Abstract
Leptin has neurotrophic actions in the hippocampus to increase synapse formation and stimulate neuronal plasticity. Leptin also enhances cognition and has antidepressive and anxiolytic-like effects, two hippocampal-dependent behaviors. In contrast, mice lacking leptin or the long form of the leptin receptor (LepRb) have lower cortical volume and decreased memory and exhibit depressive-like behaviors. A number of the signaling pathways regulated by LepRb are known, but how membrane LepRb levels are regulated in the central nervous system is not well understood. Here, we show that the lysosomal inhibitor chloroquine increases LepRb expression in hippocampal cultures, suggesting that LepRb is degraded in the lysosome. Furthermore, we show that leptin increases surface expression of its own receptor by decreasing the level of ubiquitinated LepRbs. This decrease is mediated by the deubiquitinase ubiquitin-specific protease 8 (USP8), which we show is in complex with LepRb. Acute leptin stimulation increases USP8 activity. Moreover, leptin stimulates USP8 gene expression through cAMP response element–binding protein (CREB)–dependent transcription, an effect blocked by expression of a dominant-negative CREB or with short hairpin RNA knockdown of CREB. Increased expression of USP8 causes increased surface localization of LepRb, which in turn enhances leptin-mediated activation of the MAPK kinase/extracellular signal–regulated kinase pathway and CREB activation. Lastly, increased USP8 expression increases glutamatergic synapse formation in hippocampal cultures, an effect dependent on expression of LepRbs. Leptin-stimulated synapse formation also requires USP8. In conclusion, we show that USP8 deubiquitinates LepRb, thus inhibiting lysosomal degradation and enhancing surface localization of LepRb, which are essential for leptin-stimulated synaptogenesis in the hippocampus.
Leptin is a 16-kDa cytokine that is critical for normal energy homeostasis and feeding behavior (1–3). In adults, leptin is predominately synthesized and released from white adipose tissue into the blood stream, where it acts in multiple brain regions to promote negative energy balance (4, 5). The leptin receptor (LepR) is expressed in many brain regions, including the cornu ammonis (CA)1/CA3 and dentate gyrus of the hippocampus (6–8). Leptin is actively transported into the central nervous system (CNS) (9), where it binds to the long form of the LepR (LepRb) to activate multiple signaling cascades downstream of Janus kinase 2 that are important for its actions, including signal transducer and activator of transcription 3/5, MAPK kinase/extracellular signal-regulated kinase (Erk), phosphatidylinositol 3-kinase, and Ca2+/calmodulin-dependent protein kinase kinase/Ca2+/calmodulin-dependent protein kinase type I signaling pathways (10, 11).
In addition to the regulation of energy homeostasis, leptin has neurotrophic actions, both during development and in adults (10, 12–15). Mice that do not produce leptin [obese (or ob/ob) mice] have lower brain weight and lower cortical volume, a phenotype that is rescued by leptin injections during early postnatal development (12). Leptin is also essential for the formation of appropriate neuronal connections during development (13, 16). Interestingly, both the postnatal surge in leptin levels in rodents (17) and the higher leptin levels at the end of the third trimester in humans correspond to a time of rapid hippocampal synaptogenesis. Furthermore, mice lacking functional LepRbs have a reduced number of hippocampal dendritic spines, which are the main site of glutamatergic synapses (14). This reduction in hippocampal connections is associated with a change in hippocampal-dependent behaviors, as both obese (or ob/ob) mice (lacking leptin), as well as diabetic (db/db) mice (that lack functional LepRbs) exhibit increased depressive-like behaviors and anhedonia (18–20).
Leptin also has important effects on hippocampal function in adults. It has been shown to alter hippocampal synaptic function through the trafficking of glutamate receptors and enhanced long-term plasticity (21–24). Moreover, intrahippocampal injections of leptin also enhance cognition and decrease depressive and anxiety-like behaviors (25–29), whereas targeted deletion of LepRb in the adult hippocampus induces depressive-like behaviors (30).
Interestingly, mice that are heterozygous for LepRb deletion show a partial phenotype, at least for body weight (31), suggesting that the alteration of the level of functional LepRbs can have a profound influence on leptin’s actions. The alteration of the membrane expression or stability of LepRbs would therefore also be expected to impact leptin’s effects, but little is known about how protein levels and the subcellular location of LepRb are controlled, especially in neurons. LepRb expression is regulated by the ubiquitin (Ub) signaling system in immortalized cell lines, where it is constitutively endocytosed in a clathrin-mediated manner (32) and degraded in the lysosomal pathway (33). The deubiquitinase (DUB) Ub-specific protease 8 (USP8) also alters LepRb trafficking and degradation in human embryonic kidney (HEK)293T cells (34). USP8 is expressed in the CA1, CA2/CA3, and dentate gyrus of the hippocampus (35, 36), suggesting that it may play a role in regulating LepR protein levels and localization. Whereas it is expressed in the CA1 and dentate gyrus, both during the neonatal period and in adulthood, it is only expressed in the CA2/CA3 region of the hippocampus during early neonatal development (37), suggesting that it may be more involved in early postnatal development (including during the leptin surge) in these regions.
Like leptin, ubiquitinases and DUBs also play critical roles in regulating synapse formation and function (38–41). USP8 has been shown to control synaptic strength, in part, through altering α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor expression in mammalian synapses (36), while also positively regulating dendritic spine formation (42). Given the overlapping expression of LepRbs and USP8 in the hippocampus and both their critical roles in the development and function of synapses, it raises the question of whether they interact.
Here, we show that leptin increases the expression of USP8 in the hippocampus and activates it to increase the surface expression of its own receptor (LepRb). Furthermore, we found that USP8 is in a complex with LepRb and upon activation, deubiquitinates the receptor. USP8 then increases the surface localization of LepRb, as well as leptin activation of Erk1/2 and cAMP response element–binding protein (CREB). Moreover, we found that leptin-stimulated synapse formation is dependent on USP8 expression, as targeted knockdown of USP8 blocks leptin’s effects, whereas USP8 overexpression alone is sufficient to increase synapse formation through a mechanism that requires LepRbs. This study provides evidence of how leptin activates USP8, which leads to deubiquitination of LepRbs, their increased membrane expression and leptin-induced signaling, as well as synapse formation in the hippocampus.
Materials and Methods
Drugs and DNA constructs
Full-length rat recombinant leptin (50 nM; Peprotech), MG-132 (50 µM; Selleckchem), chloroquine (50 µM; Sigma Aldrich), cycloheximide (20 µM; Sigma Aldrich), and sodium orthovanadate (1 mM; Sigma Aldrich) were used, as described in the text and figure legends. The short hairpin RNA (shRNA) targeting the USP8 sequence 5′-GGACAGGACAGTATAGATACA-3′ was cloned into the pLKO.3G vector between the EcoRI and PacI restriction sites. The constructs expressing shLepRb, shCREB, and a dominant-negative CREB (ACREB) were used as previously described (14, 43). Ub constructs were subcloned from pRK5- hemagglutinin (HA)-Ub or pRK5-HA–ubiquitin lysine 48 arginine mutant (UbK48R) (a kind gift from Dr. Michael Varnum, Washington State University) into the pCAGGS vector using Gateway Cloning (Thermo Fisher Scientific). Constructs expressing tagged proteins were constructed by amplification of LepRb from mouse cDNA and USP8 and β-actin from rat cDNA and cloned into pCAGGS destination vectors containing the designated tag using Gateway Cloning. Leptin receptor b lacking the intracellular domain (LepRΔC) was constructed by amplification of the LepRb extracellular and transmembrane-expressing domain (residues 1 to 860) from the LepRb Gateway entry vector and cloned into pCAGGS destination vectors using Gateway Cloning. The catalytically inactive USP8 [ubiquitin-specific protease 8 cysteine 749 alanine mutant (USP8C749A)] construct was mutated with primers designed by NEBaseChanger. The USP8 construct resistant to shUSP8 (V5-USP8*) was constructed silently by the mutation of the shRNA targeting sequence with primers designed by NEBaseChanger. BioID destination vectors were constructed by the subcloning of myc-BioID (Addgene) into pCAGGS destination vectors using Gibson cloning (New England Biolabs). Primers are reported in Bland et al. (44).
Cell culture
All animal work described in this manuscript followed procedures approved and in compliance with Washington State University Institutional Animal Care and Use Committee–approved protocols. Sexually-mixed hippocampal neuronal cultures were prepared from equal numbers of male and female pups, as previously described (10). Hippocampal neurons used for immunostaining and fluorescent intensity imaging were transfected with various constructs on day 6 in vitro (DIV6) and then on DIV7 to -8, treated with various reagents in media, fixed [4% paraformaldehyde in PHEMS buffer (60 mM 1,4-piperazinediethanesulfonic acid, 25 mM HEPES, 1 mM MgCl2, 5 mM EGTA), 87.6 mM sucrose, pH 7.4] for 20 minutes at room temperature, and then immunostained or mounted using Elvanol. Hippocampal neurons used for dendritic spine analysis, antivesicular glutamate transporter 1 (vGlut1) immunostaining, and electrophysiological analysis were transfected with various constructs on DIV6 and treated with leptin (50 nM) on DIV8, and on DIV11 to -12, cells were fixed, immunostained, and mounted, as previously stated, or used for electrophysiological recordings. All control conditions received the same amount of media at the time of reagent stimulation.
HEK293T cells (5 × 104 cells/cm2 for six-well plates used for biochemistry experiments) were maintained in DMEM/high glucose (HyClone), supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Gibco). HEK293T cells were transfected with various constructs, 24 hours after plating, treated 24 to 48 hours after transfection with leptin (50 nM) in Opti-Mem (Gibco), and collected at designated time points. All control conditions received the same amount of media at the time of reagent stimulation.
