SUMMARY
SETTING :
Tertiary referral center, National Institutes of Health (NIH), USA.
OBJECTIVE :
To estimate the mortality rate and its correlates among persons with pulmonary non-tuberculous mycobacteria (PNTM) disease.
DESIGN:
A retrospective review of 106 patients who were treated at the NIH Clinical Center and met American Thoracic Society/Infectious Diseases Society of America criteria for PNTM. Eligible patients were aged ⩾18 years and did not have cystic fibrosis or human immunodeficiency virus (HIV) infection.
RESULTS:
Of 106 patients followed for a median of 4.9 years, 27 (25%) died during follow-up, for a mortality rate of 4.2 per 100 person-years. The population was predominantly female (88%) and White (88%), with infrequent comorbidities. Fibrocavitary disease (adjusted hazard ratio [aHR] 3.3, 95% confidence interval [CI] 1.3–8.3) and pulmonary hypertension (aHR 2.1, 95%CI 0.9–5.1) were associated with a significantly elevated risk of mortality in survival analysis.
CONCLUSIONS:
PNTM remains a serious public health concern, with a consistently elevated mortality rate across multiple populations. Significant risk factors for death include fibrocavitary disease and pulmonary hypertension. Further research is needed to more specifically identify clinical and microbiologic factors that jointly influence disease outcome.
Keywords: epidemiology, non-tuberculous mycobacteria, lung infection, mortality
RESUME
CONTEXTE:
Centre de référence tertiaire, National Institutes of Health (NIH), Etats-Unis.
OBJECTIF:
Estimer le taux de mortalité et ses corrélations chez les personnes atteintes de maladie pulmonaire à mycobactéries non-tuberculeuses (PNTM).
SCHÉMA:
Une revue rétrospective de 106 patients qui ont été traités au centre clinique du NIH et répondaient aux critères de l’American Thoracic Society/Infectious Diseases Society of America pour la PNTM. Les patients éligibles étaient âgés de ⩾ 18 ans et n’avaient ni mucoviscidose ni infection au virus de l’immunodéficience humaine (VIH).
RÉSULTATS:
De 106 patients suivis pendant une duréemédiane de 4,9 ans, 27 (25%) sont décédés pendant le suivi, soit un taux de mortalité de 4,2 par 100 personnesannées. La population était surtout de sexe fé minin (88%) et blanche (88%), avec des comorbidités fré quentes. Une atteinte fibrocavitaire (HR ajusté [HRa] 3,3; IC95% 1,3–8,3) et une hypertension pulmonaire (HRa 2,1; IC95% 0,9–5,1) ont été associées avec un risque significativement plus élevé de mortalité dans l’analyse de survie.
CONCLUSION:
La PNTM reste une préoccupation de santé publique grave avec un taux de mortalité constamment élevé dans de nombreuses populations. Les facteurs de risque significatifs de décés incluent une atteinte fibrocavitaire et une hypertension pulmonaire. Davantage de recherche est requise pour identifier plus spécifiquement les facteurs cliniques et microbiologiques qui influencent conjointement l’ evolution de la maladie.
RESUMEN
MARCO DE REFERENCIA:
Un centro terciario de referencia de los Institutos Nacionales de Salud (NIH), Estados Unidos.
OBJETIVOS:
Calcular la tasa de mortalidad y sus factores de riesgo en las personas con enfermedad pulmonar causada por micobacterias ambientales o atípicas (PNTM).
MÉTODOS:
Se llevó a cabo un análisis retrospectivo de 106 pacientes tratados en el centro medico de NIH, que cumplían con los requisitos diagnósticos de PNTM de la American Thoracic Society/Infectious Diseases Society of America. Se consideraron aptos los pacientes a partir de los 18 años de edad, sin diagnóstico de fibrosis quística ni de infección por el virus de la inmunodeficiencia humana (VIH).
