Abstract
Parental care is essential for the survival of offspring in altricial mammalian species. However, in most mammals, virgin females tend to avoid or attack infants. Moreover, most males demonstrate avoidance and aggression toward infants, and have little to no involvement in parental care. What mechanisms suppress avoidance, and support approach towards pups, to promote maternal care? In biparental and cooperatively breeding species, what mechanisms allow non-mothers (i.e. fathers and alloparents) to demonstrate parental care? In this review, we consider the mechanisms that subserve parental care in mothers, fathers, and others (i.e. alloparents). We emphasize recent discoveries and research trends with particular emphasis on neuroendocrinology, neuroplasticity, transcriptomics, and epigenetics. Finally, we consider outstanding questions and outline opportunities for future research.
Keywords: Mothers, Fathers, Alloparents, Plasticity, Transcriptomics, Epigenetics
Parenting Under the Skin
A combination of mothers, fathers, and alloparents (see Glossary) form the earliest social networks for developing offspring. Parenting offers an opportunity to provision for and safeguard offspring while concurrently preparing them for autonomous life. Parental care is a suite of offspring-directed behaviors demonstrated by adults (or sub-adult alloparents), which may have direct consequences for the survival, growth, and psychosocial development of offspring [1] [2]. However, the complete weight of parents on offspring development can be broadened to include non-behavioral means of influence (i.e. genetic or non-behaviorally transmitted epigenetic inheritance) [3] [4] [5] [See Figure 1].
Figure 1. Parental contributions to offspring development.
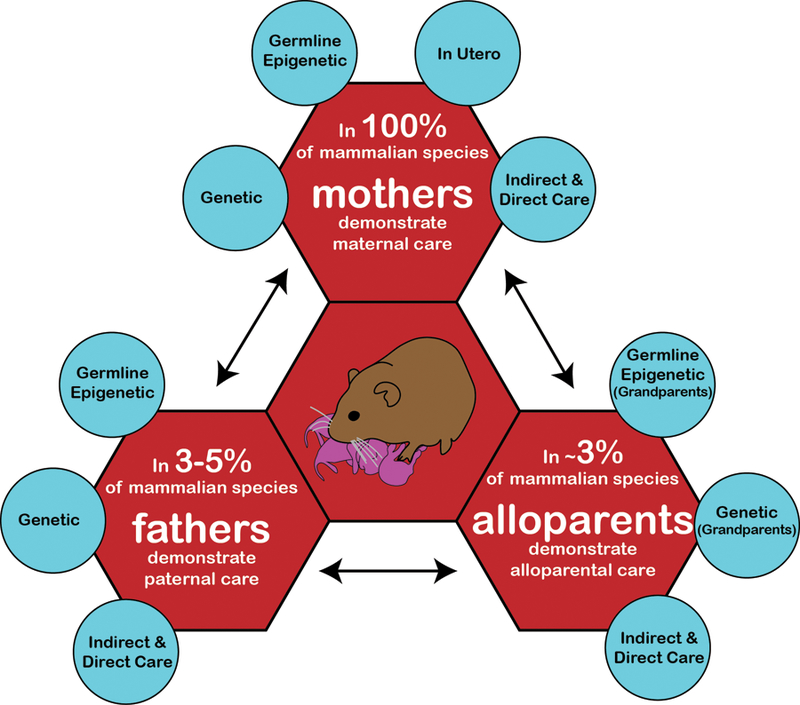
The means by which mothers, fathers, and alloparents respectively contribute to the development of offspring varies. Mothers are essential to offspring development and contribute to that development via a collection of direct and indirect routes, including genetics, transmission of life experience both epigenically at the level of the ova and in utero, as well as through parental behaviors. All mammalian fathers have the potential to contribute to offspring development through indirect genetic and germline epigenetic routes (via spermazoa), while only 3–5% of mammalian fathers demonstrate direct (e.g. grooming, contact) or indirect (e.g. defense) paternal care. Alloparental care is rare in mammals (approximately 3% of species), and the influence of alloparents varies. A unique class of alloparents are grandparents, where there is the potential to contribute to offspring development through indirect genetic and germline epigenetic routes, in addition to the influence, as in other alloparents, via direct and indirect parental care. In biparental species, mother-father interactions can mutually affect their respective parental contributions. Likewise, in cooperatively breeding species, this relationship is expanded to include mother-father, mother-alloparent, and father-alloparent exchanges.
Parents play an important role in offspring development; but what drives the offspringdirected behaviors of parents? What neuroendocrine events occur to produce what we observe as parental behavior? Answers to these questions have been primarily generated by the biobehavioral study of animal models in conjunction with a smaller number of laboratory studies in humans [6].
In this review, we highlight the proximate mechanisms that subserve parental care. We first outline the prevailing hypothesis that hormones associated with pregnancy facilitate maternal care by suppressing neural circuits associated with defense and avoidance while also promoting approach. We discuss how similar neuroendocrine mechanisms and similar neural circuitry associated with maternal care may have been repeatedly and independently coopted by several species to promote care by fathers and others (i.e. alloparents). We then move to consider recent research focused in the areas of neuroplasticity, transcriptomics, and epigenetics, in each section beginning with maternal care literature, and then extending the discussion (when evidence is available) to fathers and alloparents. Likewise, when possible, in each section we integrate findings across class Mammalia, from rodents to humans.
Mammals Vary in Parental Care According to Sex and Species
Amongst mammals, virgin females are typically averse to infant stimuli [7], and pregnancy primes females to readily demonstrate maternal behavior postpartum [7]. Hormones present during pregnancy and at parturition promote the production and let-down of breast milk, and they facilitate maternal approach and acceptance for offspring [7]. Males, which do not undergo pregnancy, often avoid infants and juveniles and may even commit infanticide under certain conditions. Yet, in cooperatively breeding mammalian species (e.g. prairie voles, humans), aversion to infant stimuli is not necessarily characteristic of virgin females nor of males, and pregnancy is not required to promote tolerance and/or active parental behavior [8] [9]. Given the central role of mothers in offspring development, the mechanisms that support maternal care have been well conserved across mammalian species. To the contrary, it is hypothesized that paternal care has evolved in multiple separate occurrences in approximately 35% of mammalian species, mainly among those that demonstrate monogamy [10]. Accordingly, one hypothesis is that the mechanisms that support parental care in males are simply the same mechanisms that support maternal care that have been repeatedly coopted by males in several species. An alternative hypothesis is that different mechanisms have evolved to support paternal behavior in each respective species.
