Abstract
The nervous and immune systems are classically studied as two separate entities. However, their interactions are key to maintaining barrier functions at tissues constantly exposed to the outside environment. Here, we focus on the role of neuronal signaling in regulating the immune system at two major barriers: the skin and respiratory tract. Barrier tissues are heavily innervated by sensory and autonomic nerves, and densely populated by resident immune cells, allowing for rapid, coordinated responses to noxious stimuli, as well as bacterial and fungal pathogens. Neural release of neurotransmitters and neuropeptides allows for quick communication with immune cells and their recruitment. Besides maintaining homeostasis and fighting infections, neuroimmune interactions are also implicated in several chronic inflammatory conditions such as atopic dermatitis, COPD, and asthma.
Keywords: Neuro-immunology, Skin, lungs, nervous system, inflammation, asthma
THE NERVOUS AND IMMUNE SYSTEMS: ALLIES WORKING UNDER DURESS
Our barrier tissues are under constant assault from a variety of environmental threats including noxious chemicals, thermal changes, mechanical injury, and microbial pathogens. The nervous and immune systems are specifically armed to combat these assailants to maintain homeostasis and to coordinate host defense. Mammalian barrier tissues including the skin, lungs, and gut, are innervated by the peripheral nervous system (PNS) that serves to detect stimuli, including harmful ones, to respond to them, and to regulate autonomic functions. The immune system responds to threats such as pathogens and irritants through antimicrobial mechanisms and clearance of damaged tissues. Whereas neural responses happen almost instantaneously, immune responses can take minutes to hours; the integration of these two systems through neuroimmune interactions creates a coordinated network ideally poised to preserve tissue integrity. Crosstalk between these systems is bidirectional, with immune cells, nerves, and neurons capable of responding to each other’s products (e.g. neurotransmitter receptors on immune cells and cytokine receptors on neurons).
In this review, we highlight studies investigating neuroimmune interactions that occur in the skin and the lungs (for reviews on neuroimmune interactions in the gut, please see for instance [1–3]). Neural mediated control of immunity is a fast-moving area, and while we cannot encompass the whole breadth of the literature in this area, we highlight several recent advances, with the goal of focusing on studies demonstrating mechanisms by which neurons control immunity by direct signaling to either tissue-resident cells or recruited immune cells. A key neuroimmune reflex we do not cover is the cholinergic anti-inflammatory reflex, which acts through the vagus nerve, splenic macrophages, and cholinergic T cells (for a review, please see [4]). We begin with an overview of general concepts of the role of neuroimmune interactions at barrier tissues. We next discuss the contribution of neuroimmune interactions in the skin, discussing its role in acute infection and chronic inflammatory diseases. The final section discusses neuroimmune interactions in the lung, with a focus on neural mediation of airway inflammation and chronic obstructive pulmonary disease.
NEUROIMMUNE INTERACTIONS AT BARRIER TISSUES
Mammalian barrier tissues (e.g. skin, cornea, respiratory tract, gastrointestinal tract) interface with the environment, with the responsibilities to discern between innocuous and noxious stimuli, and to maintain homeostasis and integrity under changing conditions. The immune and nervous systems are tasked to carry out these essential functions. The immune system uses innate and adaptive mechanisms for host defense. The nervous system uses sensory neurons to induce protective nociceptive neural reflexes and the release of regulatory molecules and neurotransmitters that modulate inflammation to combat danger. Both systems coordinate their communication with epithelial cells to maintain barrier integrity to ward off threats. The crosstalk between the nervous and immune systems is a rapidly progressing field with new studies highlighting the importance of this communication within barriers sites.
Peripheral nervous system and immune system
The peripheral nervous system (PNS) consists of the somatosensory and motor branches. The motor branch is further divided into somatic and autonomic (sympathetic, parasympathetic, enteric) nervous systems. The somatosensory nervous system is responsible for mediating sensory functions including touch, proprioception, and pain. Specialized subsets of somatosensory neurons include nociceptors and pruriceptors responsible for detecting noxious or itch-inducing stimuli, respectively. We focus attention on these neurons because their activation is typically coupled with immunity and inflammation. The autonomic system innervates a large number of tissues and serves to control involuntary activities: the sympathetic nervous system participates in the body’s response to stress, whereas the parasympathetic nervous system mostly maintains homeostasis [5]. All parts of the PNS coordinate responses to stressors and stimuli at host barrier tissues.
The skin and respiratory tract are densely populated by resident immune cells including mast cells, dendritic cells, macrophages, innate lymphoid cells, and γδ T cells (see Box 1). These cells have unique functions that are ideally poised to fight off pathogens and mediate wound healing at barrier surfaces. Neuroimmune interactions at these barrier tissues are an effective way to coordinate host-defense.
BOX 1. The Skin Resident Innate Immune Cell Population.
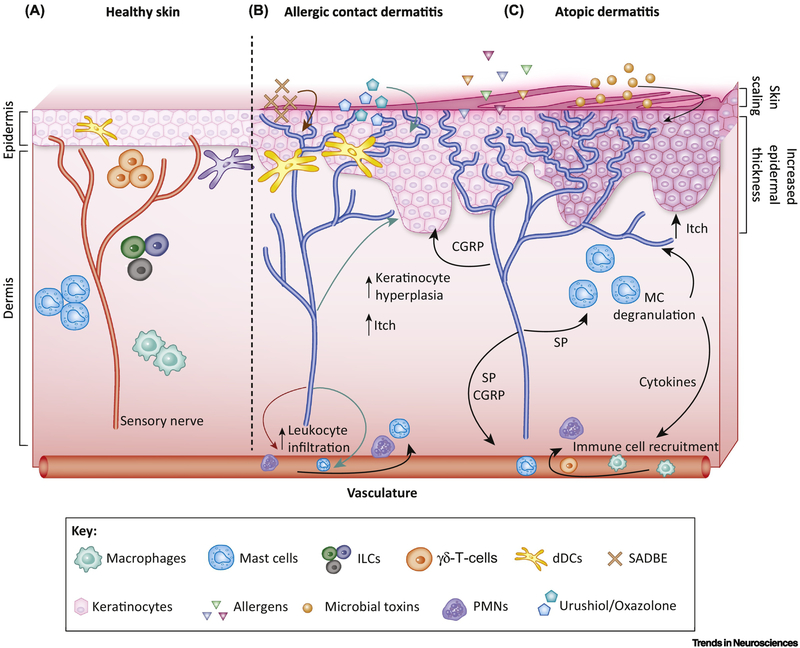
As one of the most densely innervated organs, the skin is an ideal environment for nerves to quickly communicate with resident immune cells (Figure 1a), coordinating essential functions such as host defense and homeostasis.
Macrophages are the most abundant hematopoietic skin immune cell population under steady state conditions. These cells act as key sentinels in pathogen detection and tissue damage, utilizing proinflammatory cytokines and chemokines to attract other immune cells from circulation to sites of damage [19]. Macrophages constitutively expresses neurotrophins such as nerve growth factor (NGF) and BDNF to maintain the growth and survival of neurons [20]. Macrophages can in turn respond to these factors and other secreted neuropeptides, establishing critical bidirectional crosstalk with neurons in the skin [21,22].
Skin mast cells (MCs) are in close proximity to dermal vasculature. MCs are typically associated with TH2 responses and allergic inflammation, although they have also been implicated in wound healing, pathogen defense, and contact hypersensitivity responses [19]. MCs are both potently activated by bioactive substances released by nerves (NGF, SP, etc.) and activate nerves as further discussed in Box 2.