Transfection
Primary hippocampal cultures were transfected with Lipofectamine 2000 (Life Technologies). Native media was collected before transfection and replaced with warm growth media. Lipofectamine 2000 and experimental DNA plasmids (0.5 µg per well for 24-well plates, 2 µg per well for six-well plates, and 12.5 µg/dish for 10 cm dishes) were added to cells and incubated for 30 minutes. The media was then aspirated and replaced with native media. This protocol produces a transfection efficiency of only 3% to 5% of total neurons transfected.
HEK293T cells were transfected with Lipofectamine 2000 and experimental DNA plasmids (2 µg per well for six-well plates). The mixture was added to the native media and allowed to incubate until cells were collected. This protocol produces a much higher transfection efficiency of ∼80% of total cells transfected.
Microscopy
In all of the fluorescent intensity experiments, a soluble fluorescent protein, such as tdTomato or Clover, was used to highlight the transfected neuron. Wide-field images of transfected neurons were obtained using Slidebook 5.5 Digital Microscopy Software and an Olympus IX81 inverted microscope equipped with a Hamamatsu ORCA-ER charge-coupled device camera and a 100× oil immersion lens (numerical aperture: 1.4). The soluble fill was then used to mask the transfected neuron. The masked image was then used to identify the region of interest to be analyzed (the entire neuron) for LepRb expression. Total fluorescent integrated intensity for the entire neuron was then measured.
Immunocytochemistry
Transfected neurons were treated and fixed, as described above. After fixation, cells were washed in PBS and permeabilized with 0.1% Triton X-100 detergent (Bio-Rad Laboratories); blocked with 8% BSA for 1 hour; incubated for 24 hours at 4°C with primary antibodies against USP8 (1:250; Sigma Aldrich) (45), phosphorylated (p)Erk (1:250; Cell Signaling Technology) (46), pCREB (1:250; Millipore) (47), or vGlut1 (1:250; Neuromab) (48) in IHC-Tek (IHC World); incubated for 1 hour at room temperature with the appropriate Alexa Fluor secondary IgG antibody (Life Technologies); and mounted with Elvanol. Antibodies were all validated for specificity by Western blot of HEK-expressed target protein or with no primary control in immunocytochemical experiments. The integrated staining density of pErk was measured in the whole soma, and pCREB was measured in the nucleus of transfected neurons from a minimum of 30 neurons from three separate cultures per condition using ImageJ 1.48. Images were taken as described in the Microscopy section. Background was calculated using secondary antibody incubation only and subtracted from images.
Spine quantification
Hippocampal cultures were transfected with designated DNA constructs and Clover-β-actin to allow for visualization of dendritic spine density and morphology. Confocal fluorescent images were obtained using Metamorph software and a Leica DMI6000 SD confocal microscope equipped with a Yokogawa CSU-X1 spinning disk, Hamamatsu-R2 charge-coupled device camera, and a 60× oil immersion lens (numerical aperture: 1.4). Dendritic spine density and classification were measured, as previously described (10). In summary, two to three dendritic segments were counted from a minimum of 15 neurons. Confocal images for vGlut1 and dendritic spine juxtaposition were obtained, as described earlier. The percent juxtaposition was manually measured from 50 to 75 spines on 10 different neurons from three separate cultures per condition using ImageJ 1.48.
Live immunostaining of surface Flag-LepRb was performed on neurons transfected and treated, as previously described. Neurons were incubated in growth media, supplemented with an anti-Flag (1:250; Sigma Aldrich) (49) antibody for 10 minutes, fixed, and incubated with the appropriate Alexa Fluor secondary IgG antibody for 1 hour at room temperature. They were then mounted, imaged, and analyzed, as described in the Microscopy section. Background was calculated using secondary antibody incubation only and subtracted from images.
Western blotting
Protein samples were collected by the lysing of equal numbers of cells or equal masses of tissue with radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology); supplemented with phosphatase inhibitor cocktail 2 and 3 (Sigma Aldrich), protease inhibitor cocktail, phenylmethylsulfonyl fluoride (2 mM), sodium orthovanadate (1 mM; Santa Cruz Biotechnology), and PR-619 (25 µM; Tocris); and centrifuged at 16,000 g. Samples were prepared with NuPage LDS Sample Buffer (Life Technologies) and 50 mM dithiothreitol (DTT; Thermo Fisher Scientific) and heated at 75°C for 10 minutes. Equal concentrations of protein were then loaded into Bolt 4% to 12% Bis-Tris gels (Life Technologies). Proteins were transferred to a polyvinylidene fluoride membrane (Life Technologies) overnight; blocked with 5% BSA for phosphorylated proteins or 5% milk for nonphosphorylated proteins; incubated with primary antibodies against c-Myc (1:1000; Sigma Aldrich) (50), HA (1:1000; Sigma Aldrich) (51), Erk2 (1:1000; Santa Cruz Biotechnology) (52), USP8 (1:1000; Sigma Aldrich) (45), pTyr (1:500; MilliporeSigma) (53), or V5 (1:1000; Cell Signaling Technology) (54) for 2 hours at room temperature; and then incubated with the appropriate Alexa Fluor-647 secondary IgG F(ab′)2 fragment antibody (Cell Signaling Technology) (55) for 1 hour at room temperature. Depending on the experiment, loading controls included Erk2 or the protein being analyzed [in the case of immunoprecipitation (IP) and phosphoblots]. Blots were imaged using a Chemidoc MP imaging system (Bio-Rad Laboratories) and analyzed using the ImageJ 1.48 gel analyzer tool.
IP
Protein samples were collected as described in the “Western blotting” protocol (above) from cells transfected with the designated constructs. Equal amounts of sample were incubated with myc-tag or V5-tag mouse monoclonal antibody conjugated to magnetic beads (Cell Signaling Technology) and rotated overnight at 4°C. Samples were then washed three times with PBS, eluted in NuPage LDS Sample Buffer and 50 mM DTT, heated at 75°C for 10 minutes, and then loaded into Bolt 4% to 12% Bis-Tris gels. Samples were probed in Western blot analysis, as previously described.
Active DUB labeling assay
The activity of USP8 was measured, as previously described (36). In summary, USP8 was immunoprecipitated, as described in the IP section protocol, from neurons transfected with myc-USP8. Before elution from magnetic beads, samples were incubated with the active DUB labeling HA-Ub-vinyl methylester (VME; 1 µM; Enzo Life Sciences) for 1 hour at 37°C. Samples were then eluted in NuPage LDS Sample Buffer and 50 mM DTT and probed in Western blot analysis.
BioID assay
HEK293T cells were transfected with a V5-tagged target protein and a BioID-tagged labeling protein. Biotin (50 µM; Amresco) was added to the media at the time of transfection to allow the BioID-tagged labeling protein to attach biotin to any proteins in close proximity. Protein samples were collected after 24 hours, as described in the “Western blotting” protocol (above), and equal volumes of sample were incubated with 2 µL anti-V5 antibody (Cell Signaling Technology) (54) and rotated overnight at 4°C, after which Magne Protein G beads (Promega) were added for an additional 2 hours at room temperature to immunoprecipitate the V5-tagged target protein. Samples were washed in PBS, eluted in NuPage LDS Sample Buffer and 50 mM DTT, and probed in Western blot analysis. Biotinylated proteins were stained with streptavidin, conjugated to Alexa Fluor-647 (Life Technologies) (56), and normalized to the total amount of V5-tagged protein immunoprecipitated.
Real-time quantitative RT-PCR
RNA was isolated from hippocampal cultures stimulated with leptin using the PureLink RNA Mini Kit (Invitrogen) and converted to cDNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) with on-column DNA digest using the PureLink DNase Set (Invitrogen), according to the manufacturer’s protocols. All quantitative RT-PCRs (qRT-PCRs) were carried out using Fast SYBR Green Master Mix (Applied Biosystems), according to the manufacturer’s protocols, and run on a Viia 7 System (Applied Biosystems). All primers used in our studies are listed in Bland et al. (44). Quantification of USP8 mRNA was calculated as described in Pfaffl (57) and normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Whole-cell recordings
Whole-cell recordings were performed, as previously described in Dhar et al. (14), on DIV11 to -12 hippocampal cultures, transfected with Clover-β-actin and designated constructs.
Chromatin IP and chromatin IP-sequencing
Chromatin IP (ChIP) and ChIP-sequencing (ChIP-Seq) were performed as previously described in Lesiak et al. (58).
Statistical analysis
Electrophysiological data are expressed as means ± SE and were analyzed using a one-way ANOVA and a Tukey post hoc test. All other data are expressed as the means ± SEM. Multiple comparisons were analyzed using one-way ANOVA with post hoc Tukey analysis, and single comparisons were analyzed using a two-tailed, unpaired Student t-test. Statistical significance was set to a minimum of P < 0.05.