Pregnancy Prepares Female Mammals for Maternal Care
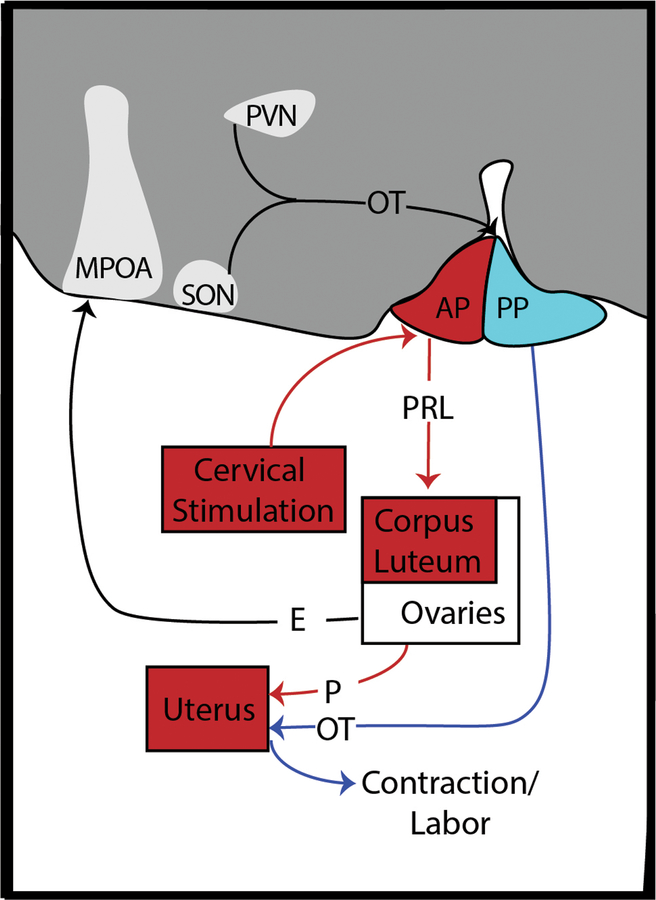
Pregnancy yields a cascade of hormones necessary for successful gestation and lactation (Figure 2). This cascade is organized in a complex series of events, triggered by sensory stimuli or endogenous cues, and it supports an effective progression of pregnancy and adoption of maternal-care behaviors. In some mammals, cervical stimulation during copulation causes prolactin (PRL) release from the anterior pituitary (AP) during early pregnancy and prevents degeneration of the corpus luteum (CL) [11]; in primates this is instead accomplished by the mid-cycle surge in luteinizing hormone (LH) secreted from the pituitary [12]. Progesterone (P) released from the CL prepares the uterine endometrium for implantation and maintains a uterine environment conducive to embryo growth [11]. Luteal secretions of P are later supported by placental lactogens to inhibit uterine contraction and maintain pregnancy [11]. Increasing P and PRL are also necessary for the development of mammary glands [13].
Figure 2. Pregnancy prepares mothers for parenthood.
Extensive work in laboratory rodents has demonstrated how pregnancy prepares mothers for parenthood through processes likely conserved across mammalian mothers. Early events in pregnancy (red lines) contribute to the successful gestation of offspring while also releasing hormones into circulation that reshape the maternal brain. Cervical stimulation during reproduction causes the subsequent release of PRL from the AP. PRL then maintains the corpus luteum, which in turn produces progesterone and insures successful gestation. Throughout pregnancy, E is released by the ovaries (black line) and restructures MPOA, which will later suppress avoidance of offspring cues while promoting approach and care behaviors. OT is produced in the PVN and SON and stored in the PP. In late pregnancy (blue lines), release of OT via the PP induces contraction and labor, while also allowing lactation. Abbreviations: AP = anterior pituitary; E = estradiol; MPOA = medial preoptic area; OT = oxytocin; P = progesterone; PP = posterior pituitary; PRL = prolactin; PVN = paraventricular nucleus of the hypothalamus; SON = supraoptic nucleus of the hypothalamus
Ovarian release of estradiol (E) increases across pregnancy, preparing the uterine endometrium for labor. Death of the CL and enzymatic conversion of P into E leads to a decline in P [14] [15], allowing oxytocin (OT) released from the posterior pituitary (PP) to induce uterine contraction and labor [11] and milk secretion [13]. Postpartum nipple stimulation causes PRL synthesis and release from the AP, which then binds to PRL receptors in the mammary gland to promote milk synthesis [13]. Contemporaneously, nipple stimulation triggers synchronized firing of magnocellular oxytocinergic neurons in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus that project to the PP, which in turn releases OT that binds to OT receptors on the mammary gland to allow milk let-down [13].
Neuroendocrine Factors Suppress Aggression and Promote Care in Mothers
The onset of maternal behavior is characterized by a positive appraisal of infant cues with an increased motivation to approach and care for offspring [7]. Maternal behavior requires the dampening of neural circuits that subserve avoidance and defense in conjunction with amplification of neural circuits associated with the acceptance of and attraction toward infant stimuli. In rodents, a central component of the former circuit is drawn from the olfactory bulb (OB), to the medial amygdala (MeA), then to the anterior hypothalamic nucleus (AHN), and finally to the periaqueductal gray (PAG) [7] [16] (Figure 3). It has been suggested that pregnancy hormones dampen this circuit, inhibiting maternal avoidance of offspring [7] via upstream action on the medial preoptic area (MPOA); i.e., the MPOA is restructured during pregnancy to then suppress activation of the avoidance circuit. The inhibition of defense circuitry can occur without pregnancy hormones through a process of repeated exposure to infant stimuli (i.e. sensitization) [7]; however, the process of sensitization occurs over several days, so its relevance to ethological settings, where offspring would typically starve during such a period in the absence of maternal nursing, remains unclear.
