Abstract
Bile duct epithelial cells, also known as cholangiocytes, regulate the composition of bile and its flow. Acquired, congenital and genetic dysfunctions in these cells give rise to a set of diverse and complex diseases, often of unknown aetiology, called cholangiopathies. New knowledge has been steadily acquired about genetic and congenital cholangiopathies, and this has led to a better understanding of the mechanisms of acquired cholangiopathies. This Review focuses on findings from studies on Alagille syndrome, polycystic liver diseases, fibropolycystic liver diseases (Caroli disease and congenital hepatic fibrosis) and cystic fibrosis-related liver disease. In particular, knowledge on the role of Notch signalling in biliary repair and tubulogenesis has been advanced by work on Alagille syndrome, and investigations in polycystic liver diseases have highlighted the role of primary cilia in biliary pathophysiology and the concept of biliary angiogenic signalling and its role in cyst growth and biliary repair. In fibropolycystic liver disease, research has shown that loss of fibrocystin generates a signalling cascade that increases β-catenin signalling, activates the NOD-, LRR- and pyrin domain-containing 3 inflammasome, and promotes production of IL-1β and other chemokines that attract macrophages and orchestrate the process of pericystic and portal fibrosis, which are the main mechanisms of progression in cholangiopathies. In cystic fibrosis-related liver disease, lack of cystic fibrosis transmembrane conductance regulator increases the sensitivity of epithelial Toll-like receptor 4 that sustains the secretion of nuclear factor-κB-dependent cytokines and peribiliary inflammation in response to gut-derived products, providing a model for primary sclerosing cholangitis. These signalling mechanisms may be targeted therapeutically and they offer a possibility for the development of novel treatments for acquired cholangiopathies.
The bile duct epithelial cells, also known as cholangiocytes, are essential in multiple physiological liver processes, including the fine regulation of bile composition and its flow1. The biliary tree is also affected by a group of diseases called cholangiopathies. Most acquired cholangiopathies are complex diseases of unknown aetiology and pathogenesis, such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC). A proportion of cholangiopathies are caused by inherited or genetically mediated dysfunction of cholangiocytes that progresses chronically, leading to end-stage liver diseases such as congenital hepatic fibrosis and cystic fibrosis-related liver disease2,3. Cholangiopathies are rare, but, as a group, they account for up to 20% of liver transplantations in the adult population4 and up to 80% in the paediatric population5. These diseases are complex, and their management usually requires referral to centres of excellence, where clinical expertise is combined with translational research. Treatment options are limited and, besides bile acids, farnesoid X receptor agonists, steroids and liver transplantation, little can be offered to individuals with these diseases at this time. However, new knowledge has been steadily acquired over the past 20 years that will hopefully translate into novel treatments in the near future.
The lack of reliable experimental models has hampered translational research of acquired cholangiopathies. However, the genetic cholangiopathies can be addressed with translational methods owing to the availability of animal and cellular models that phenocopy the disease. These approaches have clarified the functions of the defective genes, improved understanding of important and novel pathobiological concepts and resulted in innovative experimental therapeutic strategies. In addition, the mechanisms uncovered are often relevant for acquired cholangiopathies and, more broadly, for chronic liver diseases. Thus, the genetic cholangiopathies can be viewed as a pathophysiological roadmap for cholangiopathies and chronic liver diseases in general. Among the genetic and congenital cholangiopathies, this Review focuses on examples of monogenic conditions, including Alagille syndrome, polycystic and fibropolycystic liver diseases, and cystic fibrosis-related liver disease. These diseases are associated with changes in major signalling mechanisms that may have the potential to be targeted. We place these new mechanisms in the context of pathobiological mechanisms common to all cholangiopathies and discuss future directions in basic research and potential clinical translation. We do not discuss familial progressive intrahepatic cholestasis type 3 (ABCB4 deficiency) because, even if much has been learned from this model6,7, the genetic defect is localized in hepatocytes rather than cholangiocytes. Similarly, this Review does not focus on biliary atresia, as in most cases a genetic association is not present.
Biliary epithelium
To better understand genetic and malformative cholangiopathies, a working knowledge of biliary functional anatomy and development is needed. The biliary tree collects the primary bile produced by the hepatocytes and modifies its composition by alkalization and hydration, before delivering it to the intestine where it is indispensable for digestive function and endo-xenobiotic disposal3. Besides the extensive transport activities involved in generating and modifying bile that are performed by interlobular and larger bile ducts, the biliary epithelium is intimately involved in the control of liver inflammation and in the regenerative and reparative responses to injury. In response to liver injury, small bile duct cells proliferate and expand and the hepatic progenitor cell (HPC) compartment is activated8,9. HPCs, which are bipotential cells that are able to differentiate into either the biliary or the hepatocyte lineage, have two main locations: one is intrahepatic, confined to the periportal niche, in close contact with the canals of Hering8; and the other is extrahepatic, in the peribiliary glands present in the submucosal layer of the bile duct wall10. In response to chronic liver damage, particularly in the cholangiopathies, ductular epithelial cells acquire a ‘reactive’ phenotype (reactive ductular cells (RDCs)) and grow as irregular strings at the margins of the portal tract8,9. By establishing strong paracrine communications with mesenchymal cells, including myofibroblasts, inflammatory cells and endothelial cells, ductular cells orchestrate a reparative response with the side effect of fibrogenesis11.
The phenotypic differences between intrahepatic and extrahepatic bile ducts mirror their distinct embryological origin and the development of the bile duct epithelium. Whereas the extrahepatic bile ducts derive from the caudal part of the ventral foregut, the intrahepatic system originates from hepatoblasts, which are bipotent endodermal cells present in the fetal liver bud12,13. At gestational week 8, hepatoblasts abutting the mesenchyme of the nascent portal space start to differentiate towards a biliary phenotype, leading to a circular layer of single cells called the ductal plate. Initially, ductal plate cells express phenotypic markers of both the hepatocellular lineage (cytokeratin 8 (CK8) and CK18) and the biliary lineage (CK19), but when they start to duplicate and assume a tubular shape from gestational weeks 12–16, they gradually lose hepatocyte markers and gain additional biliary markers, including CK7. Non-duplicating ductal plate cells are usually removed by apoptosis13,14. Once ductal plate cells acquire a ductal morphology, these cells migrate to and incorporate into the portal mesenchyme where they enlarge1,15. During this phase, an arterial vascularization and a peribiliary plexus develop near the migrating and incorporated bile ducts16. This process is finely tuned by the combined interactions of angiogenic growth factors (mainly vascular endothelial growth factor A (VEGFA), and angiopoietin 1 (Ang1) and Ang2) and by several morphogenic signalling pathways, including Wnt–β-catenin, Hedgehog and transforming growth factor-β (TGFβ), and Notch and HIPPO–YAP17–23. Notch signalling is a clear example of how activation of these morphogens can become necessary later in life during repair of biliary damage.
Alagille syndrome and Notch signalling
Alagille syndrome is a multisystem disorder involving the liver, vasculature, heart, eyes and skeleton, and is characterized by typical facies. This syndrome is a rare (estimated to affect 1 in 30,000 to 1 in 50,000 live births) autosomal dominant dysmorphogenetic disorder caused by defective Notch signalling owing to mutations in the Notch ligand Jagged1 (JAG1)24,25 or, less frequently, in a Notch receptor (NOTCH2)26. Alagille syndrome is associated with incomplete development of the intrahepatic bile ducts, and this liver disease is characterized by bile duct paucity, with variable degrees of cholestasis, progressive jaundice, itching and failure to thrive27. Treatment is only symptomatic; in some instances, jaundice and itching slowly improve, and 20–50% of individuals with this disease require liver transplantation28.
Notch signalling
The Notch pathway is an evolutionarily conserved signalling system that regulates cell fate decisions in stem cells and has a major role in the development of the biliary tree29,30. Notch signalling enables cells to communicate with their direct neighbours by cell-to-cell ligand–receptor interactions. There are four known Notch receptors (NOTCH1–4) and five ligands (JAG1, JAG2 and Delta-like 1, 3 and 4). In the ‘canonical’ signalling pathway, ligand–receptor binding leads to proteolytic cleavage of the Notch receptor and subsequent release of the Notch intracellular domain (NICD)31. The NICD translocates into the nucleus where it interacts with recombining binding protein suppressor of hairless (RBPJκ), converting RBPJκ from a transcriptional repressor into an activator, thus promoting the transcription of Notch target genes. The main Notch target genes belong to the HES or HEY family, although several other genes, such as GATA3, which is important for T cell development, and those encoding MYC, cyclin D1 and p21 (WAF1), which are implicated in cancer, are also regulated in parallel29,32. Notch signalling can generate diverse and even opposite biological effects, depending on the specific context, gene dosage, timing and cell type33,34. During liver development, Notch-expressing hepatoblasts localized at the parenchymal interface of the nascent portal tract receive signals from adjacent mesenchymal cells expressing JAG1, and respond by upregulating the expression of HNF1B and SOX9 (FIG. 1a). These factors in turn stimulate the differentiation of hepatoblasts into ductal plate cells and eventually the duplication of the ductal plate and the incorporation of the nascent duct into the portal space. SOX9, HNF1B and HES1, among several candidate genes regulated by Notch, seem to be critical for ductular development35,36. However, there is no single overarching factor downstream of Notch that mediates ductal development. Rather, Notch is part of a large transcriptome and signalling network that, among others, includes TGFβ, Wnt–β-catenin, Hedgehog and YAP, and instructs hepatoblasts to enter the biliary lineage and mature into bile ducts37. Analysis of histological samples from patients with Alagille syndrome, studies in Notch-related animal models and genomic analysis of human HCC samples have shown that Notch signalling is involved not only in biliary ontogenesis but also after birth in biliary repair and carcinogenesis34 (FIG. 1b–d). By regulating the homotypic and heterotypic crosstalk between several types of liver cell, Notch signalling regulates biliary repair, tubulogenesis38,39 and the biliary phenotypic switch of transdifferentiating hepatocytes40–42. Furthermore, studies have shown that Notch is also involved in liver metabolism, vascular biology and immunity31, but these functions are not discussed in this Review.