Dendritic Cells (DCs) can be classified into two major populations, Langerhans Cells (LCs) and Dermal Dendritic Cells (dDCs). LCs are located in the epidermis where their dendrites interweave with keratinocytes, enabling sampling of the environment. dDCs, located in the dermis, are a heterogeneous cell population with several subsets: CD11b+, CD103+, and CD301b+ cell types. dDCs actively migrate throughout the dermis to survey the environment; they are important components of tolerance at steady state, immune regulation during skin damage, and in shaping the initial T-cell response to skin pathogens [19]. dDCs respond to CGRP, initiating skin inflammation in psoriasiform mouse models [23,24].
Dermal γδ T cells comprise 50% of the total dermal T cell population in mice. These cells, constitutively express IL-23 receptor, CCR6, and RORγT molecules (associated with Th17 cells); upon stimulation by IL-23 or IL-1β dermal γδ T cells produce IL-17A, which can augment neutrophil recruitment [19]. These cells are implicated in the pathogenesis of psoriasis and AD as discussed below [23–26].
Innate lymphoid cells (ILCs) (discussed in Box 3) are grouped into three subsets based on their developmental requirements: ILC1s, ILC2s, and ILC3s. ILCs were originally described in mucosal tissues, such as the gut, but have also been identified in the skin: ILC2s were found to be enriched in human AD lesions, and ILC3s recruited to imiquimod-induced psoriasiform lesions in mice [19,27].
Neurogenic inflammation and immune modulation
“Neurogenic inflammation” was first termed following the observations that swelling, redness, and heat produced by chemical irritants are all dependent on local innervation and that nerve stimulation leads to immediate vasodilation [6,7]. Neurogenic inflammation is mediated by sensory neuron release of neuropeptides, including calcitonin gene-related peptide (CGRP) and Substance P (SP), which act on the vasculature and induce mast cell (see Glossary) degranulation to produce edema, vasodilation, and immune cell extravasation. Besides neuropeptides, sensory neurons can also release ATP, glutamate, brain-derived neurotrophic factor (BDNF), and potassium, with many of these factors having their cognate receptors expressed by multiple immune cells (dendritic cells, T cells, neutrophils, and macrophages). Sensory neurons form close associations with many resident tissue immune cells as well, including mast cells, innate lymphocytes, and dermal dendritic cells (dDCs) [5,8] (Box 1). Expression of acetylcholine receptors (muscarinic and nicotinic) and norepinephrine receptors (including α and β adrenergic receptors) have been found on T cells, macrophages, dendritic cells, NK cells, B-cells, and other immune cells [9,10]. However, the role of Ach and NE signaling in directly mediating neurogenic inflammation is not well understood and further research is needed [11].
Although the focus of this review is primarily on the modulation of immune cells by neurons, it is important to note that neuroimmune interactions are bidirectional. Neuronal cell bodies and their nerve endings have receptors that can respond to immune cell derived cytokines, lipids, growth factors, and proteases. This can serve to help either amplify or dampen the neural response; in some cases, sensitization of the neuron can also occur. Immune cells, by themselves, are capable of synthesizing and releasing “neuromodulators” such as acetylcholine and dopamine to further regulate both immune cells and neurons, creating complex neuroimmunomodulatory circuits [8].
NEUROIMMUNE INTERACTIONS THAT REGULATE BARRIER FUNCTION
Barrier functions in the lungs and the skin are important in maintaining homeostasis and preventing infection. In mucosal surfaces such as the lung, CGRP, a neuropeptide from nociceptor neurons, has been found to regulate mucus production, which is a key aspect of barrier function [12,13]. Nociceptor neurons also mediate barrier leakiness from the lung parenchyma into the blood during bacterial infection [14]. Mechanisms such as tight junction regulation, antimicrobial peptide production, ciliary sweeping and prevention of water loss are all important lung epithelial functions. However not much is known about the neuroimmune interactions regulating these aspects of lung barrier integrity.
In the skin, keratinocytes are epithelial cells that make up the stratified epidermis of the skin (Figure 1a), and as such are important cells in barrier function (regulation of water loss, antimicrobial peptide secretion). These cells can also contribute to many inflammatory skin conditions in which barrier functions are compromised. In addition, keratinocytes mediate itch, through their release of TSLP, histamine, and ET-1, which activate surrounding pruriceptive nerves; itch promotes scratching and mechanical barrier disruption. The thickened scaly skin noted in inflammatory skin diseases can be the result of keratinocyte proliferation induced by neuropeptides (CGRP) and cytokines. Keratinocytes can also release NGF in response to SP and CGRP, mediating increased innervation, and leakiness of the skin barrier [15]. Bidirectional keratinocyte – neuron interactions represent potent feedback loops that lead to exacerbation of chronic skin conditions, but also highlight the importance of barrier function that neuroimmune interactions have in the skin.
Figure 1. Neuronal interactions with immune cells in healthy skin and in dermatitis.
a) In healthy conditions, the epidermal layer is made of tightly spaced keratinocytes, which helps to keep allergens, pathogens, and microbial toxins out. Skin resident innate immune cells including dermal DCs (dDCs), γδ T cells, ILCs, and mast cells are ideally poised to respond to signals communicated by surrounding sensory nerve fibers. b) Allergic contact dermatitis (ACD) is a T-cell mediated, type-IV hypersensitivity reaction induced by allergens/haptens that results in itchy and inflamed skin. The nociceptive ion channel, TRPA1, was found to mediate both persistent itch and inflammation (edema, leukocyte infiltration, and keratinocyte hyperplasia) in mouse models of ACD driven by squaric acid dibutylester (SADBE), urushiol, or oxazolone [33,88]. Sensory nerves are thought to modulate the immune response by interacting with antigen presenting cells (APCs) in these conditions [32]. c) Atopic dermatitis (AD) is an allergic inflammatory skin condition that results in thickened scaly skin and impaired epidermal barrier function, allowing the penetration of allergens and microbial toxins into the skin. Activated sensory nerves promote neurogenic inflammation (b, c), and secrete substance P (SP), which leads to degranulation of mast cells (MC) that release histamine and other cytokines. This pruritogenic cocktail potentiates the itch-scratch cycle characteristic of AD and also helps to further recruit immune cells to the inflamed area (darkened area surrounding each keratinocyte). Nerves also release CGRP, which mediates keratinocyte hyperplasia, resulting in increased epidermal thickness (b, c) [37]. Keratinocytes also release NGF (not shown), which leads to neuronal hyper-innervation and penetration of the sensory nerves into the topmost layers of the skin, illustrated in the figure by the nerve endings (blue) extending through the spaces in between keratinocytes into the topmost layers of the epidermis.
NEUROIMMUNE INTERACTIONS IN THE SKIN
The skin is one of the largest organs in the human body, and its integrity is necessary for homeostasis, protecting barrier function, and combating invading dangers such as pathogens. The nerve fibers innervating the skin are in close proximity to skin structural and functional cells including keratinocytes, fibroblasts, endothelial cells, Schwann cells, and resident immune cell populations [16]. The cutaneous sensory nerve fibers (CSNF), which innervate both the dermal and epidermal layers, make up the majority of skin nerve fibers. The CSNF originate from the dorsal root ganglia (DRG) in the spinal cord or from the trigeminal ganglia. DRG neurons project afferent fibers to the skin of the trunk of the body; nerve signals from these fibers synapse on to the dorsal horn of the spinal cord where signals are transduced to the brainstem and thalamus. Trigeminal ganglia neurons innervate the skin of the head and face. CSNF are responsible for sensory modalities including touch, thermosensation, mechanosensation, proprioception, stretch, itch, and pain [15,17]. The autonomic nervous system innervating the skin is largely sympathetic, and makes up a small overall percentage of the nerve fibers. These nerves are restricted to the dermal layer and innervate hair follicles, blood vessels, lymphatic vessels, apocrine and eccrine glands, and erector pili muscles [17,18]. The resident immune population ensures both protection against pathogens and maintenance of tolerance against innocuous antigens (Box 1) [19]. The skin is densely innervated, and neuroimmune interactions are important for communication with the environment and for response to changes in it. Consistently, aberrant neuroimmune interactions can be the root of several inflammatory skin conditions. We next discuss the role of the nervous system in regulating acute neurogenic inflammation, pathogenic infection, and immunity in several skin diseases.