Figure 3. Avoidance and Defense Circuit in Response to Pup Cues.
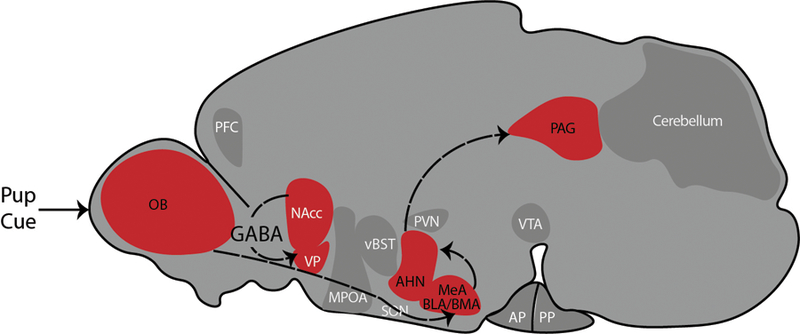
Circuit drawn in the sagittal orientation of a rodent brain, based on our synthesis of [7,16–18,76]. Virgin females detect pup cues, which then activates brain circuits which subserve avoidance and defense behaviors. A central circuit in this context is drawn from the OB, to the MeA, to the AHN and finally to the PAG. GABAergic projections from the NAcc to the VP also subserve avoidance and defense behaviors. Abbreviations: AHN = anterior hypothalamic nucleus; AP = anterior pituitary; BLA = basolateral amygdala; BMA = basomedial amygdala; MeA = medial amygdala; MPOA = medial preoptic area; NAcc = nucleus accumbens; OB = olfactory bulb; PAG = periaqueductal gray; PFC = prefrontal cortex; PP = posterior pituitary; PVN = paraventricular nucleus of the hypothalamus; SON = supraoptic nucleus of the hypothalamus; vBST = ventral bed nucleus of the stria terminalis; VP = ventral pallidum; VTA = ventral tegmental area
The MPOA is central to the acceptance of infant stimuli and attraction to them. Numan and Stolzenberg [17] proposed that the action of E, PRL, and OT at the MPOA restructures neurons and subserves maternal behavior by both dampening the avoidance/defense circuit and activating an attraction/acceptance circuit (Figure 4). The proposed circuit for approach begins with innervation of the mesolimbic dopamine (DA) system by the MPOA [17][18]. Thus, the MPOA and adjacent ventral bed nucleus of the stria terminalis (vBST) receive offspring cues and activate ventral tegmental area (VTA) DA signaling to the nucleus accumbens (NAcc), a connection associated with motivation and approach to reward related stimuli. Without DA signaling to the NAcc, GABAergic inhibition from the NAcc to the ventral pallidum (VP) subsequently inhibits approach; inversely, when DA signaling from the VTA to the NAcc does occur, the GABAergic inhibition of the NAcc on the VP is released, allowing for approach. The NAcc and VP simultaneously receive input from the basolateral/basomedial amygdala (BLA-BMA) and the prefrontal cortex (PFC), which respond to infant stimuli and lend valence (positive or negative) [17,18].
Figure 4. Maternal Care Circuitry.
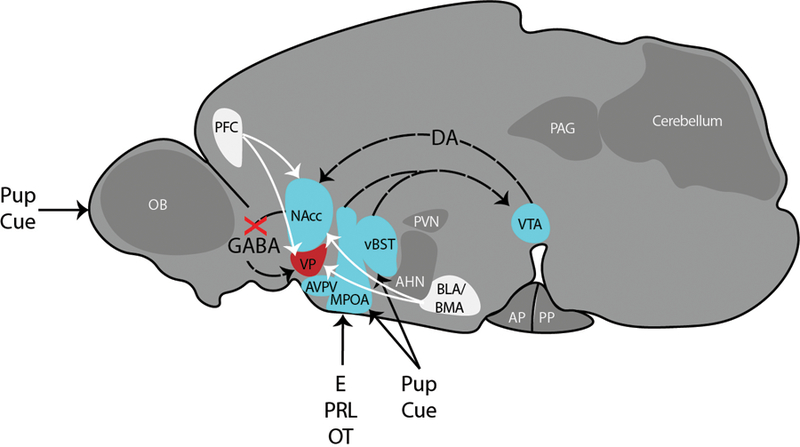
Circuit drawn in the sagittal orientation of a rodent brain based on our synthesis of [17,18,20]. Hormones of pregnancy restructure the MPOA, which along with the vBST receives inputs informing about offspring cues. The MPOA and vBST project to VTA, which in turn sends dopaminergic projections to the NAcc. In the maternal state, the GABAergic projections from the NAcc to the VP are inhibited, preventing avoidance and defense behaviors. The NAcc and VP also receive cues (white lines) of valence (both positive and negative) from the PFC and BLA-BMA. Maternal care is also supported by Tyrosine hydroxylase (TH)-expressing neurons in the AVPV. Abbreviations: AHN = anterior hypothalamic nucleus; AP = anterior pituitary; AVPV = anteroventral periventricular nucleus; BLA = basolateral amygdala; BMA = basomedial amygdala; DA = dopamine; E = estradiol; MeA = medial amygdala; MPOA = medial preoptic area; Nacc = nucleus accumbens; OB = olfactory bulb; OT = oxytocin; P = progesterone; PAG = periaqueductal gray; PFC = prefrontal cortex; PP = posterior pituitary; PRL = prolactin; PVN = paraventricular nucleus of the hypothalamus; SON = supraoptic nucleus of the hypothalamus; vBST = ventral bed nucleus of the stria terminalis; VP = ventral pallidum; VTA = ventral tegmental area
Neural activation in the VTA and NAcc in response to pup stimuli (demonstrated by c-Fos expression) is greater for lactating female mice than naïve females, and lactating females show less activation in the extended bed nucleus of the stria terminalis (BST) [19]. There is some evidence supporting that GABAergic modulation via the MPOA likely explains the suppression of BST activity in lactating female mice; that is, GABAergic projections from the MPOA to the BST may ultimately inhibit signaling from glutamatergic neurons to the VTA (a connection associated with avoidance), thus inhibiting an avoidance response toward pups [19]. Maternal care is also supported by tyrosine hydroxylase (TH)-expressing neurons in the anteroventral periventricular nucleus (AVPV) of the hypothalamus, as their ablation or optogenetic stimulation impair or promote maternal care, respectively [20]. The same actions of ablation or optogenetic stimulation of AVPV TH+ neurons also reduce or increase OT circulation, respectively [20]. Oxytocin in turn increases the salience of pup auditory cues in maternal, but not naïve female mice through action in the left auditory cortex [21]. Within the MPOA, activation of corticotropin-releasing factor receptor subtype 1 (CRFR1) reduces arched back nursing in lactating Sprague-Dawley rats, while the inhibition of CRFR1 increases maternal aggression toward a virgin female intruder (i.e. supports maternal defense of pups) [22]. Likewise, arginine vasopressin receptor 1b (V1bR) antagonism in the MPOA increases pup retrieval behavior, while antagonism in the medial posterior BST decreases licking and grooming of pups in lactating rats [23].
