immunized twice with the combination nanoparticle vaccines, gp350-ferritin plus gH/gL-ferritin (103.5) or gp350-ferritin plus gH/gL/gp42-ferritin (103.1), were slightly higher than those immunized with single nanoparticle vaccines, gp350-ferritin (102.7), gH/gL-ferritin (103.0) or gH/gL/gp42-ferritin (103.0) (Figure S5C, left panel). EBV epithelial cell neutralizing titers in animals vaccinated twice with gp350-ferritin plus gH/gL-ferritin (105.1) or gp350-ferritin plus gH/gL/gp42-ferritin (104.5) were similar to single nanoparticle vaccines, gH/gL-ferritin (104.9) or gH/gL/gp42-ferritin (104.8) (Figure S5C, right panel). The low level EBV epithelial cell neutralizing titers in mice receiving gp350-ferritin group (102.1) was likely due to overexpression of CR2 in the target cells. EBV B cell and epithelial cell neutralizing titers were boosted after the third dose of vaccine at week 14 in animals receiving combination nanoparticle vaccines, and the titers remained at high levels > 3 months after the boost (Figure S5D). Addition of gH/gL-ferritin or gH/gL/gp42-ferritin to gp350-ferritin markedly enhanced EBV epithelial cell neutralizing activity without reducing B cell neutralizing activity. In contrast, B cell neutralizing activity was not increased when gp42 was added to gH/gL-ferritin combined with gp350-ferritin, and neutralizing activity was only marginally increased when gp350-ferritin was added to gH/gL/gp42 ferritin in mice. However, it is unknown if gp42 might enhance B cell neutralization in the presence of gp350 or if gp350 would enhance neutralization in the presence of gH/gL/gp42 in humans. Thus, a vaccine that contains gp350, together with gH/gL and gp42, might induce potent neutralizing antibodies to reduce or prevent EBV infection of both B cells and epithelial cells.
gH/gL- and gH/gL/gp42-Based Vaccines Induce B Cell and Epithelial Cell Neutralizing Antibodies in non-Human Primates
To determine whether the nanoparticle vaccines could elicit antibody response in non-human primates not previously infected with EBV-related cynomolgus herpesvirus, we immunized cynomolgus macaques (Macaca fascicularis) with soluble or nanoparticle glycoprotein vaccines and evaluated their antibody responses. Monkeys were given 50 μg of soluble gH/gL, soluble gH/gL/gp42, gH/gL-ferritin, or gH/gL/gp42-ferritin formulated with SAS at weeks 0, 4 and 12, and immune responses were measured periodically until 3 months after the last vaccination. After two doses of each vaccine (week 6), serum neutralization titers in B cells in monkeys immunized with gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles were > 40-and ~ 4-fold higher than with soluble gH/gL and soluble gH/gL/gp42, (102.1 vs. 100.4, respectively, p = 0.0179; and 102.6 vs. 102.0, respectively, p = 0.1111 (Figure 5A). Similarly, gH/gL-ferritin and gH/gL/gp42-ferritin nanoparticles elicited > 25- and ~ 4-fold higher neutralizing titers in epithelial cells than the corresponding soluble glycoprotein vaccines (104.1 vs. 102.7, respectively, p = 0.0159; and 104.2 vs. 103.6, respectively, p = 0.0317) (Figure 5B). Although neutralization was boosted in monkeys immunized with a third dose of soluble gH/gL vaccines, the increase was minor in monkeys with soluble gH/gL/gp42 or nanoparticle vaccines. Importantly the serum neutralizing titers in monkeys immunized with the nanoparticles remained high for 3 months after the third dose of each vaccine (Figure 5). Analogous to the results in mice, the neutralizing titers in B cells in monkeys vaccinated with gH/gL/gp42-ferritin were higher than for those that received gH/gL-ferritin, (102.6 vs. 102.1, respectively, p = 0.0571 at week 14), while there was almost no difference in epithelial cell neutralization between the two groups. We also found that nanoparticle-based vaccines consistently elicited higher glycoprotein binding antibodies than soluble protein vaccines (Figure S6).
Figure 5.
Immunogenicity of soluble and nanoparticle gH/gL and gH/gL/gp42 immunogens in nonhuman primates. Cynomolgus macaques were immunized intramuscularly with 50 μg of soluble gH/gL (N=5), gH/gL-ferritin (N=4), soluble gH/gL/gp42 (N=5), or gH/gL/gp42-ferritin (N=5) formulated with Sigma Adjuvant System adjuvant at week 0, 4, and 12 (corresponding to injections 1, 2, and 3, respectively). (A) B cell and (B) epithelial cell neutralization titers in sera were measured at week 0, 6, 8, 14, and 24. p = 0.0179 for gH/gL-ferritin vs. soluble gH/gL, and p = 0.1111 for gH/gL/gp42-ferritin vs. soluble gH/gL/gp42 for B cell neutralizing titers at week 6. p = 0.159 for gH/gL-ferritin vs. soluble gH/gL, and p = 0.0317 for gH/gL/gp42-ferritin vs. soluble gH/gL/gp42 for epithelial cell neutraling titers at week 6. Each dot represents an individual monkey. The dotted lines represent the limit of detection. See also Figure S6.
Therefore, gH/gL- and gH/gL/gp42-nanoparticle vaccines were highly immunogenic in non-human primates and elicited potent virus neutralizing antibody responses that were maintained for at least 3 months after vaccination.
