To the Editor:
More than two decades ago, we were amongst the first to report the presence of neutrophils in the airways in severe asthma1. However, the nature of contribution of neutrophils to allergic inflammation remained a scientific enigma until quite recently. Neutrophils have long been viewed as terminally differentiated cells that clear extracellular pathogens. However, a growing body of literature indicates that neutrophils regulate innate and adaptive immune responses, and contribute to allergic diseases2–4. Adoptive transfer or recruitment of neutrophils to the skin stimulates allergic skin inflammation2. Likewise, neutrophils are required for both the sensitization and elicitation phase of allergic contact dermatitis3. Neutrophil activation occurs in vivo during anaphylaxis, and neutrophil depletion inhibits active and passive systemic anaphylaxis5. Importantly, mouse and human neutrophils each restore anaphylaxis in anaphylaxis-resistant mice, demonstrating that neutrophils are sufficient to induce anaphylaxis in mice, and suggesting that neutrophils can contribute to anaphylaxis in humans5. Tlr4KO mice are unable to mount pollen-induced allergic airway inflammation4; adoptive transfer of neutrophils to the lungs of Tlr4KO mice increases IL-33 secretion and reconstitutes pollen-induced allergic airway inflammation4. Exposure of the airway epithelium to allergenic extracts recruits reactive oxygen species (ROS)-generating neutrophils to the lungs4. Additionally, inhibiting ragweed pollen extract (RWPE)-induced neutrophil recruitment in mice by administration of CXCR2 small molecule inhibitor SB225002 attenuates allergic airway inflammation and sensitization4. Together, these studies provide compelling evidence for a role of neutrophils in the pathogenesis of allergic inflammation2–4.
An unexpected development in this story of the role of neutrophils to pathogenesis of allergic inflammation came from a report that administration of the CXCR2 inhibitor AZD5069 in humans failed to improve severe uncontrolled asthma even though it reduced the levels of neutrophils in sputum and blood6. Neutrophils have CXCR1 and CXCR2 on their surfaces, and these receptors have distinct non-redundant roles in its recruitment and activation. Thus CXCL8 monomer and dimer differentially activate and regulate function of CXCR1 and CXCR2 on neutrophils7. Inhibition of CXCR1 but not CXCR2 pathway suppresses neutrophil’s bacterial killing functions8. We identified the CXC motif in chemokines functions as a conformational switch for high affinity binding and activation of CXCR1 and CXCR2 receptors. Based on these reports4,7,8, here we hypothesized that a dual CXCR1 and CXCR2 pharmacologic inhibitor maybe effective in attenuating allergic airway inflammation.
Reparixin is a noncompetitive allosteric inhibitor of both CXCL8 receptors CXCR1 and CXCR2 that is safe for human use. Administration of reparixin in humans in a phase 2 randomized pilot study improved outcome of transplant of allogeneic islets in type 1 diabetes9. To test our hypothesis, we selected reparixin to perform a proof-of-concept study to elucidate the role of the CXCR1/CXCR2 inhibitor in an animal model of cat dander extract (CDE)-induced innate and allergic lung inflammation. While the functionality of CXCR2 has been extensively reported in mice, it was the recent demonstration of functional CXCR1 receptors in mice10 that provided the critical rationale for using mice to test our hypothesis. Wild-type (WT) naïve mice were subjected to a CDE single challenge model, CDE-SCM, Fig. 1A, by challenging them once with CDE and evaluating markers for innate inflammation were quantified 16 hrs later in BALF4. To induce allergic inflammation, WT mice were sensitized to CDE by subjecting them to CDE multiple challenge model, CDE-MCM, Fig. 2A, as previously described4. Following a rest period of one week, the mice were challenged again on Day 11, and allergic inflammation was quantified in the lungs on Day 14. Two doses of reparixin or vehicle were administered one hour before and after CDE challenge in a subset of the CDE-SCM group or CDE-MCM group on Day 11, and the effects of the inhibitor on innate or allergic lung inflammation were elucidated.
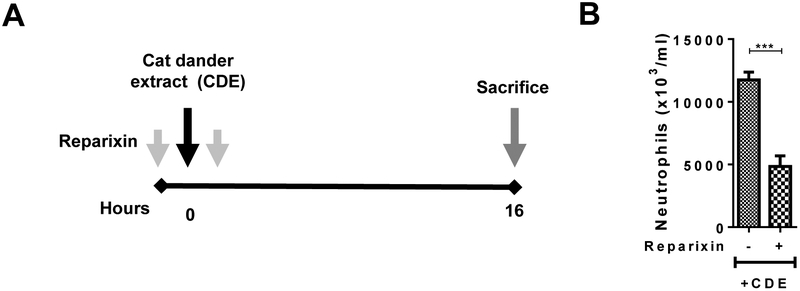
Fig. 1. Effect of Reparixin on CDE challenge-induced innate inflammation.
(A) Protocol for CDE Single Challenge Model, CDE-SCM in naïve WT mice with or without reparixin. (B) BALF neutrophil numbers in WT mice in a single CDE challenge model.
For all groups n =6–8 mice per group were used.
Data are expressed as means ± SEM. *=P< .05, **=P< .01, ***=P< .001, ****=P< .0001.