Neuroendocrine Mediation of Paternal Care
While maternal care is central to mammals, paternal care occurs naturally in only a small subset (3–5%) of mammalian species that are socially monogamous and/or cooperatively breeding [10], although suppression of the avoidance circuit and promotion of attraction toward infant stimuli may be promoted through sensitization also in males of species that are typically not paternal [24]. Much of the work on paternal care in animals has been completed in socially monogamous rodent species that are consistently biparental in the wild (e.g. prairie voles, California mice, etc.) [24]. Unlike mothers, fathers do not experience the cascade of hormones associated with pregnancy; however, some recent evidence suggests that areas of the brain associated with maternal care (e.g. the MPOA) are likely recruited and supported by endogenous hormones and neuropeptides to subserve paternal behavior [24][25] (see figure 5). Rosenblatt and colleagues established that lesion of the MPOA inhibits male sensitization to pups in Norway rats [26], and subsequent studies in rats as well as biparental prairie voles and California mice demonstrate that the MPOA, as well as the OB, BST, and extended amygdala are involved in paternal care [24]. The sensation of pup cues via the vomeronasal organ (VNO) is associated with pup-directed aggression in naïve male mice, but not in fathers, suggesting that downregulation of the VNO system is necessary for rodent paternal behavior [27]. As in females, GABAergic projections from the MPOA to the BST inhibit infanticide in male mice [28]. Hormones and neurotransmitters, including (but not limited to) OT, arginine vasopressin (AVP), corticosterone (CORT), E, testosterone (T), and PRL, also play a role in the organization and onset of male parenting in many rodent species [24]. There is some evidence that a similar suite of hormones supports paternal care in human and non-human primate species [29]. Recent evidence in mice suggests that the neuropeptide galanin (GAL) plays a critical role in male parenting, as the ablation of MPOA GAL neurons suppresses parental care in both females and males [30].
Figure 5. Paternal Care Circuitry.
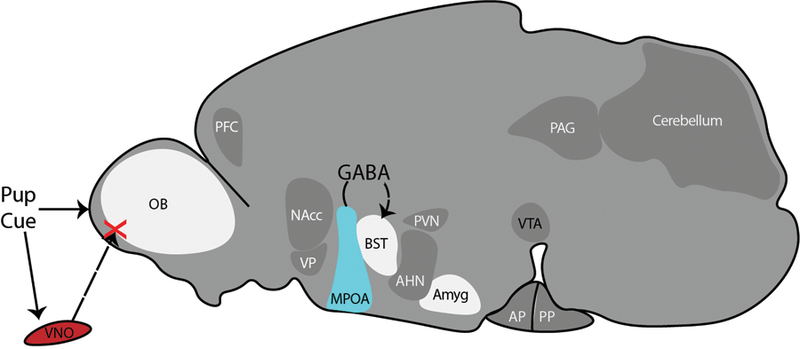
Circuit drawn in the sagittal orientation of a rodent brain based on our synthesis of [22,30,77–79]. Brain regions associated with paternal care include the OB, the BST, MPOA, and the extended amygdala. Inhibition of VNO signaling to the OB dampens paternal avoidance and defense. GABAergic signaling between the MPOA and BST, and the MPOA more generally, are associated with dampening of avoidance and promotion of paternal care. Abbreviations: AHN = anterior hypothalamic nucleus; Amgy = extended amygdala; AP = anterior pituitary; AVPV = anteroventral periventricular nucleus; BLA = basolateral amygdala; BMA = basomedial amygdala; BST = extended bed nucleus of the stria terminalis; MeA = medial amygdala; MPOA = medial preoptic area; Nacc = nucleus accumbens; OB = olfactory bulb; PAG = periaqueductal gray; PFC = prefrontal cortex; PP = posterior pituitary; PVN = paraventricular nucleus of the hypothalamus; SON = supraoptic nucleus of the hypothalamus; vBST = ventral bed nucleus of the stria terminalis; VNO = vomeronasal organ; VP = ventral pallidum; VTA = ventral tegmental area
In human studies, there is evidence for the involvement of the insula and the dopaminergic system in paternal behavior [31], perhaps respectively reflecting human fathers’ consideration of the needs of their children, and their motivational drive for this consideration. Another study of fathers demonstrated that infant cries activated the inferior frontal gyrus (IFG), which extends into the anterior insula (AI) [32]. The IFG-AI connection is involved in attentional and working memory processes and is involved in adapting to unpredictable environmental conditions [33]. AI activity in the right hemisphere is also negatively correlated with maternal report of paternal restrictiveness [32].