Acute neurogenic inflammation
Irritants, noxious stimuli, and even pathogens exposed to the skin can promote neurogenic inflammation. Nociceptors release the neuropeptides CGRP and SP from nerve terminals that act on the vasculature and mast cells to induce vasodilation, edema, and immune cell recruitment. TRPA1 and CGRP was found to mediate the effects of vesicant induced skin injury, edema and inflammation [28]. TRPA1, as well as TRPV1, are important ion channels in inflammation because they are activated downstream of cytokine signaling as well as endogenous reactive species such as nitric oxide, peroxynitrite, and oxidized lipids [29]. Besides classically described irritants and noxious stimuli promoting acute neurogenic inflammation, this neural-driven process can be a component of inflammatory skin conditions, many of which are characterized by increased levels of SP and CGRP [30]. Recent work has shown that SP driven activation of mast cells is a key molecular mechanism of neurogenic inflammation, discussed in Box 2.
Box 2. Neural Regulation of Mast Cells.
Mast cells (MCs) are long-lived tissue resident cells typically recognized for their role in IgE-mediated allergic inflammation. However, MCs reach far beyond their classical role in allergies and are capable of providing innate immune defense, regulation of adaptive immunity, and mediation of pain and itch. These functions are mediated through the rapid release of chemical mediators stored in pre-formed granules. As such, MCs are present throughout the body with a majority found within connective tissues and barrier surfaces. MC-nerve interactions were one of the earliest examples of neuroimmune relationships described, both anatomically and functionally. Several recent reviews highlight MC-neural relationships in depth [40–42].
SP is the most widely studied neuronal mediator of MC degranulation, with its “traditional” receptor identified as neurokinin-1 (NK1). However several studies pointed to the inefficacy of NK1 antagonists in blocking SP-activation of human MCs [43]. Recent studies have identified that SP can also act through a class of receptors belonging to the MAS-related family of GPCRs [44–48]. A recent study found that basic secretagogues including SP, VIP, the canonical mast cell activator 48/40, and the antimicrobial peptide LL-37, were able to induce mouse mast cell degranulation both in vivo and in vitro through the single receptor, MrgprB2, the orthologue of MrgprX2 in humans [44] (Figure 3a). The identification of this receptor will allow for specific therapeutic targeting of MrgprX2 in chronic inflammatory and allergic diseases. In another study, it was determined that MCs release different sized granules based on whether they were activated via IgE independent or dependent mechanisms. Interestingly, when SP activated MCs via MrgprX2, degranulation was characterized by the rapid release of small spherical granules. In contrast when MCs where stimulated by IgE, degranulation resulted in larger, less spherical granules that not only took a longer time to be released, but were of a different composition than those induced by SP (and other MrgprX2 agonists) [46]. This highlights the difference in IgE vs. SP-mediated activation of MCs and how different features of MC-dependent inflammation are elicited. Topical treatment with mastoparan, which activates connective tissue MCs via MrgprX2, enhanced clearance of S. aureus from infected mouse skins and accelerated healing of dermonecrotic lesions [49]. Given the known role of SP to activate MrgprX2 receptors on mast cells and the ability of bacterial pathogens to activate peptidergic neurons that express SP [50,51] it would be interesting to determine if nerve-mast cell interactions play a role in infection.
Inflammatory Skin Diseases
Neuroimmune interactions contribute to the pathology of atopic dermatitis (AD), psoriasis, and allergic contact dermatitis (ACD) (Figures 1b–c and 2a). Sensory neurons mediate both the itch associated with these disease conditions and neurogenic inflammation, which lead to exacerbation and continuation of symptoms, and denervation resulting in amelioration of symptoms [30,31]. In these conditions, more is known about how the immune system communicates with sensory nerve endings to promote itch (TSLP, Il-31, histamine), but far less is known on how these neurons control inflammation.
Figure 2. Neuroimmune interactions in skin infection and psoriasis.
Several pathogens, such as Candida albicans, Staphylococcus aureus, and Streptococcus pyogenes have been determined to interact with sensory nerves in the skin to drive neuro-immune modulation. Pathogenic activation of sensory nerves leads to release of neuropeptides that modulate immune cells during infection, affecting infection and disease outcome. a) The pathogenic yeast, C. albicans, activates sensory nerves during epicutaneous infection to release the neuropeptide CGRP, which augments the release of IL-23 from CD301b+ dermal dendritic cells (dDCs). IL-23 then drives IL-17 release from γδ T-cells, which mediates resistance against C. albicans due to induction of polymorphonuclear neutrophil (PMN) recruitment and expression of antimicrobial peptides (AMPs) [24]. In a mouse model of psoriasis, a similar neuroimmune interaction was found: sensory nerves mediate IL-23 release from dDCs which mediate IL-17 release by γδ T-cells that contribute to psoriatic inflammation and plaque formation [23]. A similar neuro-immune mechanism operates during psoriasis-like inflammation. b) S. pyogenes and S. aureus are two bacterial pathogens known to cause painful and invasive skin infections such as abscesses, cellulitis, and necrotizing fasciitis. These pathogens directly activate sensory nerves via pore-forming toxins to produce pain and to release CGRP from their nerve terminals. In S. pyogenes infection, CGRP prevents the recruitment of neutrophils (PMNs) and the subsequent killing of the bacteria, worsening the infection outcome and bacterial clearance [53]. In S. aureus infection, nociceptor release of CGRP decreases TNFα production from macrophages and lymph node hypertrophy, subsequently decreasing bacterial killing [50].
Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a T-cell mediated, type-IV hypersensitivity reaction caused by various allergens/haptens, which results in itchy, inflamed skin. Cutaneous sensory nerves are hypothesized to modulate the immune response by interacting with antigen presenting cells (APCs) [32]. Recently, it was determined that TRPV1 and TRPA1 contribute differentially to contact hypersensitization in a mouse ACD model induced by squaric acid dibutylester (SADBE): SADBE directly activated both TRPV1 and TRPA1 channels on neurons to produce itch; only TRPV1 ion channels played a role in inflammation, as ablation of TRPV1+ neurons or genetic deficiency in TRPV1 led to increased inflammation [33]. By contrast, TRPA1 was necessary for mediating both itch and inflammation (edema, leukocyte infiltration, keratinocyte hyperplasia) in ACD mouse models using urushiol (poison ivy component) and oxazolone, whereas TRPV1 channels were not involved [34] (Figure 1b).
Atopic Dermatitis
AD is a chronic inflammatory skin condition characterized by chronic pruritus, thickened scaly skin, impaired epidermal barrier function, and a TH2 skewed allergic response. Chronic itch is a debilitating symptom of this condition, with postulated mechanisms highlighted here ([35]), one being MC degranulation (see Box 2). A stark feature of AD lesions is increased hyperinnervation and penetration of the sensory neurons into the epidermis leading to increased itch and release of neuropeptides (Figure 1c). Lesions and blood samples from AD sufferers are high in SP and NGF which lead to keratinocyte hyper-proliferation ([35,36]).