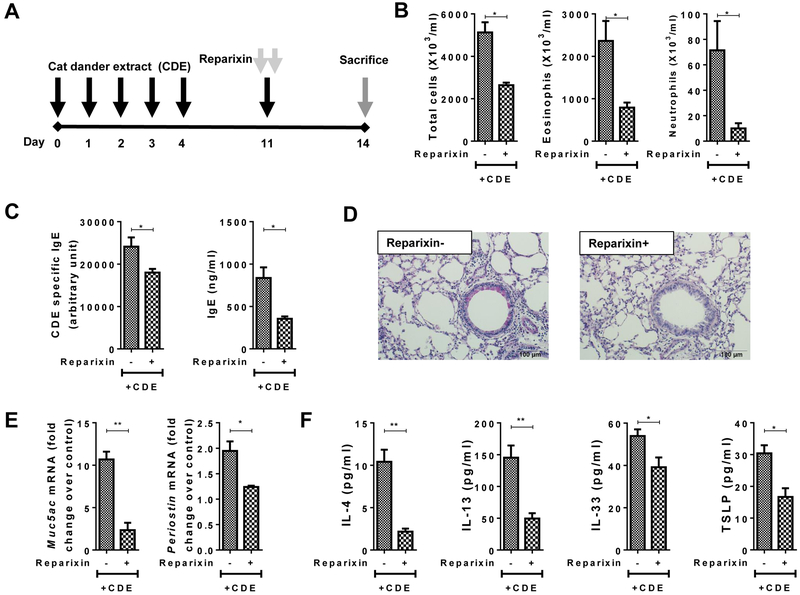
Fig. 2. Effect of Reparixin on CDE challenge-induced allergic inflammation.
(A) Protocol for CDE Multiple Challenge Model, CDE-MCM in naïve WT mice with or without reparixin. (B-F) Effect of multiple CDE challenge in the presence or absence of reparixin in WT mice. (B) BALF total inflammatory cell, eosinophil, and neutrophil numbers. (C) Serum total IgE and CDE-specific IgE. (D) Mucin secretion in airway epithelial cells. (E) Muc5ac and Periostin mRNA expression in lungs. (F) Th2 cytokines in BALF
For all groups n =4 mice per group were used.
Data are expressed as means ± SEM. *=P< .05, **=P< .01, ***=P< .001, ****=P< .0001.
In the CDE-SCM model (Fig. 1A), administration of reparixin (15 mg/kg body weight) suppressed neutrophil recruitment into the lungs (Fig. 1B). Building on this observation, we used the same dose of reparixin in the CDE-MCM model (Fig. 2A). As expected, CDE challenge in the CDE-MCM model stimulated eosinophil recruitment, allergic inflammation and sensitization (Fig. 2B–F). Administration of reparixin inhibited eosinophil, neutrophils, and total cell numbers in BALF (Fig. 2B), serum levels of total IgE and CDE-specific IgE (Fig. 2C), airway epithelial mucin secretion (Fig. 2D), levels of Th2 inflammation-associated genes periostin and muc5ac (Fig. 2E), and the BALF levels of IL-4, IL-13, IL-33, and TSLP in BALF (Fig. 2F). These results indicate that pharmacological inhibition of CXCR1/2-axis by administration of reparixin inhibits allergen-induced innate and allergic airway inflammation in mice. Taken together with the earlier report demonstrating that neutrophils recruited and activated by the CXCR1/2 axis contribute to allergic sensitization and inflammation4, our data from the present study suggest that reparixin attenuates allergic airway inflammation by inhibiting allergen-induced recruitment and activation of neutrophils.
To our knowledge, this is the first report showing that administration of dual CXCR1/2 inhibitor suppresses allergen-challenge induced neutrophilic inflammation and allergic airway inflammation. As second generation CXCR1/2 dual inhibitors such as ladarixin are developed and tested, some of these inhibitors may prove to be even more effective than reparixin in inhibiting allergic inflammation. Since asthma is a heterogeneous disease, administration of these new generation CXCR1/2 inhibitors may prove to be effective as controller therapy in neutrophil-dominant forms of asthma, such as the one described by us earlier1.
Supplementary Material
Acknowledgement.
NIAID P01 AI062885–06, NHLBI Proteomic Center, N01HV00245, Leon Bromberg Professorship at UTMB
References
- 1.Sur S, Crotty TB, Kephart GM, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? AmRevRespirDis. 1993;148(3):713–719. [DOI] [PubMed] [Google Scholar]
- 2.Oyoshi MK, He R, Li Y, et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity. 2012;37(4):747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber FC, Nemeth T, Csepregi JZ, et al. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J Exp Med. 2015;212(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosoki K, Aguilera-Aguirre L, Brasier AR, Kurosky A, Boldogh I, Sur S. Facilitation of Allergic Sensitization and Allergic Airway Inflammation by Pollen-Induced Innate Neutrophil Recruitment. Am J Respir Cell Mol Biol. 2016;54(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson F, Mancardi DA, Kita Y, et al. Mouse and human neutrophils induce anaphylaxis. The Journal of clinical investigation. 2011;121(4):1484–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Byrne PM, Metev H, Puu M, et al. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(10):797–806. [DOI] [PubMed] [Google Scholar]
- 7.Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183(5):3425–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl D, Latzin P, Hordijk P, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13(12):1423–1430. [DOI] [PubMed] [Google Scholar]
- 9.Citro A, Cantarelli E, Maffi P, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. The Journal of clinical investigation. 2012;122(10):3647–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swamydas M, Gao JL, Break TJ, et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci Transl Med. 2016;8(322):322ra310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.