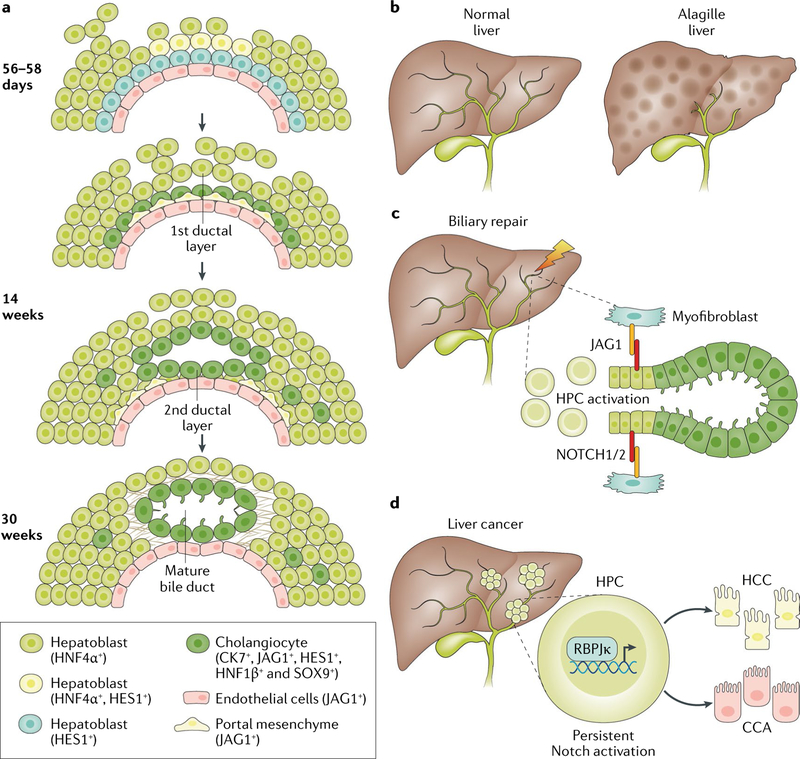
Fig. 1 |. Notch signalling in biliary development and disease.
a | Between 56 and 58 days to 14 weeks gestational age, Notch signalling in the developing liver becomes activated in hepatoblasts by interaction with Jagged-1 (JAG1)-expressing mesenchymal cells. Activation of Notch is involved in the biliary specification of the hepatoblasts in contact with the portal vein mesenchyme. This process generates ductal biliary structures that become mature at ~30 weeks of gestation. b | When Notch signalling is disrupted, as in Alagille syndrome, small branches of the biliary tree do not develop, causing ductopenia, jaundice and pruritus. In some cases, the condition improves with time, whereas in 20–50% of cases, it can progress to end-stage liver disease. c | Notch signalling is also involved in biliary morphogenesis during the repair process in the context of chronic biliary damage. In this setting, Notch has a dual function that includes the activation of hepatic progenitor cells (HPCs) and ductular cells followed by the formation of the tubular structures and crosstalk with mesenchymal cells. d | Persistent overactivation of Notch signalling in HPCs, leading to downstream RBPJκ-dependent transcriptional activity, favours malignant transformation in hepatocellular carcinoma (HCC) or cholangiocarcinoma (CCA).
Role of Notch in cholangiopathies
Studies on liver explants from patients with Alagille syndrome that had progressive liver disease provided the first clue that reactivation of Notch signalling is a key factor in the reparative response to biliary damage43. Careful pathological analysis revealed that ductopenia in patients with Alagille syndrome is associated with an imbalance in the epithelial components of the hepatic reparative machinery. In contrast to individuals with biliary atresia, in which RDCs are prevalent, individuals with Alagille syndrome show a near complete absence of RDCs, in parallel with a marked accumulation of cells with an intermediate hepatocyte–biliary (IHBC) phenotype that lack expression of the Notch-dependent transcription factor HNF1β43. This finding might indicate that HPCs were forced towards a hepatocellular fate because of defective Notch signalling, or, conversely, that transdifferentiation of hepatocytes to cholangiocytes was blocked at the IHBC level. This study also found that defective Notch signalling reduces liver fibrogenesis. On the one hand, the reduced number of RDCs in individuals with Alagille syndrome is associated with scarce deposition of fibrotic tissue in the portal space, leading to much thinner septa than in biliary atresia. On the other hand, the increased IHBC compartment is accompanied by pericellular fibrosis within the hepatic lobule (a ‘chicken wire’ fibrosis pattern), a fibrotic lesion that is also observed in alcoholic and metabolic liver injury43. These findings are consistent with the slow clinical progression to cirrhosis observed in individuals with Alagille syndrome with respect to other conditions such as biliary atresia.
Further studies have clarified that, during liver repair, direct cell–cell interaction between Notch-expressing HPCs and JAG1-expressing portal myofibroblasts favours the conversion of HPCs to RDCs as a default mechanism38,44. However, factors such as Numb, an endogenous inhibitor of Notch that targets the NICD to the proteasome and has been shown to promote a progenitor cell fate, can antagonize Notch activation in HPCs, switch off Notch-dependent biliary specification and turn on Wnt–β-catenin activation and HPC differentiation towards a hepatocyte phenotype38,45. Notch signalling also regulates ductal branching during biliary repair. The generation of branching tubular structures requires the coordinated and integrated functions of both NOTCH1 and NOTCH2 (REF.39). If only NOTCH2 is defective, biliary specification of HPCs in response to biliary injury is preserved; however, the ability to rebuild biliary tubular structures is impaired and the liver cannot restore bile duct mass39.
The clinical manifestation of Alagille syndrome is quite variable and it is not unusual to witness a reduction in disease severity over time27. This phenomenon has been also observed in mice with severe Notch defects, such as deficiency in Rbpjk and Hnf6, the latter encoding a transcription factor that promotes the expression of HNF1β and of SOX9, two essential factors for biliary development46. Work published in 2018 shows that, in these settings, functional bile ducts can be generated by transdifferentiation of hepatocytes into biliary cells and this transition can also occur in the absence of Notch as long as TGFβ signalling is intact17. This discovery not only adds an important piece to the puzzle of biliary repair but also offers hope to individuals that have poorly developed bile ducts owing to Notch mutations. In addition, mouse models with specific mutations that affect the ability of JAG1 to bind to NOTCH1 or NOTCH2 have multiple organ abnormalities typical of Alagille syndrome47–49. The clinical manifestations of Alagille syndrome can also be influenced by modifier genes. Among them, thrombospondin 2 (THBS2) is a matricellular protein that can inhibit the JAG1–Notch interaction50,51; in addition, studies in mice have shown that O-glycosyltransferase 1 (also known as POGLUT1 or RUMI) is a post-translational negative regulator of JAG1 function52, and that Fringe genes regulate Notch receptors after translation53.
Notch overactivation
Whereas defective Notch function causes ductopenia during development and hampers biliary repair from an acquired injury, persistent Notch overactivation can result in liver epithelial cell dysplasia and malignant transformation31,34,54. Development of dysplastic nodules and subsequent hepatocellular carcinoma (HCC) was observed in mice bearing Notch-dependent activation of HPCs55,56. In a mouse model tracing the fate of hepatocytes, joint activation of Notch and AKT signalling promoted ductular metaplasia of normal hepatocytes that subsequently behave as precursor cells of rapidly progressing intrahepatic cholangiocarcinoma40. It is not currently known whether intrahepatic cholangiocarcinoma in humans derives from hepatocytes through Notch-mediated hepatocyte metaplasia, but increasing evidence supports the association of intrahepatic cholangiocarcinoma with several risk factors for HCC, including cirrhosis, HCV, HBV and nonalcoholic steatohepatitis57.
Histological studies in PBC and PSC have shown that levels of several Notch ligands and receptors are upregulated in ductular cells near to neovessels58,59. Consistent with this observation, the mechanisms unveiled when studying the pathogenesis of Alagille syndrome have demonstrated that Notch signalling plays a major part in biliary repair mechanisms that promote biliary fibrosis, which is the primary factor in disease progression. Studies in animals undergoing experimental liver damage show that modulation of Notch signalling leads to reduced fibrosis deposition39. As Notch is a ‘druggable’ signalling pathway60,61, there is the possibility for pharmacological modulation of Notch signalling, for example, in primary liver tumours.
Polycystic liver disease
Polycystic liver diseases (PLDs) are inherited disorders characterized by the development of multiple (>10) fluid-filled biliary cysts scattered throughout the parenchyma62,63. PLDs are typical examples of a group of genetically mediated biliary diseases known as cholangiociliopathies64,65 that are caused by a lack or dysfunction of proteins located in the cilia. Biliary cells possess a single non-motile cilium that protrudes into the biliary lumen and performs mechanosensing, chemosensing and osmosensing functions66–70. Among biliary diseases associated with ciliary dysfunction are ductal plate malformations, such as Bardet–Biedl, Senior–Loken and Joubert syndromes71. In the past few years, dysfunction in ciliary proteins or changes in ciliary morphology have been also reported in cases of neonatal sclerosing cholangitis and biliary atresia72–74. These conditions are not further discussed in this Review as these observations await confirmation; here, we focus on PLDs.
In PLD, cystogenesis can arise exclusively in the liver (autosomal dominant PLD (ADPLD)) or in coexistence with renal cysts (autosomal dominant polycystic kidney disease (ADPKD)), depending on the mutated gene62,63,75,76 (TABLE 1). The symptoms of PLD are related to the growth of the cysts and the related hepatomegaly (and can include abdominal distension and pain, early satiety, abdominal discomfort and dyspnoea) and/or to cyst damage (for example, bleeding, infection or rupture)62,63. The diagnosis of PLD is usually confirmed by liver imaging. Current therapeutic options are only indicated for symptomatic patients and the benefits are short-term and modest. Treatments include surgical procedures (aspiration, sclerotherapy, cyst fenestration or segmental hepatic resection) for symptomatic cysts or chronic treatment with somatostatin analogues for patients with moderate–severe disease and impaired quality of life. Liver transplantation remains the only curative therapy62,63.
Table 1 |.
Genes and proteins affected in polycystic liver diseases
| Mutated genes | Protein | Localization | Function |
|---|---|---|---|
| ADPLD (frequency of ~1:100,000) | |||
| PRKCSH | Hepatocystin or glucosidase II subunit-β | ER | N-linked glycan-processing enzyme in the ER |
| SEC63 | Translocation protein Sec63 homologue (SEC63) | ER | Translocation of proteins into the ER lumen |
| LRP5 | Low-density lipoprotein receptor-related protein 5 | Plasma membrane | Canonical Wnt signalling |
| GANAB | Glucosidase II subunit-α | ER | N-linked glycan-processing enzyme in the ER |
| SEC61B | Sec61 translocon β-subunit (SEC61β) | ER | Translocation of proteins into the ER lumen |
| ALG8 | α–1,3-glucosyltransferase | ER | Protein glycosylation |
| ADPKD (frequency of ~1:500–1:1,000) | |||
| PKD1 | Polycystin-1 | Primary cilium, plasma membrane and cell junctions | Mechanoreceptor involved in calcium signalling |
| PKD2 | Polycystin-2 | Primary cilium and ER | Non-selective calcium channel |
| ARPKD, CHF and Caroli disease (frequency of ~1:20,000) | |||
| PKHD1 | Fibrocystin | Primary cilium | Tubulogenesis and/or maintenance of bile duct architecture |
ADPKD, autosomal dominant polycystic kidney disease; ADPLD, autosomal dominant polycystic liver disease; ARPKD, autosomal recessive polycystic kidney disease; CHF, congenital hepatic fibrosis; ER, endoplasmic reticulum.