Results
LepRb is targeted for degradation by the lysosomal pathway in hippocampal neurons
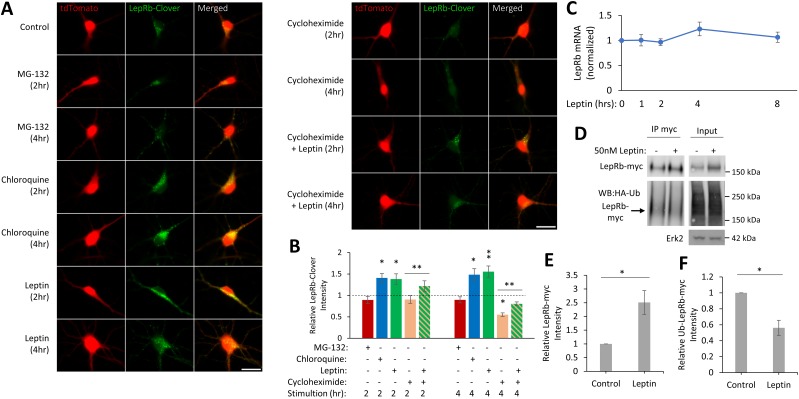
Leptin requires the surface expression of LepRb for activating downstream signaling cascades important in glutamatergic synaptogenesis (10, 14). Surface expression of LepRb has been demonstrated to be regulated by endocytosis via both ligand-dependent or constitutive mechanisms. Moreover, studies in heterologous cell lines have demonstrated that LepRb can be internalized and degraded by either the lysosomal or the proteasome pathways (32–34). These studies suggest that there are cell type-specific mechanisms for the regulation of LepRb surface expression. The mechanism by which surface expression of LepRb is regulated in neurons is unknown. To begin to identify the mechanism(s) by which LepRb expression is regulated in hippocampal neurons, we tested if LepRb is targeted for lysosome or proteasome-mediated degradation. We transfected cultured hippocampal neurons with a carboxyl-terminal, fluorescently tagged LepRb (LepRb-Clover) and tdTomato as a soluble cellular marker; then treated the cultures with either the proteasome inhibitor MG-132 (50 µM) or the lysosomal inhibitor chloroquine (50 µM); and measured the fluorescent intensity of LepRb-Clover (Fig. 1A). Neuronal cells were masked using the tdTomato channel, and the integrated intensity of the LepRb-Clover channel was measured. Inhibition of the lysosomal degradation pathway increased LepRb-Clover fluorescent intensity within 2 hours, which is further enhanced at 4 hours (2 hours: 140% ± 10%, P < 0.05; 4 hours: 148% ± 14%, P < 0.05), whereas inhibition of the proteasome does not affect LepRb-Clover fluorescent intensity (Fig. 1B).
Figure 1.
Leptin stimulation increases LepRb expression. (A) Representative fluorescent images of hippocampal cultures expressing tdTomato and LepRb-Clover. Cultures were stimulated with MG-132 (50 µM), chloroquine (50 µM), leptin (50 nM), cycloheximide (20 µM), or a combination of cycloheximide and leptin the next day for 2 h or 4 h and then fixed and imaged. Original scale bars, 25 µm. (B) Quantification of LepRb-Clover intensity normalized to control condition (dotted line; n = 30 to 49 neurons). The experiment was repeated in three independent hippocampal culture preparations. (C) qRT-PCR analysis of LEPRB mRNA levels in hippocampal neurons treated with leptin (50 nM). LEPRB transcript levels were normalized to GAPDH. (D) Representative Western blot (WB) of HEK293T cells expressing LepRb-myc and HA-Ub. Cells were treated with leptin (50 nM, 4 h) and lysed with lysis buffer containing a deubiquitinating enzyme inhibitor (PR-619; 25 μM), and LepRb-myc was immunoprecipitated. (E and F) Quantification of total (E) LepRb-myc intensity in the input lane normalized to Erk2 intensity or (F) ubiquitinated LepRb-myc intensity in the IP lane normalized to total LepRb-myc intensity in the same lane (n = 4). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control.
Leptin decreases the degradation of LepRb
Next, we show that acute leptin (50 nM) stimulation of hippocampal cultures increases LepRb-Clover intensity within 2 hours, which is further enhanced at 4 hours (2 hours: 138% ± 12%, P < 0.05; 4 hours: 155% ± 13%, P < 0.01; Fig. 1B). We also observed that the expression level of exogenously expressed LepRb-myc in HEK293T cells is enhanced (251% ± 44%, P = 0.0259) within 4 hours of leptin stimulation (Fig. 1D and 1E).
Increased expression of LepRb could be the result of increased transcription, translation, or protein stability. To test if leptin stimulation increases transcription of the LepRb gene, we monitored mRNA levels following leptin stimulation. We saw no substantial change in mRNA encoding LepRb up to 8 hours following leptin stimulation using qRT-PCR analysis (Fig. 1C).
To distinguish the role of increased protein synthesis vs degradation of LepRb with leptin stimulation, we treated hippocampal cultures expressing LepRb-Clover with leptin alone, the protein synthesis inhibitor cycloheximide (20 µM), or a combination of leptin and cycloheximide (Fig. 1A). As expected, cycloheximide treatment by itself decreases LepRb-Clover fluorescent intensity (2 hours: 90% ± 9%; 4 hours: 54% ± 4%, P < 0.05), which was returned to near-control levels with leptin treatment (2 hours: 124% ± 13%, P < 0.01; 4 hours: 166% ± 8%, P < 0.01 compared with cycloheximide alone; Fig. 1B), suggesting that leptin’s effect to increase surface expression of LepRbs is independent of synthesis.
Leptin reduces its own degradation through ubiquitination in HEK293T cells
The attachment of Ub to proteins can be used to mark proteins for both lysosome and proteasome degradation. To determine whether LepRs can be regulated in this manner, we first looked at the effect of leptin on the levels of Ub-LepRb in the HEK293T cell expressing LepRb-myc and HA-Ub and found that leptin decreases the levels of Ub-LepRb-myc (56% ± 9.4%, P = 0.019; Fig. 1D and 1F). These data suggest that leptin activation of LepRb reduces LepRb degradation through a deubiquitination-mediated mechanism in cell lines. We therefore wanted to determine whether leptins also alter deubiquitination of LepRb in hippocampal neurons. To begin to address this, we first examined the effect of leptin on deubiquitinating enzymes in hippocampal neurons.
Leptin enhances USP8 gene expression
We have previously shown that the CREB is activated by leptin (14). Interestingly, CREB Chip-Seq experiments identified DUB USP8 as one gene with CREB bound in its promotor region (Fig. 2A). qRT-PCR experiments show USP8 mRNA begins to increase in hippocampal cultures within 2 hours of leptin stimulation and remains elevated for >24 hours (2 hours: 129% ± 13%; 4 hours: 131% ± 5.4%, P < 0.01; 8 hours: 148% ± 16.3%, P < 0.05; 24 hours: 133% ± 10%, P < 0.05; Fig. 2B). Immunostaining of endogenous USP8 in hippocampal neurons transfected with soluble Clover to demark neuronal bodies and projections also shows elevated protein levels at 4 hours of leptin stimulation in hippocampal cultures (155% ± 13%, P < 0.01; Fig. 2C and 2D). This was performed by the masking of the neuronal cells using the Clover channel and measurement of the integrated intensity of the immunostained USP8 channel. Expression of ACREB or shCREB blocks the leptin-induced increase in USP8 expression (Fig. 2C and 2D), supporting that the hypothesis leptin regulates USP8 via a CREB-dependent pathway. Interestingly, the expression level of USP8 is significantly decreased (41% ± 3.8%, P = 0.027) in leptin signaling-deficient mice that lack the LepRb (db/db mice; Fig. 2E and 2F), suggesting that under normal conditions, leptin activation of LepRbs controls the expression of USP8 in the hippocampus.
Figure 2.
Leptin induces USP8 gene transcription. (A) The University of California, Santa Cruz, genome browser tracks show hippocampal CREB ChIP-Seq, embryonic cortical neuron CREB ChIP-Seq (Neocortical CREB) data relative to Reference Sequence genes, and CpG islands (green). (B) qRT-PCR analysis of USP8 mRNA levels in hippocampal neurons treated with leptin (50 nM). USP8 transcript levels were normalized to GAPDH. (C) Representative fluorescent images of hippocampal neurons expressing Clover, stimulated with leptin (50 nM), and immunostained for USP8. Original scale bar, 25 µm. (D) Quantification of USP8 integrated signal density normalized to control condition (n = 30 neurons). (E) Representative Western blot of P10 wild-type (wt) and db/db mice hippocampal protein extracts. (F) Quantification of USP8 intensity normalized to Erk2 intensity (wt: n = 4; db/db: n = 5). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control.
Leptin increases USP8 activity in hippocampal neurons
Phosphorylation and dephosphorylation of USP8 at critical tyrosine (Tyr) residues can either increase or decreases DUB activity of USP8, depending on which residues are phosphorylated (36, 59). We therefore first tested whether leptin can alter pUSP8 Tyr residues by IP of USP8 from HEK293T cells expressing a myc-USP8 (and LepRb) and measurement of the level of myc-USP8 pTyr (Fig. 3A). Leptin decreases (67% ± 11%, P = 0.05) the level of pTyr myc-USP8 within 1 hour (Fig. 3B). USP8 produces two bands on Western blots with the dephosphorylated form of USP8 migrating faster in an SDS-PAGE gel (36).
Figure 3.
Leptin stimulation decreases USP8 pTyr. (A) Representative Western blot of myc-USP8 immunoprecipitated from HEK293T cells expressing myc-USP8 and stimulated with leptin (50 nM). (B) Quantification of myc-USP8 pTyr normalized to total myc-USP8 (n = 3). (C) Representative Western blot of hippocampal neurons treated with leptin (50 nM). (D) Quantification of the ratio of the active non-pUSP8 (bottom band) to the nonactive pUSP8 (top band), normalized to control condition (n = 3). (E) Representative Western blot from hippocampal neurons treated with leptin (50 nM) or leptin and Na3VO4 (1 mM) for 1 h. Designated lysates were treated with λ protein phosphatase (λPP) for 1 h at 30°C. (F) Quantification of the ratio of the active non-pUSP8 (bottom band) to the nonactive pUSP8 (top band), normalized to control condition (n = 3). (G) Representative Western blot of myc-USP8 immunoprecipitated from hippocampal neurons expressing myc-USP8 and treated with leptin (50 nM, 1 h). Immunoprecipitated myc-USP8 was incubated with HA-Ub-VME (1 µM) for 1 h at 37°C. (H) Quantification of HA-Ub-VME-USP8 intensity normalized to total myc-USP8 intensity in the same lane (n = 4). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control condition. NS, not significant.