Antibodies Induced by EBV Glycoprotein-Nanoparticles Potently Inhibit Virus Glycoprotein-Mediated B cell and Epithelial cell Membrane Fusion
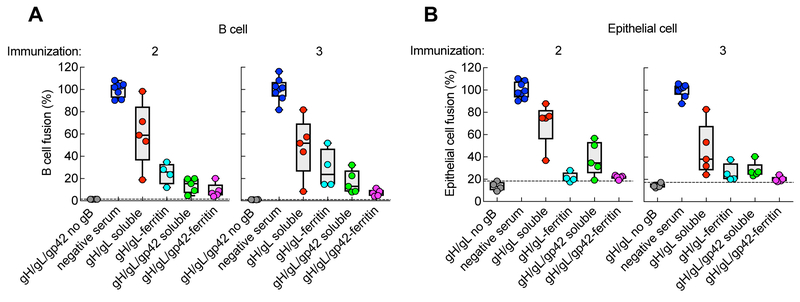
As gH/gL along with gB are components of the core fusion machinery of herpesviruses (Omerović et al., 2005; Silva et al., 2004), we measured fusion inhibitory activity in monkey immune sera by using a luciferase-based cell-cell fusion assay. Although sera from monkeys vaccinated twice with gH/gL-ferritin nanoparticles inhibited virus glycoprotein-mediated B cell membrane fusion more potently than those receiving soluble gH/gL (75% vs. 40% inhibition, respectively), inhibition was even higher (85–90% inhibition) in monkeys immunized with either gH/gL/gp42-ferritin or soluble gH/gL/gp42 (Figure 6A). This increase in fusion inhibitory activity by gH/gL/gp42-containing immunogens compared with gH/gL immunogens was expected since gp42 is required for B cell fusion and viral infection (McShane and Longnecker, 2004; Wang et al., 1998). In contrast, sera from monkeys immunized twice with either gH/gL-ferritin or gH/gL/gp42-ferritin nanoparticles showed similar virus glycoprotein-mediated inhibitory activity (80%) in an epithelial cell membrane fusion assay (Figure 6B), indicating that gp42 minimally affected epithelial cell fusion. Both gH/gL- and gH/gL/gp42-nanoparticles induced more potent epithelial cell fusion inhibition antibodies than the corresponding soluble glycoproteins (gH/gL-ferritin vs. soluble gH/gL, p = 0.0159; gH/gL/gp42-ferritin vs. soluble gH/gL/gp42, p = 0.0952) (Figure 6B). These data show that vaccination with gH/gL-ferritin or gH/gL/gp42-ferritin elicited similar levels of antibody that inhibit epithelial cell fusion, but that vaccination with gH/gL/gp42-ferritin induced more potent B cell fusion inhibitory antibodies than gH/gL-ferritin.
Figure 6.
Inhibition of B cell and epithelial cell fusion by serum from monkeys immunized with soluble gH/gL, gH/gL-ferritin, soluble gH/gL/gp42, or gH/gL/gp42-ferritin. Inhibition of B cell (A) and epithelial cell (B) fusion by sera from monkeys 2 weeks after the second and the third dose of vaccine. Sera from monkeys prior to immunization (negative sera) were used as a positive control, while assays using CHO-K1 cells expressing EBV gH/gL and luciferase with or without gp42, but not EBV gB, were used as a negative control for fusion. The percentage of fusion was calculated as (RLUSerum/RLUNegative Serum) × 100%. p = 0.0159 for gH/gL-ferritin vs. soluble gH/gL; p = 0.0952 for gH/gL/gp42-ferritin vs. soluble gH/gL/gp42 for epithelial cell fusion. The dotted lines represent the background of the assay. Data are represented as box-and-whiskers plots and each dot represents an individual monkey. The figure shown is representative of two independent experiments.
gH/gL- and gH/gL/gp42-Nanoparticles Induce Antibodies that Target a Site on gH/gL Critical for Fusion
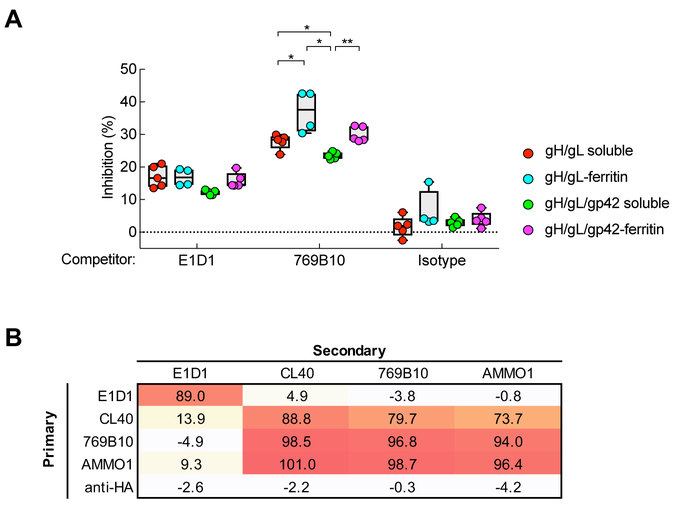
To determine the epitopes contributing to fusion inhibition in the serum from vaccinated monkeys, we carried out antibody cross-competition assays using biolayer interferometry with mAbs (Ngwuta et al., 2015). When mAb E1D1 which binds to gL (Sathiyamoorthy et al., 2016) and neutralizes epithelial cell infection (Figure S1A), was used as competitor to analyze gH/gL-binding of immune sera, we found relatively little competition across all vaccinated animals (15.4% ± 3.0%, N = 20) (Figure 7A). Serum antibodies in vaccinated monkeys therefore rarely target the site recognized by mAb E1D1.
Figure 7.