In an innervated skin model, where human AD skin samples were co-cultured with porcine DRGs, sensory nerves induced proliferation of keratinocytes dependent on CGRP (Figure 1c). Isolated AD skin samples used in this model also showed increased innervation and neurite outgrowth, CGRP release, and epidermal thickening compared to healthy skin sample controls [37]. These studies show that neuroimmune interactions in the skin could be key underlying mechanisms related to both inflammation and skin thickening in AD.
Psoriasis
Psoriasis is a skin inflammatory disorder characterized by a dysregulation in the IL-17/IL-23 axis, acanthosis, hyperkeratosis, and itch. Neuroimmune interactions mediate both induction of IL-23 signaling and inflammatory lesion formation. The role of the sensory nervous system in psoriasiform skin inflammation was first shown by cutaneous denervation of a psoriasis mouse model [31]. When the skin was surgically axotomized in KC-Tie2 psoriasiform mice, acanthosis significantly improved, CD4+ T cells and CD11c+ dendritic cells were decreased compared to the contralateral innervated side of the mouse. These characteristics were found to be largely dependent on CGRP and SP, suggesting targeting the nervous system as a treatment option for psoriasis. In a follow up study, botulinum neurotoxin A (BoNT-A) was injected intradermally in the KC-Tie2 mice [38]; this toxin cleaves SNAP25 and prevents local release of neuropeptides such as CGRP and SP. BoNT-A injection significantly improved both skin inflammation and epidermal hyperplasia. Small-scale human clinical trials have also shown BoNT-A’s effectiveness in improving plaque psoriasis [39]. Using an imiquimod-driven model of psoriasis in mice, TRPV1+ nerves were found to mediate IL-23 release by dDCs, which drive IL-17 expression by γδ T-cells [23], promoting psoriatic inflammation (Figure 2a). In all, sensory nerves play a major role in the propagation of inflammation in psoriasis, and it seems plausible that targeting neuroimmune interactions could offer a promising treatment approach.
Skin Infections
When fungal, bacterial, or viral pathogens breach the skin barrier, the immune system is recruited to the site of infection to combat the threat. Both host resistance and host tolerance mechanisms are regulated by the nervous system during infection. Pathogens have been shown to secrete molecules that directly interact with the sensory nervous system. Due to the necessity of neuroimmune interactions to barrier function, one might conjecture that these sensory nerves actively recruit immune cells to the site of infection. This is true in the case of the fungal pathogen, Candida albicans, in an epicutaneous skin infection model [24]. It was found that C. albicans directly activates nociceptive sensory nerves to release CGRP, which in turn augments the release of IL-23 from dDCs; IL-23 subsequently drives IL-17 production by γδ T-cells to mediate resistance against this fungus (Figure 2a). Therefore, nociceptors are necessary for successful protection against C. albicans skin infection. Recent work has also shown that nociceptors boost the resolution of osteoinflammation caused by C. albicans by suppressing βglucan-induced inflammation and osteoclast multinucleation through CGRP signaling [52].
Activation of sensory nerves can also potently suppress the recruitment and function of immune cells during skin infection. In a recent study, the pathogen responsible for necrotizing fasciitis, Streptococcus pyogenes, was found to activate TRPV1+ nerves, promoting the secretion of CGRP. CGRP in turn prevented the recruitment of neutrophils and subsequent S. pyogenes opsonophagocytic killing, partly by reduction of myeloperoxidase (MPO) activity (Figure 2b) [53]. When mice were depleted in the TRPV1 subset of neurons, infection severity decreased, and neutrophils to the site of infection increased. Blockade of neuronal signaling using botulinum neurotoxin or the CGRP receptor, BIBN4096, led to significantly improved neutrophil recruitment and infection outcome. Similarly, when a large portion of nociceptive neurons were depleted using Nav1.8-Cre lineage ablation in mice, monocyte recruitment and lymphadenopathy increased in a Staphylococcus aureus subcutaneous infection model; CGRP also decreased TNFα production from macrophages (Figure 2b) [50].
Neuroimmune signaling may be finely tuned to the pathogen, and even to the specific area of the skin that is affected. For example, it is possible that itch-mediating pruriceptor neurons that largely innervate the epidermis could respond to distinct pathogens compared to deeper tissue innervating pain-mediating nociceptor neurons. S. aureus subcutaneous infection causes painful abscesses, while its epicutaneous infection can contribute to chronic itch. Skin colonization by S. aureus affects over 90% of AD patients, often exacerbating this itch-inducing condition. Epicutaneous infection with S. aureus leads to inflammation via keratinocyte release of IL-36 that promotes production of IL-17 from γδ T-cells [25,26]. Our lab has determined that S. aureus is capable of interacting with nociceptor sensory nerves to produce pain during subcutaneous infections by their secretion of bacterial pore-forming toxins [50,51]. However, the question remains whether this pathogen can directly activate pruriceptor nerves at the barrier surface to induce itch and modulate neuroimmune interactions.
Besides producing pain/itch during infection, other pathogens silence pain. Determining the molecular mechanisms involved could lead to the development of novel analgesics. The pathogen Mycobacterium ulcerans produces extensive skin lesions known as Buruli ulcers that are characteristically painless. Originally it was believed that this analgesia was due to nerve damage, however a recent study showed that mycolactone, an essential polyketide toxin to this pathogen, interacts with angiotensin 2 receptors (AT2R) to hyperpolarize sensory nerves through the opening of TRAAK potassium channels [54,55]. As mycolactone can diffuse throughout the body, another study suggested that part of this analgesic effect may be due to the decreased neuro-inflammation induced by this molecule [56]. However, in this study the effects of mycolactone were tested by intrathecal delivery; whether mycolactone would reach the CNS or DRG during M. ulcerans skin infection is currently unclear. More recently, AT2R was detected on macrophages infiltrating nerve injury sites, and these AT2R+ macrophages were necessary for development of chronic neuropathic pain in peripheral tissues [57]. It would be interesting to determine whether M. ulcerans also acts on AT2R+ macrophages to block pain.
Sympathetic Nervous System and Skin Immunity
The sympathetic nervous system innervates the hair follicles and sebaceous glands in the skin, regulating stem cell regeneration, but its role in neuroimmune interactions is not as well studied. Sympathetic nerves can have important roles in immunity, especially as chronic “stress” is known to exacerbate inflammatory skin diseases such as AD [58]. Using a heterotypic chronic stress model in rats, β2 adrenergic receptors were found to mediate increased itch hypersensitivity after administration of 5-hydroxytryptamine through the release of proinflammatory factors (TNF-α and IL-1β) [59].
NEUROIMMUNE INTERACTIONS IN THE RESPIRATORY TRACT
Gas exchange with the external atmosphere occurs in the lungs. During respiration, the lung epithelial surface acts as a barrier surface that comes into direct contact with the environment [60]. With the constant risk of exposure to harmful substances, detection of these potential dangers and pulmonary immunity against them are important. Nerves are therefore critical in quickly detecting harmful substances to coordinate immune responses, which ultimately can limit the magnitude of lung infection, and help resolve inflammation. Similar to the skin, resident immune cells are also important for the quick response to barrier insult, including macrophages, in which a sub-population was recently described to be closely associated with nerves in the lungs [61]. We highlight recent studies showing roles for lung-innervating neurons in regulating immune cell function in asthma, chronic obstructive pulmonary disorder (COPD), and lung infections.
