Abstract
This paper presents the development of a multi-sensor platform capable of simultaneous measurement of dissolved oxygen (DO) concentration, glucose and lactate concentrations in a micro-chamber for real-time evaluation of metabolic flux in bovine embryos. A micro-chamber containing all three sensors (DO, glucose, and lactate) was made to evaluate metabolic flux of single oocytes or embryos at different stages of development in ≤120 μL of respiration buffer. The ability of the sensor to detect a metabolic shift from oxidative phosphorylation (OXPHOS) to glycolysis was demonstrated in embryos by an ablation of oxygen consumption and an increase in lactate production following addition of oligomycin, an inhibitor of mitochondrial adenosine triphosphate (ATP) synthesis. An increased reliance upon glycolysis relative to OXPHOS was demonstrated in embryos as they developed from morula to hatched blastocysts by a progressive increase in the lactate/oxygen flux ratio, consistent with isolated metabolic assessments reported previously. These studies highlight the utility of a metabolic multi-sensor for integrative real-time monitoring of aerobic and anaerobic energy metabolism in bovine embryos, with potential applications in the study of metabolic processes in oocyte and early embryonic development.
Keywords: Enzymatic sensor, Clark oxygen sensor, Bovine embryo, Multi-sensors, Glucose sensor, Lactate sensor
1. Introduction
The metabolic activity of a cell is closely linked to its activity and viability (Lee et al., 2009). The rate of cellular oxygen consumption is routinely used as an indicator of mitochondrial ATP production, and is often associated with changes in organismal health and phenotype (Eisenreich et al., 2013; DeBerardinis et al., 2008). However, cellular ATP production is accomplished by both aerobic and anaerobic pathways. In the aerobic pathway via oxidative phosphorylation (OXPHOS), cells catabolize glucose and other metabolic substrates to CO2 and water using oxygen to generate ATP in mitochondria. In the primary anaerobic pathway (glycolysis), cells generate ATP by the catabolism of glucose in the cytosol, releasing lactate as a byproduct (Yotter and Wilson, 2004). Shifts in cellular energy demand or substrate availability can alter the balance of these pathways and potently influence cell phenotype in health and disease (Goodpaster and Sparks, 2017; Obre and Rossignol, 2015). Therefore, a thorough understanding of cellular metabolic status and its functional implications requires assessment of both oxidative metabolism and glycolysis.
While several experimental modalities have been developed to monitor these pathways individually in cell suspensions and cultures (Pike Winer and Wu, 2014; Divakaruni et al., 2014), simultaneous measurement is more challenging. Moreover, the development of sensing techniques to analyze metabolism at the single cell level is increasingly desirable to overcome the complexities of cellular heterogeneity in small tissue samples or primary cell cultures, which presents additional challenges. Single-cell characteristics play a key role in determining population characteristics and the transient dynamics that lead to future cell expression and behavior at a population level (Vanhove et al., 2013; DeBerardinis et al., 2008). Accordingly, the development of new techniques for measuring integrated metabolic flux in single cells could have wide applications in exploring directed evolution, drug toxicity, and cancer biology (Ryan and Robards, 2006; Christofk et al., 2008).
Sensors with sensitivity at the single-cell level are also necessary for studies of organisms composed of a single or small number of cells, such as embryos during early development (Gardner et al., 2001; Gardner et al., 2013). Current methods used for selecting embryos in clinical in vitro fertilization (IVF) programs are stage-specific morphologic markers and grading systems (Ebner et al., 2003a). However, such methods are subjective and not optimal for reliably assessing the physiological status of the embryo, and they correlate poorly with subsequent developmental competence fluorometric enzymatic assays (Thompson et al., 1996).
Scanning electrochemical microscopy (SECM) provides a noninvasive means of studying the metabolism of single cells by measuring oxygen (Shiku et al., 2004; Shiku et al., 2007), glucose, and lactate (Ciobanu et al., 2008). Although this method shows high sensitivity, one of its drawbacks is its complexity. SECM requires placing the microelectrode adjacent to embryo under an inverted microscope using complex instrumentation to micro-manipulate the probe with high precision during the measurement process. This process introduces potential variability and requires specialized training for proper operation. Commercially available instruments such as Agilent Extracellular Flux Analyzer are popular for measuring oxygen consumption and extracellular acidification rate (ECAR) in cell cultures (Van der Windt et al., 2016; TeSlaa and Teitell, 2014). ECAR is an indirect index of glycolysis in cell cultures. However, these analyzers are extremely expensive and are incapable of single-cell level resolution.