Polycystins and ER-related genes
ADPKD, the most common inherited nephropathy (with a prevalence of 1 in 500–1,000), is caused by mutations in PKD1 (80–85%)77 or PKD2 (10–15%)78. The liver is affected in ~85% of patients62,65,79. PKD1 and PKD2 encode the ciliary proteins polycystin-1 (PC1; a mechanoreceptor) and PC2 (a calcium channel that is also abundant in the endoplasmic reticulum (ER)), respectively. Together, PC1 and PC2 form a functional complex that regulates intracellular Ca2+ homeostasis66. ADPLD (the PLD variant that does not affect the kidney) is a rare disease (with a prevalence of ~1 in 100,000) triggered by germline mutations in numerous genes, including PRKCSH80,81, SEC63 (REF.82), SEC61B83, GANAB83,84, ALG8 (REF.83) or LRP5 (REF.85), with PRKCSH being the most frequently mutated gene (~15% of individuals)62,76. Nevertheless, mutations in these genes explain only ~50% of ADPLD cases62,63,83. With the exception of LRP5, which encodes a plasma membrane co-receptor involved in Wnt signalling, the other ADPLD-related genes encode proteins located at the ER that participate in protein biogenesis (transport, protein folding or glycosylation)62,63,76,83.
Experimental evidence indicates that defects in PC1 biosynthesis are the rate-limiting determinant of cystogenesis in all forms of PLD83,86. The primary cilium is responsible for cholangiocyte cell polarization and the preservation of quiescence. Mutations in PKD1 or in the aforementioned ER-related genes affect PC1 expression and/or maturation, and result in impaired ciliary structure and, consequently, cholangiocyte proliferation and cystogenesis. Additionally, PC1 regulates Wnt signalling, providing a potential link between LRP5 mutations and PC1 (REF.87).
Liver cystogenesis
Hepatic cystogenesis originates from malformations of the ductal plate during embryogenesis62,88 and/or from second-hit mutations in the wild-type allele of PLD-related genes in intrahepatic cholangiocytes (loss of heterozygosity)89–92. Cystogenesis is characterized by several features75, including increased cystic cholangiocyte proliferation93, secretion94, matrix metalloproteinase activity95, autophagy96, ciliary and centrosomal abnormalities97,98, and alterations in the microRNA expression pattern99. An alternative model for initial cyst formation has arisen from work showing that biliary cysts might develop through a proliferation-independent process that involves recruitment and biliary differentiation of nearby hepatoblasts100,101. However, further postnatal growth of cysts requires cell proliferation.
Reduced levels or function of PC1 and/or PC2 directly alters cellular Ca2+ signalling and indirectly modifies Ca2+-regulated cAMP levels102. Cytoplasmic Ca2+ homeostasis is maintained by a balance between extracellular and intracellular Ca2+ levels. When ER Ca2+ stores are decreased, a mechanism called store-operated Ca2+ entry (SOCE) uses extracellular Ca2+ to replenish Ca2+ stores. In PC2-defective cholangiocytes, SOCE is inhibited and cells respond to acute reductions in Ca2+ concentration with increased cAMP production by adenylyl cyclase 5 (AC5), cAMP-mediated protein kinase A (PKA) phosphorylation, and a PKA-dependent increase in extracellular-signal-regulated kinase 1 (ERK1) and ERK2 phosphorylation103. Chemical and gene expression inhibition of stromal interacting molecule 1 (STIM1) or silencing AC5 gene expression reduced cAMP production in PC2-defective cholangiocytes104, suggesting a role for STIM1 and AC5 in ADPKD (FIG. 2). STIM1 is the molecular sensor that couples a reduction in intraluminal ER Ca2+ concentrations with the activation of Ca2+ entry from the plasma membrane105,106. STIM1 translocation from the ER to the plasma membrane activates store-operated Ca2+ channels belonging to the ORAI and transient receptor protein channel (TRPC) families105,106. Thus, in cholangiocytes, PC2 functions as a necessary component of SOCE and an inhibitor of AC5 function. In the absence of PC2, AC5 is de-repressed and more cAMP is produced103. In this context, cAMP drives PKA-dependent cell proliferation and hypersecretion93,94,103,107. Somatostatin analogues are approved by the FDA for the treatment of individuals with PLD owing to their downregulation of cAMP levels in cystic cholangiocytes62,63,108,109. Moreover, new therapeutic strategies aimed at increasing intracellular Ca2+ levels in cystic cholangiocytes are under investigation (for instance, ursodeoxycholic acid (UDCA) and transient receptor potential cation channel subfamily V (TRPV4) agonists)70,110,111. The role of PC1 in cholangiocyte ER Ca2+ homeostasis has not yet been investigated, but, as interaction with PC2 is necessary for the functions of PC1, it is likely that these changes will also be present in PC1-defective cells.
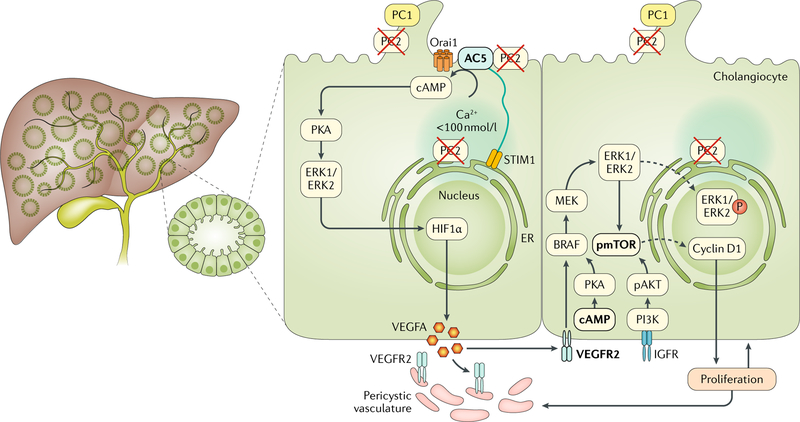
Fig. 2 |. Signalling mechanisms involved in cyst growth in ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is associated with mutations in one of two genes, PKD1 or PKD2, which encode polycystin-1 (PC1) and PC2, respectively. ADPKD is characterized by the presence of multiple cysts in the liver parenchyma that progressively dilate and grow. PC2-defective cholangiocytes are characterized by inappropriate production of cAMP and increased protein kinase A (PKA)-dependent activation of extracellular-signal-regulated kinase 1 (ERK1)/ERK2 and subsequently mechanistic target of rapamycin (mTOR), and hypoxia-inducible factor 1α (HIF1α)-increased production of cAMP is caused by changes in intracellular Ca2+ homeostasis. In PC2-defective cholangiocytes, store-operated Ca2+ entry is inhibited and cells respond to an acute reduction in endoplasmic reticulum (ER) Ca2+ levels with stromal interacting molecule 1 (STIM1)-dependent and adenylyl cyclase 5 (AC5)-dependent stimulation of cAMP production, which drives PKA-dependent activation of ERK1/ERK2. Defective PC2 also inhibits the interaction between STIM1 and Orai channels and maximizes the functional coupling of STIM1 to AC5, resulting in increased production of cAMP. PC2-defective cells respond to conditions that decrease ER Ca2+ levels and trigger oligomerization and membrane translocation of STIM1, with an overproduction of cAMP. In turn, cAMP activates the PKA–Ras–Raf–ERK pathway and stimulates vascular endothelial growth factor (VEGF) production through an mTOR–HIF1α-mediated mechanism. VEGF produced by cystic cholangiocytes increases perivascular microvascular density and cholangiocyte proliferation through binding with VEGF receptor 2 (VEGFR2). Stimulation of mTOR through AKT, for example, by insulin-like growth factor receptor (IGFR) ligand binding, can also activate ERK1/ERK2. Signalling molecules that are druggable are highlighted in bold and detailed in TABLE 2. PI3K, phosphoinositide 3-kinase; pmTOR, phosphorylated mTOR; pAKT, phosphorylated AKT.
VEGF and angiogenic signalling.
The pleiotropic growth factor VEGFA and its cognate receptors VEGFR1 and VEGFR2 are overexpressed in cystic cholangiocytes from patients with ADPKD15. VEGF has autocrine and paracrine proliferative effects on cystic cholangiocytes and vascular endothelial cells, leading to cyst expansion and pericystic vascularization15,112. Notably, cAMP is responsible for VEGF production and VEGFR2-mediated cyst growth in PC2-deficient mouse cholangiocytes via an mechanistic target of rapamycin (mTOR)–ERK1/ERK2–hypoxia-inducible factor 1α (HIF1α) signalling pathway; the pharmacological inhibition of VEGFR2 halts hepatic cystogenesis in these mice107,113,114. Similarly, insulin-like growth factor 1, which is also produced in excess by the cystic epithelium, stimulates VEGF secretion via the mTOR pathway115. Other angiogenic factors such as Ang1 have a synergic effect with VEGF in enhancing the proliferation of cystic cholangiocytes15 (FIG. 2) and are increased in PLD.
Post-translational regulation of PC2 has been linked with VEGF secretion and a cholangiocyte response to biliary damage116. PC2 is downregulated in the liver from different mouse models of biliary damage (including Mdr2−/− knockout, bile duct ligation and the 3,5-dieth-oxycarbonyl-1, 4-dihydrocollidine (DDC) diet), and by the activity of pro-inflammatory cytokines, nitric oxide donors and ER stressors. Downregulation of PC2 in these contexts triggers ERK–HIF1α-dependent VEGF secretion, and the magnitude of PC2 downregulation promotes ductular reaction or cyst formation117.
In chronic cholangiopathies, activated cholangiocytes acquire the ability to secrete VEGF and to respond to VEGF, resulting in autocrine stimulation of proliferation or in paracrine stimulation of peribiliary neoangiogenesis112,114. This process is important to support the expansion of the bile duct mass during biliary repair. However, VEGF can also stimulate recruitment or migration of fibrogenic stellate cells118, resulting in pathological repair with excess fibrosis119. The mechanisms unveiled while studying PLDs will result in an improved ability to modulate specific steps in biliary angiogenic signalling, acting on VEGFR2, PKA or mTOR.