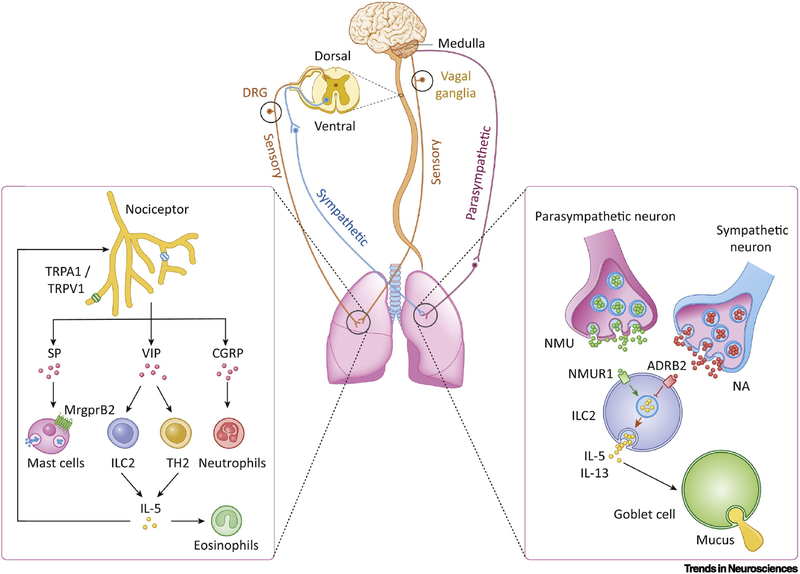
Sensory nerve lung innervation largely originates from vagal afferents whose cell bodies reside in the nodose and jugular ganglia; remaining sensory nerve innervation comes from the DRG [62]. Nociceptive afferent nerve endings are located in the lung parenchyma and near the airways; this poises them to detect noxious stimuli such as allergens, irritants, and pathogens, contained in inhaled air, expelling them through cough [5]. Sympathetic nerve innervation originates from the upper six thoracic segments of the spinal cord, which synapse with the sympathetic ganglia; postganglionic fibers then innervate the lung. The cholinergic parasympathetic nerves originate from the vagal nuclei in the medulla; the superior and recurrent laryngeal vagal nerve branches synapse at the parasympathetic ganglia to innervate the airways [62] (Figure 3). The sympathetic nervous system controls bronchodilation and mucous production, whereas the parasympathetic nervous system controls bronchoconstriction. Regulation of oxygen and carbon dioxide levels, as well as neural reflexes such as coughing, results from these systems. The necessity of neuroimmune interactions in the lung was shown through vagatomy, which worsens lung infections, inflammation, and injury, while increasing pro-inflammatory cytokine levels in circulation in an Escherichia coli induced acute lung injury model [63].
Figure 3. Neuroimmune interactions in the lungs.
The lungs (center) are innervated by the parasympathetic, sympathetic, and sensory components of the peripheral nervous system. Parasympathetic nerves travel to the lungs via the vagus nerve, which originate in the medulla. Sympathetic nerves originate in the ventral horn of the spinal cord. Nociceptive sensory nerves either innervate the lungs via the vagus nerve from the nodose and jugular ganglia or from the dorsal root ganglion located in the spinal cord. Left: Lung-innervating nociceptor neurons can be activated by TRPV1 and/or TRPA1 stimulation in response to a variety of stimulants such as chemicals and irritants. Release of the neuropeptide calcitonin gene-related peptide (CGRP) can inhibit neutrophil recruitment and surveillance [14,86,89], whereas vasoactive intestinal peptide (VIP) activates ILC2s [64] and TH2 cells [77]. TH2 cells produce IL-5, which is a potent activator of eosinophils. Substance P (SP) binds to mas-related G-protein coupled receptor member B2 (MrgprB2) on mouse mast cells [44], resulting in their degranulation and thus the release of histamine and other cytokines. Right: Lung-innervating autonomic neurons modulate ILC2 function. Parasympathetic nerves release neuromedin U (NMU) which acts on NMU receptor 1 (NMUR1) expressed on type 2 innate lymphoid cells (ILC2s) to trigger release of interleukin 5 (IL-5) and interleukin 13 (IL-13). IL-5 and IL-13 can then act on goblet cells to promote mucus release [3,65,66]. Noradrenaline (NA) release from sympathetic nerves, which binds to β2-adrenoreceptor (ADRB2) on ILC2s, inhibits the release of these cytokines [67,68].
Sensory neuron-immune interactions in asthma and airway inflammation
Neuroimmune interactions have been extensively studied in asthma, an airway allergic reaction characterized by airway hyper-responsiveness and inflammation [73]. Nociceptor sensory nerves were shown to play an important role in the etiology of asthma in several studies, utilizing ovalbumin (OVA) sensitization mouse models, as discussed below. The nociceptive TRPA1 ion channel was first shown to play a critical role in driving immune cell cytokine production and airway hyper-reactivity in this model [74]. Treatment of mice with capsaicin to induce loss of TRPV1+ nociceptor neurons reduced both eosinophil infiltration and inflammation in OVA induced airway inflammation [75]. Targeted genetic ablation of TRPV1+ neurons in the nodose/jugular vagal ganglia, which provides major sensory innervation to the lungs, or silencing of these neurons using a tetanus-toxin reporter, was shown to significantly reduce bronchial hyper-responsiveness in the OVA mouse model [76]. When NaV1.8+ nociceptors were genetically ablated or pharmacologically inhibited using the membrane impermeant sodium channel blocker, QX-314, immune cell infiltration and bronchial hyper-responsiveness were reduced [77]. Sensory neuron expression of VIP was thought to contribute to activation of both CD4+ TH2 cells (Figure 3, left) and innate lymphoid type 2 cells (ILC2s) (Box 3) [77]. Coexposure of the pollutants PM2.5 and formaldehyde in the OVA mouse model of asthma exacerbated the activation of TRPV1 signaling pathways, leading to increased inflammation. Treatment with capsazepine (TRPV1 antagonist) decreased both neuropeptide production and oxidative stress [73].
Box 3. Neural Regulation of Innate Lymphoid Cells.
Innate Lymphoid Cells (ILCs) are a heterogeneous population of cells that are diverse in their cytokine production, effector functions, and tissue locations. ILCs characteristically do not express TCR or BCR, and have characteristics that resemble innate lymphocytes. Based on their developmental requirements, cytokine and cell surface marker expression, ILCs are grouped into three populations: ILC1s, ILC2s, and ILC3s [27].
Recent work has shown that neurons closely interact with ILC2s, though it is likely that there are other major interactions that occur between neurons and the other ILC subtypes. ILC2s are potent sources of cytokines that contribute to the type 2 inflammatory response; in particular, ILC2s are associated with wound healing, allergy, and parasitic worm infection at mucosal surfaces. Vasoactive intestinal peptide (VIP) was first shown to interact through VPAC2 receptors expressed on ILC2s in the gut, suggesting that neural-ILC interactions could partake at mucosal surfaces [64] (Figure 3). Further studies indicated a role for Neuromedin U (NMU), a neuropeptide produced by cholinergic neurons, in the regulation of ILC2s; both NMU+ nerve fibers and ILCs are in close proximity with each other in the lungs, with ILC2s selectively expressing NMU receptor 1 (NMUR1). Activation of ILC2s by NMU induced rapid expression of type-2 inflammatory cytokines (IL-5 and IL-13) which act on goblet cells to induce goblet cell hyperplasia and mucous production (Figure 3b); this mechanism of neural-driven ILC2 activation was critical to mediating both airway hypersensitivity and inflammation following mouse exposure to allergens and parasitic worms [3,65,66]. In contrast, ILC2s are inhibited by the sympathetic nervous system through the β2AR. Catecholaminergic neurons, which secrete molecules such as norepinephrine or noradrenaline (which bind β2AR), were recently shown to act as off-switches that dampen ILC2 responses. β2AR deficiency resulted in exaggerated type-2 inflammation and ILC2 responses in the lungs; β2AR agonist treatment led to reduced inflammation and impaired ILC2 responses [14,67,68] (Figure 3b). These recent studies show that there are multiple tracks by which the nervous system is poised to trigger barrier protection programs that can either switch on or off ILC2 responses.
