Abstract
Objective:
To determine whether clinical and patient-reported outcomes differ in children receiving blenderized diets compared with conventional formula.
Study design:
We conducted a prospective cohort study of 70 children age 1–18 years receiving blenderized diets versus conventional formula via feeding tube. We assessed rates of hospitalization and emergency room (ER) visits at Boston Children’s Hospital in 2017; and Likert scale addressing satisfaction with feeding regimen; Pediatric Gastroesophageal Reflux Disease Symptom and Quality of Life Questionnaire (PGSQ); Pediatric Quality of Life Inventory (PedsQL), and PedsQL Gastrointestinal Symptoms Scale (GI-PedsQL).
Results:
Participants receiving blenderized diets (N=42, 60%) did not differ in demographics or comorbid diagnoses from those receiving conventional formula (N=28, 40%). Rates of total ER visits (0.8±1.5 vs. 1.4±2.7, P = .05), total admissions (0.8±1.2 vs. 1.7±2.3, P=0.01) and respiratory admissions (0.2±0.5 vs. 0.6±0.8, P=0.04) per year were significantly lower in participants receiving blenderized diets, and respiratory ER visits trended toward significance (0.1±0.4 vs. 0.4±0.8, P=0.08). Compared with those receiving conventional formula, participants on blenderized diets reported higher satisfaction ratings (Likert scale 4.3 ± 1.0 vs 3.3 ± 1.2, P=0.001), lower symptom (0.7±0.8 vs. 1.2±1.1, P=0.03) and total (0.8±0.8 vs. 1.2±1.0, P=0.02) scores on PGSQ and higher scores on GI-PedsQL indicating less nausea and vomiting (64.0±22.6 vs. 49.0±37.9, P=0.02), abdominal pain (65.0±26.8 vs. 56.4±33.9, P=0.04), diarrhea (87.9±15.5 vs. 73.6±26.3, P=0.004) and fewer total symptoms (70.2±16.3 vs. 62.3±19.6, P=0.03).
Conclusions:
Blenderized diets are associated with decreased health care utilization, improved symptom scores and increased patient satisfaction compared with conventional formulas.
Children with medical complexity are a vulnerable, expensive, and rapidly growing population. Accounting for <1% of the pediatric population, they accumulate one-third of all pediatric medical expenditures, approaching $50–110 billion dollars annually.1 Efforts directed at decreasing rates of hospitalization have potential for major cost savings – recent modeling suggests that as little as a 10% reduction in hospital days could translate to $2.9 billion cost savings nationally.2 The primary drivers of cost burden, specifically cardiovascular and pulmonary diseases including pneumonia and aspiration pneumonitis,3 as well as common comorbidities of asthma and obesity,4 can be modified by improving quality of dietary intake. However, optimizing diet quality in medically complex children for the purpose of improving health outcomes has not been systematically addressed.
Many children with medical complexity are fed conventional formulas via gastrostomy tube.4, 5 Several characteristics of conventional formulas—that they are rich in processed carbohydrate, high in saturated fat, and devoid of fiber, fruits, vegetables and other foods—have been strongly implicated in the pathogenesis of cardiovascular disease6, 7 and pulmonary diseases such as chronic obstructive pulmonary disease8 and asthma9 in adults and neurotypical children. In addition, emulsifiers and other preservatives required for shelf stability may be linked with inflammatory diseases.10 Despite the rising evidence implicating individual diet components of conventional formulas to chronic disease in an otherwise healthy population, the fragile population of medically complex children who receive 100% of their caloric needs in this fashion has been largely ignored.
Blenderized diets, the provision of wide range of pureed table foods such as fruits, vegetables, meat, and legumes via enteral tube, are emerging as popular alternatives to conventional formulas.11, 12 This movement is largely driven by parental concern of poor dietary quality of conventional formulas, and the fundamental urge to share a family mealtime experience with their medically complex children.12 Despite the widespread popular use,11 these diets have not yet been universally accepted by the medical community. Concerns have been raised about the variability across blenderized diets, potential for nutrient deficiencies and excesses, and lack of data to demonstrate safety and clinical superiority of these data. Therefore, in this prospective, observational cohort study of children with medical complexity, we sought to characterize clinical and patient-reported outcomes of children receiving blenderized diets.
Methods
We prospectively recruited 70 children age 1–18 years who require enteral feeding support from gastroenterology clinics and gastrointestinal procedure unit from January 2017 – October 2018. Participants were classified in the blenderized diet group if ≥50% of their diet was consumed from homemade table food or commercially prepared blenderized diets, e.g. Nourish/Liquid Hope, Nutritional Medicinals, LLC, Centerville, OH or Real Foods Blends, Real Food Blends, LLC, Chicago, IL. Participants were classified as conventional formula if they received the majority of their diet via standard milk-based or elemental formulas. Diet classification was performed based on dietary intake as of December 2017. One participant enrolled in the study while receiving formula for the duration of 2017, then re-enrolled in the study on a blenderized diet. For this patient, healthcare utilization in 2017 was not included under the blenderized classification. This study was approved by the Boston Children’s Hospital Institutional Review Board, and informed consent was obtained from parents or guardians of all study participants, with individual subject assent as appropriate.