This paper presents the development of a microfabricated multi-sensor system for real-time measurement of aerobic and anaerobic energy metabolism in single cells. The capabilities of this system were demonstrated by monitoring changes in glycolytic flux and mitochondrial respiration of single bovine embryos following pharmacological inhibition and during development from morula to hatch blastocysts. The micro-chamber design enables the sensors to measure tiny changes in analyte concentration from 0.001 to 10 fmol/s with high specificity comparable to published values using other methods (Sugimura et al., 2012; Santos et al., 2017). Potential applications of this integrated multi-sensor system include non-invasive monitoring of oocyte and embryo metabolism during development to improve outcomes in assisted reproductive technologies, and the biological study of single-cell energetics relevant to the fields of cancer and microbiology.
2. Materials and Methods
2.1. Materials and Reagents
SU8–2050 and SU8 developer were purchased from MicroChem Corp (MA, USA). Megaposit MF −26A developer and S1813 photoresist were purchased from Capitol Scientific, Inc (Austin, TX). Glass substrates, 5% w/w Nafion perfluorinated resin, glucose oxidase (GOx), lactate oxidase (LOx), D- (+)-glucose, sodium L-lactate, phosphate buffer saline (PBS), Tween-20, oligomycin, and bovine serum albumin (BSA) were purchased from Sigma Aldrich (St. Louis, Missouri). Sodium sulfite (Na2SO3) was purchased from Eisen-Golden laboratories (Berkeley, California). G-MOPS medium (respiration buffer) and paraffin oil (OVOIL™) were purchased from Vitrolife.
2.2. Electrodes and Chamber Designs and Manufacturing
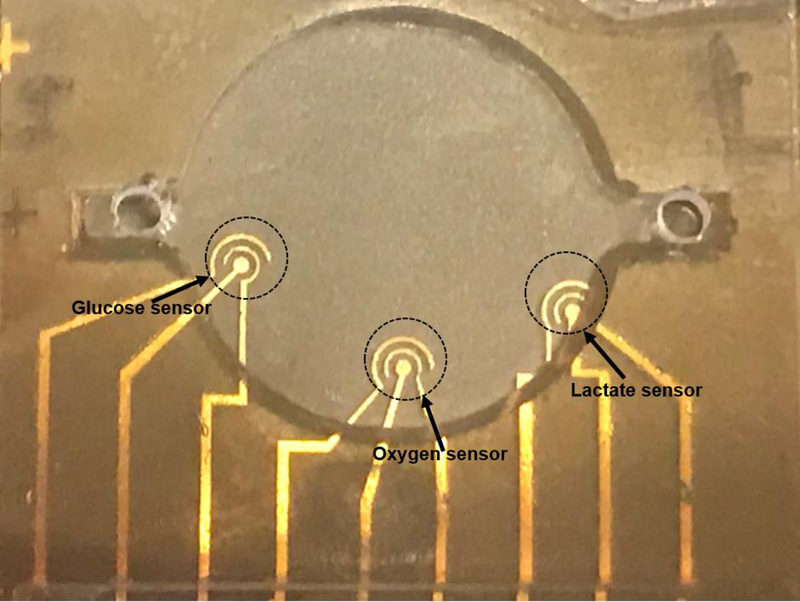
A multi-sensor chip was fabricated on a glass substrate of 24.5 mm x 24.5 mm through standard photolithography and lift-off techniques. The three-electrode configuration with working electrode (WE), quasi reference electrode (RE), and counter electrode (CE) was used for all sensor configurations. Details of photolithography and metal evaporation are included in S.1 in Supplementary Material. A 12 mm diameter and 3 mm deep micro-chamber containing all three sensors was made using SU8 with details provided in S.1.3 in Supplementary Material. A glass lid with two drilled holes (inlet and outlet) was glued on the chamber (Fig. 1).