Detection of antibodies that compete with known EBV neutralizing monoclonal antibodies in immunized monkey sera. (A) Cross-competition of immune sera to gH/gL mAbs E1D1 and 769B10. The percent inhibition of monkey sera antibody to bind gH/gL by mAb E1D1, 769B10, or isotype control antibody is shown. Data are represented as box-and-whiskers plots and each dot represents an individual monkey. * p < 0.05; ** p < 0.01. (B) Table of antibody cross-competition of mAb 769B10 by mAbs E1D1, CL40, AMMO1, and anti-HA (control). Antibody competition was measured by biolayer interferometry with recombinant gH/gL protein. Biosensors immobilized with gH/gL protein were pre-saturated with antibodies (primary) and then measured binding of secondary antibodies. See also Figure S7.
We isolated mAb 769B10 from a human donor (see Experimental Procedures). This mAb neutralizes EBV infection of B cells and epithelial cells (Figure S7A) and inhibits fusion in both B cells and epithelial cells (Figure S7B). Recently another monoclonal antibody (AMMO1) has been reported that like 769B10 neutralizes EBV infection of B cells and epithelial cells, inhibits fusion, and binds to a site on gH/gL that partially overlaps the CL40 mAb epitope (Snijder et al., 2018). Importantly, mAb 769B10 showed very high affinity to gH/gL (KD = 30.7 pM) (Figure S7C, left panel) and competed for gH/gL-binding with mAb AMMO1 and CL40 (Figure 7B) which bind at or near the gp42 binding site on gH/gL(Sathiyamoorthy et al., 2017; Snijder et al., 2018). The affinity of the 769B10 to gH/gL/gp42 was >10-fold weaker than to gH/gL (KD = 458 pM) (Figure S7C, right panel), presumably due to the presence of gp42 whose binding site on gH/gL overlaps with CL40 and likely with 769B10 (Figure 7B). Thus, mAb 769B10 shares similar binding to gH/gL with mAb CL40 and AMMO1. These data suggest that the epitope recognized by CL40, 769B10, and AMMO1 represents a site of vulnerability for virus neutralization (Snijder et al., 2018).
When mAb 769B10 was used as a competitor, binding to gH/gL was substantially reduced in serum from animals vaccinated with gH/gL-nanoparticles (37.0% ± 6.4%) or with gH/gL/gp42-nanoparticles (30.0% ± 2.3%). Competition was higher in monkeys vaccinated with ferritin nanoparticles than those vaccinated with soluble gH/gL (27.8% ± 2.3%) or soluble gH/gL/gp42 (23.5% ± 1.0%) (p = 0.0159 for gH/gL nanoparticle vs. soluble gH/gL; p = 0.0079 for gH/gL/gp42 nanoparticle vs. soluble gH/gL/gp42) (Figure 7A). These data indicate that gH/gL- and gH/gL/gp42-nanoparticles induced antibodies that target the site on gH/gL critical for virus fusion, namely the site recognized by mAbs CL40,769B10, and AMMO1.
DISCUSSION
By defining the specificity of neutralizing antibodies to EBV glycoproteins during natural infection, we identified viral targets that can serve as immunogens to optimize protective immunity. We found that substantial immunity was directed to the core viral fusion machinery. This knowledge allowed us to elicit neutralizing antibodies that block EBV infection of the two major cell types targeted by the virus. Here, we showed that immunogens based on gH/gL and gH/gL/gp42 elicited robust virus neutralizing and fusion inhibitory antibody responses in non-human primates.
Viral glycoproteins gH/gL (Chesnokova and Hutt-Fletcher, 2011) and gp42 (Kirschner et al., 2007) are essential for fusion of EBV to the cell membrane. gp42 binds to HLA-DR on B cells (Spriggs et al., 1996) and is required for virus infection of B cells (Li et al., 1995, 1997; Wang et al., 1998). In contrast, gH/gL binds to integrins (Chesnokova et al., 2009) and ephrin receptor A2 (Chen et al., 2018; Zhang et al. 2018) on epithelial cells, and is required for virus attachment to epithelial cells (Molesworth et al., 2000) and cell-to-cell fusion (Shannon-Lowe et al., 2006). The gH/gL- and gH/gL/gp42-nanoparticles reported here spontaneously form noncovalent dimer or trimer complexes on the particle surface, displaying the glycoproteins for immune recognition. Immunization of mice or monkeys with gH/gL- and gH/gL/gp42-nanoparticles elicited higher titers of virus neutralizing antibodies that were effective in both epithelial cells and B cells than the corresponding soluble glycoproteins. This difference in the titers between the vaccines might be due to more effective cross-linking of B cell receptors with the nanoparticle vaccines than with the soluble vaccines due to optimal spacing of antigenic epitopes in the former. The levels of epithelial cell neutralizing antibody titers in these immunized animals exceeded titers observed in naturally infected humans. The levels of B cell neutralizing antibodies was similar or slightly higher in vaccinated animals compared to naturally infected humans, even though gp350, the dominant target of neutralizing antibodies was not in the vaccine. The addition of gp42 to gH/gL-nanoparticles increased B cell neutralizing titers by 4- to 8-fold, suggesting that incorporation of gp42 may be preferred. Although gp42 has been reported to inhibit viral entry into epithelial cell (Wang et al., 1998), we did not find that addition of gp42 affected induction of epithelial cell neutralizing antibody responses, despite the possibility that gp42 might have shielded an epitope on gH/gL from the immune system.
Immune sera from animals vaccinated with either gH/gL- or gH/gL/gp42-nanoparticles contained substantial levels of antibody directed to the CL40/769B10/AMMO1 epitope, targeting the gp42-binding site on gH/gL which mediates potent virus neutralization (Chesnokova and Hutt-Fletcher, 2011) and fusion inhibition (Sathiyamoorthy et al., 2017). This specificity of gH/gL-directed antibodies may not be common, since we did not observe a substantial contribution of antibodies in human plasma targeting gH/gL in a B cell infection assay. Induction of antibody specifically targeting the gp42-binding site on gH/gL would allow an EBV vaccine to protect multiple cell types from virus infection. Thus, gH/gL- and gH/gL/gp42-nanoparticles direct the immune system to epitopes on gH/gL and gp42 critically involved in virus entry into cells, resulting in robust virus neutralizing and fusion inhibitory antibody responses that are effective in protecting both B cells and epithelial cells from infection.