CHF and Caroli disease
Fibropolycystic liver disease is a collective term for a group of different diseases characterized by bile duct dysmorphogenesis and fibrosis120,121, by ductal plate malformation and that result from mutations in the PKHD1 gene, which encodes the protein fibrocystin (FPC; also known as polyductin). FPC is localized in the primary cilia, basal bodies and centromeres of several epithelial ductal structures, such as pancreatic and renal ducts, salivary glands and biliary cells122,123. The distinctive feature of fibropolycystic liver diseases is the presence of cyst-like dilatations of the biliary tree surrounded by a macrophage-dominated immune infiltrate and dense fibrosis124. Mutation of PKHD1 can generate three different diseases: autosomal recessive polycystic kidney disease (ARPKD), congenital hepatic fibrosis (CHF) and Caroli disease125. Despite sharing some common pathological traits, such as biliary cyst development, these diseases differ in several aspects (for example, age of onset). CHF is a rare disease (diagnosed in 1 in 20,000 live births) characterized by cystic malformation of the biliary tree and a prominent peribiliary fibrotic response113. ARPKD is characterized by a very early onset (at late pregnancy or at birth), massive kidney involvement, severe outcomes and eventually death due to kidney and liver enlargement and lung hypoplasia126. Whereas ARPKD and CHF usually affect newborn babies and children, individuals with Caroli disease are usually young adults (around the fourth decade)127. Patients with Caroli disease have a prominent liver involvement characterized by fibrosis deposition and recurrent cholangitis, which can lead to development of cholangiocarcinoma127. The precise function of FPC is still largely unknown but it is thought to be involved in various cellular functions, including regulation of proliferation, secretion, differentiation, tubulogenesis, planar cell polarity and cell–matrix interactions93,95,123,128,129. Membrane-bound FPC undergoes Notch-like proteolytic processing, with translocation of the C terminus fragment to the nucleus where it can affect transcriptional regulation130; however, the signals stimulating this cleavage are not clear. Pharmacological treatments to slow disease progression are not available and therapy is aimed at controlling the consequences of portal hypertension, which relies on hepatic resection or liver transplantation in the most advanced cases131,132.
Fibropolycystic diseases are characterized by ductal dysgenesis with generation of biliary microhamartomas (irregularly shaped bile duct dilatation originating from ductal plate malformations and embedded in fibrous stroma) and segmental dilations that display a fetal-like phenotype, ductal plate remnants that remain in connection with the biliary system, and progressive accumulation of peribiliary fibrosis leading to portal hypertension and hepatic failure133. Dysgenic bile ducts, which originate from duct plate malformations with increased recruitment of nearby hepatoblasts100, progressively enlarge after birth in association with dense and slow-growing worsening fibrosis and the accumulation, over the course of the disease, of a rich portal inflammatory infiltrate recruited within the fibrotic area. However, overt necro-inflammatory damage to the biliary epithelium is absent134. This pathogenetic sequence is different from that observed in PBC, in which the biliary epithelium is damaged by inflammation due to immune activation, and cholangiocyte necrosis or apoptosis is the cause of portal scarring. Rather, the context is reminiscent of PSC, in which stricture and dilatation of the biliary tree become associated with extensive peribiliary fibrosis.
β-Catenin signalling
Cystic structures are characterized by a high mitotic index98 and by perturbations in several signalling mechanisms. In particular, in orthologous rodent models of ARPKD, cellular levels of cAMP are increased135, whereas intracellular Ca2+ levels are downregulated93. Data published in the past few years show that, in mice with mutations in Pkhd1 (Pkhd1del4/del4, in which expression of Pkhd1 is at ~30% of the wild-type level), cAMP, in addition to its known effects on biliary proliferation and secretion, also modulates the hepatic fibro-inflammatory response by stimulating β-catenin nuclear import136,137. β-Catenin has a double role in cellular physiology, acting as a structural protein in adherence junctions and as a transcriptional regulator as part of the Wnt–β-catenin pathway138. In the absence of Wnt signals, this protein is retained in the cytoplasm in an inactive form as part of the β-catenin destruction complex. Upon Wnt stimulation, the destruction complex is inhibited and β-catenin is able to translocate to the nucleus, where it binds to transcription factors such as TCF and LEF and regulates target gene expression139. Notably, in the Pkhd1del4/del4 mouse, higher concentrations of cAMP and increased PKA activity induces the phosphorylation of β-catenin at Ser675, which stabilizes the protein, prevents its catabolism and enables its nuclear transfer where it acts as a transcription factor137 (FIG. 3). This mechanism is responsible for the increased secretion into the portal microenvironment of several chemokines (including CXC-chemokine ligand 1 (CXCL1), CXCL10 and CXCL12) that are involved in the development of the fibroinflammatory response typical of CHF124,136. These chemokines are responsible for the pericystic recruitment of inflammatory cells, mostly M1 macrophages and then, at later stages of the disease, M2 macrophages124,136. Macrophages secrete TGFβ and TNF, which stimulate cystic cholangiocytes to express αVβ6 integrin, a protein of fundamental importance for fibrogenesis owing to its activation of the latent form of TGFβ124. Furthermore, CXCL10 secretion by FPC-defective cholangiocytes is mediated not only by β-catenin nuclearization but also by the secretion of IL-1β through the activation of the NLRP3 inflammasome complex136 (FIG. 3). Active IL-1β secretion in epithelial cells leads to a self-perpetuating feedforward loop that sustains an inflammatory response. This vicious cycle of inflammation is the basis of autoinflammatory conditions, either caused by abnormal production of IL-1β owing to NLRP3 mutation or deficient antagonism of the IL-1β receptor. IL-1β is the major driver of these diseases and has the potential to cause both systemic and organ-specific immunopathology140,141.
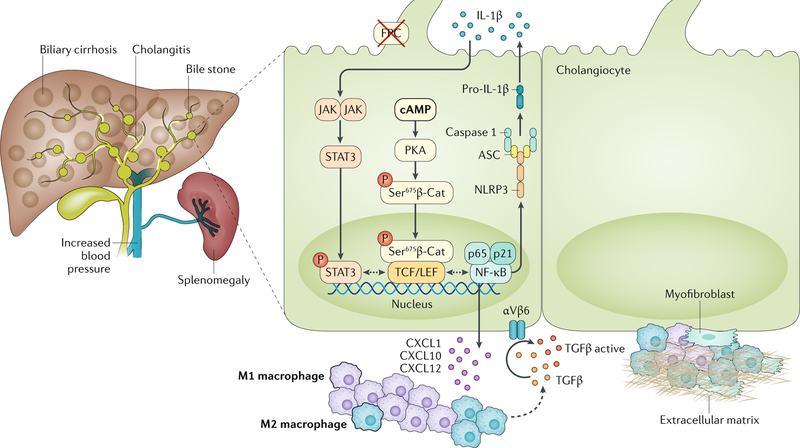
Fig. 3 |. Novel mechanisms of biliary fibrosis in ARPKD.
Liver disease in autosomal recessive polycystic kidney disease (ARPKD), which is caused by mutations in the PKHD1 gene (encoding fibrocystin (FPC)), is characterized by cystic dysgenesis of the intrahepatic bile ducts that retain an immature phenotype reminiscent of the embryonic biliary structures (ductal plate malformations). The disease is associated with progressive portal fibrosis, leading to portal hypertension and related complications. In FPC-defective cholangiocytes, increased levels of cAMP activate protein kinase A (PKA)-dependent phosphorylation of β-catenin (β-Cat) at Ser675 that leads to the nuclear translocation of pSer675β-catenin and transcriptional activation. This mechanism mediates the secretion of CXC-chemokine ligand 1 (CXCL1), CXCL10 and CXCL12 that recruit inflammatory cells, mostly M1 and then M2 macrophages, around the cystic epithelium. Macrophages secrete transforming growth factor-β (TGFβ) and TNF and stimulate the expression of αVβ6 integrins on cystic cholangiocytes that in turn activate latent TGFβ. CXCL10 secretion is further increased by the production of IL-1β through the activation of the Janus kinase (JAK)–signal transducer and activator of transcription 3 (STAT3) pathway. IL-1β secretion is mediated by an activated inflammasome. Signalling molecules that are druggable are highlighted in bold and detailed in TABLE 2. ASC, apoptosis-associated speck-like protein containing a CARD; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing 3.
Relevance to acquired cholangiopathies
The pathophysiological mechanisms involved in CHF and Caroli disease are relevant for acquired cholangiopathies as they demonstrate the roles of β-catenin in biliary inflammation and cholangiocyte dysfunction in biliary fibrosis. Furthermore, the identification of these signalling pathways has clarified the sequence of events leading from cholangiocyte dysfunction to biliary fibrosis, through increased intracellular cAMP levels, β-catenin-mediated secretion of pro-inflammatory cytokines and chemokines, early recruitment of macrophages, activation of latent TGFβ and accumulation of myofibroblasts. These pathobiological mechanisms are potential therapeutic targets for the treatment of cholangiopathies characterized by the accumulation of peribiliary fibrosis accompanied by inflammation and possibly by cholangiocyte hyperplasia. Several studies have examined the use of pasireotide and octreotide, two somatostatin analogues that are able to bind to the somatostatin receptors and block cAMP signalling; both compounds were able to slow hepatic cyst growth and, to a lesser extent, the deposition of peribiliary fibrosis in PCK rats, a rodent model orthologous to human ARPKD carrying mutations in the Pkhd1 gene108,135. To inhibit cell proliferation directly, Cdc25A+/− mice (heterozygous for a defective version of the gene encoding M-phase inducer phosphatase 1, which is responsible for the regulation of all cell cycle phases) were cross-bred with Pkhd1del2/del2 mice. The offspring of these animals had reduced cystogenesis and pericystic fibrosis owing to the effect on CDC25A that partially reverted the pathogenic effect of FPC depletion142. Treatment of PCK rats with agonists of peroxisome proliferator-activated receptor-γ (PPARγ), such as pioglitazione or telmisartan, inhibited the activation of the ERK1–ERK2 and mTOR–S6 kinase signalling pathways and reduced cyst area, cholangiocyte proliferation and the extent of pericystic fibrosis143,144. Another promising approach to treat ARPKD and CHF is to target macrophages. Administration of clodronate, a bisphosphonate able to inhibit monocyte–macrophage transdifferentiation, reduced cyst growth and the accumulation of pericystic inflammatory infiltrate and fibrosis in Pkhd1del4/del4 mice124. Using the same mouse model, inhibition of CXC-chemokine receptor 3 (CXCR3; the receptor for CXCL10, a chemokine involved in macrophage recruitment) showed similar effects to clodronate, further indicating that the presence of a macrophage infiltrate is pivotal for the pathogenesis of fibropolycystic diseases136. Similar approaches aiming to inhibit the recruitment of inflammatory cells were applied also to chronic liver diseases, such as PBC. Starting from the observation that patients with PBC have increased plasma levels of CXCL10 (REF.145), a clinical trial using NI-0801, a monoclonal anti-CXCL10 antibody, was proposed. Unfortunately, this clinical trial failed to reach the main endpoint of the trial: a reduction in the levels of liver function tests146. Using a rodent model of PSC (Mdr2−/− mice), two groups demonstrated that the use of cenicriviroc, an inhibitor of CCR2 and CCR5, is able to reduce the peribiliary accumulation of T cells and macrophages with a concomitant reduction in peribiliary fibrosis and normalization of liver function tests147,148; on this basis, a clinical trial involving human patients with PSC is ongoing.