Pulmonary neuroendocrine cells (PNECs) are rare airway epithelial cells with poorly understood functions. In rodents, these cells can form highly innervated, clustered neuroepithelial bodies. In an Ascl1 mutant mouse model, which prevents PNEC formation in airway epithelium, ILC2s and goblet cell hyperplasia were both decreased under allergic asthma conditions. PNEC release of CGRP and GABA was determined to be necessary for ILC2 cytokine expression and goblet cell hyperplasia, respectively [69].
All three subtypes of ILCs have been found in the skin under steady state conditions [70]. The importance of skin ILCs to the pathology of inflammatory skin conditions is just emerging, and neural-ILC connections have yet to be established. Skin ILC2s in atopic dermatitis (AD) were found to be regulated by TSLP, IL-25, and IL-33, all of which are up-regulated in human AD lesions [27,71]. Psoriasis is associated with epidermal thickening (IL-22) and neutrophilic invasion (IL-17A/F); these cytokines were found to be largely attributable to skin populations of ILC3s and γδ T cells rather than Th17 cells [72]. Given the strong neural contribution to both AD and psoriasis, it is possible that skin ILC subtypes are also regulated by nerves during inflammation.
Recent studies have shown a role for TRP channels in other models of asthma. TRPA1 and TRPV1 channels were also shown to mediate the induction of airway hyperreactivity (AHR) caused by TDI (toluene-2,4-diisocyanate), a model known to induce immune mediated asthma in mice. MCs were shown to also be crucial for AHR with speculation by the authors on whether degranulation of MCs induced by SP was a key mechanism; however follow up studies are needed to confirm the neuroimmune interaction occurring in this context [78]. Nerves and MCs have been shown to interact closely in several tissues, including the skin and lungs. Recent work has uncovered a critical role for the MrgprX2 receptor in human mast cells (and MrgprB2 in mouse mast cells) in detecting SP released from nerves to mediate mast cell degranulation (see Figure 3 left and Box 2).
Autonomic neuroimmune interactions in asthma and airway inflammation
The parasympathetic nervous system interacts with immune cells through the action of acetylcholine on muscarinic receptors, to induce airway inflammation. Activation of muscarinic receptors promotes release of several cytokines and growth factors involved in asthma and COPD pathology. During allergic airway inflammation, epithelial damage promotes reflex mechanisms by exposing vagal nerve endings in the submucosa to the airway lumen leading to Ach release from vagal parasympathetic neurons. M1 receptor (M1R) activation on epithelial cells can induce Leukotriene-B4 (LTB4) release, stimulating neutrophil, eosinophil, and monocyte chemotaxis [79]. Muscarinic 3 receptors (M3R) on structural cells in the lung also play a pro-inflammatory role; genetic ablation of M3R prevents neutrophilic airway inflammation in response to cigarette smoke exposure [80]. Muscarinic agonists can also act on macrophage M3R and M5R, promoting both their chemotaxis and release of LTB4 [79] in the lung. Recent work also highlights a key interaction of neurons with innate lymphoid cells (ILCs) at mucosal surfaces (Box 3). Type 2 ILCs were found to express high levels of the neuropeptide receptor NMUR1. Neuromedin U (NMU), a neuropeptide, whose main source is cholinergic neurons, acts on the NMUR1 receptor to increase ILC2 proliferation and cytokine production during helminth and allergen challenge in both the lungs and the gut [3,65,66].
The sympathetic nervous system also modulates immune cells via noradrenaline mediated activation of β2 adrenergic receptors (β2AR) on ILCs and other immune cells [14,67,68,81]. β2AR agonists are potent smooth muscle relaxers and can inhibit immune cell recruitment, activation, cytokine release and their survival [82]. Paradoxically, β2AR is also needed to induce a full asthma phenotype in mice, as its activation on airway epithelial cells was necessary for inducing the cardinal features of asthma (inflammation, mucus production, and airway hyperresponsiveness) by regulating responses to IL-13 [83]. By contrast, β2AR signaling inhibited activation of ILC2 in mouse models of asthma and inflammation, leading to dampening of key cytokines that drive airway inflammation [67]. In this way, the autonomic nervous system has distinct mechanisms by which it can control airway immunity and responsiveness.
Neuroimmune interactions during COPD
COPD, a progressive disease involving pulmonary inflammation and obstruction, causes an increase in M1R and M3R expression in airway structural and sputum cells as a result of prolonged Ach release. In a mouse model of COPD caused by exposure to cigarette smoke, treatment with iotropium, which is a long acting muscarinic antagonist, decreased the levels of several inflammatory mediators (IL-6, TNF-α, LTB4) in the lungs [84]. In a rat model of resistive breathing (RB) that models severe COPD, tiotropium used prior to induction of RB reduced inflammatory infiltrates and attenuated lung injury and protein in BALF (bronchoalveolar lavage fluid) [85] compared to untreated rats. Further studies will be needed to clarify the neuroimmune interactions in COPD.
Neuroimmune interactions during lung infections
Bacteria, viruses, and fungi cause lung inflammation, irritation (cough), and AHR. Neuroimmune interactions at the lung/air barrier surface also occur during lung infections. During S. aureus induced lethal bacterial pneumonia, TRPV1+ nociceptors suppress immunity against this bacterium through CGRP release; this neuropeptide decreases the recruitment and surveillance of neutrophils that mediate killing of bacterial pathogens (Figure 3, left). As a result, nociceptor ablation increases survival, cytokine induction, and bacterial clearance in the lung [14]. Of note, CGRP is also expressed by PNECs in the lung in addition to sensory nerves. PNECs were found to play a role in regulating immune cell recruitment in the lungs [69,86]. Future work will be required to dissociate neural vs. PNEC derived CGRP in different lung infections.
In influenza A viral infections, the sympathetic nervous system increases proinflammatory cytokines and exacerbates infection. Peripheral sympathectomy (using 6-hydroxydopamine), reduced morbidity and mortality in lethal influenza A virus-induced pneumonia due to a decrease in the influx of monocytes, neutrophils, and NK cells, as well as a diminished innate cytokine response [87].
Concluding Remarks and Future Perspectives
Barrier tissues including the skin and respiratory tract are constantly exposed to the outside environment, and to threats that come with that to our health and internal homeostasis. These tissues are therefore heavily innervated, with the nervous system poised to quickly detect insults and in turn recruit the immune system and communicate with it. This establishes critical neuroimmune crosstalk that is necessary for maintenance of barrier function and host defense. Although there are some shared features between the lung and skin in their overall protection strategy, it is not currently clear whether there are specific shared mechanisms between the lungs and the skin. Neuropeptide regulation of immune cells and neurogenic inflammation seem to be commonalities between the two barriers, however specific similarities and differences in their functionalities are currently speculative. Moreover, the skin directly interfaces with the external environment, whereas the lung is a mucosal tissue, and each of the two barriers has distinct subtypes of immune cells and epithelial makeup. It will be interesting to see whether parallels in neuroimmune interactions between the two barrier sites emerge as the field progresses.
There are still many remaining questions to be answered concerning neuroimmunity in the skin and respiratory tract, including the logic of why certain neurotransmitters or neuropeptides mediate immune cell proliferation or activation, whereas others suppress immune cell function. The role of neuroimmune interactions in barrier surfaces during development is another important area for future investigations. Defining the key molecular mechanisms underlying the communication between the immune and nervous systems could lead to novel therapeutic targets. Given that neurologists have already developed highly specific pharmacologic agonists and antagonists for many neurotransmitter receptors to treat neurologic diseases, these drugs may be repurposed to modulate neuroimmune signaling in the skin or respiratory tract, which could open up exciting opportunities for possible treatments of inflammatory and infectious diseases. \
Outstanding Questions.