The largest clinical trial to date of a prophylactic EBV vaccine used soluble gp350 and failed to prevent infection, although the rate of infectious mononucleosis was reduced (Sokal et al., 2007). Since gH/gL and gp42 also contribute to B cell neutralization, and gH/gL is the principal target of epithelial cell neutralizing antibodies, these additional glycoproteins will likely increase the breadth of protection and enhance the efficacy of an EBV vaccine. Comparison of the immune response to gH/gL/gp42-ferritin with gp350-ferritin showed that gH/gL/gp42-ferritin induced about 2.5-fold higher B cell neutralizing titers and 250-fold higher epithelial cell neutralizing titers than gp350-ferritin. Thus, a gH/gL/gp42-ferritin vaccine may be superior to a gp350-ferritin vaccine or a combined gH/gL/gp42-ferritin and gp350-ferritin vaccine might also be considered. Our previous findings, that anti-ferritin antibodies elicited by an influenza HA-ferritin vaccine do not diminish subsequent responses to a different influenza HA-ferritin vaccine (Kanekiyo et al., 2013), suggest that combined ferritin-based EBV vaccines may not interfere with each other in inducing immune responses. Monoclonal antibodies that inhibit fusion and block gH/gL have been shown to inhibit infection of other herpesviruses. Therefore, the ability of gH/gL nanoparticles to elicit potent antibodies that block fusion and neutralize EBV infection in a cell-type independent manner demonstrates that such an approach may be important for an effective EBV vaccine as well as for other herpesviruses.
STAR METHODS
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Jeffrey I. Cohen (jcohen@niaid.nih.gov).
Experimental Model and Subject Details
Human specimens
Serum and PBMCs were obtained from de-identified healthy blood bank donors age 18 or older and whose gender and precise age were not available to the investigators. All donors provided written informed consent on a National Cancer Center Institutional Review Board approved protocol at the National Institutes of Health. Of the 38 blood donors, 34 were EBV seropositive and 4 were seronegative. EBV seropositivity was determined by the Captia EBV VCA (P-18) IgG Kit (Trinity Biotech). Human intravenous immunoglobulin (Gammunex) was obtained from Talecris Biotherapeutics, Inc.
Non-human primates
Two to four year-old outbred male cynomolgus macaques from a specific-pathogen-free colony that had not been infected with, or vaccinated against, any pathogen were used for experiments. The animals were obtained by mating, reared, and studied at the Tsukuba Primate Research Center, National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN), after approval by the Committee on the Ethics of Animal Experiments of NIBIOHN in accordance with the guidelines for animal experiments at NIBIOHN. Animals were individually housed.
Mice
Six to seven-week old female BALB/c mice were purchased from Charles River Laboratories. Mice were housed in sterile microisolator cages (5 animals per cage) and were specific-pathogen-free for multiple viruses, bacteria, and parasites. All experiments were carried out in accordance with federal regulations and NIH guidelines and were approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases.
Cell lines
HeLa (human female) cells and 293T (human female) cells were cultured in DMEM medium. CHO-K1 (hamster female) cells were cultured in MEM medium, and gastric carcinoma AGS (human female) cells were cultured in F-12 medium. 293/2089 cells contain the B95–8/F EBV genome that expresses GFP and were cultured in DMEM supplemented with 100 μg/ml hygromycin (Delecluse et al., 1998). 293T14 cells that express T7 polymerase were cultured in DMEM medium supplemented with 100 μg/ml zeocin. SVKCR2 (human male) epithelial cells stably express gp350 receptor CR2 (Li et al., 1992) and were cultured in DMEM and F-12 (1:1) medium supplemented with 400 μg/ml G418 and 10 ng/ml cholera toxin (Li et al., 1992). Raji cells (a human male EBV-positive Burkitt lymphoma cell line) were propagated in RPMI 1640 medium. Akata BX1 (human female) cells (Molesworth et al., 2000) that contain the Akata EBV genome and express GFP were cultured in RPMI 1640 medium supplemented 500 μg/ml G418. Daudi (human male) B cells that express T7 polymerase were cultured in RPMI 1640 medium supplemented with 1mg/ml G418. Expi293F (human female) cells were cultured in Expi293 expression medium (Thermo Fisher Scientific). All cell cultures were supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin.
EBV mAbs
EBV mAbs 72A1, F-2–1 and E1D1 (the latter two were a gift from Lindsey Hutt-Fletcher) were purified from the media of hybridoma cells by protein G chromatography.
Plasmids
Plasmids expressing gp350-Renilla luciferase and gp42-Renilla luciferase have been described previously (Sashihara et al., 2009). EBV gH was amplified with forward primer (Table S1) 5’-ATACTCGAGATGCAGTTGCTCTGTG-3’ and reverse primer 5’-CCCAAGCTTGAGTGTGCTCTTTCTTCATCA-3’, PCR products were digested with Xho I and Hind III, and cloned into the corresponding sites of plasmid pRen3S (Sashihara et al., 2009). The resulting plasmid contains the ectodomain of gH fused to the Renilla luciferase gene. EBV gL was amplified with forward primer 5’-CCCAAGCTTATGCGTGCTGTTGGTGTATT-3’ and reverse primer 5’-CCCTCTAGACTAGCCCCCGCGATGCC-3’, digested with Hind III and Xba I and cloned into plasmid pcDNA3.1.