Outcomes
Dietary intake data was obtained from detailed food journal and/or interview with registered dietitian, and calculated nutrient composition using Cronometer, a web-based nutrient database (Revelstoke, British Columbia). Vitamin and mineral intake was compared with ageand sex- appropriate dietary reference intake (recommended dietary allowances for B12, folate, calcium, vitamin D, iron, zinc, and adequate intake for manganese).13–16 Weight and height were recorded from the most recent outpatient gastroenterology, nutrition or gastrointestinal procedure unit visits (performed May 2017 through October 2018).
Health care utilization rates at Boston Children’s Hospital over the one year period of January 1, 2017 to December 31, 2017 were obtained by retrospective chart review. The one year duration was selected to ensure equal lengths of follow-up in both groups and inclusion of all four seasons to prevent bias as admissions typically are highest in winter. The numbers of hospitalizations and emergency room (ER) visits were quantified, and classified as respiratory if the primary presenting complaint could be attributed to this category, for example pneumonia, pneumonitis, or respiratory distress.
A subgroup of patients had 1 year of follow up on conventional formula and an additional year of follow up in the year following transition to blends. In this small cohort, using a paired t test, we compared admissions and ER visits in the year before and after starting the blend in the same patient.
To address quality of life measures, participants’ caregivers completed a Likert scale for satisfaction with enteral regimen (1 to 5, with 5 most satisfied), and three validated questionnaires: Pediatric Gastroesophageal Reflux Disease Symptom and Quality of Life Questionnaire (PGSQ), Pediatric Quality of Life Inventory (PedsQL) and PedsQL Gastrointestinal Symptoms Scale (GI-PedsQL).
Statistical Analyses
Data are presented as N (%) or mean ± standard deviation. Baseline characteristics and nutrient composition were compared using the Fisher exact test (categorical) or the student t-test (continuous). Mantel-Haenszel test was used to compare rates of comorbidities by diet type stratified by enteral tube type. For macronutrient composition, we compared variances between the groups using the Levene F-test.
We calculated propensity scores from underlying medical comorbidities using logistic regression, with outcome variable of diet category, and exposure of individual comorbidities, to create a covariate for use in regression analysis. For health care utilization rate, we created this propensity score from those comorbidities associated with health care utilization on univariate analysis (cardiac, prematurity, and respiratory). We compared health care utilization rates (number of ER visits, respiratory ER visits, admissions and respiratory admissions) between the 2 groups (blenderized vs conventional formula) using zero-inflated negative binomial regression model to account for zero inflation and over-dispersion, controlling for age and propensity score. We compared quality of life outcomes (Likert scale, PGSQ, PedsQL and PedsQL) using linear regression with covariates of age and propensity score. P-value ≤ 0.05 was set as the threshold for statistical significance. Analyses were performed using SPSS Statistics 24 (Revelstoke, British Columbia) or SAS version 9.4 (Cary, North Carolina).
Results
Patient Characteristics
Forty two (60%) participants received blenderized diets and 28 (40%) received conventional formula. The groups did not differ in age, sex, underlying comorbidities, race, parental education, income or insurance (P> 0.09, Table I). The majority of participants receiving blenderized diets were fed via gastrostomy, with a smaller proportion fed post-pyloric using a gastrojejunostomy or primary jejunostomy as shown in Table 1. Feeding tube type differed significantly compared with those receiving formula (P=0.002, Table 1). When diet category was stratified by feeding tube type, comorbidity prevalence did not differ significantly (Table 2; available at www.jpeds.com). Frequency of bolus, continuous or combination feeding methods are shown in Table 1.