Cystic fibrosis-related cholangiopathy
Cystic fibrosis is a common monogenic disease (with a prevalence of 1 in 2,500 and 1 in 3,500 in Europe and the United States, respectively) that predominantly affects white individuals149,150. The condition is an autosomal recessive disease caused by mutations affecting the function of cystic fibrosis transmembrane conductance regulator (CFTR)151, a chloride channel expressed by secretory epithelia including the biliary epithelium in the liver152,153 (FIG. 4a). Cystic fibrosis is a multiple organ disease, with the lungs being the most severely affected followed by the pancreas, intestines and the liver150. Owing to the improvement of supportive treatments for the lung disease, the life expectancy in patients with cystic fibrosis has greatly increased and the number of adult individuals with cystic fibrosis-related cholangiopathy (also known as cystic fibrosis liver disease (CFLD)) is rising154,155. CFLD affects 5–10% of patients with cystic fibrosis and has a heterogeneous spectrum of manifestations that ranges from abnormal biochemical liver tests (elevated levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γGT) or alkaline phosphatase (ALP)) to biliary architecture changes, cholestasis and, finally, to clinically severe sclerosing cholangitis, focal biliary cirrhosis and multi-lobular biliary cirrhosis complicated by portal hypertension156,157. Furthermore, the development of cirrhosis and portal hypertension aggravates the respiratory function of these individuals, and CFLD is the third most common cause of mortality after cardiorespiratory and transplant complications157. The variability of the liver phenotype and the fact that these individuals are often asymptomatic makes the early diagnosis of CFLD challenging158.
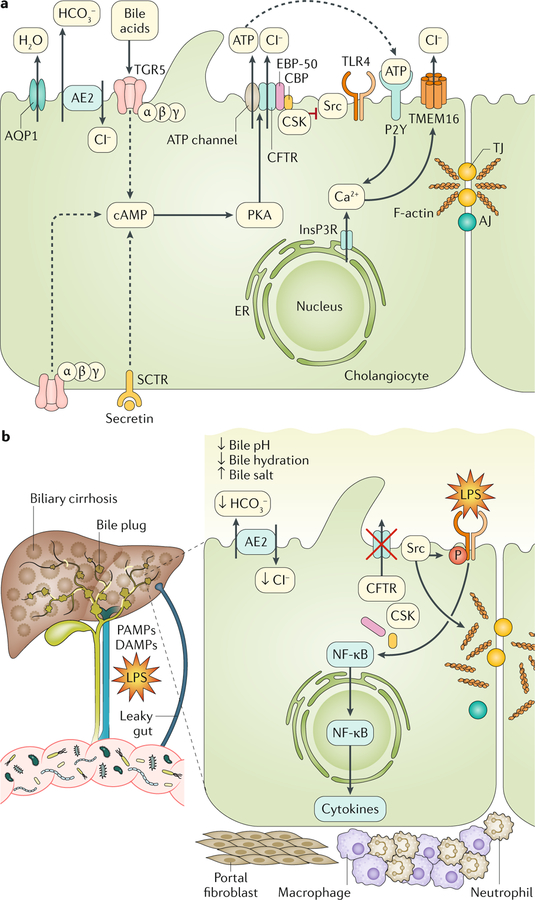
Fig. 4 |. CFTR function in cholangiocytes.
a | Cystic fibrosis transmembrane conductance regulator (CFTR) is located on the apical membrane of cholangiocytes where it has a major role in modifying bile properties (fluidity and pH). Bicarbonate (HCO3−) secretion into bile is necessary to sustain bile flow, to enable clearance of xenobiotics and to accomplish digestive needs (digestion and absorption of fats) within the intestine. Secretin is the main hormone that controls the secretory functions of the biliary epithelium. Secretin interacts with the G protein-coupled secretin receptor (SCTR) expressed on the basolateral membrane of cholangiocytes and triggers the production of cAMP, which activates protein kinase A (PKA). In turn, PKA phosphorylates the R domain of CFTR and opens the chloride conductivity channel. Apical chloride secretion is mediated by bicarbonate exchange through the anion exchanger 2 (AE2), generating an electrolyte–osmotic gradient that favours the passive movement of water through aquaporins (AQPs). These mechanisms are responsible for the normal hydration and alkalization of bile. CFTR-dependent biliary secretion is also triggered in response to TGR5 receptor–PKA signalling when TGR5 is stimulated by bile acids. TGR5, a membrane-bound bile acid receptor coupled to a stimulatory G protein, is expressed in the liver by several non-parenchymal cells (including the sinusoidal epithelium, Kuppfer cells and hepatic stellate cells) and by cholangiocytes. On cholangiocytes, TGR5 is localized both on the apical membrane and on the primary cilium and is strongly activated by taurolithocholic acid and taurocholate. In vitro data in mouse cholangiocytes have shown that CFTR can mediate the apical release of ATP that binds to P2Y purinergic receptors on the apical membrane of biliary cells and activates the Ca2+–calmodulin chloride channel (TMEM16). CFTR also regulates the function of proteins involved in biliary innate immunity and endotoxin tolerance (such as Src family tyrosine kinase and its regulatory proteins CBP and CSK). b | Mutations in the gene encoding CFTR cause a chronic cholangiopathy that can eventually progress to biliary cirrhosis. When CFTR is absent, biliary secretion is impaired and the protein complex formed with Src and its regulatory proteins is disrupted, resulting in the self-activation of Src. Src activation is responsible for the phosphorylation of Toll-like receptor 4 (TLR4), which enhances its response to lipopolysaccharide (LPS) and increases nuclear factor-κB (NF-κB) activation and cytokine secretion. This inflammatory milieu also affects the F-actin cytoskeleton and tight junction (TJ) integrity, altering the epithelial barrier function. CFTR loss also promotes changes in the gut that favour the colonization of a more pro-inflammatory microbiota and the translocation of their products to the liver. The altered biliary epithelial innate immunity and changes in the gut microbiota synergistically lead to the progression of cystic fibrosis-related liver disease. Signalling molecules that are druggable are detailed in TABLE 2. AJ, adherens junction; DAMP, damage-associated molecular pattern; ER, endoplasmic reticulum; InsP3R, inositol-1,4,5-trisphosphate receptor; PAMP, pathogen-associated molecular pattern.
UDCA is the current treatment of choice for patients with CFLD (10–20 mg per kg body weight per day) and this drug has been shown to reduce serum levels of ALT, AST, γGT and ALP159,160. However, doses of UDCA higher than commonly used (25–30 mg per kg body weight per day) have raised safety concerns in patients with PSC; furthermore, the beneficial effects of UDCA on the progression of CFLD has not been proven by long-term randomized clinical studies161. Small-molecule treatments aimed at correcting the basal defect of CFTR have been approved in the past 7 years for the treatment of specific cystic fibrosis genotypes (ivacaftor, lumacaftor–ivacaftor and ivacaftor–tezacaftor) and several more therapies are in development. These molecules can be categorized as compounds that are able to rescue the specific mutation dysfunction (correctors, such as lumacaftor and tezacaftor) or as compounds that are able to restore CFTR channel activity (potentiators, such as ivacaftor). These treatments can be used in combination, depending on the functional and/or genetic defect151,162,163. However, most of the clinical outcome data about their efficacy are restricted to the pulmonary function.
The pathogenesis of CFLD is still unclear, and further studies are needed to design specific interventions; however, progress has been made in the past few years. Furthermore, novel stem cell technologies, such as induced pluripotent stem cells and 3D culture of epithelial organoids, have increased the availability of patient-derived cells to model the disease, and these advances will hopefully accelerate the translation of novel findings into treatment164–166. CFTR plays an essential role in the regulation of bile secretion by the biliary tree1 (FIG. 4a). These fundamental mechanisms are impaired in cystic fibrosis, and bile flow and the secretion of chloride and bicarbonate are substantially reduced. Biliary secretion is defective in all individuals with cystic fibrosis and, although different mutations might have different effects on CFTR function, there is no clear correlation between the mutation genotype and the progression of liver disease, suggesting that other comorbidities or modifier genes might contribute155,167.
Role of CFTR in epithelial innate immunity
CFTR has a role in multiple cell functions168,169; for example, CFTR can act as a hub protein that forms macromolecular complexes with several other proteins at the apical membrane of epithelia. CFTR has been reported to interact directly or indirectly with proteins that regulate its channel activity (such as AKAP and PKA), integrate signalling pathways (such as adenosine 2b receptors or the β2-adrenergic receptor) and coordinate other transport activities (for example, amiloride-sensitive sodium channel (ENaC), ROMK, AE2 and AQP1). Usually, PDZ-domain-containing proteins (such as NHERF1–4, CAL and CAP70) mediate these interactions by connecting CFTR to other proteins in the complex and to the sub-membrane actin cytoskeleton that stabilizes the complex168,169.
One such example is the discovery of the interaction between CFTR and Src family tyrosine kinases (SFKs) in cholangiocytes170 (FIG. 4a). This physical interaction is important for the regulation of innate immune responses and to maintain endotoxin tolerance in cholangiocytes. CFTR, in association with NHERF1, binds CBP and CSK that retain Src kinase in an inactive state170. When this complex is not assembled, unbound Src self-activates and phosphorylates Toll-like receptor 4 (TLR4), the pattern recognition receptor for lipopolysaccharides (LPS)170,171. Phosphorylation of TLR4 is important for the activation of its signalling pathway and the recruitment of downstream signal-competent adaptor proteins (for example, MyD88) and kinases (such as IRAK4 and IRAK1) in response to LPS binding172,173. However, in the normal biliary epithelium, TLR4 phosphorylation is markedly and constitutively downregulated, yielding endotoxin tolerance140. This tolerance is important to avoid inappropriate inflammatory responses, given that the biliary epithelium expresses a full set of TLRs (TLR2, TLR3, TLR4 and TLR5) and is continuously exposed to microbial components coming from the gut174,175.