RESULTADOS:
Se practicó el seguimiento de 106 pacientes durante una mediana de 4,9 años. Veintisiete pacientes fallecieron durante el seguimiento (25%), lo cual significa una tasa de mortalidad de 4,2 por 100 personas-año. La población fue en su mayor parte de sexo femenino (88%) y de etnia blanca (88%), con enfermedades concomitantes poco frecuentes. En el análisis de supervivencia, la presencia de enfermedad fibrocavernomatosa (HR ajustado [HRa] 3,3; IC95% 1,3–8,3) y de hipertensión pulmonar (HRa 2,1; IC95% 0,9–5,1) se asoció con un riesgo de mortalidad significativamente mayor.
CONCLUSIONES:
La PNTM sigue siendo un grave problema de salud pública, que da lugar de manera constante a una alta tasa de mortalidad en muchas poblaciones. Los principales factores de riesgo de mortalidad incluyen la enfermedad fibrocavernomatosa y la hipertensión pulmonar. Se precisan nuevos estudios que determinen los factores clínicos y microbiológious específicos que influyen de manera conjunta sobre el desenlace clínico de la enfermedad.
IN THE UNITED STATES, pulmonary non-tuberculous mycobacteria (PNTM) disease is an emerging public health problem.1–3 Among persons aged ⩾65 years, the annual prevalence of PNTM has been increasing at a rate of 8.2% per year.1 Antibiotic treatment for PNTM is difficult, toxic, and costly,4 and treatment failure rates are high.5
Studies with estimates and predictors of mortality for PNTM are limited. In a 5-year study of PNTM treatment without macrolides, all-cause mortality was 36%.6 The introduction of macrolide therapy in the 1990s improved treatment,7 but few estimates are available on mortality data since that time. Five-year mortality was estimated at 40% in Denmark8 and 24% in Japan.9 In the US population, PNTM age-adjusted mortality was estimated at 0.1 per 100 000 person-years (py).10 To obtain current estimates for PNTM-associated mortality in the United States, we conducted a retrospective analysis of patients with PNTM referred to a tertiary care research hospital.
METHODS
Study population
Subjects were adult human immunodeficiency virus (HIV) negative patients enrolled in an Institutional Review Board-approved natural history study of PNTM at the National Institutes of Health (NIH). Patients in this cohort had moderate to severe disease, and had typically received treatment for NTM prior to referral to the NIH.11 All patients in this analysis met American Thoracic Society diagnostic criteria for PNTM disease.7 Patients were excluded from analysis if they were aged ˂18 years or had been diagnosed with cystic fibrosis before the age of 18: persons with cystic fibrosis were likely to have different risk factors for mortality than those without.
Data were abstracted between November 2010 and January 2011, and included all study visits up to that point. Available medical records, including autopsy findings, when available, were reviewed (by KNO) to assign immediate and contributing causes of death. The National Death Index was searched to identify any patients whose survival status was unknown at the time of data abstraction.
Data abstraction
Demographic, clinical, radiographic, microbiologic, and treatment data were abstracted from medical and laboratory records. The baseline visit was defined as the date of the first mycobacterial culture test performed at the NIH; measurements for variables of interest obtained within 12 months (±6 months) of that date were considered baseline values. Diagnosis of pulmonary hypertension (PH) was ascertained through medical records; when available, transthoracic echocardiograms were reviewed to confirm this diagnosis.
Computerized tomography (CT) images were assessed (by LF) to determine the presence of bronchiectasis and/or cavities at baseline. Because a continuum of dilated bronchus and cavity formation is frequent, cavities were considered present when isolated and not contiguous with a dilated bronchus (Figure 1). Coronal and sagittal reformations were used to help differentiate dilated bronchi from cavities.
Figure 1.