Table 1:
Clinical characteristics of study population
| Formula (N=28) |
Blenderized (N=42) |
P-value | |
|---|---|---|---|
| Age | 5.7 ± 5.0 | 4.8 ± 3.6 | 0.38 |
| Male | 18 (64%) | 24 (57%) | 0.62 |
| Race | |||
| White | 19 (68%) | 25 (60%) | 0.09 |
| Black | 3 (11%) | 0 | |
| More than one race | 4 (14%) | 5 (12%) | |
| No survey data | 2 (7%) | 12 (29%) | |
| Underlying comorbidities | |||
| Neurologic | 14 (50%) | 29 (69%) | 0.14 |
| Metabolic | 16 (57%) | 21 (36%) | 0.63 |
| Oropharyngeal malformations | 14 (50%) | 20 (48%) | 1.00 |
| Cardiac | 14 (50%) | 15 (36%) | 0.32 |
| Prematurity | 9 (32%) | 17 (41%) | 0.62 |
| Respiratory | 9 (32%) | 6 (14%) | 0.14 |
| Cancer | 0 | 1 (2%) | 1.00 |
| Other | 9 (32%) | 22 (52%) | 0.14 |
| Tube type | |||
| Gastrostomy (G) | 13 (46%) | 35 (83%) | 0.002 |
| G-J | 14 (50%) | 5 (12%) | |
| Jejunostomy (J) | 1 (4%) | 2 (5%) | |
| Feeding method | |||
| Continuous | 15 (54%) | 15 (36%) | 0.09 |
| Bolus | 8 (29%) | 23 (55%) | |
| Mixed (continuous/bolus) | 5 (18%) | 4 (10%) | |
| Anthropometrics | |||
| Height z-score | −1.69 ± 1.69 | −1.57 ± 1.18 | 0.74 |
| Weight z-score | −1.74 ± 1.89 | −1.34 ± 1.29 | 0.34 |
| BMI z-score | −0.83 ± 1.79 | −0.29 ± 1.09 | 0.16 |
| Parental education | |||
| Some high school | 0 | 0 | 0.23 |
| Graduated from high school/GED | 1 (4%) | 0 | |
| Some college, all college or vocational | 15 (54%) | 13 (30%) | |
| Post college graduate courses or degree | 10 (36%) | 17 (40%) | |
| No survey data | 2 (7%) | 12 (29%) | |
| Household income | |||
| <$50,000 | 3 (11%) | 4 (10%) | 0.32 |
| $50,000–$100,000 | 11 (39%) | 12 (29%) | |
| >$100,000 | 9 (32%) | 14 (33%) | |
| Prefer not to answer | 3 (11%) | 0 | |
| No survey data | 2 (7%) | 12 (29%) | |
| Insurance | |||
| Private only | 7 (25%) | 8 (19%) | 0.20 |
| Government only | 7 (25%) | 8 (19%) | |
| International | 2 (7%) | 0 | |
| Mix of government and private | 12 (43%) | 26 (62%) | |
Results are mean ± standard deviation or N (%)
Table 2, Online:
Presence of Comorbidities Stratified by Diet and Feeding Tube Type
| Comorbidity Category | Formula (N=28) |
Blenderized (N=42) |
P-value | ||||
|---|---|---|---|---|---|---|---|
| G (N=13) |
G-J (N=14) |
J (N=1) |
G (N=35) |
G-J (N=5) |
J (N=2) |
||
| Neurologic | 6 (46%) | 8 (57%) | 0 | 24 (69%) | 3 (60%) | 2 (100%) | 0.20 |
| Metabolic | 8 (62%) | 8 (57%) | 0 | 19 (54%) | 1 (20%) | 1 (50%) | 0.52 |
| Oropharyngeal malformations | 5 (38%) | 9 (64%) | 0 | 16 (46%) | 3 (60%) | 1 (50%) | 0.85 |
| Cardiac | 7 (54%) | 7 (50%) | 0 | 12 (34%) | 2 (40%) | 1 (50%) | 0.46 |
| Prematurity | 3 (23%) | 6 (43%) | 0 | 15 (43%) | 2 (40%) | 0 | 0.48 |
| Respiratory | 4 (31%) | 5 (36%) | 0 | 4 (11%) | 2 (40%) | 0 | 0.43 |
| Cancer | 0 | 0 | 0 | 1 (3%) | 0 | 0 | 0.61 |
| Other | 3 (23%) | 5 (36%) | 1 (100%) | 17 | 4 | 1 (50%) | 0.09 |
Results are N (% of column)
P-value for Mantel-Haenszel test
Nutrient Composition
Of the 28 participants receiving conventional formula, 16 (57%) were prescribed elemental formulas, 10 (36%) standard milk-based formulas, and 2 (7%) more than one formula. Of the 42 participants receiving blenderized diets, 17 (40%) use blenderized table foods with conventional formula base, 14 (33%) use commercially-prepared blenderized diets (Nourish N=9, Liquid Hope N=3, Real Foods Blends N=2), and 11 (26%) use home-made blenderized diets. A sample home-made blenderized diet recipe is presented in Table 3 (available at www.jpeds.com). For those patients in whom start date of blenderized feeds was documented (N=32), mean duration of blenderized feeds was 19.4 ± 15.8 months.
Table 3, Online:
Sample Blenderized Diet Batch Recipe
| Ingredient | Amount |
|---|---|
| Chicken breast, cooked | 2 oz |
| Sweet potato, cooked & mashed | ½ cup |
| Green beans, cooked or canned | ½ cup |
| Mixed tropical fruit | ¼ cup |
| Banana | 1 small |
| Macaroni pasta | 123 g |
| Cream of Wheat, plan | 1 oz |
| Whole milk* | 1 cup |
| Olive oil | 1 Tbsp |
Makes 1060 kcal, 950 mL
Can substitute for non-dairy milk such asRipple milk
Full diet histories were available in 62 participants (N= 36 blenderized, N= 26 formula), and 8 (N=6 blenderized, N=2 formula) had limited diet history from which we could not calculate nutrient intake. Dietary fiber intake was significantly higher in blenderized diets (14.6± 7.3 vs. 4.6 ± 5.1 g/d, P<0.001). Total energy and macronutrient distribution, as well as variability in each macronutrient component, did not differ between the two groups (Table 4). Vitamin D intake was lower from blenderized diets compared with formula (64 ± 42 vs. 96 ± 57 % of dietary reference intake, P=0.01). Though the groups differed in folate, iron and zinc intake, both diets exceeded dietary reference intake (Table 4).