Fig. 1.
Complete design with 3 mm thick SU8 micro-chamber covered by a glass lid.
2.3. Sensors Surfaces Modification
2.3.1. Oxygen Electrode Surface Modification
Among different surface preparation methods, Nafion was chosen as a solid-state electrolyte as well as a membrane. Using Nafion only is more compatible with integration and multiplexed sensing applications than using other electrolytes and membranes (Obeidat and Chen, 2016). A solid electrolyte layer was formed on the electrode surface by applying 0.1 μL of Nafion solution to the surface of the WE and allowing it to dry for 20 min.
2.3.2. Glucose Electrode Surface Modification
The GOx film solution was prepared by dissolving 5 mg of GOx and 50 mg of BSA in 500 μL of 1 mM PBS containing 0.02% v/v Tween-20. The roles of BSA in the process of enzyme immobilization have been well documented in the literature (Smith et al., 1975; Beddows et al., 1982; Shah et al., 2006) for its abilities to stabilize the enzyme layer without causing any biochemical reactions during the immobilization process.
The glucose WE was covered by 0.1 μL of the GOx solution and was left to dry for 30 min at room temperature. Then a 0.1 μL of 5% w/w Nafion was used to cover the GOx film surface and allowed to dry for 15 min at room temperature. The functionalized electrodes were refrigerated (4°C) in phosphate buffer until use.
2.3.3. Lactate Electrode Surface Modification
The LOx film solution was prepared by dissolving 2.5 mg of LOx and 50 mg of BSA in 500 μL of 1 mM PBS containing 0.02% v/v Tween-20. The roles of BSA used for LOx film immobilization are the same as those for the GOx film described in Section 2.3.2.
The lactate electrode was covered by LOx film by adding 0.1 μL of the LOx solution to the WE surface and was left to dry for 30 min at room temperature. Nafion was diluted with ethanol, with one-part Nafion to nine parts ethanol. Then a 0.1 μL of Nafion was added to the LOx film surface and allowed to dry for 15 min at room temperature. The functionalized electrodes were placed in a refrigerator (4°C) in phosphate buffer until use.
2.4. Bovine Embryos and Their Stimuli
Bovine oocytes were obtained from ovaries collected at a slaughterhouse and fertilized and incubated in embryo culture (G-MOPS) at 38.5°C. Oligomycin (1 μM) was used as an ATP synthase inhibitor. The initial volume of the respiration medium used in all experiments was 120 μL. Stock solutions of oligomycin were made up in 100% ethanol to provide 1 μM concentration in the 120 μL respiration chamber with a 1 μL volume.
2.5. Electrochemical Instrumentation
A potentiostat (eDAC, Quadstat EA164H, Colorado Springs, CO) was used to perform all electrochemical measurements. Data were collected using the Potentiostat were analyzed using a set of custom-built tools written in MATLAB (The MathWorks, Inc.) for data calibration and conversion from measured units in μA/min to the equivalent respiration rate in fmol/s. (see S.5 in Supplementary Material).
2.6. Sensor Activation Voltages
A set of experiments were performed on the sensors in the micro-chamber to determine their activation voltages. The details of the experiments and the results can be found in Supplementary Material.
2.7. Sensors Calibration
2.7.1. Oxygen Sensor Calibration
To determine the calibration curve and linearity of the DO sensor, the dissolved oxygen concentration was changed by adding 0.1 M Na2SO3 to the saturated solution in incremental steps with continuous stirring to produce different oxygen concentrations for generating the calibration data (Obeidat et al., 2018). All O2 concentration measurements were made at 38.5°C and validated using a calibrated Oakton DO6+ dissolved oxygen meter. The dissolved oxygen reduction current was measured at −0.6 V at 0.5 min after each addition of Na2SO3. The dissolved oxygen concentration in the G-MOPS respiration buffer corresponding to the measured current in the calibration curve was calculated at 158 μM based on the average barometric pressure of the experiment location (Fort Collins, Colorado (84.8 kPA)) at the temperature of 38.5°C, corrected for the slightly lower oxygen solubility of the G-MOPS respiration buffer (0.92) compared to water (Compton et al., 2011).