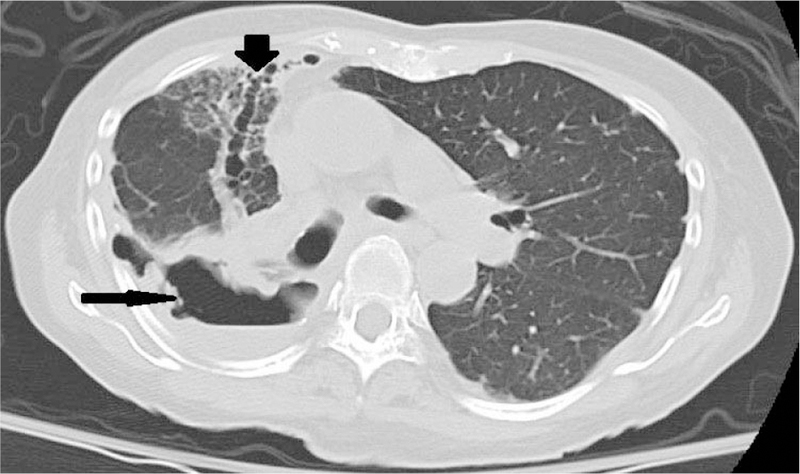
Fibrocavitary lung disease. Large cavities (thin arrows) with surrounding inflammation adjacent to marked bronchiectasis airways (thick arrow). Note significant mediastinal shift to the right due to fibrosis.
Mycobacterial species were grouped as following for analysis: any patient with Mycobacterium abscessus or M. massiliense (both subspecies of M. abscessus) identified was considered ‘M. abscessus group’. Any patient identified with M. avium complex (MAC), M. intracellulare, M. avium, or M. chimaera was classified as ‘MAC’. Concomitant organisms were defined as organisms identified at the time of mycobacterial culture. We defined chronic colonization as the isolation of potentially pathogenic bacteria in sputum culture on two or more occasions at least 3 months apart in a 1-year period.12
To describe treatment regimens and to exclude antibiotics given for short periods to treat concurrent infections or that were discontinued early due to intolerance, we included only oral or inhaled antibiotics taken for at least 3 months, and intravenous antibiotics taken for at least 1 month during the follow-up period.
Survival analysis
We analyzed demographic, clinical, and microbiological data to identify predictors of all-cause mortality among persons with PNTM. Specifically, we considered sex, age, mycobacterial species, semi-quantitative acid-fast bacilli culture data, concomitant organisms, body mass index (BMI, kg/m2), fibrocavitary disease, comorbidities, forced expiratory volume in 1 second (FEV1), percentage predicted (FEV1%pred), and C-reactive protein (CRP) levels. Total follow-up time was defined as the interval between the first mycobacterial culture performed at the NIH and the date of death or date of abstraction for surviving patients.
Statistical analyses were performed using SAS software, version 9.2 (Statistical Analysis System, Cary, NC, USA) and GraphPad PRISM, version 6 (GraphPad, La Jolla, CA, USA). The univariate association of demographic, clinical, and microbiologic factors with risk of mortality was assessed using Cox proportional hazards regression models. Variables significant on univariate analysis at P ˂ 0.1 were then included in multivariate Cox proportional hazards models, and hazard ratios (HRs) were calculated using the Cox proportional hazard model. Statistical significance was assessed at P ˂ 0.05. For continuous factors of interest, survival curves were calculated by grouping patients with values above and below the median value of the entire cohort. Kaplan-Meier survival curves were calculated, with the significance of difference in the survival distribution function estimated using the log rank (Mantel-Cox) test.
RESULTS
Baseline patient characteristics and mortality
Overall, 106 patients met our study criteria for analysis. Of these, 93 (87%) were White and 92 (86.8%) were women. The median age at diagnosis was 55 years. Median BMI was 20.5 for women and 23.8 for men. Baseline FEV1%pred was 75% and median CRP was 0.48 mg/l (Table 1). Overall, 46 (46%) had fibrocavitary disease at baseline (Table 1, Figure 1). In addition, 19 (18%) patients had heart disease and 12 (11%) patients had PH (Table 2). Nine of the 12 patients with PH had transthoracic echocardiograms at the NIH that confirmed the diagnosis of PH.
Table 1.
Baseline patient characteristics (n = 106)*
| Characteristic | n (%) |
|---|---|
| Female sex | 92 (86.8) |
| Race\ethnicity | |
| White | 93 (87.7) |
| Asian | 8 (7.6) |
| Black | 4 (3.8) |
| Hispanic | 1 (0.9) |
| Fibrocavitary disease | 46 (46) |
| Age at diagnosis, years, median [IQR]† | 55 [48–64] |
| Follow-up time at the NIH, years, median [IQR]† | 4.9 [2.3–9.5] |
| Baseline BMI, kg/m2, median [IQR]† | |
| Women | 20.5 [18.9–23.1] |
| Men | 23.8 [21.6–25.2] |
| CRP, mg/l, median [IQR]† | 0.48 [0.4–2.6] |
| FEV1 (%pred), median [IQR]† | 75.5 [61–93] |
Missing data are as follows: fibrocavitary disease: n=5, no CT performed 12 months (±6 months) from baseline culture; CRP: n=18, no CRP level checked 12 months (±6 months) from baseline culture; age of diagnosis: n=8, no age reported on patient questionnaire.