To make recombinant vaccinia virus, EBV gp350, gH, gL and gp42 were amplified by PCR with the following primers, digested with restriction enzymes, and cloned into pRb21 vector (Blasco and Moss, 1995). The gp350 gene was amplified using forward primer 5’-TTTGCTAGCATGGAGGCAGCCTTGCTTGTG-3’ and reverse primer 5’-GGGAGGCCTTTATCAATAGGTCTCGGCGTC-3’, and the PCR product was digested with Nhe I and Stu I; the gH gene was amplified with forward primer 5’-GGGCTGCAGATGCAGTTGCTCTGTGTTTTTTGC-3’ and reverse primer 5’-GGGAAGCTTCTAAAGGAAAAACATAACAATC-3’, and the PCR product was digested with Pst I and Hind III; the gL gene was amplified using forward primer 5’-TTTGAATTCATGCGTGCTGTTGGTGTATTTC-3’ and reverse primer 5’-CCCAGGCCTCTAGCCCCCGCGATGCCATG-3’, and the PCR product was digested with EcoR I and Stu I; and the gp42 gene was amplified using forward primer 5’-GGGGAATTCATGGTTTCATTTAAGCAGG-3’ and reverse primer 5’-TTTAGGCCTTTAGCTATTTGATCTTTGAC-3’, and the PCR product was digested with EcoR I and Stu I.
The EBV gH, gL and gp42 genes were codon optimized, synthesized (Genscript) and cloned into expression vectors. To generate a gH-ferritin fusion protein, the full length ectodomain was inserted into the N-terminus of an H. pylori ferritin vector (Kanekiyo et al., 2015). Plasmids expressing gH-ferritin/F2A/gL/F2A/gp42 and gH-ferritin/F2A/gL were codon-optimized and synthesized (Genscript). Soluble EBV gH, soluble gp42 fused to a human CD5 leader sequence, and soluble gH fused with an Avi-His tag were cloned into the CMV/R 8κb VRC 8405 vector.
Recombinant Vaccinia Viruses (VVs)
BSC-1 cells were infected with VV deleted for most of vp37 (vRB12), and after 1 hr the viral inoculum was removed, and the cells were transfected with pRB21 containing vp37 and either an EBV glycoprotein gene or no inserted EBV gene. After 2 days, cells were lysed by three cycles of freezing and thawing followed by sonication (to release virus) and centrifugation. BSC-1 cells were infected with serial dilutions of the supernatant from centrifugation and plaques were picked after 2–3 days. The parental virus (vRB12) cannot form plaques in the absence of the vp37 gene (Blasco and Moss, 1995), so virus forming plaques is derived from recombination with plasmid pRB21. Recombinant viruses with EBV glycoprotein genes were confirmed by PCR and further purified by 2 additional rounds of plaque purification. EBV glycoprotein genes in recombinant VVs were confirmed by DNA sequencing.
Immunofluorescent Staining
HeLa cells grown on glass coverslips were infected with VVs expressing EBV glycoproteins gp350, gH/gL or gp42. For staining, cells were washed with PBS, fixed with paraformaldehyde, stained with primary antibody 72A1, E1D1, or F-2–1 and secondary antibody Alexa Fluor 488 goat anti-mouse IgG (H+L chain) (Thermo Fisher Scientific), washed 3 times in PBS, and mounted with DAPI Fluoromount-G medium (Southern Biotech). Slides were visualized with a Leica SP5 confocal microscope.
Luciferase Immunoprecipitation System (LIPS) Assay
293T cells were co-transfected with plasmids pRen3S-gH and pcDNA3.1-gL and lysates were used in LIPS assays. Antibody titers to EBV gH/gL, gp350, and gp42 were determined by LIPS assay as previously described (Coghill et al., 2016; Sashihara et al., 2009). Briefly, cell lysates containing EBV glycoprotein-Renilla luciferase fusion proteins were incubated with sera or IVIG, immunoprecipitated with protein A/G beads, incubated with coelenterazine substrate, and light units (LU) were quantified using a luminometer to obtain a measure of the amount of antibody in the sample. LU data were obtained from the mean of the triplicates.
GFP-Based EBV Neutralization Assays
Neutralization of EBV infection in B cells has been described previously (Sashihara et al., 2009). For neutralization of epithelial cells, human plasma, IVIG, mAbs, or media were serially diluted in 2-fold steps and 25 μl of the diluted sample was incubated with EBV-GFP derived from Akata BX-1 cells (Molesworth et al., 2000) for 2 hr. The mixture was added to SVKCR2 or AGS cells in 96-well plates and incubated for 3 days in a 37°C incubator. Cells were washed with PBS, treated with trypsin, and fixed in 2% paraformaldehyde in PBS. GFP-positive cells were quantified using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) and BD CSampler software. The dilution of human serum, IVIG or mAb, which inhibits infectivity by 50% (IC50) based on reduction of the number of GFP-positive cells, was calculated by non-linear regression analysis using GraphPad PRISM software. Neutralizing activity was considered absent when the software program failed to fit the results to an appropriate regression curve.
Depletion of Glycoprotein Antibody Using VV-Infected HeLa Cells
Confluent HeLa cells in T175 flasks were infected with VV expressing EBV glycoproteins or VV with no insert at an MOI of 8 for 1 hr at 37°C in the presence of 40 μg/ml cytosine β-D-arabinofuranoside (AraC) to minimize cytopathic effects and maximize accumulation of glycoproteins on infected cells. The inoculum was removed and replaced with fresh media in the presence AraC. Infected cells were scraped after overnight incubation, washed with medium, and incubated with IVIG on ice for 1 hr to deplete antibodies. The mixture was centrifuged and the supernatant was collected for another round of depletion. The depletion process was repeated four times.