What is the integrative logic of neural control of immunity in barrier surfaces? Although it is well established that both the sensory and autonomic branches of the peripheral nervous system control immunity, the biological advantages for why certain peripheral neural signals activate immune responses while others suppress them remains unclear. Understanding how neural signals are integrated by the immune system as a whole and within individual cell-types is an important future research topic.
Can neural-targeted therapies be a viable option to treat inflammatory diseases? Chronic inflammatory diseases such as rheumatoid arthritis, asthma, atopic dermatitis, and colitis are mostly treated with immune-targeted drugs. What are the effects of individually treating the immune or neural components of these diseases? Would a two-pronged approach targeting both systems alleviate immunopathology more quickly?
Can information of how pathogens impact neurons be used to develop novel approaches to treat infection? Does the location of pathogen invasion affect neuroimmune interactions (i.e. types of immune cells recruited, neuroimmune modulation mechanism)? Does the same pathogen differentially induce neuroimmune interactions at different barrier surfaces (i.e. gut, lungs, skin)? These questions could lend insight into future treatments as antibiotic resistant pathogens are on the rise.
How does the microbiota impact neuroimmune interactions at barrier tissues? Emerging evidence shows that both microbes and pathogens regulate neural function. Gut, lung, or skin commensal microbes may have a major impact on neuroimmune signaling.
Highlights.
Barrier tissues, such as the respiratory tract and skin, are major sites where swift communication between the peripheral nervous system and immune system occurs.
Recent insights uncover the molecular mechanisms by which nerves regulate tissue resident immune cells, including innate lymphoid cells (ILCs) and mast cells.
Bacterial, fungal, and parasitic pathogens can directly signal to peripheral sensory nerves to induce neuroimmune interactions during infection of barrier tissues.
Neuroimmune interactions are involved in the exacerbation of many chronic inflammatory diseases including asthma, COPD, atopic dermatitis, and psoriasis.
Immune cells in the lungs and skin can be positively or negatively modulated by the nervous system depending on the type of peripheral sensory or autonomic nerves – representing neuroimmune regulatory switches.
Acknowledgements
The Chiu lab is funded by NIH grant F31 AI138384-01A (K.J.B), NIH/NCCIH DP2AT009499 (I.M.C.), NIH/NIAID R01AI130019 (I.M.C.), and Chan-Zuckerberg Initiative (I.M.C.). Select images were adapted from Servier Medical Art (www.servier.com).
GLOSSARY
- Antigen Presenting Cells
cells that present antigens complexed with major histocompatibility complexes (MHCs) on their surfaces to T cells
- BALF
the fluid that results from the diagnostic procedure by which cells and other components from bronchial and alveolar spaces are obtained for various studies or diagnoses. It is typically performed to diagnose lung diseases
- Dermal Dendritic Cells (dDCs)
an antigen presenting cell located in the skin dermal layer, which processes and presents antigens on its cell surface to T-cells. dDCs act as messengers between the innate and the adaptive immune systems
- Eosinophils
a granulocytic immune cell primarily responsible for combating extracellular parasites and usually associated with allergic responses
- Innate Lymphoid Cells (ILCs)
Innate, tissue resident innate immune cells lacking antigen receptors, often having functions analogous to helper T cells including protective immunity, regulation of homeostasis and inflammation, and tissue repair at barrier surfaces
- Leukotrienes
a family of eicosanoid inflammatory mediators produced by the oxidation of arachidonic acid and released by leukocytes
- Mast Cells
long-lived tissue-resident cells with an important role in many inflammatory settings including host defense against parasitic infections and allergic reactions. Mast cells degranulate upon stimulation, and release important inflammatory mediators stored in granules including cytokines, histamine, serotonin, and proteases
- TH2 cells
orchestrate protective type 2 immune responses such as those targeting extracellular parasites or facilitating wound repair. However, TH2 cells have also been found to contribute to several allergic and inflammatory diseases, like asthma
- γδ T-cells
T cells that have T cell receptors with a γ chain and a chain, with the primary function of recognizing lipid antigens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lai NY et al. (2017) Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Intern. Med 282, 5–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veiga-Fernandes H and Mucida D (2016) Neuro-immune interactions at barrier surfaces. Cell 165, 801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallrapp A et al. (2017) The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlov VA and Tracey KJ (2017) Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci 20, 156–166 [DOI] [PubMed] [Google Scholar]
- 5.McMahon SB et al. (2015) Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci 16, 389–402 [DOI] [PubMed] [Google Scholar]
- 6.Bruce AN (1913) Vaso-Dilator Axon-Reflexes. Q. J. Exp. Physiol 6, 339–354 [Google Scholar]
- 7.Jancsó N et al. (1967) Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother 31, 138–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinho-Ribeiro FA et al. (2017) Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 38, 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavan SS et al. (2017) Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 46, 927–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney M and Ganta C (2014) Autonomic Nervous System and Immune System Interactions. Compr. Physiol 4, 1177–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlereth T et al. (2014) Inflammation in CRPS: Role of the sympathetic supply. Auton. Neurosci 182, 102–107 [DOI] [PubMed] [Google Scholar]
- 12.Webber SE et al. (1991) The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br. J. Pharmacol 102, 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson SD et al. (2004) Proximal airway mucous cells of ovalbumin-sensitized and - challenged Brown Norway rats accumulate the neuropeptide calcitonin gene-related peptide. Am. J. Physiol. Lung Cell. Mol. Physiol 287, L286–295 [DOI] [PubMed] [Google Scholar]
- 14.Baral P et al. (2018) Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med 24, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwendinger-Schreck J et al. (2015) Interactions Between Keratinocytes and Somatosensory Neurons in Itch In Pharmacology of Itch pp. 177–190, Springer, Berlin, Heidelberg: [DOI] [PubMed] [Google Scholar]
- 16.Peters EMJ et al. (2012) The neuroimmune connection interferes with tissue regeneration and chronic inflammatory disease in the skin: Stress and neuroimmune plasticity. Ann. N. Y. Acad. Sci 1262, 118–126 [DOI] [PubMed] [Google Scholar]
- 17.Laverdet B et al. (2015) Skin innervation: Important roles during normal and pathological cutaneous repair. Histol. Histopathol 30, 11610. [DOI] [PubMed] [Google Scholar]
- 18.Vetrugno R et al. (2003) Sympathetic skin response: basic mechanisms and clinical applications. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc 13, 256–270 [DOI] [PubMed] [Google Scholar]
- 19.Tay SS et al. (2014) The Skin-Resident Immune Network. Curr. Dermatol. Rep 3, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manti S et al. (2017) Chapter Twelve - The Role of Neurotrophins in Inflammation and Allergy In Vitamins and Hormones 104 (Litwack G, ed), pp. 313–341, Academic Press; [DOI] [PubMed] [Google Scholar]
- 21.Lim JE et al. (2017) A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep 7, 9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leal EC et al. (2015) Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol 185, 1638–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riol-Blanco L et al. (2014) Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashem SW et al. (2015) Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 43, 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H et al. (2017) Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe 22, 653–666.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa S et al. (2017) Staphylococcus aureus Virulent PSMα Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 22, 667–677.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS et al. (2013) TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med 5, 170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achanta S et al. (2018) TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation. Toxicol. Lett 293, 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viana F (2016) TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J. Physiol 594, 4151–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi JE and Nardo AD (2018) Skin neurogenic inflammation. Semin. Immunopathol 40, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrowski SM et al. (2011) Cutaneous Denervation of Psoriasiform Mouse Skin Improves Acanthosis and Inflammation in a Sensory Neuropeptide-Dependent Manner. J. Invest. Dermatol 131, 1530–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen HH et al. (2017) High-concentration topical capsaicin may abolish the clinical manifestations of allergic contact dermatitis by effects on induction and elicitation. Med. Hypotheses 99, 53–56 [DOI] [PubMed] [Google Scholar]