IQR (25th percentile, 75th percentile).
IQR= interquartile range; NIH= National Institutes of Health; BMI= body mass index; CRP = C-reactive protein; FEV1 = forced expiratory volume in 1 second; %pred = per cent predicted.
Table 2.
Comorbidities (n = 106)
| n (%) | |
|---|---|
| Heart disease | 19 (18) |
| Pulmonary hypertension | 12 (11) |
| Immune dysregulation* | 7 (7) |
| Asthma | 11 (11) |
| Primary ciliary dyskinesia | 2 (2) |
| Gastroesophageal reflux | 28 (26) |
Includes Sjogren’s syndrome, immunoglobulin A deficiency, systemic lupus erythematosis, alpha-1 anti-trypsin disease, Graves’ disease, common variable immunodeficiency and hypogammaglobulinaemia.
Twenty-seven (25%) patients died during the follow-up period. The median age at death was 67 years. The median length of follow-up from the baseline NIH visit was 4.9 years (Table 1). The overall mortality rate was 4/100 py. Cumulative mortality was 10% at 3 years and 18% at 5 years. Of the 24 patients with medical records available from the time of death, 8 of whom had autopsies, PNTM was determined to be the primary cause of death for 16 (67%). Three patients had respiratory failure not due to PNTM, and an additional 3 had unknown causes of death, 2 of whom had stable PNTM and 1 had progressive PNTM. One patient died of sepsis and one of aspiration pneumonia.
An average of 9.6 positive mycobacterial cultures per patient was collected over the follow-up period. MAC was the most common organism identified at first positive NTM culture (Table 3); overall, 65 (61%) patients grew at least one MAC organism. Of the 56 MAC isolates identified at the species level (9 others were identified generally as MAC), 15 (27%) were identified as M. avium and 38 (68%) as M. intracellulare, including one confirmed as M. chimaera. Four patients (7%) had both M. avium and M. intracellulare/chimaera. M. abscessus group cultures (M. abscessus and M. massiliense) were identified in 37 (35%) patients. Other rapidly growing mycobacteria (M. fortuitum, M. chelonae) were identified in only four patients (Table 3). The most frequent concomitant organism was Aspergillus sp., found in 30 (29%) patients, followed by Pseudomonas sp., found in 24 (23%), and Penicillium sp. (n = 23, 22%).
Table 3.
Species distribution for mycobacterial isolates (n = 106)*
| Organism | n (%) |
|---|---|
| MAC† | 65 (61) |
| M. abscessus group‡ | |
| M. abscessus | 32 (30) |
| M. massiliense | 4 (3.8) |
| M. abscessus + M. massiliense | 1 (0.9) |
| M. fortuitum | 3 (2.8) |
| M. chelonae | 1 (0.9) |
| M. mucogenicum | 2 (1.8) |
| M. simiae | 2 (1.8) |
| M. kansasii | 1 (0.9) |
As some patients had more than one isolate at baseline, the total exceeds 100%.
Includes M. avium, M. intracellulare, and M. chimaera; of 65 patients with MAC, 4 patients had both M. avium and M. intracellulare.
Includes M. abscessus and M. massiliense.
MAC = M. avium complex.
Antibiotic data were available for 90 of the 106 patients (Table 4). The most frequently used antibiotics were the macrolides, azithromycin and clarithromycin (97%), as well as ethambutol (60%), rifampin/rifabutin (47%), and moxifloxicin (47%).
Table 4.