Recombinant Proteins
Expi293F cells (Life Technologies) were co-transfected with plasmids expressing soluble gH and gL or soluble gH, gL and gp42 to produce soluble gH/gL or gH/gL/gp42 proteins, respectively. Expi293F cells were co-transfected with plasmids expressing gH-ferritin and gL or gH-ferritin, gL, and gp42 to produce gH/gL-ferritin or gH/gL/gp42-ferritin nanoparticles, respectively. Proteins in the supernatant of cell culture were purified by GNA-Immobilized Lectin beads (EY Laboratories Inc.), and eluted proteins were purified by size exclusion chromatography using Superose 6 10/300 Gl and HiPrep 16/60 Sephacryl S-500 HR for soluble proteins and nanoparticles, respectively. Purified proteins were concentrated and filtered through 0.22 uM filters for biochemical studies and vaccination. EBV gH-AviHis/gL proteins were purified from supernatant of Expi293F cells co-transfected with plasmids expressing gHAviHis and gL by Ni-NTA agarose (Thermo Fisher Scientific) followed by size exclusion chromatography using Superdex 200 10/300 GL column.
Negative Stain Transmission Electron Microscopy
Samples were diluted to ~0.05 mg/ml, adsorbed to a freshly glow-discharged carbon-film grid for 15 s, washed with buffer containing 10 mM HEPES, pH 7.0, and 150 mM NaCl, and stained with 0.75% uranyl formate. Images were collected at a nominal magnification of 100,000x (pixel size of 0.22 nm) on an FEI Tecnai T20 electron microscope operated at 200 kV and equipped with a 2k × 2k Eagle CCD camera
Cryoelectron Microscopy
Sample preparation, data collection and 3D reconstruction were conducted as described previously (Yap et al., 2017). A total of 3,612 and 4,224 particles of gH/gL-ferritin and gH/gL/gp42-ferritin, respectively were included in the final 3D reconstruction. The final reconstructions were estimated to be 20 Å and 23 Å, respectively based on the gold-standard Fourier shell correlation criterion of 0.143 (Henderson et al., 2012). Both maps were low-pass filtered to 23 Å. EM density maps of gH/gL-ferritin and gH/gL/gp42-ferritn were deposited into EMDB with accession number EMD-7799 and EMD-7798, respectively.
Immunizations
Six to seven-week old female Balb/c mice were immunized (n = 5) intramuscularly with 0.5 μg of soluble glycoproteins or nanoparticles in 100 μl of 50% (v/v) mixture of SAS adjuvant at week 0, 3 and 14. Blood was drawn at weeks 2, 5, 13, 16, 20, 24 and 28 and sera were collected for analysis of binding antibody and neutralizing antibody titers.
Nineteen male cynomolgus macaques (3 ± 0.75 years old) from a pathogen-free colony that were seronegative for EBV (based on our gH/gL LIPS assay that detects cynomolgus lymphocryptovirus) were immunized intramuscularly with 50 μg of soluble glycoproteins or nanoparticles in 1.0 ml of 50% (v/v) mixture of SAS at weeks 0, 4 and 12. Blood was drawn at weeks 0, 6, 8, 14, and 24 and sera were collected for analysis of binding antibody and neutralizing antibody titers.
Flow Cytometry, Single-Cell Sorting, Ig Amplification, and Antibody Expression
Cryopreserved peripheral blood mononuclear cells from healthy blood bank donors with high serum EBV neutralization titers were stained with anti-human mAbs CD3, CD56, CD14, CD27, and CD38 from BioLegend; IgG and IgM were from BD Biosciences, and CD19 was from Beckman Coulter. An EBV gH/gL probe was expressed, biotinylated, and labeled with fluorochromes as described previously for other viral glycoprotein probes (Whittle et al., 2014). Aqua dead cell stain was used to discriminate live/dead cells (Thermo Fisher Scientific). To sort single cells, gH/gL-specific B cells were stained as above, and CD19+ IgG+ gH/gL+ B cells were sorted into 96-well plates using a FACSAria II (BD Biosciences). Reverse transcription was performed on sorted cells, and multiplexed PCR was used to amplify Ig heavy and light chain genes, as described previously (Tiller et al., 2008). PCR products were sequenced by Beckman Coulter or Genewiz and analyzed using the ImMunoGeneTics information system (IMGT) (Brochet et al., 2008). Heavy and light chain sequences were synthesized and cloned by GenScript into IgG1, kappa expression vectors. To produce recombinant antibody 769B10, Expi293 cells were transfected with plasmids encoding Ig heavy and light chains with ExpiFectamine (Thermo Fisher Scientific). Antibody 769B10 was purified from the cell supernatant using rmp Protein A Sepharose Fast Flow (GE Healthcare) and the activity of purified 769B10 was confirmed by its binding specificity to gH/gL and neutralizing activity.
Fusion Inhibition Assays
Epithelial cell fusion was measured as previously reported (Omerović et al., 2005; Silva et al., 2004; Sorem and Longnecker, 2009). Briefly, HEK 293T14 cells that express T7 RNA polymerase were incubated with CHO-K1 cells that had been transfected with plasmids encoding gB, gH, gL under the control of the CMV IE promoter, and a plasmid encoding luciferase under the control of the T7 polymerase promoter. B cell fusion assays were performed as previously described (Sorem and Longnecker, 2009). Daudi-T7 B lymphocytes that stably express T7 RNA polymerase were incubated with CHO-K1 cells that had been transfected with plasmids encoding gB, gH, gL and gp42 under the control of a CMV IE promoter, and a plasmid encoding luciferase under the control of the T7 polymerase promoter. To quantify the ability of sera to inhibit epithelial cell or B cell fusion, HEK 293T14 epithelial cells or Daudi-T7 B cells were mixed with transfected CHO-K1 cells and incubated in the presence of sera from immunized monkeys overnight. The next day the cells were lysed and luciferase activity was quantified using a luminometer.