2.7.2. Lactate Sensor Calibration
A 10 mM lactate solution was prepared using L-lactate powder taking into consideration its molecular weight (112.06 gram/mole) and calculations required to convert grams into mol/L. Solutions with different lactate concentrations were prepared by diluting a known lactate solution in DI water. The current generated from the oxidation of hydrogen peroxide was measured at 0.4 V at 38.5°C.
2.7.3. Glucose Sensor Calibration
A 20 mM glucose solution was prepared using D-glucose powder, taking in considering its molecular weight (180 gram/mole) and calculations required to convert grams into mol/L. Different glucose concentrations solutions were prepared by diluting a known glucose concentration solution in DI water. The current generated from the oxidation of hydrogen peroxide was measured at 0.4 V at 38.5°C.
2.8. Measurement Setup
2.8.1. Oxygen, Glucose and Lactate Measurements
The multi-sensors chip embedded in the micro-chamber described in Section 2.2 and Supplementary Material was used. The temperature of the medium was maintained at 38.5°C by placing the device on a stage warmer for a stereomicroscope. The applied potential during all the amperometric experiments was set at −0.6 V for measuring DO and at 0.4 V for measuring lactate and glucose (cyclic voltammetry results are included in S.3 in Supplementary Material). The electrodes were rinsed using DI water and electrochemically pulse cleaned for 1 min before and after each test. Sterilized DI water was used for pulse cleaning to avoid any toxic effect on cells from any other cleaning chemicals. The amplitude of pulses used for cleaning was 1.2 V (from −0.6V to 0.6V) and the pulse duration was 2 ms.
G-MOPS respiration buffer (120 μL) was first placed in the micro-chamber and overlaid with 120 μL of paraffin oil to seal the micro-chamber. The choice of 120 μL is based on the tradeoff of two criteria. First of all, for detecting single cell respiration with sufficient sensitivity, the volume in the micro-chamber should be as small as possible; secondly, given the size of the micro-chamber, the volume in the micro-chamber should be sufficient enough to cover the cell to be measured. A technician selected and moved the embryos into the micro-chamber, and the readings were done with the sensor operator blinded to the quality or type of sample. Embryos of different stages were tested during these experiments. A single embryo was transferred into the micro-chamber by pipetting through the paraffin oil layer on top of the WE, while viewing through a stereoscope. Analytes were measured for 8–10 min each by reading DO, glucose, and lactate consecutively, with the embryo moved from one WE to another for specific analyte reading. (See S.4 in Supplementary Material).
3. Results and Discussion
3.1. Sensors Calibration
3.1.1. Oxygen Sensor Calibration
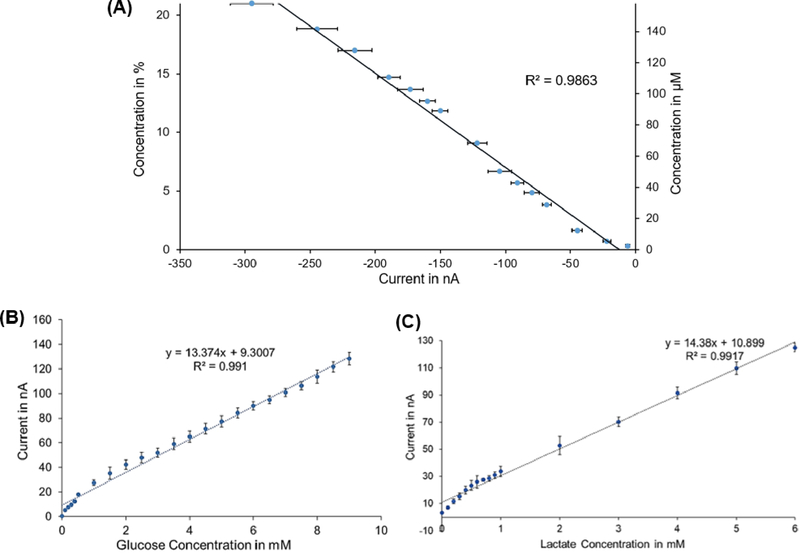
A calibration curve (Fig. 2A) was obtained for the oxygen sensor, which demonstrated its ability to measure a dissolved oxygen range of 0 to 170 μM, with a sensitivity of 1.93 nA/μM (13.9 nA/%). Sensor output current increased linearly with the increase in O2 concentration with a correlation coefficient of 0.986 and had a limit of detection (LOD) of 1.22 μM.