Table 4:
Nutrient Composition
| Formula (N=26) |
Blenderized (N=36) |
P-value | |
|---|---|---|---|
| Macronutrients | |||
| Kcal/kg | 74.0 ± 26.0 | 79.4 ± 32.0 | 0.48 |
| Total kilocalories (kcal) | 1152 ± 540 | 1134 ± 469 | 0.89 |
| % cal from carbohydrate | 46 ± 6 | 45 ± 9 | 0.67 |
| % cal from protein | 14 ± 5 | 14 ± 3 | 0.60 |
| % cal from fat | 40 ± 5 | 42 ± 7 | 0.47 |
| Carbohydrate total (g) | 132 ± 66 | 122 ± 46 | 0.49 |
| Fiber (g) | 4.6 ± 5.1 | 14.6 ± 7.3 | <0.001 |
| Protein (g/kg/d) | 2.3 ± 1.0 | 2.6 ± 1.3 | 0.48 |
| Fat (g/kg/d) | 3.3 ± 1.1 | 3.8 ± 1.9 | 0.18 |
| Micronutrients | |||
| B12 (% DRI) | 418 ± 197 | 292 ± 351 | 0.11 |
| Folate (% DRI) | 148 ± 68 | 107 ± 69 | 0.02 |
| D (% DRI) | 96 ± 57 | 64 ± 42 | 0.01 |
| Calcium (% DRI) | 126 ± 47 | 103 ± 49 | 0.07 |
| Iron (% DRI) | 227 ± 134 | 144 ± 83 | 0.009 |
| Manganese (% DRI) | 154 ± 148 | 206 ± 139 | 0.16 |
| Zinc (% DRI) | 249 ± 101 | 159 ± 133 | 0.006 |
Results are mean ± standard deviation
DRI = Dietary Reference Intake
Health Care Utilization Rates
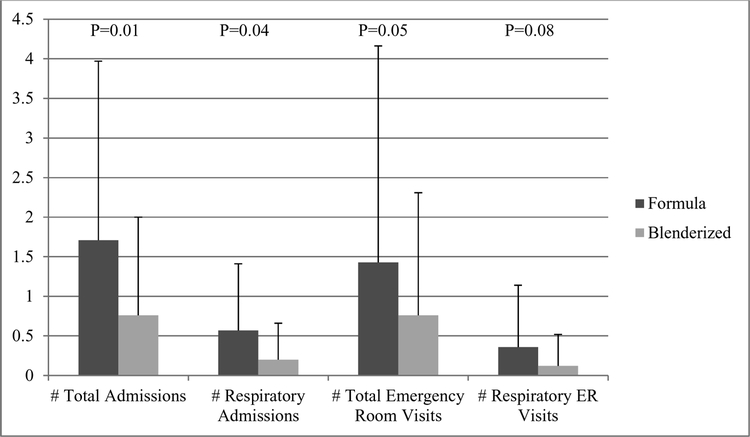
Using the zero-inflated negative binomial regression model, rates of total ER visits per 1 year (0.8 ± 1.5 vs. 1.4 ± 2.7, P=0.05), total admissions (0.8 ± 1.2 vs. 1.7 ± 2.3, P=0.01) and respiratory admissions (0.2 ± 0.5 vs. 0.6 ± 0.8, P=0.04) were significantly lower in participants receiving blenderized diets, controlling for age and propensity score (Figure). Respiratory ER visits were lower, though this did not reach statistical significance (0.1 ± 0.4 vs. 0.4 ± 0.8, P=0.08). During the one year observation period, there were a total of 79 hospitalizations (48 in formula group, 31 in blend group) and 71 emergency visits (40 in formula group, 31 in blend group). A total of 32 patients (8 in formula group, 24 in blenderized group) were not admitted during the 1 year period, and 45 (19 in formula group, 26 in blenderized group) never went to the ER during the 1 year period.
Figure:
Health care utilization at Boston Children’s Hospital in 2017 by diet type, adjusted for age and propensity score for comorbidities. Dark gray bars = conventional formula; light gray bars = blenderized feeds
Compared with participants receiving post-pyloric feeds, participants receiving blenderized diets via gastrostomy had fewer ER visits (0.8 ± 1.6 vs. 1.6 ± 2.8, P=0.05), respiratory ER visits (0.1 ± 0.4 vs. 0.4 ± 0.7, P=0.04), total admissions (0.7 ± 1.3 vs. 1.9 ± 2.4, P=0.008) and respiratory admissions (0.2 ± 0.5 vs. 0.7 ± 0.9, P=0.02) per year, adjusting for age and propensity score.