Next, to determine if leptin also decreases the endogenous pUSP8 in hippocampal cultures, we measured the ratio between the faster migrating USP8 band (dephosphorylated USP8) and the slower migrating band (pUSP8; Fig. 3C). Leptin increases (1.29 ± 0.01, P < 0.05) the USP8/pUSP8 ratio within 60 minutes (Fig. 3D). To determine further if this shift in molecular weight is a result of dephosphorylation, we treated cellular lysates with λ protein phosphatase (λPP). Treatment with λPP greatly increased [λPP: 2.58 ± 0.3, P < 0.01; λPP + leptin (1 hour): 3.16 ± 0.4, P < 0.01] the USP8/pUSP8 ratio in both control and leptin-stimulated cultures, whereas treatment with the pTyr inhibitor Na3VO4 (60) inhibited leptin dephosphorylation of USP8 (Fig. 3E and 3F).
As others have shown that dephosphorylation of USP8 can enhance its activity (36), we next wanted to determine whether leptin increases the activity of USP8 in hippocampal neurons. To examine this, we immunoprecipitated USP8 from hippocampal cultures expressing myc-USP8 and treated with leptin for 1 hour (Fig. 3G). The immunoprecipitated myc-USP8 was then labeled with the DUB activity probe HA-Ub-VME which will only bind to active USP8 (61). Leptin increases (161% ± 10%, P < 0.05) the binding of HA-Ub-VME to myc-USP8 (Fig. 3H), suggesting increased USP8 activity with leptin stimulation. Taken together, these data show that leptin both decreases pTyr of USP8 and enhances its activity in hippocampal neurons.
USP8 is in a complex with LepRb
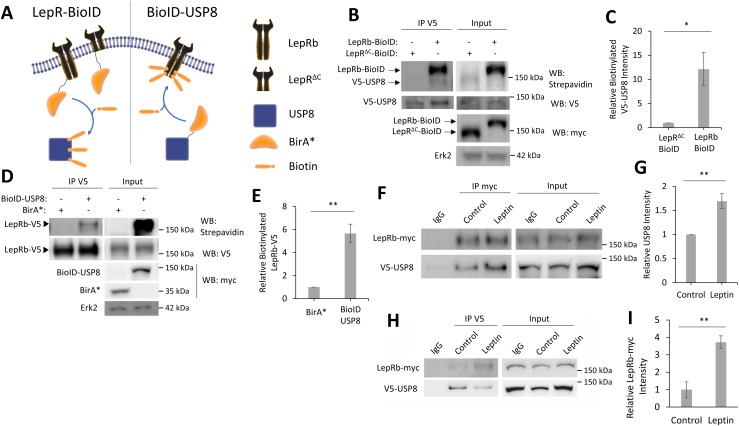
One challenge in finding the targets of DUBs is determining if they deubiquitinate the target protein themselves or if the deubiquitination occurs through an intermediate DUB. To test if USP8 is associated with, or in close proximity to, LepRb, we used a BioID method (62) combined with co-IP assays. As a result of poor transfection efficiency and very low expression of LepRb-expressing constructs in hippocampal neurons, we performed these biochemical analyses in HEK293T cells, which show a more robust expression of LepRb using these same constructs. First, to determine if USP8 is in close proximity to LepRb, we used BioID (Fig. 4A). This method uses a construct that fuses the promiscuous biotin ligase myc-BirA* (which will be further listed as BioID) to a protein of interest, which can then add biotin to any proteins that are in close proximity to the BioID-tagged protein (≤20 nm labeling radius) (63). We first expressed a LepRb-BioID fusion protein or a truncated form of LepRb (LepRΔC-BioID), which only includes the extracellular and transmembrane domain of LepRb (residues 1 to 860), to normalize for nonspecific biotinylation. In the presence of biotin, LepRb-BioID will covalently attach biotin to proteins that are either in a complex with LepRb-BioID or in close proximity. As LepRΔC-BioID has no intracellular domain to form complexes with other proteins, this construct was used to normalize for nonspecific biotinylation by the BioID tag. To determine if USP8 is in a complex with LepRb, we coexpressed LepRb-BioID and V5-USP8 in HEK293T cells and incubated them for 24 hours in media supplemented with biotin. To measure the ability of LepRb-BioID to biotinylation V5-USP8, we immunoprecipitated V5-USP8 and measured its biotinylation levels [Fig. 4B; see Bland et al. (44)]. LepRb-BioID robustly biotinylates V5-USP8 (12.1 ± 3.4-fold over LepRΔC-BioID, P = 0.032), suggesting that USP8 is localized in close proximity to LepRb but not LepRΔC-BioID (Fig. 4C). To determine further the proximity of these two proteins, we performed the reciprocal experiment by attaching BioID to USP8 and measuring its ability to biotinylate LepRb. This was performed by coexpression of BioID-USP8 and LepRb-V5, IP of LepRb-V5, and measurement of its level of biotinylation. As USP8 is not membrane bound, nonspecific biotinylation by BioID was normalized by the expression of a soluble form of BioID alone (BirA*). BioID-USP8 robustly biotinylates LepRb-V5 compared with soluble BirA* alone (5.7 ± 0.8-fold over BirA*, P = 0.0021; Fig. 4D and 4E). We also show that under basal conditions, V5-USP8 coimmunoprecipitates with LepRb-myc, and LepRb-myc coimmunoprecipitates with V5-USP8 in HEK293T cells (Fig. 4F and 4H); the interaction is enhanced (163% ± 15.3%, P = 0.0397, and 373% ± 36.6%, P = 0.009, respectively) following leptin stimulation (Fig. 4G and 4I). This suggests that under basal conditions, there is some USP8 bound to LepRb, and leptin stimulation promotes more binding of USP8. This further supports the hypothesis that LepRb and USP8 can occur in a complex.
Figure 4.
USP8 is in a complex with LepRb. (A) Diagram of BioID experiment. (B) Representative Western blot of V5-USP8 immunoprecipitated from HEK293T cells expressing the designated BioID constructs and V5-USP8. Biotin (50 μM) was added at time of transfection. (C) Quantification of IP biotinylated V5-USP8 normalized to total V5-USP8 in the same lane (n = 3). (D) Representative Western blot of LepRb-V5 immunoprecipitated from HEK293T cells expressing either BirA* or BioID-USP8 and LepRb-V5. Biotin (50 μM) was added at time of transfection. (E) Quantification of IP biotinylated LepRb-V5 normalized to total LepRb-V5 in the same lane (n = 3). (F–H) Representative Western blot of HEK293T cells expressing LepRb-myc and V5-USP8. Anti-IgG, (F) anti-myc, and (H) anti-V5 antibody-linked magnetic beads were used to coimmunoprecipitate LepRb-myc and V5-USP8 ± leptin stimulation (50 nM, 4 h). (G and I) Quantification of (G) coimmunoprecipitated V5-USP8 intensity normalized to LepRb-myc intensity and (I) coimmunoprecipitated LepRb-myc intensity normalized to V5-UPS8 intensity in the same lane (n = 3). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control.
USP8 deubiquitinates LepRb
As USP8 occurs in a complex with LepRb, and leptin stimulation increases USP8 expression and activation while decreasing LepRb ubiquitination, we tested if USP8 deubiquitinates LepRbs. To measure the level of Ub-LepRb, we expressed LepRb-myc and HA-Ub in HEK293T cells, immunoprecipitated LepRb-myc, and measured its level of ubiquitination by Western blot [Fig. 5A; see Bland et al. (44)]. Coexpression with V5-USP8 decreases (35% ± 1.2%, P < 0.01) ubiquitinated LepRb-myc levels, which enhances leptin-stimulated deubiquitination of LepRb (25% ± 2.1%, P < 0.05; Fig. 5B). We show that shUSP8 greatly decreases USP8 levels [48% ± 5%, P < 0.001; see Bland et al. (44)]. LepRb-myc shows increased levels of ubiquitination when coexpressed with shUSP8 (124% ± 6.3%, P = 0.0195; Fig. 5C and 5D). We also show that the catalytically inactive USP8 mutant (V5-USP8C749A) (64) does not decrease basal levels of Ub-LepRb-myc and inhibits the decrease of LepRb-myc ubiquitination mediated by leptin (Fig. 5E and 5F). To demonstrate further that LepRb is ubiquitinated, we immunoprecipitated HA–ubiquitin wild type (Ubwt) and then blotted for LepRb-Myc. Under both control and leptin-stimulated conditions, immunoprecipitated Ub coimmunoprecipitated a high molecular smear of LepRb-Myc [see Bland et al. (44)]. No significant change in the amount of LepRb-Myc coimmunoprecipitated was seen following leptin stimulation. This is most likely caused by the fact that a single molecule HA-Ub conjugated to LepRb would be sufficient to allow for IP of LepRb-Myc.
Figure 5.
USP8 deubiquitinates LepRb. (A) Representative Western blot of LepR-myc immunoprecipitated from HEK293T cells expressing LepRb-myc, HA-Ubwt, or HA-UbK48R and V5-USP8 ± leptin stimulation (50 nM, 4 h). (B) Quantification of IP Ubwt-LepRb-myc and UbK48R-LepRb-myc intensities normalized to total LepRb-myc in the same lane (n = 4). (C) Representative Western blot of LepR-myc immunoprecipitated from HEK293T cells expressing LepRb-myc, HA-Ubwt, and shUSP8. (D) Quantification of IP Ubwt-LepRb-myc intensity normalized to total LepRb-myc in the same lane (n = 3). (E) Representative Western blot of LepR-myc immunoprecipitated from HEK293T cells expressing LepRb-myc, HA-Ubwt, and V5-USP8C749A ± leptin stimulation (50 nM, 4 h). (F) Quantification of IP Ubwt-LepRb-myc intensity normalized to total LepRb-myc in the same lane (n = 3). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control. NS, not significant; wt, wild type.