Cross-Competition Analysis with Biolayer Interferometry
Antibody cross-competition was performed as described previously (Ngwuta et al., 2015) with some modifications. Briefly, biotinylated gH/gL protein (1 μg/mL) was immobilized on streptavidin biosensors (FortéBio) in assay buffer (1% BSA in PBS). After the reaction was equilibrated with assay buffer, the biosensors were dipped in competitor antibodies (30 μg/mL in assay buffer) for 300 s followed by analyte antibodies (30 μg/mL in assay buffer) for 300 s with a short baseline step (60 s) in between the two antibody steps. Percent inhibition of antibody binding by competing mAbs was carried out by an equation: inhibition (%) = 100 - [(analyte antibody binding in the presence competitor mAb) / (analyte antibody binding in the presence of isotype control mAb)] × 100. For serum antibody competition, biotinylated gH/gL or gH/gL/gp42 proteins (1 μg/ml) were immobilized on streptavidin biosensors (Fortébio) and equilibrated with assay buffer. The biosensors were then dipped in competitor antibodies (30 μg/mL in assay buffer) for 300 s followed by serial dilutions of monkey serum (seven 2-fold dilutions in assay buffer starting at 1:10) for 300 s with a short baseline step (60 s) between the two antibody steps. The maximum response unit for each dilution was plotted against the corresponding dilution factor and the area under the curve was calculated for each dilution series. Percent inhibition of serum antibody binding by competing mAbs was calculated based on the area under curve. All assays were performed at 30°C with agitation of 1,000 rpm in an Octet HTX instrument (FortéBio).
Dynamic Light Scattering
Dynamic light scattering (DLS) measurements were performed at 25°C using a DynaPro Plate Reader II (Wyatt Technology). The samples were diluted in PBS, adjusted to 0.5 mg/ml, and spun at 15,700 × g for 45 min prior to analysis. The assay was performed in triplicate. The data were analyzed using DYNAMICS version 7.1.7 software (Wyatt Technology). The reported values are average with an SD of 3 independent measurements.
Quantification and Statistical Analysis
Spearman’s rank correlation and p values based on Wilcoxon test were calculated by statistical software R.
Supplementary Material
Highlights.
gH/gL antibodies in plasma neutralize EBV infection of B cells and epithelial cells
EBV gH/gL or gH/gL/gp42 nanoparticles induce potent neutralizing antibody responses
Vaccine-induced antibodies neutralize EBV infection of B cell and epithelial cells
Vaccine-induced antibodies block virus-mediated cell fusion by targeting EBV gH/gL
ACKNOWLEDGMENTS
We thank Jing Qin for statistical analyses, Sundar Ganesan and Juraj Kabat for help with microscopy, Bernard Moss for plasmids and vaccinia viruses, Lindsey Hutt-Fletcher for mAbs E1D1, F-2–1, and CL40, and Akata BX1 and SVKCR2 cells, Henri-Jacques Delecluse and Bill Sugden for 293/2089 cells, Shannon Kenney for AGS cells, Richard Longnecker for plasmids encoding EBV gH, gL, gB, gp42, and T7 luciferase and for 293-T14 and Daudi cells, Andrew McGuire for AMMO1 antibody, Jeffrey Boyington and Peter Kwong for help with DLS experiments, and David Ambrozak and VRC Flow Cytometry Core for help with B cell sorting. This research was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases and in part with Federal funds from the Frederick National Laboratory for Cancer Research, National Institutes of Health, under contract HHSN261200800001E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
M.K., W.B., G.J.N. and J.I.C. are named as inventors on a patent application describing the data presented in this paper, which have been filed by the National Institutes of Health. G.N. is an employee of Sanofi.
SUPPLEMENTAL INFORMATION
Document S1. Figures S1–S7 and Table S1
Data and Software Availability
The IgH and IgL variable region sequences of monoclonal antibody 769B10 are deposited in GenBank with accession number MK375259 and MK375260. The cryo-EM density maps of gH/gL-ferritin and gH/gL/gp42-ferritin are deposited into EMDB under accessions EMDB-7799 and EMDB-7798, respectively.