This regulatory mechanism has important implications for the pathogenesis of CFLD. In vitro data show that cholangiocytes isolated from Cftr−/− mice are hyperresponsive to gut-derived endotoxins, and, when exposed to LPS, these cells elicit an abnormal inflammatory response with the production of several pro-inflammatory cytokines176. In cultured mouse Cftr−/− cholangiocytes, Src tyrosine kinase activity and TLR4 phosphorylation are substantially increased (FIG. 4b). Pharmacological inhibition of SFKs (with PP2 for example) or targeting inflammation using PPARγ agonists (pioglitazone and rosiglitazone) decreased the inflammatory phenotype in Cftr−/− cholangiocytes both in vivo and in vitro170,177. Similar findings were confirmed in human cholangiocytes derived from an individual carrying the most common mutation in cystic fibrosis (ΔF508) using induced pluripotent stem cell technology164. Remarkably, the association of the SFK inhibitor with CFTR modulators (lumacaftor and ivacaftor) that correct the ΔF508 misfolding mutation further ameliorated the secretory defect in human cystic fibrosis cholangiocytes. Taken together, these results support a role for CFTR in controlling innate immunity responses in the biliary epithelium, challenge the current view of CFLD as a classic ‘channelopathy’ and suggest potential synergistic targets for therapy (TABLE 2).
Table 2 |.
Actionable therapeutic targets in genetic cholangiopathies
| Disease | Target | Agent | Refs |
|---|---|---|---|
| PLD | Somatostatin receptors (inhibitor) | Octreotide | 108,135 |
| Lanreotide | 62,63 | ||
| Pasireotide | 108 | ||
| AC5 (inhibitor) | SQ22,536 | 104 | |
| VEGFR2 (inhibitor) | SU5416 | 113,114 | |
| BRAF | Sorafenib | 107 | |
| Intracellular calcium and toxic bile acid removal | UDCA | 110 | |
| pmTOR | Rapamycin | 115 | |
| CHF/CD | Somatostatin receptors (inhibitor) | Pasireotide | 108 |
| Octreotide | 108,135 | ||
| Intracellular calcium and toxic bile acid removal | UDCA | 111 | |
| Matrix metalloproteases | Marimastat | 95 | |
| PPARγ (agonist) | Pioglitazione | 144 | |
| Telmisartan | 143,144 | ||
| Macrophages (depletion) | Clodronate | 124 | |
| CXCR3 expressed by macrophages | AMG-487 | 136 | |
| CFLD | PPARγ (agonist) | Pioglitazione | 177 |
| Rosiglitazone | 177 | ||
| CFTR function (correctors and potentiators) | Ivacaftor, lumacaftor and tezacaftor | 162,163 | |
| Src tyrosine kinase family (inhibitor) | PP2 | 164,170 |
In polycystic liver disease (PLD), octreotide, lanreotide and pasireotide are currently under evaluation in phase II–III clinical trials (octreotide in , lanreotide in and pasireotide in ). In cystic fibrosis, ivacaftor and the combinations of ivacaftor plus lumacaftor and ivacaftor plus tezacaftor are currently in use by patients with specific mutations. However, no data are available about their effect on cystic fibrosis-related liver disease (CFLD). AC5, adenylyl cyclase 5; CD, Caroli disease; CFTR, cystic fibrosis transmembrane conductance regulator; CHF, congenital hepatic fibrosis; CXCR3, CXC-chemokine receptor 3; pmTOR, phosphorylated mechanistic target of rapamycin; PPARγ, peroxisome proliferator activated receptor-γ; UDCA, ursodeoxycholic acid; VEGFR, vascular endothelial growth factor receptor.
Microbiome and gut–biliary axis in CFLD
The gut and liver share important anatomical and functional connections and their crosstalk has been implicated in the pathogenesis of several liver diseases178,179. Data published in the past few years indicate that CFLD exemplifies this axis. The CFTR genetic defect is present in both the biliary epithelium and the intestine, and increased intestinal permeability and dysbiosis have been described in mouse models and in patients with cystic fibrosis180–182. Early studies in mouse models of cystic fibrosis showed that induction of colitis with dextran sodium sulfate, which increases intestinal permeability and the portal release of bacterial products, causes biliary damage and inflammation in Cftr−/− mice but not in wild-type littermates. Interestingly, the biliary damage is attenuated by treatment with broad-spectrum antibiotics, supporting a contribution of the intestinal microbiota in the establishment of the disease176. More studies are needed to clarify the pathophysiological role of the gut microbiota in the development of CFLD. The sequence of events discovered in CFLD is relevant for acquired cholangiopathies, such as PSC. In fact, they demonstrate that changes in microbiota and intestinal permeability can cause a cholangiopathy in the presence of an altered regulation of epithelial innate immunity. The involvement of the gut microbiota in the pathogenesis of PSC is strongly supported by the association of PSC with IBD and ulcerative colitis (~60–80% of individuals with PSC)183, by genome-wide association study data that have identified genetic variants in PSC that are associated with ulcerative colitis (such as those encoding GPR35)184 or influence biliary bacterial composition (such as those encoding FUT2)185, and by the presence of bacterial products in the liver explants of patients with PSC186. Conversely, in vitro data have shown that biliary cells isolated from patients with PSC have aberrant TLR–nuclear factor-κB (NF-κB) immune responses to intestinal endotoxins with increased production of pro-inflammatory cytokines, such as IL-8 and TNF187.
Conclusions
Studies in inherited and congenital cholangiopathies have generated important new information that has not yet been discussed in a unified view. Importantly, investigations into these conditions have yielded insights into cholangiocyte pathobiology that are relevant also to acquired cholangiopathies. The central mechanism of cholangiocyte pathobiology2,3,188 is persistent inflammation with ongoing biliary damage and repair, resulting in progressive fibrosis, changes in bile production and evolution to biliary cirrhosis. Recent studies demonstrated that these processes require the reactivation of morphogenetic mechanisms typical of biliary development, such as Notch signalling (for Alagille syndrome), and the Hedgehog pathway and Wnt–β-catenin signalling (for CHF). Defective Notch signalling causes congenital ductopenia, but also impairs liver repair after damage when compensatory biliary hyperplasia in response to cholestasis does not occur, leading to extravasation of bile in the interstitial tissue (bile lakes). Conversely, the blunted ductular reaction dampens the fibrotic response. Unrestrained Notch activity might be involved in liver carcinogenesis and, specifically, in the formation of intrahepatic cholangiocarcinomas through biliary transdifferentiation of hepatocytes. We have also learned that there is considerable redundancy in the mechanisms regulating the formation of ductular cholangiocytes, as other pathways may, in the long run, vicariate for Notch effects by stimulating hepatocyte transdifferentiation into biliary cells.
Studies in PLDs have revealed a fundamental role of the cilium, an organelle that was previously neglected. The cilium hosts many proteins that are able to sense physical and chemical properties of the biliary milieu, and its dysfunction is associated with increased cholangiocyte proliferation and profound changes in cholangiocyte intracellular signalling and in the ability of cholangiocytes to sense biliary composition and flow. Using PC2-defective mice, we have unveiled novel mechanisms in which loss of Ca2+ homeostasis in the ER might increase cAMP levels via AC5, when PC2 expression is absent or heavily reduced. This novel mechanism, ultimately leading to increased cholangiocyte proliferation, is also activated when PC2 is downregulated by inflammatory factors, as in acquired cholangiopathies.
By studying PLD, we have understood the role played by VEGF and VEGFR2 in regulating autocrine biliary proliferation and paracrine peribiliary vascularization. Angiogenic factors also play a fundamental role in biliary repair by increasing cholangiocyte proliferation and provide a clue for neoangiogenesis. Future studies will clarify the role and whether this mechanism can be targeted to modify cholangiocyte repair.
The role of the biliary epithelium in innate immunity has been the focus of intensive investigation in recent years, driven by interest in autoimmune and inflammatory cholangiopathies. In spite of these research efforts, the mechanism of these cholangiopathies remains elusive. It is important to consider that cholangiocytes, in addition to being the target of immune cell aggression and presenting antigens to immune cells, might also be the originators of an inflammatory and immune reaction. This concept is highlighted by biliary diseases caused by a genetically determined defect in FPC (CHF and Caroli disease) or in CFTR. It is worth mentioning that loss of homeostasis in epithelial cells, as a consequence of a mutation or exposure to damaging agents, generates a sequence of signals directed towards the re-establishment of new homeostatic set points. In this scenario, epithelial cells secrete numerous molecules and factors that are able to instruct immune cells or to generate an inflammatory reaction (the epi-immunome). The studies discussed in this Review have also led to the identification of several possible actionable targets and experimental treatments, as highlighted in TABLE 2. We expect that future research into the pathobiology of these fascinating diseases will be further rewarding and will also reveal important insights on the function of the healthy biliary epithelium.
Key points.
Reactivation of morphogen signalling (such as Notch, Wnt–β-catenin and Hedgehog) takes place during biliary repair and orchestrates the balance between biliary remodelling and fibrogenesis and transdifferentiation and carcinogenesis.
Polycystins control fundamental Ca2+–cAMP-dependent cell signalling processes in cholangiocytes and, when defective, these proteins enhance cell proliferation and activate angiogenic signalling that leads to cystogenesis.
Polycystin-2 can be modulated in response to biliary inflammation and mediates vascular endothelial growth factor secretion and cholangiocyte proliferation in acquired cholangiopathies.
Genetic defects in fibrocystin are associated with altered β-catenin signalling, which generates an auto-inflammatory response with secretion of chemokines that are able to attract macrophages, resulting in biliary fibrogenesis.
Cystic fibrosis transmembrane conductance regulator (CFTR) regulates cholangiocyte innate immunity and maintains Toll-like receptor tolerance; loss of CFTR predisposes the biliary epithelium to inflammation and damage in response to gut-derived microbial components.
Pathological mechanisms identified in genetic cholangiopathies can be applied to acquired cholangiopathies and might represent potential targets for the next generation of treatments.