Fig. 2.
(A) Oxygen sensor calibration curve: left y-axis represents concentration in %, right y-axis is concentration in μM. (B) Glucose sensor calibration curve. (C) Lactate sensor calibration curve. (Error bars in each curve are standard deviations (SD) between 6 data points)
3.1.2. Glucose Sensor Calibration
The calibration curve generated for the glucose sensor (Fig. 2B) demonstrated a wide dynamic range of 0 to 9 mM with good sensitivity (between 12.89 and 14.1 nA/mM) and linearity (r = 0.98 – 0.99), and a LOD of 0.5 μM.
3.1.3. Lactate Sensor Calibration
The calibration curve (Fig. 2C) obtained for the lactate sensor demonstrated a wide dynamic range of 0 to 6 mM with good sensitivity (12.1 – 14.38 nA/Mm) and linearity (r = 0.99), and a LOD of 0.4 μM.
3.2. Characterization of Bovine Embryo Energy Metabolism
3.2.1. Effect of Oligomycin on Embryo Metabolism
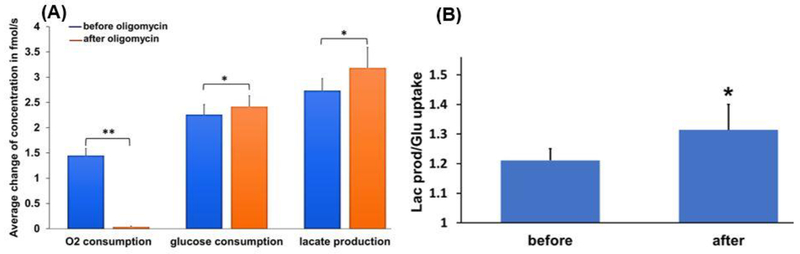
To determine the specific ability of the multi-sensor to monitor a metabolic shift from OXPHOS to glycolysis in real-time, the metabolism of 6 embryos (at the blastocyst stage) were measured before and after adding oligomycin. Oligomycin inhibits the mitochondrial ATP synthase by blocking H+ transport through the complex, thereby forcing cells to rely on non-mitochondrial (primarily glycolytic) ATP production for survival (TeSlaa and Teitell, 2014). Fig. 3A shows the averages of the oxygen and glucose consumption and lactate production before and after the addition of oligomycin.
Fig. 3.
(A) Oxygen and glucose consumptions and lactate production of bovine embryos before and after adding oligomycin. (B) Flux ratio (Lac prod/Glu uptake) of lactate production and glucose uptake for bovine embryos before and after adding oligomycin. (mean ± SD, n= 6 embryos at blastocyst stage). *paired t-test: P≤ 0.01, **paired t-test: P≤ 0.001.
As expected, oligomycin stopped oxygen consumption, but increased lactate production, without a significant effect on glucose uptake. To better describe this observed effect on cellular metabolism, we calculated the flux ratio of lactate production to glucose uptake before and after adding oligomycin (Fig. 3B). This ratio increased significantly (P≤0.01) after the addition of oligomycin, demonstrating a greater rate of lactate release relative to glucose consumption after inhibition of mitochondrial ATP production, consistent with a “switch” from OXPHOS to glycolysis to maintain cellular ATP production. These results highlight that embryo glucose uptake alone is not a reliable measure of glycolytic energy production, since the pyruvate generated in glycolysis can be oxidized in mitochondria (glucose oxidation) or converted to and released as lactate (anaerobic glycolysis).
3.2.2. Evaluation of Embryo Metabolism Throughout Development
To investigate the metabolic characteristics of embryos during development, a total of 106 embryos in various stages were evaluated. Sixty-nine embryos graded good to excellent in quality (see S.2 in Supplementary Material), were used for metabolism studies. Glucose and oxygen consumption and lactate production are expressed as fmol per embryo per second ± SD. The patterns of oxygen and metabolite flux during development from (8 to 32) cells to the hatched blastocyst stage was analyzed by one-way analysis of variance (ANOVA). Differences between means were examined using Tukey HSD (pairwise comparison among stages). Dead and degenerate oocytes and embryos (negative controls) had low measurements when compared with viable embryos of any development stage.