REFERENCES
- Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, and Zinkernagel RM (1993). The influence of antigen organization on B cell responsiveness. Science. 262, 1448–1451. [DOI] [PubMed] [Google Scholar]
- Blasco R, and Moss B (1995). Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158, 157–162. [DOI] [PubMed] [Google Scholar]
- Brochet X, Lefranc MP, and Giudicelli V (2008). IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, and Longnecker R (2018). Ephrin receptor A2 is a functional entry receptor for Epstein–Barr virus. Nat. Microbiol 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, and Hutt-Fletcher LM (2011). Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol 85, 13214–13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Nishimura SL, and Hutt-Fletcher LM (2009). Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc. Natl. Acad. Sci. U. S. A 106, 20464–20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill AE, Bu W, Nguyen H, Hsu WL, Yu KJ, Lou PJ, Wang CP, Chen CJ, Hildesheim A, and Cohen JI (2016). High levels of antibody that neutralize b-cell infection of Epstein-barr virus and that bind ebv gp350 are associated with a lower risk of nasopharyngeal carcinoma. Clin. Cancer Res. 22, 3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI (2000). Epstein-Barr virus infection. N. Engl. J. Med 343, 481–492. [DOI] [PubMed] [Google Scholar]
- Cohen JI (2015). Epstein–barr virus vaccines. Clin. Transl. Immunol 4, e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, and Nabel GJ (2011). Epstein-Barr virus: an important vaccine target for cancer prevention. Sci. Transl. Med 3, 107fs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, and Hammerschmidt W (1998). Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci U S A 95, 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis R, Vogelstein B, and Dintzis H (1982). Specific cellular stimulation in the primary immune response: experimental test of a quantized model. Proc Natl Acad Sci USA 79, 884–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, and Jooss K (2005). Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol 23, 584–590. [DOI] [PubMed] [Google Scholar]
- Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro P. a, and Fearon DT (1984). Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc. Natl. Acad. Sci. U. S. A 81, 4510–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Sali A, Baker ML, Carragher B, Devkota B, Downing KH, Egelman EH, Feng Z, Frank J, Grigorieff N, et al. (2012). Outcome of the first electron microscopy validation task force meeting. In Structure, pp. 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GJ, Lazarowitz SG, and Hayward SD (1980). Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc. Natl. Acad. Sci 77, 2979–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Bu W, Joyce MG, Meng G, Whittle JRR, Baxa U, Yamamoto T, Narpala S, Todd J-P, Rao SS, et al. (2015). Rational Design of an Epstein-Barr Virus Vaccine Targeting the Receptor-Binding Site. Cell 162, 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ. (2013). Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AN, Omerovic J, Popov B, Longnecker R, and Jardetzky TS (2006). Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J Virol 80, 9444–9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AN, Lowrey AS, Longnecker R, and Jardetzky TS (2007). Binding-Site Interactions between Epstein-Barr Virus Fusion Proteins gp42 and gH/gL Reveal a Peptide That Inhibits both Epithelial and B-Cell Membrane Fusion. J. Virol 81, 9216–9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Turk SM, and Hutt-Fletcher LM (1995). The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol 69, 3987–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, and Hutt-Fletcher LM (1997). Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol 71, 4657–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QX, Young LS, Niedobitek G, Dawson CW, Birkenbach M, Wang F, and Rickinson AB (1992). Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356, 347–350. [DOI] [PubMed] [Google Scholar]
- McShane MP, and Longnecker R (2004). Cell-surface expression of a mutated Epstein-Barr virus glycoprotein B allows fusion independent of other viral proteins. Proc. Natl. Acad. Sci. U. S. A 101, 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesworth SJ, Lake CM, Borza CM, Turk SM, and Hutt-Fletcher LM (2000). Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. J Virol 74, 6324–6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang GY, et al. (2015). Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omerović J, Lev L, and Longnecker R (2005). The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with epithelial and B cells. J. Virol 79, 12408–12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara J, Burbelo PD, Savoldo B, Pierson TC, and Cohen JI (2009). Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology 391, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Hu YX, Möhl BS, Chen J, Longnecker R, and Jardetzky TS (2016). Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nat. Commun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Jiang J, Möhl BS, Chen J, Zhou ZH, Longnecker R, and Jardetzky TS (2017). Inhibition of EBV-mediated membrane fusion by anti-gHgL antibodies. Proc. Natl. Acad. Sci 201704661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, and Delecluse H-J (2006). Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc. Natl. Acad. Sci. U. S. A 103, 7065–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AL, Omerovic J, Jardetzky TS, and Longnecker R (2004). Mutational analyses of Epstein-Barr virus glycoprotein 42 reveal functional domains not involved in receptor binding but required for membrane fusion. J. Virol 78, 5946–5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder J, Ortego MS, Weidle C, Stuart AB, Gray MD, McElrath MJ, Pancera M, Veesler D, and McGuire AT (2018). An Antibody Targeting the Fusion Machinery Neutralizes Dual-Tropic Infection and Defines a Site of Vulnerability on Epstein-Barr Virus. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Léonard P, Moreels A, Haumont M, Bollen A, Smets F, and Denis M (2007). Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis 196, 1749–1753. [DOI] [PubMed] [Google Scholar]
- Sorem J, and Longnecker R (2009). Cleavage of Epstein-Barr virus glycoprotein B is required for full function in cell-cell fusion with both epithelial and B cells. J. Gen. Virol 90, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MK, Armitage RJ, Comeau MR, Strockbine L, Farrah T, Macduff B, Ulrich D, Alderson MR, Müllberg J, and Cohen JI (1996). The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. J. Virol 70, 5557–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, and Poodry CA (1982). Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol 43, 730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, and Wardemann H (2008). Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 329, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, and Palefsky JM (2003). Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med 9, 307–314. [DOI] [PubMed] [Google Scholar]
- Wang X, Kenyon WJ, Li Q, Müllberg J, Hutt-Fletcher LM, Mullberg J, and Hutt-Fletcher LM (1998). Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol 72, 5552–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle JRR, Wheatley AK, Wu L, Lingwood D, Kanekiyo M, Ma SS, Narpala SR, Yassine HM, Frank GM, Yewdell JW, et al. (2014). Flow Cytometry Reveals that H5N1 Vaccination Elicits Cross-Reactive Stem-Directed Antibodies from Multiple Ig Heavy-Chain Lineages. J. Virol 88, 4047–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, and Hutt-Fletcher LM (2007). Point mutations in EBV gH that abrogate or differentially affect B cell and epithelial cell fusion. Virology 363, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap ML, Klose T, Urakami A, Hasan SS, Akahata W, and Rossmann MG (2017). Structural studies of Chikungunya virus maturation. Proc. Natl. Acad. Sci 114, 13703–13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama H, Imai S, Shimizu N, and Takada K (1997). Epstein-Barr virus infection of human gastric carcinoma cells: Implication of the existence of a new virus receptor different from CD21. J. Virol 71, 5688–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, et al. (2018). Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nat. Microbiol 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.