Some recent work in humans has focused on the influence of hormones (e.g. OT, AVP), traditionally associated with parenting, on brain activity in parents [34]. Intranasal OT has been used as a manipulation in fathers, and has been shown to increase activity in the caudate and visual cortex [35]. Intranasal AVP has shown rather minimal effects on brain activity in both new fathers [36] and expectant ones [35,36].
Neuroendocrine Mediation of Alloparental Care
Alloparental care is demonstrated in a small percentage of mammals (about 3%), including humans [8][9]. Most work considering the neurobiology of alloparental care has been done in rodents (e.g. prairie voles, dwarf hamsters) and non-human primates (e.g. marmosets, tamarins, and titi monkeys) [8][9]. Across many species, there is a positive association between OT receptor expression in the NAcc and alloparental behavior [9]. There are mixed findings with regard to the relationship between alloparental care and T, E, glucocorticoids, and PRL; and the respective effect of these hormones is likely dependent on species, age, sex, and reproductive status [9].
Only a small number of human studies have focused on neural or neuroendocrine aspects of caregiving by non-parents. In a study of foster mothers, rise in urinary OT during a cuddle interaction with the foster infant was correlated with experimenter coding of maternal delight while playing with her foster infant, as well as with the maternal parietally maximal P3 response in reaction to a photo of their foster infant [37]. This relationship became more personalized to the foster infant (as opposed to other infants) over the course of the study [37]. Despite the clear societal importance of alloparents [38], and a call to extend research on OT and the parental brain to grandparents [39], we are not aware of any other empirical studies on the topic at this time.
Pregnancy and Parenthood Reshape Neural Structures and Function
The transition into parenthood is associated with extensive changes in affect, behavior and cognition. This transition is associated with increased positive affect and emotions in some human and animal mothers, but increased depression and anxiety in others (see Agrati & Lonstein, 2016 for review) [40].
Cognition can be extensively altered during the transition from the non-maternal to the maternal state, particularly as it relates to memory formation [41]. Many of these cognitive effects are reflected in the hippocampus [41]. Maternal experience in rats was recently demonstrated to increase immature neuron density within the hippocampus and to enhance acquisition of working memory [42]. Maternity may also reduce CORT reactivity to stressors in female rats, while also reducing neurogenesis in the dentate gyrus (DG) [43]. There is significant change in the auditory nerve and cochlear nucleus during the transition into maternity, which translates into faster detection of auditory stimuli (e.g. pup vocalizations) in maternal mice than in naïve conspecifics [44]. Similarly, maternal mice demonstrate stronger responses to behaviorally relevant odors supported by functional changes in the OB [45].
Research in humans has also identified pregnancy-induced changes in maternal brain activity [46]. As in rodent mothers, motherhood in humans is associated with enhanced responsiveness to infant auditory cues, and many human studies have focused on neural responses to infant cries. Generally, new mothers have enhanced responsiveness to infant auditory cues in neural areas associated with reward, social information or salience, and emotion regulation [47,48].
Other recent work in human mothers has focused on broader cognitive changes due to pregnancy and postpartum, rather than exclusively those associated with infant interactions [48]. In many cases, the changes that have been reported reflect reduced cognitive abilities [49]. While the rodent literature often reports improvements in spatial memory in new mothers [50], these data contrast with the commonly held idea of “pregnancy brain” or “mom brain” in humans, in which many new mothers report cognitive impairment. Meta-analyses have concluded that there are small but significant memory impairments for pregnant and postpartum women including recall and naturalistic prospective memory, and that these are more likely as pregnancy progresses [51]. This progressing cognitive change may be even more pronounced in mothers of children with disabilities [52]. The mechanisms for these changes may include reductions in gray matter, primarily in areas along the midline, including cingulate, prefrontal and temporal cortex [53]. Notably, these same changes were not seen in fathers [53].
Additional mechanisms for maternal changes in cognition and memory seem to point to involvement of metabolic hormones. A new body of work in human mothers shows amplified cognitive changes in women who experience gestational diabetes [54], with potential candidate mediators including insulin-signaling pathways or hormones like leptin which has been shown to affect hippocampal signaling in the offspring [55].
Less is known about the neuroplasticity associated with paternity, even in animal models. In male California mice, fathers showed lower anxiety-like behavior in the elevated-plus maze than non-fathers, but only during the period of highest interactions with pups; moreover, fathers maintained more new neurons in the DG compared to non-fathers [56]. Further study of California mouse fathers demonstrated an up-regulation of estrogen receptor beta in the hippocampus [57].
Variation in Gene Expression Drives Differential Expression of Parental Care
The advent of techniques such as microarrays and next generation sequencing has allowed for wide-ranging exploration of gene expression changes due to parenting, in both the maternal and paternal brain.
In a series of studies, Gammie and colleagues used microarrays to study gene expression changes in outbread hsd:ICR mouse mothers (typically seven days postpartum) as compared to virgin females [58][59]. In the MPOA, microarray results were enriched for genes associated with mental health disorders involving social deficits, such as schizophrenia and bipolar disorder [58]. In the lateral septum (LS), differentially expressed genes included those in reward and neural plasticity pathways [59].
Gammie and colleagues synthesized their studies, finding that the top 700 genes which changed in postpartum maternal brain had also been identified in pathways for several processes including social bonding and reward; addiction, depression and other mental health disorders; as well as central nervous system plasticity and development [60]. A particularly interesting analysis of transcriptional regulators and miRNAs suggested that maternal miRNAs may migrate across multiple brain regions [61]. In addition, strong evidence was found for involvement of the gene Nr1d1 in the maternal postpartum brain; Nr1d1 is associated with addiction processes and other mental health outcomes[62].