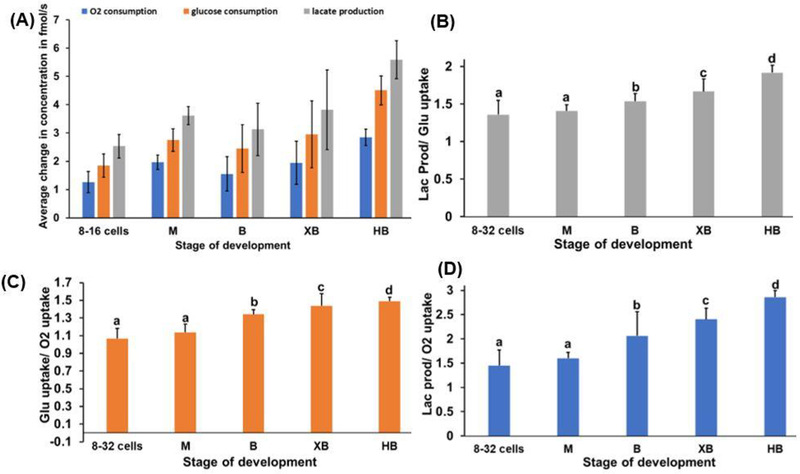
Throughout the observed stages of embryo development, every parameter of metabolic flux (oxygen, glucose, and lactate) significantly increased (P<0.001, ANOVA). (See Table S.1 and Fig. S.4 in Supplementary Material for pairwise comparison) A non-significant change in mean oxygen consumption (P>0.05) was observed from early-stage embryos (8 to 32 cells) to morulae and from morulae to blastocysts (Fig. 4A) (See also Table S.1 and Fig. S.4 in Supplementary Material); but, oxygen uptake increased significantly from the blastocyst to expanding blastocyst stages and from expanding to hatching blastocyst stages (P≤0.05). Glucose consumption followed a similar pattern to oxygen uptake with a significant increase from blastocyst to expanding blastocyst stages (P≤0.05) and a highly significant increase from expanded to hatched blastocysts (P≤0.05). Lactate production significantly increased from blastocyst to expanded blastocyst and from expanded to hatched blastocysts (P<0.05). (See Table S.1 and Fig. S.4 in Supplementary Material)
Fig. 4.
(A) Oxygen and glucose consumptions and lactate production of bovine embryos at various stages. (B) Flux ratio between lactate production and glucose uptake (Lac prod/Glu uptake). (C) Flux ratio between glucose and oxygen uptakes (Glu uptake/O2 uptake). (D) Flux ratio between lactate production and oxygen consumption (Lac prod/O2 uptake). In all figures: mean ± SD; dead oocytes or embryos (n= 12), 8 to 32 cells (n= 12), morula (M, n=7), blastocyst (B, n= 6), expanded blastocyst (XB, n= 17), and hatched blastocyst (n=8, HB). a, b, c, d within columns: Tukey HSD (pairwise comparison), values with different superscripts are significantly different (P≤ 0.001).