We next determined what type of ubiquitination USP8 removes from LepRb. Ub attachment can occur as a monomer or as branched poly-Ub chains. Ub that is branched from the lysine 48 residue (K48) of Ub generally targets proteins for degradation, whereas mono-Ub can signal proteins for internalization and sorting (65). To determine if USP8 can remove K48-branched Ub from LepRb, which would protect the receptor from degradation, we used a mutant form of Ub lacking the K48 branch site (UbK48R; Fig. 5A). When LepRb-myc is ubiquitinated with UbK48R, V5-USP8-mediated deubiquitination of LepRb-myc is inhibited but not fully abolished (Ubwt: 65% ± 1.2%, P < 0.001; UbK48R: 39% ± 8.9%, P = 0.0299; Fig. 5B). This inhibition suggests that USP8 targets K48-branched Ub bound to LepRb. As UbK48R did not completely abolish deubiquitination of LepRb by USP8, this suggests that USP8 also has the ability to remove multiple forms of Ub from LepRb, other than K48-branched chains. Leptin-stimulated decrease in Ub-LepRb-myc is also inhibited with UbK48R expression (Fig. 5B), suggesting that leptin-mediated LepRb deubiquitination mainly involves the removal of K48-branched Ub chains.
USP8 enhances LepRb surface localization and leptin signaling in hippocampal neurons
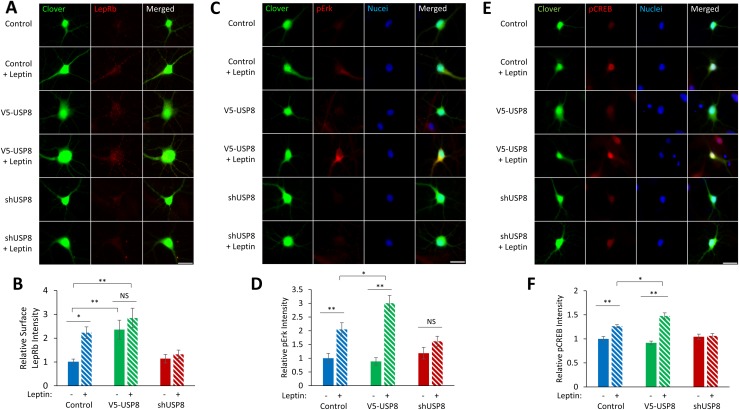
We next determined the functional significance of USP8 deubiquitination of LepRb. Under basal conditions, there are low levels of surface-localized LepRb resulting from the majority of LepRb being localized to the Golgi complex or in a post-Golgi intracellular compartment (66). Leptin stimulation, as well as USP8 overexpression, increases total LepRb expression [Fig. 1A; see Bland et al. (44)], but to determine if they increase the surface pool of receptors, we expressed LepRb that carries an extracellular tag on its amino terminus (Flag-LepRb) in hippocampal cultures and performed live immunostaining for surface Flag-LepRb (Fig. 6A). Four-hour leptin stimulation, as well as coexpression of V5-USP8, increases surface Flag-LepRb localization, whereas shUSP8 inhibits a leptin-stimulated increase in surface LepRb localization (leptin: 223% ± 25%, P < 0.05; V5-USP8: 236% ± 40%, P < 0.01; V5-USP8 + leptin: 285% ± 41%, P < 0.01; shUSP8: 114% ± 18%; shUSP8 + leptin: 132% ± 18%; Fig. 6B). Leptin stimulation and USP8 overexpression also increased the number of Flag-LepRb surface puncta, which was blocked with shUSP8 (data not shown).
Figure 6.
USP8 increases surface expression of LepRb and enhances leptin-stimulated signaling cascade activation. (A) Representative fluorescent images of hippocampal neurons expressing Clover, Flag-LepRb, and either V5-USP8 or shUSP8 ± leptin stimulation (50 nM, 4 h). Cultures were live immunostained for surface Flag-LepRb. (B) Quantification of the Flag-LepRb integrated signal density normalized to control condition (n = 28 to 30 neurons). (C and E) Representative fluorescent images of hippocampal neurons expressing Clover and either V5-USP8 or shUSP8 ± leptin stimulation (50 nM, 15 min). Neurons were immunostained for (C) pErk or (E) pCREB. (D and F) Quantification of the (D) pErk integrated signal density or the (F) pCREB integrated signal density normalized to control condition (n = 30 neurons). All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01. Original scale bars, 25 µm.
We next tested the functional consequences of the increased surface LepRb localization. Increased surface expression of LepRb could result in either increased sensitivity or increased amplitude of the effect of leptin stimulation in the neuron. Our laboratory has previously reported that leptin activates pErk1/2 and pCREB but not phosphorylation of signal transducer and activator of transcription 3 in hippocampal cultures, an effect dependent on LepRb expression (14). To test for increased amplitude of leptin’s effects, we stimulated hippocampal cultures with leptin for 15 minutes and immunostained for pErk1/2 (pErk) and pCREB. Leptin increases pErk and pCREB levels, which is further enhanced with expression of V5-USP8 (pErk: leptin: 205% ± 24%, P < 0.01; V5-USP8: 89% ± 13%; V5-USP8 + leptin: 301% ± 28%, P < 0.05 compared with control + leptin; pCREB: leptin: 126% ± 3%, P < 0.01; V5-USP8: 92% ± 4%; V5-USP8 + leptin: 148% ± 6%, P < 0.05 compared with control + leptin), and inhibited or completely abolished shUSP8 (Fig. 6C–6F). To test whether USP8 increased sensitivity to leptin, we performed a leptin dose response ± USP8 expression and then measured Erk activation. Although UPS8 expression increased the amplitude, as described above, its expression did not shift the sensitivity of LepRb to different leptin concentrations [Bland et al. (44)]. These data suggest that USP8 expression is necessary for the surface localization of LepRbs, and USP8 expression amplifies LepRb activation of downstream signaling cascades by leptin in hippocampal neurons.
USP8 increases synapse formation in hippocampal neurons
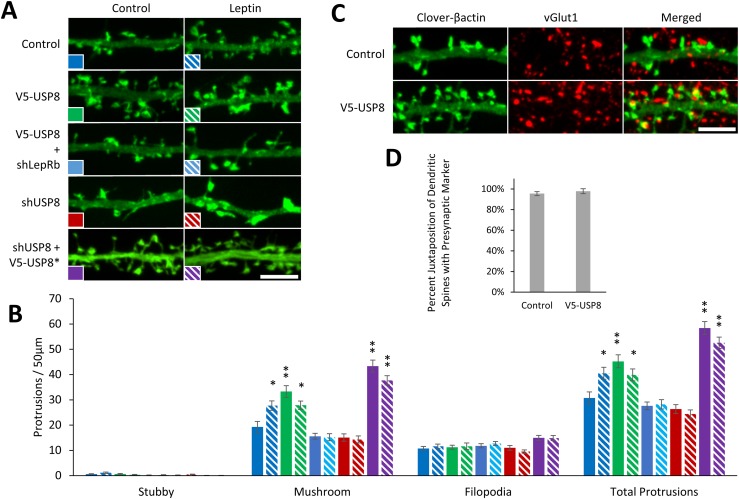
Leptin increases the number of functional excitatory glutamatergic synapses in hippocampal neurons, an effect attenuated by LepRb shRNA knockdown (14). The alteration of surface expression of LepRb could therefore impact synaptogenesis in the hippocampus. Thus, we tested the effect of USP8 on leptin-stimulated synaptogenesis. To visualize and measure the density of dendritic spines and spine morphology, hippocampal cultures were transfected on DIV6 with a fluorescently tagged actin (Clover-β-actin), which is enriched in dendritic spines, treated with leptin on DIV8, and fixed and imaged on DIV11 (Fig. 7A). We show that USP8 overexpression in hippocampal neurons increases mature mushroom-shaped dendritic spine density (mushroom spine density/50 μm ± SEM; control: 19.3 ± 2.1; control + leptin: 27.7 ± 1.9, P < 0.05; V5-USP8: 33.3 ± 2.3, P < 0.01; V5-USP8 + leptin: 27.9 ± 1.6, P < 0.05; Fig. 7B). Interestingly, USP8 overexpression did not enhance leptin-stimulated dendritic spine formation but actually occluded it. These data might suggest some unknown homeostatic regulation of synaptic numbers following USP8 overexpression. However, enhancement of mushroom-shaped spine density by USP8 is blocked with shLepRb (mushroom spine density/50 μm ± SEM; control: 19.3 ± 2.1; control + leptin: 27.7 ± 1.9, P < 0.05; V5-USP8 + shLepR: 15.6 ± 1.2; V5-USP8 + shLepR + leptin: 15.2 ± 1.4), suggesting that LepRbs are required for the USP8 increase in dendritic spines. Moreover, we showed that leptin cannot induced dendrite spine formation following shRNA-mediated knockdown of USP8, an effect that can be rescued by expression of a shRNA-resistant USP8 mutant (V5-USP8*; mushroom spine density/50 μm ± SEM; control: 19.3 ± 2.1; control + leptin: 27.7 ± 1.9, P < 0.05; shUSP8: 15.1 ± 1.5; shUSP8 + leptin: 14.3 ± 1.4; shUSP8 + V5-USP8*: 43.3 ± 2.4, P < 0.01; shUSP8 + V5-USP8* + leptin: 37.7 ± 1.8; P < 0.01; Fig. 7B).