Acknowledgements
This work was supported by the National Institutes of Health (RO1DK096096 (M.S.), RO1DK-079005–07 (M.S.) and RO1DK101528 (C.S.)); by DK034989 Silvio O. Conte Digestive Diseases Research Core Center (M.S., C.S. and R.F.); by PSC Partners Seeking a Cure (M.S.); by a grant from Connecticut Innovations (16-RMA-YALE-26) (M.S.); by a grant from Cystic Fibrosis Foundation (FIOROT18GO) (R.F.); by the University of Padua, Progetti di Ricerca di Dipartimento (PRID) 2017 (L.F.); by the Spanish Ministry of Economy and Competitiveness and “Instituto de Salud Carlos III” grants PI14/00399, PI17/00022 and Ramon y Cajal Programme RYC-2015–17755 (M.J.P.) and by PI15/01132, PI18/01075 and Miguel Servet Programme CON14/00129 co-financed by “Fondo Europeo de Desarrollo Regional” (FEDER) (J.M.B.); by CIBERehd, Spain (M.J.P. and J.M.B.); by IKERBASQUE, Basque foundation for Science, Spain (M.J.P. and J.M.B.); by “Diputación Foral de Gipuzkoa” DFG18/114 (M.J.P.), DFG15/010 and DFG16/004 (J.M.B.); by BIOEF (Basque Foundation for Innovation and Health Research): EiTB Maratoia BIO15/CA/016/BD (J.M.B.); by the Department of Health of the Basque Country (2015111100 (M.J.P.) and 2017111010 (J.M.B.)); and by AECC Scientific Foundation (J.M.B.).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks S. Karpen and the other anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Strazzabosco M & Fabris L Functional anatomy of normal bile ducts. Anat. Rec 291, 653–660 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazaridis KN, Strazzabosco M & Larusso NF The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127, 1565–1577 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Strazzabosco M, Fabris L & Spirli C Pathophysiology of cholangiopathies. J. Clin. Gastroenterol 39, S90–S102 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis KN & LaRusso NF The cholangiopathies. Mayo Clin. Proc 90, 791–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spada M, Riva S, Maggiore G, Cintorino D & Gridelli B Pediatric liver transplantation. World J. Gastroenterol 15, 648–674 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava A Progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol 4, 25–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falguieres T, Ait-Slimane T, Housset C & Maurice M ABCB4: insights from pathobiology into therapy. Clin. Res. Hepatol. Gastroenterol 38, 557–563 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Van Haele M & Roskams T Hepatic progenitor cells: an update. Gastroenterol. Clin. North Am 46, 409–420 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Duncan AW, Dorrell C & Grompe M Stem cells and liver regeneration. Gastroenterology 137, 466–481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardinale V et al. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 55, 2041–2042 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Fabris L, Spirli C, Cadamuro M, Fiorotto R & Strazzabosco M Emerging concepts in biliary repair and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol 313, G102–G116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strazzabosco M & Fabris L Development of the bile ducts: essentials for the clinical hepatologist. J. Hepatol 56, 1159–1170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober EA & Lemaigre FP Development of the liver: insights into organ and tissue morphogenesis. J. Hepatol 68, 1049–1062 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Si-Tayeb K, Lemaigre FP & Duncan SA Organogenesis and development of the liver. Dev. Cell 18, 175–189 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Fabris L et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43, 1001–1012 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Lemaigre FP Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 137, 62–79 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Schaub JR et al. De novo formation of the biliary system by TGFbeta-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabris L et al. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology 47, 719–728 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Bhattaram P et al. Organogenesis relies on SoxC transcription factors for the survival of neural and mesenchymal progenitors. Nat. Commun 1, 9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yimlamai D et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SH, Camargo FD & Yimlamai D Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 152, 533–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemaigre FP Molecular mechanisms of biliary development. Prog. Mol. Biol. Transl Sci 97, 103–126 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Li L et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet 16, 243–251 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Oda T et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet 16, 235–242 (1997). [DOI] [PubMed] [Google Scholar]
- 26.McDaniell R et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet 79, 169–173 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lykavieris P, Hadchouel M, Chardot C & Bernard O Outcome of liver disease in children with Alagille syndrome: a study of 163 patients. Gut 49, 431–435 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamath BM et al. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 18, 940–948 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Artavanis-Tsakonas S, Rand MD & Lake RJ Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Lai EC Notch signaling: control of cell communication and cell fate. Development 131, 965–973 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Geisler F & Strazzabosco M Emerging roles of Notch signaling in liver disease. Hepatology 61, 382–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borggrefe T & Oswald F The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell. Mol. Life Sci 66, 1631–1646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bray SJ Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol 7, 678–689 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Morell CM, Fiorotto R, Fabris L & Strazzabosco M Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin. Res. Hepatol. Gastroenterol 37, 447–454 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Zong Y et al. Notch signaling controls liver development by regulating biliary differentiation. Development 136, 1727–1739 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodama Y, Hijikata M, Kageyama R, Shimotohno K & Chiba T The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology 127, 1775–1786 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Gerard C, Tys J & Lemaigre FP Gene regulatory networks in differentiation and direct reprogramming of hepatic cells. Semin. Cell Dev. Biol 66, 43–50 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Boulter L et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med 18, 572–579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorotto R et al. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J. Hepatol 59, 124–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan B et al. Cholangiocarcinomas can originate from hepatocytes in mice. J. Clin. Invest 122, 2911–2915 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekiya S & Suzuki A Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J. Clin. Invest 122, 3914–3918 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morell CM et al. Notch signaling and progenitor/ ductular reaction in steatohepatitis. PLOS ONE 12, e0187384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabris L et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am. J. Pathol 171, 641–653 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie G et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology 58, 1801–1813 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strazzabosco M & Fabris L The balance between Notch/Wnt signaling regulates progenitor cells’ commitment during liver repair: mystery solved? J. Hepatol 58, 181–183 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Walter TJ, Vanderpool C, Cast AE & Huppert SS Intrahepatic bile duct regeneration in mice does not require Hnf6 or Notch signaling through Rbpj. Am. J. Pathol 184, 1479–1488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson ER et al. Mouse model of Alagille syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology 154, 1080–1095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofmann JJ et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development 137, 4061–4072 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masek J & Andersson ER The developmental biology of genetic Notch disorders. Development 144, 1743–1763 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Mitchell E, Gilbert M & Loomes KM Alagille syndrome. Clin. Liver Dis 22, 625–641 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Tsai EA et al. THBS2 is a candidate modifier of liver disease severity in Alagille syndrome. Cell. Mol. Gastroenterol. Hepatol 2, 663–675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thakurdas SM et al. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi). Hepatology 63, 550–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan MJ et al. Bile duct proliferation in Jag1/fringe heterozygous mice identifies candidate modifiers of the Alagille syndrome hepatic phenotype. Hepatology 48, 1989–1997 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Strazzabosco M & Fabris L Notch signaling in hepatocellular carcinoma: guilty in association! Gastroenterology 143, 1430–1434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villanueva A et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 143, 1660–1669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dill MT et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology 57, 1607–1619 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Guest RV et al. Notch3 drives development and progression of cholangiocarcinoma. Proc. Natl Acad. Sci. USA 113, 12250–12255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nijjar SS, Crosby HA, Wallace L, Hubscher SG & Strain AJ Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularization. Hepatology 34, 1184–1192 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Nijjar SS, Wallace L, Crosby HA, Hubscher SG & Strain AJ Altered Notch ligand expression in human liver disease: further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects. Am. J. Pathol 160, 1695–1703 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersson ER & Lendahl U Therapeutic modulation of Notch signalling—are we there yet? Nat. Rev. Drug Discov 13, 357–378 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Morell CM & Strazzabosco M Notch signaling and new therapeutic options in liver disease. J. Hepatol 60, 885–890 (2014). [DOI] [PubMed] [Google Scholar]
- 62.van Aerts RMM, van de Laarschot LFM, Banales JM & Drenth JPH Clinical management of polycystic liver disease. J. Hepatol 68, 827–837 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Gevers TJ & Drenth JP Diagnosis and management of polycystic liver disease. Nat. Rev. Gastroenterol. Hepatol 10, 101–108 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Masyuk T, Masyuk A & LaRusso N Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr. Opin. Gastroenterol 25, 265–271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strazzabosco M & Somlo S Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology 140, 1855–1859 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masyuk AI et al. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masyuk AI et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am. J. Physiol. Gastrointest. Liver Physiol 295, G725–G734 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masyuk AI et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol 299, G990–G999 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masyuk AI et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol 304, G1013–G1024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gradilone SA et al. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 139, 304–314 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hildebrandt F, Benzing T & Katsanis N Ciliopathies. N. Engl. J. Med 364, 1533–1543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bezerra JA et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology 68, 1163–1173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rock N & McLin V Liver involvement in children with ciliopathies. Clin. Res. Hepatol. Gastroenterol 38, 407–414 (2014). [DOI] [PubMed] [Google Scholar]
- 74.van de Laarschot LFM & Drenth JPH Genetics and mechanisms of hepatic cystogenesis. Biochim. Biophys. Acta Mol. Basis Dis 1864, 1491–1497 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Perugorria MJ et al. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nat. Rev. Gastroenterol. Hepatol 11, 750–761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perugorria MJ & Banales JM Genetics: novel causative genes for polycystic liver disease. Nat. Rev. Gastroenterol. Hepatol 14, 391–392 (2017). [DOI] [PubMed] [Google Scholar]
- 77.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77, 881–894 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Mochizuki T et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 (1996). [DOI] [PubMed] [Google Scholar]
- 79.Harris RA, Gray DW, Britton BJ, Toogood GJ & Morris PJ Hepatic cystic disease in an adult polycystic kidney disease transplant population. Aust. NZ J. Surg 66, 166–168 (1996). [DOI] [PubMed] [Google Scholar]
- 80.Drenth JP, te Morsche RH, Smink R, Bonifacino JS & Jansen JB Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat. Genet 33, 345–347 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Li A et al. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am. J. Hum. Genet 72, 691–703 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davila S et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet 36, 575–577 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Besse W et al. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J. Clin. Invest 127, 1772–1785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porath B et al. Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am. J. Hum. Genet 98, 1193–1207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cnossen WR et al. Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cystogenesis. Proc. Natl Acad. Sci. USA 111, 5343–5348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fedeles SV et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet 43, 639–647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S et al. The polycystin complex mediates Wnt/ Ca2+ signalling. Nat. Cell Biol 18, 752–764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raynaud P et al. A classification of ductal plate malformations based on distinct pathogenic mechanisms of biliary dysmorphogenesis. Hepatology 53, 1959–1966 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janssen MJ, Salomon J, Te Morsche RH & Drenth JP Loss of heterozygosity is present in SEC63 germline carriers with polycystic liver disease. PLOS ONE 7, e50324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janssen MJ et al. Secondary, somatic mutations might promote cyst formation in patients with autosomal dominant polycystic liver disease. Gastroenterology 141, 2056–2063 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Pei Y et al. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol 10, 1524–1529 (1999). [DOI] [PubMed] [Google Scholar]
- 92.Watnick TJ et al. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol. Cell 2, 247–251 (1998). [DOI] [PubMed] [Google Scholar]