The observed increases in oxygen consumption, glucose uptake, and lactate production of bovine embryos as they progress toward the blastocyst stages are consistent with previous studies (Thompson et al., 1996; Guerif et al., 2013), and parallel the well-established increase in embryo energy demand as cell numbers increase (Hardy et al., 1989; Overstrom et al., 1992). The mean values of glucose and lactate flux in bovine embryos in the present study are similar to those previously reported for bovine (Thompson et al., 1996) and human embryos (Hardy et al., 1989; Wales et al., 1987). However, observed rates are lower than those reported for equine embryos (Lane et al., 1996) and bovine and human embryos incubated in lactate-free culture media (Guerif et al., 2013; Gott et al., 1990), suggesting variations due to both species and culture media composition. Indeed, levels of lactate may attenuate rates of glycolysis in vitro (Leese, 1992), and at least one previous study demonstrated that rate of glucose metabolism is linearly related to its concentration in the media (Wales et al., 1987). The degree of increase in glucose consumption and lactate production by embryos from the morula to blastocyst stages in the present study is similar to previous studies utilizing glucose tracer methods in bovine embryos (Rieger et al., 1992a) and microfluorometry in equine embryos (Lane et al., 1996), but lower than some reports in bovine and human embryos (Thompson et al., 1996; Guerif et al., 2013). These discrepancies could be explained in part using later stage (Day 6) morula in the present study which can behave similarly to blastocysts used in the latter studies (Thompson et al., 1996; Lane et al., 1996). Table 1 provides a comprehensive comparison between the multi-sensor system described herein and the published results on methods, targeting species and cells, specific analytes to be measured, and the related sensor performance.
Table 1:
Comparison between different systems that measure metabolic activity in oocytes and embryos at different stages such as: 1–32 cells, morula (M), blastocyst (B), expanded blastocyst (XB), and hatched blastocyst (HB).
| Reference | Method | Species | Sample type/stage | Analytes assessed | Range detected per stage (fmol/s) |
|---|---|---|---|---|---|
| Gardner, D.K. and Leese, H.J., 1986 | Ultramicrofluorescence | Mouse | Embryos: 1–8 cells, M, B | Glucose and pyruvate consumption | Glucose: 1-cell: 0.027; 2–8 cells: 0.083; M: 0.42; B:1.11. |
| Gardner, D.K. and Leese, H.J., 1990 | Ultramicrofluorometric | Mouse | Embryos: B | Glucose consumption and lactate production | Glucose:1.2±0.05 |
| Lactate: 2.18±0.16 | |||||
| Lane, M. and Gardner, D.K., 1996 | Microfluorescence | Mouse | Embryos: blastocyst | Glucose consumption | 1.1–2 |
| Gardner, D.K. et al. 1993 | Microfluorescence | Sheep | Oocytes (O) Embryos:2–16 cells, M, B, HB | Pyruvate and glucose consumptions | Glucose: O and 2–8: 0.28; 16: 1.67; M: 3.94; B:6.14; HB: 15.1 |
| Lane, M. et al. 2001 | Microfluorometry | Horse | Embryos: M, B, XB | Pyruvate and glucose consumptions, and lactate production | Glucose: M: 41.5–51.8; B:56.6–102.9; XB: 35–201.47. |
| Lactate: M: 56.25–65.3; B: 60.2–82.13; XB: 110.17–261.63 | |||||
| Gardner, D.K. et al. 2001 | Ultramicrofluorescence | Human | Embryos: B | Pyruvate and glucose consumptions | Glucose: 34.9 |
| Gardner, D.K. et al. 2011 | Microfluorimetry | Human | Embryos: B | Glucose consumption | 14–50 |
| Hardy, K. et al. 1989 | Fluorescence | Human | Embryos | Glucose consumption | 2.2–6.6 |
| Gott, A.L. et al. 1990 | Fluorescence | Human | Embryos: 2–16, M, B | Pyruvate and glucose consumptions, and lactate production | Glucose: 2–16 cells: 3.33; 32 cells: 3.61; M: 5; B: 12.5. |
| Lactate: 2–16 cells: 11.67, 32 cells: 18.05; M: 22.2; B: 27.78. | |||||
| Sturmey, R.G. and Leese H.J., 2003 | Fluorescence | Pig | Oocytes Embryos:1–4, M, B, XB |