- 33.Feng J et al. (2017) Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun 8, 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu B et al. (2013) TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 27, 3549–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollanazar NK et al. (2016) Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin. Rev. Allergy Immunol 51, 263–292 [DOI] [PubMed] [Google Scholar]
- 36.Tominaga M and Takamori K (2014) Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol 41, 205–212 [DOI] [PubMed] [Google Scholar]
- 37.Roggenkamp D et al. (2013) Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J. Invest. Dermatol 133, 1620–1628 [DOI] [PubMed] [Google Scholar]
- 38.Ward NL et al. (2012) Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J. Invest. Dermatol 132, 1927–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschenbeck KA et al. (2018) Neuromodulatory treatment of recalcitrant plaque psoriasis with onabotulinumtoxinA. J. Am. Acad. Dermatol 79, 1156–1159 [DOI] [PubMed] [Google Scholar]
- 40.Forsythe P (2019) Mast Cells in Neuroimmune Interactions. Trends Neurosci. 42, 43–55 [DOI] [PubMed] [Google Scholar]
- 41.Gupta K and Harvima IT (2018) Mast cell-neural interactions contribute to pain and itch. Immunol. Rev 282, 168–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian H et al. (2016) Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol 138, 700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voisin T et al. (2017) Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int. Immunol 29, 247–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeil BD et al. (2015) Identification of a mast-cell-specific receptor crucial for pseudoallergic drug reactions. Nature 519, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatemoto K et al. (2006) Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun 349, 1322–1328 [DOI] [PubMed] [Google Scholar]
- 46.Gaudenzio N et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Invest 126, 3981–3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian H et al. (2011) PMX-53 as a Dual CD88 Antagonist and an Agonist for Mas-Related Gene 2 (MrgX2) in Human Mast Cells. Mol. Pharmacol 79, 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian H et al. (2016) Roles of MAS-related G protein coupled receptor-X2 (MRGPRX2) on mast cell-mediated host defense, pseudoallergic drug reactions and chronic inflammatory diseases. J. Allergy Clin. Immunol 138, 700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arifuzzaman M et al. (2019) MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv 5, eaav0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu IM et al. (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blake KJ et al. (2018) Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat. Commun 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maruyama K et al. (2017) Nociceptors Boost the Resolution of Fungal Osteoinflammation via the TRP Channel-CGRP-Jdp2 Axis. Cell Rep. 19, 2730–2742 [DOI] [PubMed] [Google Scholar]
- 53.Pinho-Ribeiro FA et al. (2018) Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 173, 1083–1097.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marion E et al. (2014) Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell 157, 1565–1576 [DOI] [PubMed] [Google Scholar]
- 55.Song O-R et al. (2017) A Bacterial Toxin with Analgesic Properties: Hyperpolarization of DRG Neurons by Mycolactone. Toxins 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isaac C et al. (2017) Mycolactone displays anti-inflammatory effects on the nervous system. PLoS Negl. Trop. Dis 11, e0006058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shepherd AJ et al. (2018) Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc. Natl. Acad. Sci 115, E8057–E8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arck P and Paus R (2006) From the brain-skin connection: the neuroendocrine-immune misalliance of stress and itch. Neuroimmunomodulation 13, 347–356 [DOI] [PubMed] [Google Scholar]
- 59.Peng X-Y et al. (2015) Adrenergic β2-receptor mediates itch hypersensitivity following heterotypic chronic stress in rats. Neuroreport 26, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 60.Whitsett JA and Alenghat T (2015) Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol 16, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakarov S et al. (2019) Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, [DOI] [PubMed] [Google Scholar]
- 62.Belvisi MG (2002) Overview of the innervation of the lung. Curr. Opin. Pharmacol 2, 211–215 [DOI] [PubMed] [Google Scholar]
- 63.Su X et al. (2010) Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. Baltim. Md 1950 184, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nussbaum JC et al. (2013) Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardoso V et al. (2017) Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klose CSN et al. (2017) The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moriyama S et al. (2018) β2-adrenergic receptor–mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 [DOI] [PubMed] [Google Scholar]
- 68.Veiga-Fernandes H and Artis D (2018) Neuronal–immune system cross-talk in homeostasis. Science 359, 1465–1466 [DOI] [PubMed] [Google Scholar]
- 69.Sui P et al. (2018) Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebbo M et al. (2017) Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol 17, 665–678 [DOI] [PubMed] [Google Scholar]
- 71.Salimi M et al. (2013) A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med 210, 2939–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pantelyushin S et al. (2012) Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest 122, 2252–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song J et al. (2017) Mediating Role of TRPV1 Ion Channels in the Co-exposure to PM2.5 and Formaldehyde of Balb/c Mice Asthma Model. Sci. Rep 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caceres AI et al. (2009) A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. U. S. A 106, 9099–9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogerio AP et al. (2011) C-fibers, but not the transient potential receptor vanilloid 1 (TRPV1), play a role in experimental allergic airway inflammation. Eur. J. Pharmacol 662, 55–62 [DOI] [PubMed] [Google Scholar]
- 76.Tränkner D et al. (2014) Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl. Acad. Sci. U. S. A 111, 11515–11520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talbot S et al. (2015) Silencing Nociceptor Neurons Reduces Allergic Airway Inflammation. Neuron 87, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devos FC et al. (2016) Neuro-immune interactions in chemical-induced airway hyperreactivity. Eur. Respir. J 48, 380–392 [DOI] [PubMed] [Google Scholar]
- 79.Kolahian S and Gosens R (2012) Cholinergic regulation of airway inflammation and remodelling. J. Allergy 2012, 681258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kistemaker LEM et al. (2015) Muscarinic M3 receptors on structural cells regulate cigarette smoke-induced neutrophilic airway inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 308, L96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ağaç D et al. (2018) Adrenergic Signaling at the Interface of Allergic Asthma and Viral Infections. Front. Immunol 9, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Profita M et al. (2012) β₂ long-acting and anticholinergic drugs control TGF-β1-mediated neutrophilic inflammation in COPD. Biochim. Biophys. Acta 1822, 1079–1089 [DOI] [PubMed] [Google Scholar]
- 83.Nguyen LP et al. (2017) β2-Adrenoceptor signaling in airway epithelial cells promotes eosinophilic inflammation, mucous metaplasia, and airway contractility. Proc. Natl. Acad. Sci. U. S. A 114, E9163–E9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wollin L and Pieper MP (2010) Tiotropium bromide exerts anti-inflammatory activity in a cigarette smoke mouse model of COPD. Pulm. Pharmacol. Ther 23, 345–354 [DOI] [PubMed] [Google Scholar]
- 85.Toumpanakis D et al. (2017) Tiotropium bromide exerts anti-inflammatory effects during resistive breathing, an experimental model of severe airway obstruction. Int. J. Chron. Obstruct. Pulmon. Dis 12, 2207–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Branchfield K et al. (2016) Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351, 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grebe KM et al. (2010) Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. Baltim. Md 1950 184, 540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu B et al. (2013) TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 27, 3549–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sui P et al. (2018) Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, [DOI] [PMC free article] [PubMed] [Google Scholar]