Another research group used RNA-Seq to examine gene expression changes in a number of brain regions (hypothalamus, hippocampus, neocortex, and cerebellum) [63] in mouse females at day 14 and 16 prepartum, as well as days 1, 3, and 10 postpartum, as compared to virgin females. Similarly to the studies from Gammie’s group, a number of genes showed correlated changes across all four areas. The pathways enriched in all four brain regions included map kinase pathways, focal adhesion, metabolic pathways, and neuroactive ligand-receptor interaction.
The paternal brain has been primarily studied in non-traditional laboratory animals. Prairie voles are socially monogamous, biparental rodents [64]. Seelke and colleagues examined differential gene expression in the MPOA between age-matched virgin males, paired males (males that had mated but were not yet parents), and fathers [65]. Fathers showed lower gene expression of many plasticity-related genes, including genes in the Ras1-Rap pathway, as well as genes associated with NMDA receptor subunits.
Collectively, transcriptomic studies of the parental brain have led to new specific targets and systems of interest. They have highlighted overlap with other, non-social processes. Conspicuously missing, however, are gene expression studies of alloparents, and of gene expression in brain areas other than the MPOA in fathers.
Epigenetic Mechanisms Connect Life Experience and Subsequent Parental Behavior
The inhibition of defense circuitry associated with pup exposure can also be achieved in the absence of pregnancy hormones, through sensitization, as discussed above. The field of epigenetics has offered an additional approach by which to explore how non-hormonal mechanisms subserve parental behavior; i.e. how molecular mechanisms driving chromatin remodeling can subsequently influence gene expression and the neurobiological substrates of parental care [11]. In a series of studies by Stolzenberg and colleagues, it was demonstrated that histone deacetylase inhibition in the context of repeated pup exposure induces significant and persistent gene expression in the MPOA that subserves the rapid onset of maternal behavior in female mice, even in the absence of E [18,66–68].
An epigenetic approach has the potential to elucidate the molecular mechanisms underlying the developmental origins of variation in parental behavior; i.e. how life experiences shape varying parental phenotypes [11]. The early life experience of parenting, for example, can modify how one parents later in life. For example, recent work by Peña and colleagues illustrate the molecular pathways by which mothers influence maternal care in subsequent generations [69]. Epigenetics can also help explain more passive pathways of parental influence. The experiences of mothers during pregnancy (e.g. environmental stress or trauma) have the potential to shape offspring development, for example increasing their likelihood of developing metabolic disorders or affective disorders like PTSD, anxiety, and depression [70,71]. Moreover, the experiences that mothers have prior to offspring conception also have the potential to similarly alter offspring development [70].
Concluding Remarks and Future Perspectives
Maternal care is essential to the development of mammalian offspring, and in many species the care of fathers and alloparents is also critical. The research presented here has primarily been conducted in rodents, and while there is reason to believe the neural substrates of maternal behavior should generalize well across species, direct evidence speaking to this point is limited, particularly when it comes to humans. Research aimed to address this knowledge gap would be invaluable to our understanding of human parenting behavior. It will also be crucial for elucidating mental disorders related to the maternal transition, such as postpartum depression, which often cannot be modeled effectively in rodents.
Most of our understanding of the parental brain is inferred from research conducted in mothers alone. Accordingly, the neural substrates of paternal and alloparental care remains substantially understudied. In human society, fathers and alloparents often play key roles in the upbringing of children, and subsequently their affective, behavioral and cognitive development. Another gap that should be acknowledged is that with some exceptions [72], there is little research into parental care that includes LGBT+ parents.
When considering the trajectory of the field for coming years, it is useful to note that parenting occurs in the context of both endogenous (e.g. hormonal) and exogenous (e.g. social) factors. Regarding the former, increased attention is being paid to interactions between the brain and other aspects of maternal physiology, such as the immune system [73] and the placenta [74], although these cutting-edge topics are particularly under-represented in human research. Regarding the latter, there is a need to expand our understanding of inter-parental social dynamics, and how those dynamics influence parental behavior and respective neurobiology [75].
Outstanding Questions.
How well do neural substrates of maternal care in rodent models generalize to humans, and how can that inform the treatment of mental disorders associated with pregnancy and parenthood (e.g. postpartum depression)?
How well to the neural substrates of maternal care generalize to those of paternal and alloparental care?
How is it that in cooperatively breeding species (e.g. prairie voles, humans), a process of sensitization is not required for non-mothers to tolerate and demonstrate care toward infant stimuli? How can intra- and inter-species comparisons help elucidate this question?
What is the full extent of the relationship between the medial preoptic area (MPOA) and the bed nucleus of the stria terminalis (BST)? How does that relationship affect other brain areas and neural / behavioral processes downstream, like the ventral tegmental area (VTA)?
What components of peripheral physiology (e.g. the gut, the immune system, etc.) interact with the nervous system to shape or modify parental behavior?
How do accumulated parental experience and inter-parental social dynamics influence parental care behavior and its neural substrates?
What are adjustments to research practices are needed to make future research more inclusive and better representative of the full diversity of human and non-human social systems?
Highlights.
Many aspects of the neural substrates of maternal care are well conserved across mammalian species. Much of the maternal care circuitry can be organized into two opposing circuits, mediating avoidance/defense vs. approach towards offspring cues, and transition to maternal-care behavior is characterized by suppression of the avoidance/defense arm of this circuitry.
Paternal and alloparental care, which evolved independently in multiple mammalian species, are likely subserved by neural substrates coopted from the maternal brain.
Traditional neuroendocrine approaches, combined with newer transcriptomic and epigenetic methods have deepened our understanding of the neural substrates of parenting and the neural plasticity associated with the transition into parenthood.
Acknowledgement
FDR was supported by the Bay Area Pre-doctoral Training Consortium in Affective Science (NIH 5T32MH020006–20) at the time this manuscript was written. Work in the laboratory of KLB is supported by NIH OD011107, NIH HD092055, NIH MH108319, NIH MH115680, and the Good Nature Institute.
Glossary
- Alloparent
an individual that is not a genetic parent (i.e. biological mother or father), who demonstrates parental behavior toward an immature conspecific.