Figure 7.
USP8 regulates dendritic spine formation in hippocampal neurons. (A–D) DIV6 hippocampal neurons were transfected with a fluorescent Clover-β-actin and the indicated constructs. Neurons were stimulated with leptin (50 nM) on DIV8 and fixed on DIV11 to DIV12 for spine density and presynaptic and postsynaptic juxtaposition experiments. (A) Representative fluorescent images of dendrite segments. Dendritic spine density was measured by hand using ImageJ with the NeuronJ plugin. (B) Quantification of dendritic spine density (n = 15 neurons). (C) Representative fluorescent images of hippocampal cultures immunostained for the presynaptic marker vGlut1. (D) Quantification of juxtaposition of dendritic spines and vGlut1 puncta averaged from 50 to 75 spines from 10 neurons. All experiments were repeated in three independent culture preparations and presented as the means ± SEM. *P < 0.05, **P < 0.01 compared with control. Original scale bars, 5 µm.
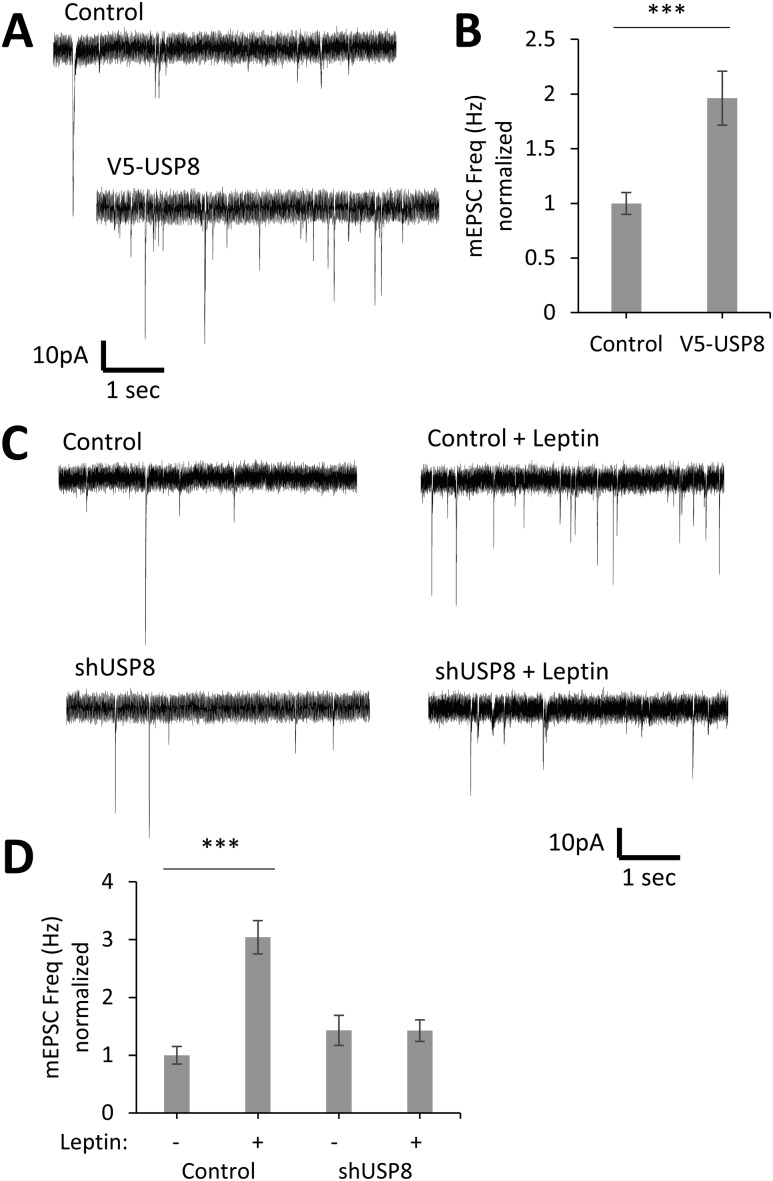
To determine if USP8 induces an increase in functional synapses, we measured the percent of dendritic spines that are juxtaposed to the presynaptic marker vGlut1 (Fig. 7C), and we also measured the frequency, amplitude, and decay time of miniature excitatory postsynaptic currents (mEPSCs) in hippocampal cultures (Fig. 8). USP8 maintains the same number of spines juxtaposed to vGlut1 puncta as control condition (Fig. 7D) and increases (196% ± 25%, P < 0.001, n = 31) the frequency of mEPSCs but does not affect the amplitude and decay time (Fig. 8A and 8B). In contrast, knockdown of USP8 inhibits the leptin-stimulated increase in mEPSC frequency [percent control of mEPSC frequency (Hz) ± SE; control: 100% ± 15%, n = 29; control + leptin: 304% ± 29%, P < 0.001, n = 35; shUSP8: 143% ± 26%, not significant (NS), n = 30; shUSP8 + leptin: 143% ± 19%, NS, n = 29], as well as amplitude [percent control of mEPSC amplitude (−pA) ± SE; control: 100% ± 3%, n = 29; control + leptin: 133% ± 8%, P < 0.001, n = 35; shUSP8: 105% ± 4%, NS, n = 30; shUSP8 + leptin: 106% ± 4%, NS, n = 29; Fig. 8C and 8D]. It should be noted that the modest increase in amplitude seen following leptin stimulation varies from culture to culture (data not shown) for as-yet unknown reasons, whereas the effect on frequency is robust and seen in all cultures. Taken together with the dendritic spine measurements, these data strongly suggest that USP8 increases the formation of functional synapses and is required for leptin-mediated synaptogenesis.
Figure 8.
USP8 regulates synapse formation in hippocampal neurons. (A–D) DIV6 hippocampal neurons were transfected with a fluorescent Clover-β-actin and the indicated constructs. Neurons were stimulated with leptin (50 nM) on DIV8, and electrophysiological recordings were performed on DIV11 to -12. (A and C) Representative electrophysiological recordings. (B and D) Quantification of mEPSC frequency (Hz) normalized to control condition (control: n = 29; V5-USP8: n = 31; control + leptin: n = 35; shUSP8: n = 29; shUSP8 + leptin: n = 30). All experiments were repeated in three independent culture preparations and presented as the means ± SE. ***P < 0.001 compared with control.
Discussion
The CNS actions of leptin primarily require the LepRb, and the size of leptin’s effects are influenced by the number of LepRb (31, 67). However, very little is known about how LepRb protein expression and surface localization are regulated in neurons. Here, we show five findings. First, leptin increases the levels of its own receptor in hippocampal neurons; second, this is through increased deubiquitination of the LepRb receptor, which protects it from targeting for degradation rather than a change in synthesis. Third, leptin induces both the expression and activity of the DUB USP8 in hippocampal neurons. Fourth, USP8 forms a complex with the LepRb and deubiquitinates it, resulting in a reduction in its degradation. Fifth, these actions of USP8 are required for leptin-induced hippocampal synapse formation, indicating that this regulation is critical for one of leptin’s key actions in developing hippocampal neurons.
Leptin enhances LepRb expression
Leptin has been shown to alter LepRb gene expression and protein levels. Early reports from Chinese hamster ovary cell lines show that leptin stimulation decreases LepRb expression (33). However, in vivo peripheral leptin injections increase LepRb mRNA and leptin-binding sites in the hypothalamus, including in the arcuate nucleus (68, 69). Leptin also alters LepRb expression in hypothalamic, pituitary, and ovarian cultures (70). Here, we show that short-term (hours) leptin treatment increases the expression of LepRb protein in both HEK cells and cultured hippocampal neurons. Interestingly, LepRb mRNA levels do not change in hippocampal neurons following leptin stimulation. Changes in LepRb levels have physiological implications, as demonstrated by the partial phenotype of the heterozygous db/db mice (31), suggesting that changes in the levels of LepRb impact the size of leptin’s effects and therefore, the physiological effects mediated by leptin. Interestingly, hypothalamic LepR expression can also be modulated by diet and nutritional state, conditions associated with changes in circulating leptin (71). However, the mechanisms underlying how leptin alters the levels of its own receptor are not well understood. As we found that the effects of leptin are not blocked by inhibiting protein synthesis, our data suggested that the effect was through decreased degradation of the receptor.
Leptin enhances USP8 expression
One mechanism by which the surface LepR expression is controlled is by constitutive endocytosis and degradation via the lysosomal pathway (32, 33, 72). Here, we show that leptin promotes the deubiquitination of LepRb. As ubiquitination targets the LepRb for degradation, deubiquitination of LepRb enhances its expression and promotes the maintenance of LepRb at the plasma membrane of hippocampal neurons.
The DUB USP8 had been shown to regulate LepRb trafficking and degradation in cell lines (34), but its role in the hippocampus had not been determined. Here, we show time that leptin increases USP8 expression levels, which would be expected to increase the level of deubiquitination. Furthermore, USP8 expression and activity are also regulated by post-translational modification (42, 73), and we found that leptin both decreases USP8 pTyr and increases USP8 activity.
USP8 is expressed in other brain regions critical for leptin’s actions, such as the hypothalamus (6–8, 37). It is therefore possible that USP8 deubiquitinates LepRbs in these brain regions, as well increases surface expression of LepRbs and enhances other leptin-dependent behaviors, such as feeding, energy expenditure, and glucose homeostasis.