A subgroup of 22 participants were followed for 1 year before and after starting blends (for a total of 2 complete years of data). Using a paired t-test, we found that, with individual patients, total number of total admissions decreased significantly after blenderized feeds were started (1.5 ± 1.7 pre-blend vs. 0.7 ± 1.0 post-blend, P=0.05). Because of the small number of patients and the even smaller number of the subtypes of ER visits and admissions, there was inadequate power to draw conclusions regarding differences in other hospital visits. In this subgroup of patients, there was also no difference in the anthropometrics in the year before and after starting blends (P > 0.20).
Quality of Life Assessments
Fifty eight participants (N=32 blenderized and N=26 formula) completed a baseline Likert questionnaire addressing satisfaction ratings with feeding regimen. Compared with those receiving conventional formula, participants on blenderized diets reported higher satisfaction ratings (Likert scale 4.3 ± 1.0 vs. 3.3 ± 1.2, P=0.001, with higher scores indicating greater satisfaction). Moreover, they reported fewer gastroesophageal reflux disease symptoms, as evidenced by lower symptom (0.7 ± 0.8 vs. 1.2 ± 1.1, P=0.007) and total (0.8 ± 0.8 vs. 1.2 ± 1.0, P=0.02) scores on PGSQ. Furthermore, participants on blenderized diets reported higher total scores on the GI-PedsQL indicating overall improved gastrointestinal function (70.2 ± 16.3 vs. 62.3 ± 19.6, P=0.03). Importantly, GI-PedsQL subscores demonstrated higher scores indicating less nausea and vomiting (64.0 ± 22.6 vs. 49.0 ± 37.9, P=0.02), less abdominal pain (65.0 ± 26.8 vs. 56.4 ± 33.9, P=0.04), abdominal upset (71.1 ± 26.0 vs. 58.9 ± 32.7, P=0.02), less diarrhea (87.9 ± 15.5 vs. 73.6 ± 26.3, P=0.004), less worry about stool (91.5 ± 12.8 vs. 81.4 ± 30.0, P=0.05), and less limitation to food and drink (46.1 ± 29.6 vs. 29 ± 27.6, P=0.006), (Table 5). The remainder of PGSQ, PedsQL and GI-PedsQL did not differ between the groups (Table 5).
Table 5:
Differences in Quality of Life measures between participants on formula and blenderized feeds*
| Formula | Blenderized | P-value | |
|---|---|---|---|
| Likert Scale | |||
| Satisfaction with feeding regimen | 3.3 ± 1.2 (N=26) | 4.3 ± 1.0 (N=32) | <0.001 |
| Pediatric Gastroesophageal Reflux Disease Symptom and Quality of Life Questionnaire (PGSQ) | |||
| Total symptoms | 1.2 ± 1.1 (N=23) | 0.7 ± 0.8 (N=29) | 0.007 |
| Every day impact | 1.3 ± 1.0 (N=21) | 0.9 ± 0.9 (N=29) | 0.06 |
| School items | 0.6 ± 1.0 (N=13) | 0.7 ± 0.9 (N=17) | 0.83 |
| Total score | 1.2 ± 1.0 (N=23) | 0.8 ± 0.8 (N=29) | 0.02 |
| Pediatric Quality of Life Inventory (PedsQL) | |||
| Physical | 53.1 ± 30.3 (N=24) | 63.7 ± 31.3 (N=28) | 0.31 |
| Psychosocial | 62.2 ± 20.1 (N=23) | 63.7 ± 20.1 (N=28) | 0.65 |
| Total | 58.0 ± 20.4 (N=24) | 63.4 ± 22.5 (N=28) | 0.34 |
| PedsQL Gastrointestinal Symptoms Scale (GI-PedsQL) | |||
| Stomach pain | 56.4 ± 33.9 (N=22) | 65.0 ± 26.8 (N=28) | 0.04 |
| Stomach upset | 58.9 ± 32.7 (N=22) | 71.1 ± 26.0 (N=26) | 0.02 |
| Food & drink limits | 18.6 ± 27.6 (N=22) | 46.1 ± 29.6 (N=26) | 0.006 |
| Trouble swallowing | 48.4 ± 29.8 (N=21) | 55.9 ± 27.5 (N=28) | 0.45 |
| Heartburn/reflux | 63.9 ± 26.5 (N=22) | 67.4 ± 23.9 (N=28) | 0.28 |
| Nausea/vomiting | 49.0 ± 37.9 (N=20) | 64.0 ± 22.6 (N=27) | 0.02 |
| Gas | 61.6 ± 31.1 (N=22) | 66.1 ± 14.3 (N=28) | 0.19 |
| Constipation | 74.5 ± 24.5 (N=20) | 73.7 ± 28.4 (N=28) | 0.96 |
| Blood in stool | 88.7 ± 20.1 (N=21) | 95.1 ± 13.8 (N=28) | 0.19 |
| Diarrhea | 73.6 ± 26.3 (N=21) | 87.9 ± 15.5 (N=28) | 0.004 |
| Total symptoms | 59.7 ± 21.7 (N=22) | 69.7 ± 16.9 (N=28) | 0.01 |
| Worry about stool | 81.4 ± 30.0 (N=18) | 91.5 ± 12.8 (N=23) | 0.05 |
| Worry about abdominal pain | 79.6 ± 33.6 (N=19) | 88.1 ± 24.2 (N=21) | 0.10 |
| Medications | 74.6 ± 17.6 (N=17) | 78.8 ± 25.1 (N=19) | 0.48 |
| Communication | 36.5 ± 39.1 (N=20) | 39.3 ± 41.3 (N=23) | 0.68 |
| Total GI score | 62.3 ± 19.6 (N=22) | 70.2 ± 16.3 (N=28) | 0.03 |
Results are mean ± SD
P-value adjusts for age and propensity score
For Likert, higher scores indicate improved satisfaction (scale 1 to 5)
For PGSQ, higher scores indicate higher symptom burden
For PedsQL and GI-PedsQL, higher scores indicate less limitation
A subset of questions in the PGSQ, PedsQL and GI-PedsQL may not be appropriate for all ages and/or developmental abilities