Antibiotics received during follow-up (n = 90)*
| Antibiotic | n (%) |
|---|---|
| Azithromycin/clarithromycin | 87 (97) |
| Ethambutol | 54 (60) |
| Rifampin/rifabutin | 42 (47) |
| Moxifloxacin | 42 (47) |
| Linezolid | 36 (40) |
| Amikacin (intravenous or inhaled) | 28 (31) |
| Meropenem | 17 (19) |
| Clofazimine | 13 (14) |
| Ciprofloxacin | 12 (13) |
| Tigecycline | 4 (4.4) |
| Levofloxacin | 2 (2.2) |
| Imipenem | 1 (1.1) |
Only included for antibiotics given for at least 3 months; complete antibiotic information was missing for 16 patients.
Survival analysis
In the univariate proportional hazard models, fibrocavitary disease, PH, low FEV1%pred, and high CRP were significantly associated with an elevated risk of mortality (Table 5). Age, sex, BMI, NTM species, semi-quantitative culture scores, concomitant organisms (Aspergillus, etc.), and chronic colonization with other potentially pathogenic organisms (e.g., Pseudomonas) were not significantly associated with mortality. In the adjusted proportional hazard model, only fibrocavitary disease remained significant, with an adjusted HR (aHR) of 3.3 (95% confidence interval [CI] 1.3–8.3). PH was also strongly associated with mortality, with a two-fold increased risk of death in this group (aHR 2.1, 95%CI 0.9–5.1), although this was not significant after adjustment for fibrocavitary disease. FEV1%pred and CRP were not included in the multivariate model due to the considerable amount of missing data for these variables.
Table 5.
HRs for selected predictive factors (n = 106*)
| Baseline factors associated with death | HR (95%CI) | aHR (95%CI)* |
|---|---|---|
| Fibrocavitary disease | 3.3 (1.3–8.3) | 3.3 (1.3–8.3) |
| Pulmonary hypertension | 2.3 (1.0–5.6) | 2.1 (0.9–5.1) |
| FEV1%pred ˂76 | 4.6 (1.6–13.8) | NA |
| CRP ˃0.48 mg/l | 2.4 (1.0–5.7) | NA |
Missing data are as follows: fibrocavitary disease n=5, FEV1 n=14, CRP n=18, BMI n= 4; only fibrocavitary disease and PH were included in the multivariable model due to missing data on FEV1 and CRP.
HR=hazard ratio; CI=confidence interval; aHR=adjusted HR; FEV1=forced expiratory volume in 1 second; %pred = per cent predicted; NA = not available; CRP=C-reactive protein; BMI=body mass index; PH pulmonary=hypertension.
The survival curves for patients with and those without fibrocavitary disease were significantly different (P=0.006) (Figure 2A). Those with fibrocavitary disease had a median survival of 9.0 years, while those without fibrocavitary disease had a median survival of 13.1 years. The survival curves for patients with and without baseline PH were also significantly different (P=0.048), with a median survival of 6.5 years in those with PH compared with at least 18.4 years in those without (Figure 2B).
Figure 2.