LepRb is deubiquitinated by USP8
Here, we show that LepRb is deubiquitinated by the DUB USP8 in hippocampal neurons. As it has been shown for many different proteins, deubiquitination of surface proteins prevents their internalization and degradation, whereas deubiquitination is required for proteins to progress from multivesicular bodies into the lysosome to be degraded (74, 75). As enhanced USP8 expression in hippocampal cultures enhances LepRb surface expression, it is most likely that USP8 targets and deubiquitinates surface LepRbs, thus preventing their endocytosis and degradation. We also found that USP8 can form a complex with the LepRb, at least in a cell line, suggesting the enzyme may be in a prime site to deubiquitinate the receptor.
Furthermore, we have demonstrated that USP8 increases LepRb surface localization, as well as promotes activation of two of its key signaling pathways: MAPK kinase/Erk1/2 and CREB. Taken together, our results demonstrate a feed-forward mechanism by which leptin can enhance expression of its own receptor and thus, increase the magnitude of its own signaling.
We also show that leptin stimulates a decrease in ubiquitinated LepRbs, and our data suggest that this is, at least in part, through USP8. However, we cannot rule out that Ub E3 ligases may not also play a role. The E3 ligase RNF41 has been implicated in the recycling of LepRb to the surface (34), but its direct ubiquitination of LepRb or its role in synapse formation has yet to been determined.
Leptin-induced synaptogenesis requires USP8
We have previously shown that leptin stimulates synapse formation in hippocampal neurons (10, 14). Furthermore, this seems to be critical in vivo as well, as dendritic spines are reduced in db/db animals (14). Interestingly, we found that USP8 is required for leptin-induced synaptogenesis. Given that we have shown that leptin stimulates USP8 to increase the expression of its own receptor, the fact that the effects of leptin are lost in the absence of USP8 is most likely a result of enhanced ubiquitination and degradation of LepRb leading to a reduction in LepRb signaling. As LepRbs are required for leptin-induced synaptogenesis, a reduction in LepRb expression would be expected to reduce leptin’s effects.
Our data support previous findings that the increase of USP8 alone is sufficient to promote dendritic spine formation in hippocampal neurons (42), yet we show that there is a reciprocal requirement for LepRbs. The mechanism of this enhancement may include regulation of the expression and activity of AMPA receptors, as USP8 and leptin have both been shown to enhance AMPA receptor activity and surface expression (36, 76). It is therefore possible that leptin enhancement of USP8 expression and activity leads to decreased ubiquitination and enhanced surface expression of AMPA receptors as well. We and others (77) have previously shown that synaptic activity acts as an additional neurotrophic signal to stimulate synaptogenesis and synaptic maturation stability. Thus, it is possible that leptin may produce some of its neurotrophic actions by increasing synaptic activity via the AMPA receptor.
Physiological implication of the contribution of USP8 to the development of synapses in the hippocampus
The Ub signaling system controls a diverse set of neuronal functions, including synapse development and function (38, 39, 78). The removal of Ub from synaptic proteins by DUBs also plays a critical role in synaptic function (40, 41), such as regulation of synaptic activity and structure (79, 80) and regulation of glutamate and GABA-receptor expression (81, 82). Mutations in DUBs have been linked to multiple neurologic disorders, such as Parkinson disease, Angelman syndrome, and ataxia (83–85). The DUB USP8 is highly expressed in multiple brain regions, including the CA1 region and dentate gyrus of the hippocampus, and is also localized in the synapse (35, 36). As multiple synaptic proteins, including Ras protein-specific guanine nucleotide-releasing factor 1, epidermal growth factor receptors, and AMPA receptors, have been shown to be regulated by USP8 deubiquitination (36, 64, 86), USP8 clearly plays an important role in synaptic function.
We show here that USP8 targets LepRb in the hippocampus. Many studies over the past decade have shown that leptin signaling influences hippocampal-dependent behaviors, including having procognitive effects by enhancing learning and memory (87) and having anti-depressive and anxiolytic effects (20, 27). Peripheral and intrahippocampal leptin injections have positive effects on memory tasks, which are dose dependent (25, 88). The actions of leptin in the CNS require the expression of LepRb, which has been observed in the hippocampus (6, 89) with obese db/db mice that lack LepRb expression, showing impairments in spatial learning tasks (90). This correlates well with human studies, as diet-induced obesity has been shown to be associated with decreases in cognitive function, including effects on executive decision making and short-term memory (91, 92). These deficits could arise from leptin insensitivity, both peripherally and centrally, which has been shown to occur in diet-induced obesity (93). Furthermore, leptin signaling has been implicated in multiple neurologic disorders, with decreased levels of LepRb mRNA observed in the hippocampus of patients with Alzheimer’s disease (94), protection of dopamine neurons in a Parkinson disease model (95), and increased levels of circulating leptin presenting in children with autism spectrum disorders (96). As the dendritic spine and synapse number are closely correlated with cognitive function and many neurologic disorders (97–99), furthering our knowledge on the formation of these structures is critical.
In summary, we have identified a signaling pathway by which leptin stimulates dendritic spine and synapse formation in the hippocampus by positively regulating LepRb expression. This occurs through increased expression and activation of USP8, which deubiquitinates LepRb, leading to increased LepRb surface localization and enhanced leptin-stimulated intracellular signaling. Taken together, these results reveal a function of USP8 in synapse formation during early hippocampal development. With the consideration that both LepRb and USP8 are expressed in other brain regions, including the hypothalamus, our findings could have broad implications as to how LepRb expression is regulated, which would, in turn, influence the magnitude of leptin’s effects in these brain regions.
Acknowledgments
We thank Professor Michael Varnum for the gifts of ubiquitin-expressing constructs.
Financial Support: This work was supported by National Institute of Mental Health Grant MH086032 (to G.A.W.), National Institute of Child Health and Human Development Grant HD092369 (to G.A.W.), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK083452 (to S.M.A.).
Author Contributions: T.B., G.S.S., and G.A.W. conducted the experiments and analyzed the results. T.B. wrote the initial draft of the manuscript. G.A.W. and S.M.A. assisted in the design of the study and in writing the manuscript. M.Z. conducted and analyzed the results of electrophysiological recording experiments in Fig. 8. S.I. conducted the CREB ChIP-Seq experiment in Fig. 2. C.D. generated all of the neuron cultures and maintained the animal colony. All authors reviewed the results and approved the final version of the manuscript.
Glossary
Abbreviations:
- ACREB
a dominant-negative cAMP response element–binding protein
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CA
cornu ammonis
- ChIP
chromatin immunoprecipitation
- ChIP-Seq
chromatin immunoprecipitation sequencing
- CNS
central nervous system
- CREB
cAMP response element–binding protein
- db/db mice
diabetic mice
- DIV
day in vitro
- DTT
dithiothreitol
- DUB
deubiquitinase
- Erk
extracellular signal–regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HA
hemagglutinin
- HEK
human embryonic kidney
- IP
immunoprecipitation
- K48
lysine 48 residue
- LepR
leptin receptor
- LepRb
long form of the leptin receptor
- LepRbΔC
leptin receptor b lacking the intracellular domain
- mEPSC
miniature excitatory postsynaptic current
- NS
not significant
- p
phosphorylated
- qRT-PCR
quantitative RT-PCR
- shRNA
short hairpin RNA
- Tyr
tyrosine
- Ub
ubiquitin
- UbK48R
ubiquitin lysine 48 arginine mutant
- Ubwt
ubiquitin wild type
- USP8
ubiquitin-specific protease 8
- USP8C749A
ubiquitin-specific protease 8 cysteine 749 alanine mutant
- vGlut1
antivesicular glutamate transporter 1
- VME
vinyl methylester
- λPP
λ protein phosphatase
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. [DOI] [PubMed] [Google Scholar]
- 2. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):546–549. [DOI] [PubMed] [Google Scholar]
- 3. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. [DOI] [PubMed] [Google Scholar]
- 4. Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, Warren MacKellar W, Rosteck PR Jr, Schoner B, Smith D, Tinsley FC, Zhang X-Y, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. [DOI] [PubMed] [Google Scholar]
- 5. Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes. 2012;36(12):1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 7. Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7(15–17):2635–2638. [DOI] [PubMed] [Google Scholar]
- 8. Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518(4):459–476. [DOI] [PubMed] [Google Scholar]
- 9. Banks WAA, Kastin AJJ, Huang W, Jaspan JBB, Maness LMM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17(2):305–311. [DOI] [PubMed] [Google Scholar]
- 10. Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM. Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci. 2014;34(30):10022–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flak JN, Myers MG Jr. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 2016;30(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256(3):600–602. [DOI] [PubMed] [Google Scholar]
- 13. Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhar M, Zhu M, Impey S, Lambert TJ, Bland T, Karatsoreos IN, Nakazawa T, Appleyard SM, Wayman GA. Leptin induces hippocampal synaptogenesis via CREB-regulated microRNA-132 suppression of p250GAP. Mol Endocrinol. 2014;28(7):1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283(26):18238–18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bouret SG. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. [DOI] [PubMed] [Google Scholar]
- 17. Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101(5):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collin M, Håkansson-Ovesjö ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res. 2000;81(1–2):51–61. [DOI] [PubMed] [Google Scholar]
- 19. Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101(3):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, Nakao K. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152(7):2634–2643. [DOI] [PubMed] [Google Scholar]
- 21. Moult PR, Harvey J. Regulation of glutamate receptor trafficking by leptin. Biochem Soc Trans. 2009;37(6):1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21(24):RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25(6):991–996. [DOI] [PubMed] [Google Scholar]
- 24. Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27(6):1420–1425. [DOI] [PubMed] [Google Scholar]
- 26. Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27(11):2738–2749. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl). 2010;207(4):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7(6):643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harvey J, Shanley LJJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33(5):1029–1032. [DOI] [PubMed] [Google Scholar]
- 30. Guo M, Huang T-Y, Garza JC, Chua SC, Lu X-Y. Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. Int J Neuropsychopharmacol. 2013;16(4):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, Boozer CN, Leibel RL. Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274(4):R985–R990. [DOI] [PubMed] [Google Scholar]
- 32. Belouzard S, Rouillé Y. Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J. 2006;25(5):932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uotani S, Bjørbaek C, Tornøe J, Flier JS. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48(2):279–286. [DOI] [PubMed] [Google Scholar]
- 34. De Ceuninck L, Wauman J, Masschaele D, Peelman F, Tavernier J. Reciprocal cross-regulation between RNF41 and USP8 controls cytokine receptor sorting and processing. J Cell Sci. 2013;126(16):3770–3781. [DOI] [PubMed] [Google Scholar]
- 35. Bruzzone F, Vallarino M, Berruti G, Angelini C. Expression of the deubiquitinating enzyme mUBPy in the mouse brain. Brain Res. 2008;1195:56–66. [DOI] [PubMed] [Google Scholar]
- 36. Scudder SL, Goo MS, Cartier AE, Molteni A, Schwarz LA, Wright R, Patrick GN. Synaptic strength is bidirectionally controlled by opposing activity-dependent regulation of Nedd4-1 and USP8. J Neurosci. 2014;34(50):16637–16649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. d’Amora M, Angelini C, Aluigi MG, Marcoli M, Maura G, Berruti G, Vallarino M. Expression pattern of mUBPy in the brain and sensory organs of mouse during embryonic development. Brain Res. 2010;1355:16–30. [DOI] [PubMed] [Google Scholar]
- 38. DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27(1):223–246. [DOI] [PubMed] [Google Scholar]
- 39. Tai H-C, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9(11):826–838. [DOI] [PubMed] [Google Scholar]
- 40. Todi SV, Paulson HL. Balancing act: deubiquitinating enzymes in the nervous system. Trends Neurosci. 2011;34(7):370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kowalski JR, Juo P. The role of deubiquitinating enzymes in synaptic function and nervous system diseases. Neural Plast. 2012;2012:892749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kerrisk Campbell M, Sheng M. USP8 deubiquitinates SHANK3 to control synapse density and SHANK3 activity-dependent protein levels. J Neurosci. 2018;38(23):5289–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling [published correction appears in Mol Cell Neurosci. 2012;49(2):250]. Mol Cell Neurosci. 2010;43(1):146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bland T, Sahin G, Zhu M, Dillon C, Impey S, Appleyard SM, Wayman GA. Data from: USP8 deubiquitinates the leptin receptor and is necessary for leptin-mediated synapse formation. Washington State University 2019. Accessed 23 July 2019. https://ipn.vetmed.wsu.edu/docs/librariesprovider7/faculty-docs/bland_usp8-manuscript_supplemental_resubmission.pdf?sfvrsn=6ca24d3b_2. [DOI] [PMC free article] [PubMed]
- 45. RRID:AB_2801489, https://scicrunch.org/resolver/AB_2801489.