Discussion
Our results demonstrate superior clinical outcomes in children receiving blenderized diets compared with children receiving conventional formula. Specifically, total ER visits were reduced by 43%, total admissions by 53% and respiratory admissions by 67%. We observed lower rates of healthcare utilization with blenderized diets consumed via gastrostomy even when compared with post-pyloric feeding regimens, which is a commonly prescribed approach to address feeding intolerance. Despite identical comorbidity profiles and similar degree of reported impairment in functioning, participants receiving blenderized feeds were more likely to be fed via gastric tube, suggesting improved tolerance of feeds. Together, this suggests blenderized diets are a well-tolerated, safe and relatively low cost intervention (compared with expensive medications) to improve health outcomes in this population, with potential for significant reduction in health care cost.
There are several potential mechanisms that may explain the beneficial effect on health outcomes observed by blenderized diets. The first is increased viscosity.17 Gastroesophageal reflux disease is common in children with neurologic impairment,18 which in turn can increase the risk of asthma, chronic cough and recurrent pneumonia.18–20 Increasing viscosity using half-solid feeds has been shown to reduce regurgitation episodes,21 full column gastroesophageal reflux22, 23 and number of fever episodes due to aspiration pneumonitis in neurologically impaired, gastrostomy-fed adults.24 This may mediate the significant effect observed in our study on respiratory-related health outcomes.
The second potential mechanism for improved health is improved nutrient profile.25 Although our data show similar energy content and macronutrient composition between formula and blenderized diets, the quality of macronutrient composition differs substantially. Specifically, conventional formulas utilize rapidly-digestible carbohydrate sources such as maltodextrin and corn syrup solids whereas diets with slowly digestible carbohydrates from legumes and whole grains, found in blenderized diets, reduce risk of airway inflammation seen in ventilator-associated tracheobronchitis compared with standard formula.26, 27 The provision of a diet containing actual fruits and vegetables, rather than simply vitamins as is present in conventional formulas, may reduce airway hyperreactivity28or reactive airway disease.29,30 In addition to a multitude of health benefits, dietary fiber, which is absent in many conventional formulas but emphasized in blenderized diets, acutely decreases airway inflammation.30
Lastly, blenderized feeds provide increased dietary diversity. Gallagher et al demonstrated that this increase in dietary diversity increases intestinal microbial diversity in children transitioning from conventional formula to blenderized diets.25 A diverse intestinal microbiota is associated with decreased risk of asthma,31 systemic inflammation,32 and obesity (a risk factor for restrictive lung disease),32, 33 which is associated with risk of COPD-type symptoms and asthma.34 Dietary fiber may be an important driver of this relationship, as it shapes intestinal microbiome composition, and affects short chain fatty acid production which in turn directs immune cell differentiation toward non-allergic phenotypes, important for decreasing airway hypersensitivity.35
As reported by Vieira et al, one of the concerns of blenderized feeds is the worry that patients are not receiving complete nutrition.36 In our study, we show that, in fact, patients are receiving identical calorie and micronutrient profiles to formula-fed patients with the exception of vitamin D. Therefore, like many otherwise healthy children on a regular diet, additional vitamin D supplementation may be indicated. Based on this study, providers should be reassured that complete nutrition is clearly possible using blenderized feeds. In contrast to Gallagher et al and Tanchoco et al, who reported that an increase in calories from blenderized diets is required to maintain body weight, we did not observe this effect.25,37 This is likely due to differences in underlying patient population, target growth trajectory, or dietary quality affecting metabolism and/or intestinal microbiome composition.