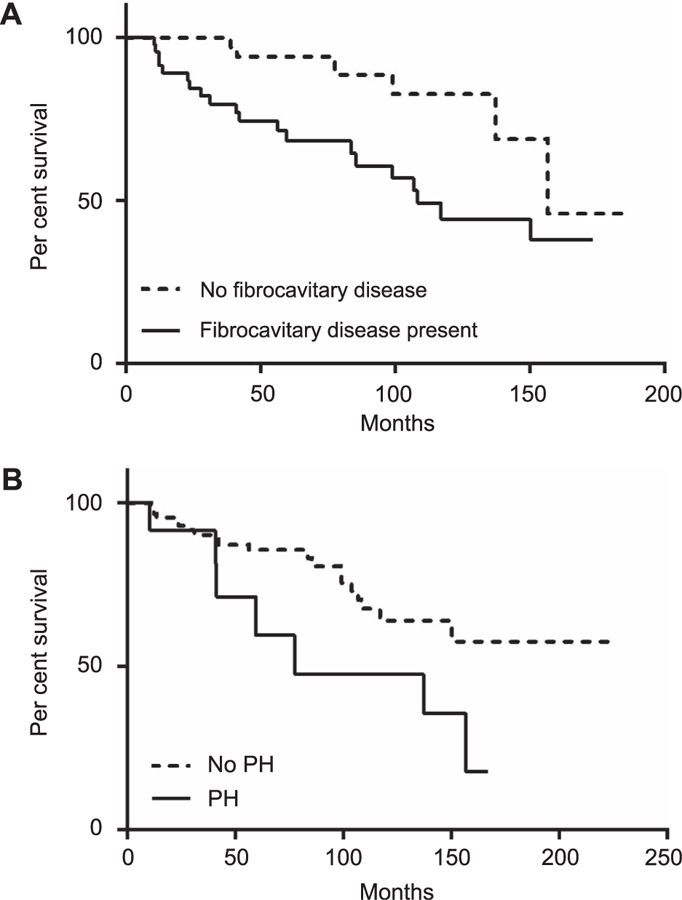
A) Survival curves by baseline fibrocavitary disease status, P=0.006. Median survival from first visit at NIH was 9.0 years for patients with fibrocavitary disease, and 13.1 years for those without fibrocavitary disease. B) Survival curves stratified by baseline PH status, P=0.048. Median survival was 6.5 years for patients with PH, and ˃18.4 years for those without PH, as the estimated survival was greater than 50% at the end of follow-up. NIH=National Institutes of Health; PH=pulmonary hypertension.
DISCUSSION
We evaluated risk factors for all-cause mortality in a cohort of predominantly White female patients with PTNM disease, reflecting the female predominance of PNTM in the United States.2 Among these patients followed for an average of nearly 5 years, 25% died, resulting in a mortality rate of 4/100 py. This mortality rate is comparable to those from other recent studies. Among 634 PNTM patients in Japan, mortality was 25% over a median follow-up of 4.7 years.9 Another Japanese study of patients with pulmonary MAC reported an overall 5-year all-cause mortality of 28%.13 In Denmark, 5-year cumulative mortality was 40%.8 However, that cohort had an overall higher rate of comorbid conditions such as chronic obstructive pulmonary disease, cystic fibrosis, and HIV, likely contributing to the markedly higher mortality. Finally, a Finnish study found a 4-year case-fatality rate of 20% in a cohort of 121 non-HIV-infected patients.14 Similar to our population, Japanese studies have comprised mostly women.9,13,15,16 In contrast, European populations in which PNTM has been studied have tended to be predominantly male.8,14,17 The predominance of MAC in our study is similar to that found in Japan and in some European studies.18
Mortality associated with PNTM in this cohort was higher than that found for pulmonary tuberculosis (TB) in the United States: in a follow-up study of persons reported with TB, the overall adjusted mortality rate was 8/1000 py.19 In addition, the median age at death of 67 years in our cohort was 13 years less than the average life expectancy for women in the United States in 2010, highlighting the excess burden of mortality from PNTM in the United States.
Our adjusted proportional hazards model found a significantly increased risk of mortality among patients with fibrocavitary disease (aHR 3.3, 95%CI 1.3–8.3), as well as an increased risk of death among those with PH (aHR 2.1, 95%CI 0.9–5.1). Increased mortality in association with radiographic evidence of cavitary disease is consistent with multiple prior studies.9,13,20–22 In a large retrospective cohort study in Japan, fibrocavitary disease with and without nodular bronchiectasis was a significant negative prognostic factor (HR 1.7, 95%CI 1.2–2.4). Interestingly, in that study the majority of patients with cavitary disease were male smokers, consistent with the known presentation of cavities in younger male smokers.9,23–25 In contrast, our study population was predominantly female and older than the more commonly reported patient population with fibrocavitary disease. Fibrocavitary disease may represent either more virulent NTM infections that are more difficult to treat, or more susceptible hosts who have more severe disease. In a retrospective review of patients with M. abscessus infection, those with cavitary lesions had significantly greater microbiologic failure, as measured by lack of sputum conversion.26 A retrospective review comparing CT patterns between patients with M. xenopi and those with MAC found more cavities in M. xenopi disease (46% vs. 16%, P=0.01).24 In a study of incidence and prognostic factors, M. xenopi was found to be the strongest negative prognostic factor among adults in Denmark with PNTM.8 These studies are consistent with the hypothesis that fibrocavitary PNTM may be associated with specific microbiologic factors. For example, in one study, microbiologic isolates from cavitary mycobacterial disease had faster intracellular macrophage growth as well as higher levels of elicited cytokines than from the nodular bronchiectasis forms. Furthermore, after infecting mice intranasally with these isolates, severe lung inflammation was only observed in those who were infected with an isolate from cavitary disease.27