- Epigenetics
the field of research concerned with the molecular processes that alter gene expression without the modification of the genome itself.
- Neuroendocrine
related to the field of research concerned with the interactions between the nervous and endocrine systems.
- Neuroplasticity
the process of the remodeling of neural substrates and their function.
- Transcriptomics
the field of research concerned with the set of RNA transcripts produced by the genome (i.e. the transcriptome), often with the specific aim of determining sources of differential gene expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkeybile AM and Bales KL (2017) Intergenerational transmission of sociality: The role of parents in shaping social behavior in monogamous and non-monogamous species. J. Exp. Biol 220, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curley JP and Champagne FA (2016) Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol 40, 52–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinnally EL and Capitanio JP (2015) Paternal early experiences influence infant development through non-social mechanisms in rhesus macaques. Front. Zool 12, S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dias BG and Ressler KJ (2014) Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci 17, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun K and Champagne FA (2014) Paternal influences on offspring development: Behavioural and epigenetic pathways. J. Neuroendocrinol 26, 697–706 [DOI] [PubMed] [Google Scholar]
- 6.Bales KL (2017) Parenting in animals. Curr. Opin. Psychol 15, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Numan M (2012) Maternal Behavior: Neural Circuits, Stimulus Valence, and Motivational Processes. Parenting 12, 105–114 [Google Scholar]
- 8.Hrdy SB (2016) Variable postpartum responsiveness among humans and other primates with “cooperative breeding”: A comparative and evolutionary perspective. Horm. Behav 77, 272–283 [DOI] [PubMed] [Google Scholar]
- 9.Kenkel WM et al. (2017) The Neurobiological Causes and Effects of Alloparenting. Dev Neurobiol 77, 214–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiman DG (1977) Monogamy in Mammals. Q. Rev. Biol 52, 39–69 [DOI] [PubMed] [Google Scholar]
- 11.Stolzenberg DS and Champagne FA (2016) Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Horm. Behav 77, 204–210 [DOI] [PubMed] [Google Scholar]
- 12.Stouffer RL et al. (2013) Endocrine and local control of the primate corpus luteum. Reprod. Biol 13, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas W and Woodside B (2016) Physiological mechanisms, behavioral and psychological factors influencing the transfer of milk from mothers to their young. Horm. Behav 77, 167–181 [DOI] [PubMed] [Google Scholar]
- 14.Ravanos K et al. (2015) Factors implicated in the initiation of human parturition in term and preterm labor: A review. Gynecol. Endocrinol 31, 679–683 [DOI] [PubMed] [Google Scholar]
- 15.Zakar T and Hertelendy F (2007) Progesterone withdrawal: key to parturition. Am. J. Obstet. Gynecol 196, 289–296 [DOI] [PubMed] [Google Scholar]
- 16.Bridges RS (2015) Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol 36, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numan M and Stolzenberg DS (2009) Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front. Neuroendocrinol 30, 46–64 [DOI] [PubMed] [Google Scholar]
- 18.Stolzenberg DS and Numan M (2011) Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev 35, 826–847 [DOI] [PubMed] [Google Scholar]
- 19.Matsushita N et al. (2015) Comparison of c-Fos expression in brain regions involved in maternal behavior of virgin and lactating female mice. Neurosci. Lett 590, 166–171 [DOI] [PubMed] [Google Scholar]
- 20.Scott N et al. (2015) A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–522 [DOI] [PubMed] [Google Scholar]
- 21.Marlin BJ et al. (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klampfl SM et al. (2018) Maternal stress and the MPOA: Activation of CRF receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology 133, 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayerl DS et al. (2016) Antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats. Horm. Behav 79, 18–27 [DOI] [PubMed] [Google Scholar]
- 24.Bales KL and Saltzman W (2016) Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm. Behav 77, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saltzman W and Ziegler TE (2014) Functional Significance of Hormonal Changes in Mammalian Fathers. J. Neuroendocrinol 26, 685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblatt JS et al. (1996) Maternal behavior in male rats: Effects of medial preoptic area lesions and presence of maternal aggression. Horm. Behav 30, 201–215 [DOI] [PubMed] [Google Scholar]
- 27.Tachikawa KS et al. (2013) Behavioral transition from attack to parenting in male mice: A crucial role of the vomeronasal system. Ann. Intern. Med 158, 5120–5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuneoka Y et al. (2015) Distinct preoptic-BST nuclei dissociate paternal and infanticidal behavior in mice. EMBO J 34, 2652–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storey AE and Ziegler TE (2016) Primate paternal care: Interactions between biology and social experience. Horm. Behav 77, 260–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z et al. (2014) Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rilling JK and Mascaro JS (2017) The neurobiology of fatherhood. Curr. Opin. Psychol 15, 26–32 [DOI] [PubMed] [Google Scholar]
- 32.Mascaro JS et al. (2014) Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc. Cogn. Affect. Neurosci 9, 1704–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tops M and Boksem MAS (2011) A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials 2, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S and Strathearn L Oxytocin and Maternal Brain Plasticity., New Directions for Child and Adolescent Development (2016) [DOI] [PMC free article] [PubMed]
- 35.Li T et al. (2017) Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Horm. Behav 93, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T et al. (2018) Explaining individual variation in paternal brain responses to infant cries. Physiol. Behav 193, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bick J et al. (2013) Foster Mother-Infant Bonding: Associations Between Foster Mothers’ Oxytocin Production, Electrophysiological Brain Activity, Feelings of Commitment, and Caregiving Quality. Child Dev 84, 826–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrdy SB (2017) Cooperative Breeding and the Paradox of Facultative Fathering DOI: 10.1016/B978-012374285-8.00026-3 [DOI]
- 39.Huffmeijer R et al. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations - A mini-review., Gerontology (2012) [DOI] [PubMed]