In addition to improved objective health outcomes, our study demonstrates improved patient-reported symptom scores with blenderized diets. We observed lower burden of vomiting, as well as lower symptom and total scores on the Pediatric Gastroesophageal Reflux Disease Symptom and Quality of Life Questionnaire (PGSQ). This complements the study by Pentiuk et al showing reduced gastrointestinal symptoms of retching in pediatric patients given blenderized feeds after fundoplication, and extends the previous observations by Hurt et al in adults and Gallagher et al in children.12,25,38 Together, these data support the hypothesis that blenderized diets reduce reflux burden by way of increased viscosity. In addition, we report that participants on blenderized diets had fewer symptoms of diarrhea, less abdominal pain, less abdominal upset, and less worry about stooling, all of which may be explained by the higher fiber content of blenderized feeds. Contrary to findings from Hurt et al, we did not observe a difference in constipation burden, which may reflect differences in underlying characteristics of our patient populations, or our sample size.12 We also found that parents reported less limitation to food and drink intake with blenderized diets. This may reflect improved gastrointestinal tolerance of dietary components, increased perception of diversity of diet, or increased oral intake, as suggested by Gallagher et al.25 Our study supports that blenderized feeds not only have a beneficial impact on lung health but reduce GI symptoms in a medically complex patient population.
Apart from the health benefit, ratings of caregiver satisfaction with feeding regimen ratings were significantly higher with blenderized diets. Parents are able to satisfy the fundamental urge to feed one’s child, sharing a mealtime experience with profound effects on family bonding. They may be driven by wanting a more holistic approach to feeding.12 In addition, parents may be more satisfied with the diet type because they are observing improved clinical outcomes with blenderized diets.
Limitations
Strengths of our data include a prospectively recruited, large cohort of medically complex children in whom we have detailed classification of health outcomes and quality of life. There are several minor limitations. Although study participants were prospectively recruited, we were only able to assess hospitalizations or emergency room visits within Boston Children’s Hospital.Because of the medical complexity of our patients, the majority of our patients receive care atBCH and require management in a tertiary care facility, and therefore we are likely recording the majority if not all of health care utilization. Although we believe that use of blenderized diets improves health outcomes, we cannot exclude confounding by indication – that patients who are overall doing well are more likely to be prescribed blenderized diets. Similarly, a higherproportion of participants receiving blenderized diets were fed via gastrostomy vs. post-pyloric feeds. We believe this represents improved tolerance to gastric feeds and may constitute part ofthe causal pathway by which health care utilization decreases. The similar comorbidity profile including prevalence of pulmonary disease between the groups, even when stratifying by tubetype, as well as similar physical and emotional scores on quality of life surveys (other than symptoms that would be expected to improve in response to diets), argue against systematic differences in underlying disease burden. Moreover, all outcomes data were adjusted bypropensity score. Generalizability of these data may be impacted by the high education, income level and rates of insurance coverage observed in this study. Lastly, we captured dietary recall atthe time of the study visit, but energy content and macronutrient distribution for both formulaand blenderized patients may have varied during the follow-up period which may have had an impact on hospitalizations and ER visits. We would anticipate, however, this would likely bias toward the null hypothesis of no difference between the groups making this limitation less likely.
Acknowledgements
We thank the study participants and their families for their enthusiasm, time, and dedication, and Ms Kylie Henderson for her contributions to their care.
Supported by National Institutes of Health (R01 DK097112-01). B.H. an academic collaborator on a research study for which financial support was provided by AbbVie. R.R. is a consultant for Takeda Pharmaceuticals. The other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented in abstract form at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Annual Meeting, October 24–27, 2018, Hollywood, Florida.
References
- 1.Berry JG, Hall M, Hall DE, Kuo DZ, Cohen E, Agrawal R, et al. Inpatient growth and resource use in 28 children’s hospitals: a longitudinal, multi-institutional study. JAMA Pediatr. 2013;167:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry JG, Hall M, Neff J, Goodman D, Cohen E, Agrawal R, et al. Children with medical complexity and Medicaid: spending and cost savings. Health Aff (Millwood). 2014;33:2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon TD, Berry J, Feudtner C, Stone BL, Sheng X, Bratton SL, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry JG, Agrawal R, Kuo DZ, Cohen E, Risko W, Hall M, et al. Characteristics of hospitalizations for patients who use a structured clinical care program for children with medical complexity. J Pediatr. 2011;159:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130:e1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 2015 8th edition. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 7.Ma J, Strub P, Lv N, Xiao L, Camargo CA Jr., Buist AS, et al. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur Respir J. 2016;47:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazdanpanah L, Paknahad Z, Moosavi AJ, Maracy MR, Zaker MM. The relationship between different diet quality indices and severity of airflow obstruction among COPD patients. Med J Islam Repub Iran. 2016;30:380. [PMC free article] [PubMed] [Google Scholar]