Although we cannot determine in this study whether fibrocavitary changes are a pre-existing condition or a consequence of PNTM disease, these results should inform future research on the pathogenesis of cavitation in the context of PNTM as well as the treatment of PNTM with cavitation. There are reports of success with surgical resection of treatment-refractory PNTM.25,28,29 In one study, 70% of the 23 patients undergoing surgical resection were cavity-predominant on CT scan. Although one postoperative death and one late out-patient death were observed, all survivors experienced sputum conversion without relapse.28 Another study reported that sputum conversion was maintained in 81% of patients, with a higher rate of complications in patients with fibrocavitary disease.25 Among PNTM patients at a tertiary care referral center in Brazil, cure rates were lower among those with MAC or M. abscessus and cavitary disease.30 Our study does not provide conclusive evidence that fibrocavitary disease is more virulent per se, but it is clearly associated with more severe disease and mortality.
We found comorbid PH to be an independent risk factor for all-cause mortality in patients with PNTM. To the best of our knowledge, this finding has not yet been reported in the literature, although, as expected, severe underlying comorbid disease is a significant predictor of mortality.8,9,14,31 With regard to PH, one study reported 10 cases of chronic thromboembolic PH complicated by PNTM.32 NTM-infected segments were significantly more common in areas where blood flow was obstructed than where it was not. Improvement after re-vascularization with pulmonary endarterectomy was higher in arterial segments that were successfully re-perfused than in those that were not (100% vs. 57.1%, not statistically significant).32 This observation may provide a possible mechanism for the association between PH and mortality in PNTM disease, and suggests a possible target for intervention.
The primary limitation of this study was our inability to link detailed clinical and microbiologic information longitudinally. One study examined serial isolates from nine patients with persistent pulmonary M. abscessus infection, and identified a shift from smooth to rough colony morphology as well as a loss of glycopeptidolipid among later disease isolates.33 Whether or not microbial virulence and resistance are linked to clinical outcomes in patients with PNTM is critical to determine in prospective studies.
A second limitation of our study relates to the nature of the patients referred to the tertiary center. Those with refractory disease may not be representative of patients across the spectrum of disease. However, the fact that our mortality rates were similar to those of other reports with similar comorbidity profiles suggests that our population is similar to those represented in a number of other recent studies.
In summary, we have studied all-cause mortality in a PNTM population in the United States. Death from PNTM exceeds mortality rates in the general population, with the majority of deaths being attributed to PNTM per se. The specific clinical and microbiologic factors that interact to influence disease outcome have not been fully elucidated. The increased mortality associated with fibrocavitary disease and PH suggests possible targets for more aggressive interventions.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Conflicts of interest: none declared.
The views expressed in this article are those of the authors and do not necessarily reflect those of the US Department of Health and Human Services.