- 40.Agrati D and Lonstein JS (2016) Affective changes during the postpartum period: Influences of genetic and experiential factors. Horm. Behav 77, 141–152 [DOI] [PubMed] [Google Scholar]
- 41.Pawluski JL et al. (2016) Neuroplasticity in the maternal hippocampus: relation to cognition and effects of repeated stress. Horm. Behav 77, 86–97 [DOI] [PubMed] [Google Scholar]
- 42.Barha CK et al. (2015) Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol. Aging 36, 2391–2405 [DOI] [PubMed] [Google Scholar]
- 43.Workman JL et al. (2016) Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis. Neuropharmacology 105, 443–453 [DOI] [PubMed] [Google Scholar]
- 44.Miranda JA et al. (2014) Adult plasticity in the subcortical auditory pathway of the maternal mouse. PLoS One 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinograd A et al. (2017) Functional Plasticity of Odor Representations during Motherhood. Cell Rep 21, 351–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman R (2017) The Neurobiology of Human Attachments. Trends Cogn. Sci 21, 80–99 [DOI] [PubMed] [Google Scholar]
- 47.Kim P Human Maternal Brain Plasticity: Adaptation to Parenting., New Directions for Child and Adolescent Development (2016) [DOI] [PMC free article] [PubMed]
- 48.Barba-Müller E et al. (2018) Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Arch. Womens. Ment. Health DOI: 10.1007/s00737-018-0889-z [DOI] [PMC free article] [PubMed]
- 49.Shin N-Y et al. (2018) Disturbed retrieval network and prospective memory decline in postpartum women. Sci. Rep 8, 5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawluski JL et al. (2016) Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav 77, 86–97 [DOI] [PubMed] [Google Scholar]
- 51.Anderson MV and Rutherford MD (2012) Cognitive reorganization during pregnancy and the postpartum period: An evolutionary perspective. Evol. Psychol 10, 659–687 [PubMed] [Google Scholar]
- 52.Song J et al. (2016) Cognitive aging in parents of children with disabilities. Journals Gerontol. Psychol. Sci 71, 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoekzema E et al. (2017) Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci 20, 287–296 [DOI] [PubMed] [Google Scholar]
- 54.John C et al. (2018) Maternal Cognitive Impairment Associated with Gestational Diabetes Mellitus—A Review of Potential Contributing Mechanisms. Int. J. Mol. Sci 19, 3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumon C et al. (2018) The adipocyte hormone leptin sets the emergence of hippocampal inhibition in mice. Elife 7, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyer MM et al. (2016) Neurogenesis and anxiety-like behavior in male California mice during the mate’s postpartum period. Eur. J. Neurosci 43, 703–709 [DOI] [PubMed] [Google Scholar]
- 57.Hyer MM et al. (2017) Estrogen-dependent modifications to hippocampal plasticity in paternal California mice (Peromyscus californicus). Horm. Behav 96, 147–155 [DOI] [PubMed] [Google Scholar]
- 58.Driessen TM et al. (2014) Genes showing altered expression in the medial preoptic area in the highly social maternal phenotype are related to autism and other disorders with social deficits. BMC Neurosci 15, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisinger BE et al. (2013) Large Scale Expression Changes of Genes Related to Neuronal Signaling and Developmental Processes Found in Lateral Septum of Postpartum Outbred Mice. PLoS One 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gammie SC et al. (2016) Genetic and neuroendocrine regulation of the postpartum brain. Front. Neuroendocrinol 42, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saul MC et al. (2016) MicroRNA expression is altered in lateral septum across reproductive stages. Neuroscience 312, 130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao C et al. (2014) Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens 8, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray S et al. (2016) An examination of dynamic gene expression changes in the mouse brain during pregnancy and the postpartum period. G3 6, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Getz LL et al. (1981) The mating system of the prarie vole Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav. Ecol. Sociobiol 8, 189–194 [Google Scholar]
- 65.Seelke AMH et al. (2018) Fatherhood alters gene expression within the MPOA. Environ. Epigenetics [DOI] [PMC free article] [PubMed]
- 66.Mayer HS et al. (2018) Histone deacetylase inhibitor treatment induces postpartum-like maternal behavior and immediate early gene expression in the maternal neural pathway in virgin mice. Horm. Behav DOI: 10.1016/j.yhbeh.2018.02.011 [DOI] [PMC free article] [PubMed]
- 67.Stolzenberg DS et al. (2014) Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology 155, 3674–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stolzenberg DS et al. (2012) Experience-facilitated improvements in pup retrieval; evidence for an epigenetic effect. Horm. Behav 62, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peña CJ et al. (2013) Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology 154, 4340–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yehuda R and Lehrner A (2018) Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry 17, 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Susser ES et al. (1998) Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. Am. J. Epidemiol 147, 213–216 [DOI] [PubMed] [Google Scholar]
- 72.Abraham E et al. (2014) Father’s brain is sensitive to childcare experiences. Proc. Natl. Acad. Sci. U. S. A 111, 9792–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haim A et al. (2017) A survey of neuroimmune changes in pregnant and postpartum female rats. Brain. Behav. Immun 59, 67–78 [DOI] [PubMed] [Google Scholar]
- 74.Creeth HDJ et al. (2018) Imprinted genes influencing the quality of maternal care. Front. Neuroendocrinol DOI: 10.1016/j.yfrne.2018.12.003 [DOI] [PubMed]
- 75.Rogers FD et al. (2018) Longitudinal Trajectories and Inter-parental Dynamics of Prairie Vole Biparental Care. Front. Ecol. Evol 6, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abraham E and Feldman R (2018) The neurobiology of human allomaternal care; implications for fathering, coparenting, and children’s social development. Physiol. Behav [DOI] [PubMed]
- 77.Hrdy SB (2009) Meet the alloparents. In Mothers and Others: The Evolutionary Origins of Mutual Understanding
- 78.Kohl J et al. (2017) The neurobiology of parenting: A neural circuit perspective. BioEssays 39, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bales KL et al. Social touch during development: Long-term effects on brain and behavior., Neuroscience and Biobehavioral Reviews (2018) [DOI] [PubMed]