- 9.Root MM, Houser SM, Anderson JJ, Dawson HR. Healthy Eating Index 2005 and selected macronutrients are correlated with improved lung function in humans. Nutr Res. 2014;34:277–84. [DOI] [PubMed] [Google Scholar]
- 10.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epp L, Lammert L, Vallumsetla N, Hurt RT, Mundi MS. Use of Blenderized Tube Feeding in Adult and Pediatric Home Enteral Nutrition Patients. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2017;32:201–5. [DOI] [PubMed] [Google Scholar]
- 12.Hurt RT, Edakkanambeth Varayil J, Epp LM, Pattinson AK, Lammert LM, Lintz JE, et al. Blenderized Tube Feeding Use in Adult Home Enteral Nutrition Patients: A Cross-Sectional Study. Nutr Clin Pract 2015;30:824–9. [DOI] [PubMed] [Google Scholar]
- 13.Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)1997. [PubMed] [Google Scholar]
- 14.Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)1998. [PubMed] [Google Scholar]
- 15.Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC)2001. [PubMed] [Google Scholar]
- 16.In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)2011. [PubMed] [Google Scholar]
- 17.Sullivan MM, Sorreda-Esguerra P, Platon MB, Castro CG, Chou NR, Shott S, et al. Nutritional analysis of blenderized enteral diets in the Philippines. Asia Pac J Clin Nutr. 2004;13:385–91. [PubMed] [Google Scholar]
- 18.Mousa HM, Rosen R, Woodley FW, Orsi M, Armas D, Faure C, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011;52:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88:75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton RE, Wheatley R, Minford J. Respiratory tract infections due to direct and reflux aspiration in children with severe neurodisability. Dev Med Child Neurol. 1999;41:329–34. [DOI] [PubMed] [Google Scholar]
- 21.Wenzl TG, Schneider S, Scheele F, Silny J, Heimann G, Skopnik H. Effects of thickened feeding on gastroesophageal reflux in infants: a placebo-controlled crossover study using intraluminal impedance. Pediatrics. 2003;111:e355–9. [DOI] [PubMed] [Google Scholar]
- 22.Kanie J, Suzuki Y, Akatsu H, Kuzuya M, Iguchi A. Prevention of late complications by half-solid enteral nutrients in percutaneous endoscopic gastrostomy tube feeding. Gerontology. 2004;50:417–9. [DOI] [PubMed] [Google Scholar]
- 23.Nishiwaki S, Araki H, Shirakami Y, Kawaguchi J, Kawade N, Iwashita M, et al. Inhibition of gastroesophageal reflux by semi-solid nutrients in patients with percutaneous endoscopic gastrostomy. JPEN J Parenter Enteral Nutr. 2009;33:513–9. [DOI] [PubMed] [Google Scholar]
- 24.Shizuku T, Adachi K, Furuta K, Niigaki M, Miyaoka Y, Katoh S, et al. Efficacy of half-solid nutrient for the elderly patients with percutaneous endoscopic gastrostomy. J Clin Biochem Nutr. 2011;48:226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher K, Flint A, Mouzaki M, Carpenter A, Haliburton B, Bannister L, et al. Blenderized Enteral Nutrition Diet Study: Feasibility, Clinical, and Microbiome Outcomes of Providing Blenderized Feeds Through a Gastric Tube in a Medically Complex Pediatric Population. JPEN J Parenter Enteral Nutr 2018;42:1046–1060. [DOI] [PubMed] [Google Scholar]
- 26.Vanschoonbeek K, Lansink M, van Laere KM, Senden JM, Verdijk LB, van Loon LJ. Slowly digestible carbohydrate sources can be used to attenuate the postprandial glycemic response to the ingestion of diabetes-specific enteral formulas. Diabetes Educ. 2009;35:631–40. [DOI] [PubMed] [Google Scholar]
- 27.Mesejo A, Montejo-Gonzalez JC, Vaquerizo-Alonso C, Lobo-Tamer G, Zabarte-Martinez M, Herrero-Meseguer JI, et al. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: a prospective, open-label, blind-randomized, multicenter study. Critical Care. 2015;19:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. 2012;96:534–43. [DOI] [PubMed] [Google Scholar]
- 29.Arvaniti F, Priftis KN, Papadimitriou A, Papadopoulos M, Roma E, Kapsokefalou M, et al. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22:283–9. [DOI] [PubMed] [Google Scholar]
- 30.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients. 2017;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunology. 2017;6:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–66. [DOI] [PubMed] [Google Scholar]
- 36.Vieira MMC, Santos VFN, Bottoni A, Morais TB. Nutritional and microbiological quality of commercial and homemade blenderized whole food enteral diets for home-based enteral nutritional therapy in adults. Clinic Nutr 2018;37:177–81. [DOI] [PubMed] [Google Scholar]
- 37.Tanchoco CC, Castro CA, Villadolid MF, Casino G, Rodriguez MP, Roa C, et al. Enteral feeding in stable chronic obstructive pulmonary disease patients. Respirology. 2001;6:43–50. [DOI] [PubMed] [Google Scholar]
- 38.Pentiuk S, O’Flaherty T, Santoro K, Willging P, Kaul A. Pureed by gastrostomy tube diet improves gagging and retching in children with fundoplication. JPEN J Parenter Enteral Nutr. 2011;35:375–9. [DOI] [PubMed] [Google Scholar]