References
- 1.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Non-tuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49: e124–e129. [DOI] [PubMed] [Google Scholar]
- 4.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med 2009; 103: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection Chest 2004; 126: 566–581. [DOI] [PubMed] [Google Scholar]
- 6.The Research Committee of the British Thoracic Society. Pulmonary disease caused by Mycobacterium avium- intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis 2002; 6: 628–634. [PubMed] [Google Scholar]
- 7.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 8.Andrejak C, Thomsen VO, Johansen IS, et al. Non-tuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010; 181: 514–521. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012; 185: 575–583. [DOI] [PubMed] [Google Scholar]
- 10.Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Non-tuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative study. PLOS ONE 2014; 9: e91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary non-tuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008; 178: 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary mycobacterium avium-intracellulare complex disease. Int J Tuberc Lung Dis 2012; 16: 408–414. [DOI] [PubMed] [Google Scholar]
- 14.Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Jarvinen A. Prognostic value of American Thoracic Society criteria for non-tuberculous mycobacterial disease: a retrospective analysis of 120 cases with four years of followup. Scand J Infect Dis 2013; 45: 194–202. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014; 11: 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Okumura M, Iwai K, Ogata H, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008; 47: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 17.Gommans EP, Even P, Linssen CF, et al. Risk factors for mortality in patients with pulmonary infections with non-tuberculous mycobacteria: a retrospective cohort study. Respir Med 2015; 109: 137–145. [DOI] [PubMed] [Google Scholar]
- 18.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013; 42: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 19.Miller TL, Wilson FA, Pang JW, et al. Mortality hazard and survival after tuberculosis treatment. Am J Public Health 2015; 105: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura M, Iwai K, Ogata H, et al. [Clinical studies on the pathogenetic factors of cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease]. Nihon Kokyuki Gakkai Zasshi 2006; 44: 3–11. [Japanese] [PubMed] [Google Scholar]
- 21.Harada S, Harada Y, Ochiai S, et al. [A clinical study of deceased cases of pulmonary M. avium complex (MAC) disease—: in contrast with survived cases followed-up for 5 years or longer]. Kekkaku 2002; 77: 709–716. [Japanese] [PubMed] [Google Scholar]
- 22.Shu CC, Lee CH, Hsu CL, et al. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung 2011; 189: 467–474. [DOI] [PubMed] [Google Scholar]
- 23.Yano Y, Kitada S, Mori M, et al. Pulmonary disease caused by rapidly growing mycobacteria: a retrospective study of 44 cases in Japan. Respiration 2013; 85: 305–311. [DOI] [PubMed] [Google Scholar]
- 24.Carrillo MC, Patsios D, Wagnetz U, Jamieson F, Marras TK. Comparison of the spectrum of radiologic and clinical manifestations of pulmonary disease caused by Mycobacterium avium complex and Mycobacterium xenopi. Can Assoc Radiol J 2014; 65: 207–213. [DOI] [PubMed] [Google Scholar]
- 25.Kang HK, Park HY, Kim D, et al. Treatment outcomes of adjuvant resectional surgery for nontuberculous mycobacterial lung disease. BMC Infect Dis 2015; 15: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung YJ, Bittaye SO, Tsai JR, et al. Risk factors for microbiologic failure among Taiwanese adults with Mycobacterium abscessus complex pulmonary disease. J Microbiol Immunol Infect 2015; 48: 437–445. [DOI] [PubMed] [Google Scholar]
- 27.Sohn H, Kim HJ, Kim JM, Jung KO, Koh WJ, Shin SJ. High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease. Microb Pathog 2009; 47: 321–328. [DOI] [PubMed] [Google Scholar]
- 28.Koh WJ, Kim YH, Kwon OJ, et al. Surgical treatment of pulmonary diseases due to non-tuberculous mycobacteria. J Korean Med Sci 2008; 23: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Hasegawa N, Ishizaka A, et al. Early pulmonary resection for Mycobacterium avium complex lung disease treated with macrolides and quinolones. Ann Thorac Surg 2006; 81: 2026–2030. [DOI] [PubMed] [Google Scholar]
- 30.de Mello KG, Mello FC, Borga L, et al. Clinical and therapeutic features of pulmonary nontuberculous mycobacterial disease, Brazil, 1993–2011. Emerg Infect Dis 2013; 19: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Jarvinen A. Clinical symptoms and survival in non-smoking and smoking HIV-negative patients with non-tuberculous mycobacterial isolation. Scand J Infect Dis 2011; 43: 188–196. [DOI] [PubMed] [Google Scholar]
- 32.Kuroda F, Tanabe N, Igari H, et al. Non-tuberculous mycobacterium diseases and chronic thromboembolic pulmonary hypertension. Intern Med 2014; 53: 2273–2279. [DOI] [PubMed] [Google Scholar]
- 33.Park IK, Hsu AP, Tettelin H, et al. Clonal diversification, changes in lipid traits and colony morphology in Mycobacterium abscessus clinical isolates. J Clin Microbiol 2015; 53: 3438–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]