- 46. RRID:AB_331646, https://scicrunch.org/resolver/AB_331646.
- 47. RRID:AB_310153, https://scicrunch.org/resolver/AB_310153.
- 48. RRID:AB_2750766, https://scicrunch.org/resolver/AB_2750766.
- 49. RRID:AB_259529, https://scicrunch.org/resolver/AB_259529.
- 50. RRID:AB_439680, https://scicrunch.org/resolver/AB_439680.
- 51. RRID:AB_260092, https://scicrunch.org/resolver/AB_260092.
- 52. RRID:AB_627547, https://scicrunch.org/resolver/AB_627547.
- 53. RRID:AB_309678, https://scicrunch.org/resolver/AB_309678.
- 54. RRID:AB_2687461, https://scicrunch.org/resolver/AB_2687461.
- 55. RRID:AB_10693544, https://scicrunch.org/resolver/AB_10693544.
- 56. RRID:AB_2336066, https://scicrunch.org/resolver/AB_2336066.
- 57. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lesiak A, Pelz C, Ando H, Zhu M, Davare M, Lambert TJ, Hansen KF, Obrietan K, Appleyard SM, Impey S, Wayman GA. A genome-wide screen of CREB occupancy identifies the RhoA inhibitors Par6C and Rnd3 as regulators of BDNF-induced synaptogenesis. PLoS One. 2013;8(6):e64658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kasahara K, Aoki H, Kiyono T, Wang S, Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N, Inagaki M. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun. 2018;9(1):758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982;107(3):1104–1109. [DOI] [PubMed] [Google Scholar]
- 61. Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9(10):1149–1159. [DOI] [PubMed] [Google Scholar]
- 62. Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim DI, Birendra KC, Zhu W, Motamedchaboki K, Doye V, Roux KJ. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci USA. 2014;111(24):E2453–E2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alwan HAJ, van Leeuwen JEM. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem. 2007;282(3):1658–1669. [DOI] [PubMed] [Google Scholar]
- 65. Mabb AM, Ehlers MD. Ubiquitination in postsynaptic function and plasticity. Annu Rev Cell Dev Biol. 2010;26(1):179–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wauman J, Zabeau L, Tavernier J. The leptin receptor complex: heavier than expected? Front Endocrinol (Lausanne). 2017;8(FEB):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutafton in the mouse. Science. 1966;153(3740):1127–1128. [DOI] [PubMed] [Google Scholar]
- 68. Haltiner AL, Mitchell TD, Harris RBS. Leptin action is modified by an interaction between dietary fat content and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1250–R1255. [DOI] [PubMed] [Google Scholar]
- 69. Mitchell SE, Nogueiras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C, Williams LM. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol. 2009;587(14):3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Di Yorio MP, Bilbao MG, Pustovrh MC, Prestifilippo JP, Faletti AG. Leptin modulates the expression of its receptors in the hypothalamic-pituitary-ovarian axis in a differential way. J Endocrinol. 2008;198(2):355–366. [DOI] [PubMed] [Google Scholar]
- 71. Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000;875(1-2):89–95. [DOI] [PubMed] [Google Scholar]
- 72. Belouzard S, Delcroix D, Rouillé Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem. 2004;279(27):28499–28508. [DOI] [PubMed] [Google Scholar]
- 73. Meijer IMJ, Kerperien J, Sotoca AM, van Zoelen EJJ, van Leeuwen JEM. The Usp8 deubiquitination enzyme is post-translationally modified by tyrosine and serine phosphorylation. Cell Signal. 2013;25(4):919–930. [DOI] [PubMed] [Google Scholar]
- 74. Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315(5809):201–205. [DOI] [PubMed] [Google Scholar]
- 75. Clague MJ, Coulson JM, Urbé S. Cellular functions of the DUBs. J Cell Sci. 2012;125(2):277–286. [DOI] [PubMed] [Google Scholar]
- 76. Moult PR, Cross A, Santos SD, Carvalho A-L, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J. Leptin regulates AMPA receptor trafficking via PTEN inhibition. J Neurosci. 2010;30(11):4088–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57(1):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding M, Shen K. The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. BioEssays. 2008;30(11-12):1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89(1):115–126. [DOI] [PubMed] [Google Scholar]
- 80. Cartier AE, Djakovic SN, Salehi A, Wilson SM, Masliah E, Patrick GN. Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci. 2009;29(24):7857–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lappe-Siefke C, Loebrich S, Hevers W, Waidmann OB, Schweizer M, Fehr S, Fritschy JM, Dikic I, Eilers J, Wilson SM, Kneussel M. The ataxia (axJ) mutation causes abnormal GABAA receptor turnover in mice. PLoS Genet. 2009;5(9):e1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kowalski JR, Dahlberg CL, Juo P. The deubiquitinating enzyme USP-46 negatively regulates the degradation of glutamate receptors to control their abundance in the ventral nerve cord of Caenorhabditis elegans. J Neurosci. 2011;31(4):1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome [published correction appears in Nat Genet. 1997;15(4):411]. Nat Genet. 1997;15(1):70–73. [DOI] [PubMed] [Google Scholar]
- 84. Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT Jr. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111(2):209–218. [DOI] [PubMed] [Google Scholar]
- 85. Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet. 2002;32(3):420–425. [DOI] [PubMed] [Google Scholar]
- 86. Gnesutta N, Ceriani M, Innocenti M, Mauri I, Zippel R, Sturani E, Borgonovo B, Berruti G, Martegani E. Cloning and characterization of mouse UBPy, a deubiquitinating enzyme that interacts with the ras guanine nucleotide exchange factor CDC25(Mm)/Ras-GRF1. J Biol Chem. 2001;276(42):39448–39454. [DOI] [PubMed] [Google Scholar]
- 87. Van Doorn C, Macht VA, Grillo CA, Reagan LP. Leptin resistance and hippocampal behavioral deficits. Physiol Behav. 2017;176:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haleem DJ, Haque Z, Inam QU, Ikram H, Haleem MA. Behavioral, hormonal and central serotonin modulating effects of injected leptin. Peptides. 2015;74:1–8. [DOI] [PubMed] [Google Scholar]
- 89. Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387(2-3):113–116. [DOI] [PubMed] [Google Scholar]
- 90. Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113(3):607–615. [DOI] [PubMed] [Google Scholar]
- 91. Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. [DOI] [PubMed] [Google Scholar]
- 92. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;19(8):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. [DOI] [PubMed] [Google Scholar]
- 94. Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, Jicha G, Casadesus G, Smith MA, Zhu X, Lee HG. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128(1):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282(47):34479–34491. [DOI] [PubMed] [Google Scholar]
- 96. Al-Zaid FS, Alhader AA, Al-Ayadhi LY. Altered ghrelin levels in boys with autism: a novel finding associated with hormonal dysregulation. Sci Rep. 2014;4(1):6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10(10):981–991. [DOI] [PubMed] [Google Scholar]
- 98. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50(1):10–20. [DOI] [PubMed] [Google Scholar]