- 93.Banales JM et al. The cAMP effectors Epac and protein kinase A (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49, 160–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Banales JM et al. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. Am. J. Pathol 173, 1637–1646 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urribarri AD et al. Inhibition of metalloprotease hyperactivity in cystic cholangiocytes halts the development of polycystic liver diseases. Gut 63, 1658–1667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masyuk AI et al. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology 67, 1088–1108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masyuk TV et al. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology 125, 1303–1310 (2003). [DOI] [PubMed] [Google Scholar]
- 98.Masyuk TV et al. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. Am. J. Pathol 184, 110–121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee SO et al. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J. Clin. Invest 118, 3714–3724 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beaudry JB et al. Proliferation-independent initiation of biliary cysts in polycystic liver diseases. PLOS ONE 10, e0132295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benhamouche-Trouillet S et al. Proliferation-independent role of NF2 (Merlin) in limiting biliary morphogenesis. Development 145, dev162123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chebib FT, Sussman CR, Wang X, Harris PC & Torres VE Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat. Rev. Nephrol 11, 451–464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spirli C et al. Altered store operated calcium entry increases cyclic 3′,5′-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology 55, 856–868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Spirli C et al. Adenylyl cyclase 5 links changes in calcium homeostasis to cAMP-dependent cyst growth in polycystic liver disease. J. Hepatol 66, 571–580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Deng X & Gill DL Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci. Signal 3, pe42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y et al. STIM protein coupling in the activation of Orai channels. Proc. Natl Acad. Sci. USA 106, 7391–7396 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spirli C et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology 56, 2363–2374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Masyuk TV et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 58, 409–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khan S, Dennison A & Garcea G Medical therapy for polycystic liver disease. Ann. R. Coll. Surg. Engl 98, 18–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D’Agnolo HM et al. Ursodeoxycholic acid in advanced polycystic liver disease: a phase 2 multicenter randomized controlled trial. J. Hepatol 65, 601–607 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Munoz-Garrido P et al. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. J. Hepatol 63, 952–961 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brodsky KS, McWilliams RR, Amura CR, Barry NP & Doctor RB Liver cyst cytokines promote endothelial cell proliferation and development. Exp. Biol. Med 234, 1155–1165 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Amura CR et al. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/–) mice. Am. J. Physiol. Cell Physiol 293, C419–C428 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Spirli C et al. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology 138, 360–371 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spirli C et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 51, 1778–1788 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spirli C et al. Posttranslational regulation of polycystin-2 protein expression as a novel mechanism of cholangiocyte reaction and repair from biliary damage. Hepatology 62, 1828–1839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Torrice A et al. Polycystins play a key role in the modulation of cholangiocyte proliferation. Dig. Liver Dis 42, 377–385 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Novo E et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am. J. Pathol 170, 1942–1953 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoshiji H et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut 52, 1347–1354 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brancatelli G et al. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 25, 659–670 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Veigel MC et al. Fibropolycystic liver disease in children. Pediatr. Radiol 39, 317–327 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Menezes LF et al. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int 66, 1345–1355 (2004). [DOI] [PubMed] [Google Scholar]
- 123.Zhang MZ et al. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc. Natl Acad. Sci. USA 101, 2311–2316 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Locatelli L et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 63, 965–982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harris PC & Torres VE Polycystic kidney disease. Annu. Rev. Med 60, 321–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bergmann C Genetics of autosomal recessive polycystic kidney disease and its differential diagnoses. Front. Pediatr 5, 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yonem O & Bayraktar Y Clinical characteristics of Caroli’s syndrome. World J. Gastroenterol 13, 1934–1937 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mai W et al. Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol. Biol. Cell 16, 4398–4409 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Al-Bhalal L & Akhtar M Molecular basis of autosomal recessive polycystic kidney disease (ARPKD). Adv. Anat. Pathol 15, 54–58 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Kaimori JY et al. Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum. Mol. Genet 16, 942–956 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cerwenka H Bile duct cyst in adults: interventional treatment, resection, or transplantation? World J. Gastroenterol 19, 5207–5211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moslim MA, Gunasekaran G, Vogt D, Cruise M & Morris-Stiff G Surgical management of Caroli’s disease: single center experience and review of the literature. J. Gastrointest. Surg 19, 2019–2027 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Desmet VJ Ludwig symposium on biliary disorders—part I. Pathogenesis of ductal plate abnormalities. Mayo Clin. Proc 73, 80–89 (1998). [DOI] [PubMed] [Google Scholar]
- 134.Masyuk TV et al. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am. J. Pathol 165, 1719–1730 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Masyuk TV, Masyuk AI, Torres VE, Harris PC & Larusso NF Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology 132, 1104–1116 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Kaffe E et al. beta-Catenin and interleukin-1beta-dependent chemokine (C-X-C motif) ligand 10 production drives progression of disease in a mouse model of congenital hepatic fibrosis. Hepatology 67, 1903–1919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spirli C et al. Protein kinase A-dependent pSer(675)-beta-catenin, a novel signaling defect in a mouse model of congenital hepatic fibrosis. Hepatology 58, 1713–1723 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Niehrs C The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol 13, 767–779 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Dunach M, Del Valle-Perez B & Garcia de Herreros A p120-catenin in canonical Wnt signaling. Crit. Rev. Biochem. Mol. Biol 52, 327–339 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Strazzabosco M et al. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim. Biophys. Acta 1864, 1374–1379 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park H, Bourla AB, Kastner DL, Colbert RA & Siegel RM Lighting the fires within: the cell biology of autoinflammatory diseases. Nat. Rev. Immunol 12, 570–580 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masyuk TV et al. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology 142, 622–633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshihara D et al. Telmisartan ameliorates fibrocystic liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. PLOS ONE 8, e81480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoshihara D et al. PPAR-gamma agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am. J. Physiol. Renal Physiol 300, F465–F474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chuang YH et al. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J. Autoimmun 25, 126–132 (2005). [DOI] [PubMed] [Google Scholar]
- 146.de Graaf KL et al. NI-0801, an anti-chemokine (C-X-C motif) ligand 10 antibody, in patients with primary biliary cholangitis and an incomplete response to ursodeoxycholic acid. Hepatol. Commun 2, 492–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guicciardi ME et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol 69, 676–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu D, Cai SY, Mennone A, Vig P & Boyer JL Cenicriviroc, a cytokine receptor antagonist, potentiates all-trans retinoic acid in reducing liver injury in cholestatic rodents. Liver Int 38, 1128–1138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Castellani C & Assael BM Cystic fibrosis: a clinical view. Cell. Mol. Life Sci 74, 129–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Sullivan BP & Freedman SD Cystic fibrosis. Lancet 373, 1891–1904 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Veit G et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 27, 424–433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cohn JA et al. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology 105, 1857–1864 (1993). [DOI] [PubMed] [Google Scholar]
- 153.Kinnman N et al. Expression of cystic fibrosis transmembrane conductance regulator in liver tissue from patients with cystic fibrosis. Hepatology 32, 334–340 (2000). [DOI] [PubMed] [Google Scholar]
- 154.Ooi CY & Durie PR Cystic fibrosis from the gastroenterologist’s perspective. Nat. Rev. Gastroenterol. Hepatol 13, 175–185 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Debray D et al. Cystic fibrosis-related liver disease: research challenges and future perspectives. J. Pediatr. Gastroenterol. Nutr 65, 443–448 (2017). [DOI] [PubMed] [Google Scholar]
- 156.Rowland M et al. Outcome in patients with cystic fibrosis liver disease. J. Cyst. Fibros 14, 120–126 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Chryssostalis A et al. Liver disease in adult patients with cystic fibrosis: a frequent and independent prognostic factor associated with death or lung transplantation. J. Hepatol 55, 1377–1382 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Debray D, Kelly D, Houwen R, Strandvik B & Colombo C Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J. Cyst. Fibros 10 (Suppl. 2), S29–S36 (2011). [DOI] [PubMed] [Google Scholar]
- 159.Beuers U Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol 3, 318–328 (2006). [DOI] [PubMed] [Google Scholar]
- 160.Lindblad A, Glaumann H & Strandvik B A two-year prospective study of the effect of ursodeoxycholic acid on urinary bile acid excretion and liver morphology in cystic fibrosis-associated liver disease. Hepatology 27, 166–174 (1998). [DOI] [PubMed] [Google Scholar]
- 161.Cheng K, Ashby D & Smyth RL Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst. Rev 9, CD000222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fajac I & Wainwright CE New treatments targeting the basic defects in cystic fibrosis. Presse Med 46, e165–e175 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Strug LJ, Stephenson AL, Panjwani N & Harris A Recent advances in developing therapeutics for cystic fibrosis. Hum. Mol. Genet 27, R173–R186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fiorotto R et al. Src kinase inhibition reduces inflammatory and cytoskeletal changes in DeltaF508 human cholangiocytes and improves cystic fibrosis transmembrane conductance regulator correctors efficacy. Hepatology 67, 972–988 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huch M et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sampaziotis F et al. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat. Protoc 12, 814–827 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Staufer K, Halilbasic E, Trauner M & Kazemi-Shirazi L Cystic fibrosis related liver disease—another black box in hepatology. Int. J. Mol. Sci 15, 13529–13549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guggino WB The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc. Am. Thorac. Soc 1, 28–32 (2004). [DOI] [PubMed] [Google Scholar]
- 169.Li C & Naren AP CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr. Biol 2, 161–177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fiorotto R et al. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology 64, 2118–2134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akira S Toll-like receptors and innate immunity. Adv. Immunol 78, 1–56 (2001). [DOI] [PubMed] [Google Scholar]
- 172.Chattopadhyay S & Sen GC Tyrosine phosphorylation in Toll-like receptor signaling. Cytokine Growth Factor Rev 25, 533–541 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Medvedev AE et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem 282, 16042–16053 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Szabo G, Dolganiuc A & Mandrekar P Pattern recognition receptors: a contemporary view on liver diseases. Hepatology 44, 287–298 (2006). [DOI] [PubMed] [Google Scholar]
- 175.Chen XM et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol 175, 7447–7456 (2005). [DOI] [PubMed] [Google Scholar]
- 176.Fiorotto R et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology 141, 1498–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Scirpo R et al. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62, 1551–1562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schnabl B & Brenner DA Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wiest R, Albillos A, Trauner M, Bajaj JS & Jalan R Targeting the gut-liver axis in liver disease. J. Hepatol 67, 1084–1103 (2017). [DOI] [PubMed] [Google Scholar]
- 180.De Lisle RC & Borowitz D The cystic fibrosis intestine. Cold Spring Harb. Perspect. Med 3, a009753 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Flass T et al. Intestinal lesions are associated with altered intestinal microbiome and are more frequent in children and young adults with cystic fibrosis and cirrhosis. PLOS ONE 10, e0116967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lynch SV et al. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes 4, 41–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hirschfield GM, Karlsen TH, Lindor KD & Adams DH Primary sclerosing cholangitis. Lancet 382, 1587–1599 (2013). [DOI] [PubMed] [Google Scholar]
- 184.Ellinghaus D et al. Genome-wide association analysis in primary sclerosing cholangitis and ulcerative colitis identifies risk loci at GPR35 and TCF4. Hepatology 58, 1074–1083 (2013). [DOI] [PubMed] [Google Scholar]
- 185.Folseraas T et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol 57, 366–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Katt J et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 58, 1084–1093 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Mueller T et al. Enhanced innate immune responsiveness and intolerance to intestinal endotoxins in human biliary epithelial cells contributes to chronic cholangitis. Liver Int 31, 1574–1588 (2011). [DOI] [PubMed] [Google Scholar]
- 188.Banales JM et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol 16, 269–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]