Oxygen, pyruvate and glucose consumptions, and lactate production | Oxygen: O: 6.9–9.16; 1–8 cells: 5.6–7.78; M: 3.33; B: 16.11; XB: 5 |
| Glucose: O: 1.3; 1–8 cells: 0.6–0.8; M: 2, B: 4.8; XB: 2.8. | |||||
| Lactate: O: 1.5±0.5; 1–8 cells: 0.5–1.4; M: 2±0.2; B: 2.2±0.3; XB: 3.3±0.5 | |||||
| Guerif et al., 2013 | Fluorescence | Cow | Embryos: 2–8 cells, M, B, XB, HB | glucose and pyruvate consumptions, and lactate production | Glucose:2–8 cells: 0.65–0.72; M: 2±0.36; B: 3.975±0.32; XB:9.5±0.5; HB: 15.63±0.8 |
| Lactate:2–8 cells: 0.46–1; M:4.08±0.33; B: 6.56±0.39; XB: 14.2±0.66; HB:23.8±1.24 | |||||
| Thompson, J.G. et al. 1996 | Fluorescence | Cow | Embryos: 1–8 cells, 16 cells M, M, B | glucose and pyruvate and oxygen consumptions, and lactate production | Glucose:1–8 cells: 0.417–0.86; 16 cells M: 0.83±0.11; M: 2.94±0.91; B: 4.05±0.25. |
| Lactate:1–8 cells: 0.306–1.27; 16 cells M: 1.72±0.38; M:--, B: 8.86±1.27. | |||||
| Oxygen:1–8 cells:0.067–0.052; 16 cells M: 0.075±0.016; M: 0.1±0.01; B:0.25±0.036 | |||||
| Sugimura, S. et al., 2012 | SECM | Cow | Oocytes | Oxygen consumption | Up to 8 |
| Date, Y. et al., 2011 | Electrochemical (Amperometry) | Mouse | Embryos/ two cells, M, B | Oxygen consumption | Two cells: 1.36±0.33 |
| M:1.38±0.58 | |||||
| B: 3.44±2.07 | |||||
| Wu, C., et al., 2007 | Electrochemical (Amperometry) | Cow | Embryos/ M | Oxygen consumption | 3.77 ± 0.11 to 7.36 ± 0.29 |
| This work | Electrochemical (Amperometry) | Cow | Embryos: 8–32, M, B, XB, HB | Glucose and oxygen consumptions, and lactate production | Glucose: 8–32:1.123±0.23; M:1.648±0.23; B:2.26 ± 0.26; XB:2.92 ± 0.49; HB:.5.63±0.55. |
| Lactate:8–32:1.528±0.24; M:2.169±0.19; B:2.77±0.27; XB:3.74±0.5; HB:6.96±0.66. | |||||
| Oxygen: 8–32 cells: 0.765±0.2; M: 1.178±0.15; B: 1.484±0.3; XB: 1.94±0.3; HB: 3.58±0.37 |
3.2.3. Metabolic Flux Ratios Reveal Shifts in Glucose Utilization during Embryo Development
A primary advantage of simultaneous measurement of metabolite flux using the multi-sensor system is its ability to express different rates relative to one another. This provides internally-controlled indices of substrate utilization that are more sensitive than absolute flux rates of individual metabolites. Accordingly, to further investigate the nature of increased metabolic flux observed in embryos during development, we evaluated relative flux of lactate production/glucose uptake, the glucose uptake/oxygen uptake, and the lactate production/oxygen uptake at different stages of development (Figs. 4B, 4C, and 4D). Results show that while increases in all three flux ratios were observed (reflecting enhanced glucose metabolism), the amount of lactate produced relative to oxygen consumed nearly doubled from morula to hatched blastocyst stages (P≤0.001), indicating an increasing contribution of anaerobic glycolysis to embryo ATP production during development. These findings are in general agreement with studies demonstrating increases in glucose and oxygen uptake and lactate production of bovine (Rieger et al., 1992a; Guerif et al., 2013), human (Hardy et al. 1989; Gott et al., 1990; Wales et al., 1987), equine (Lane and Gardner, 1996) and sheep embryos (Gardner et al., 1993) at the expanding blastocyst stage.
Overall, the results of our studies are consistent with a link between embryo metabolic activity and development, and specifically a transition in the relative contribution of OXPHOS and glycolysis to energy production during later stages of development. However, as noted above, it is important to consider that embryos being studied in vitro are subject to the stress of being placed in an artificial environment, including the potential for nutrient imbalance and oxidative stress (Gardner and Harvey, 2015; Wale and Gardner, 2012), which have the potential to influence embryo genomic imprinting, development rate and metabolism (Leese, 2002; Leese et al., 2008; Lane et al., 2005). Therefore, it is critical to understand how specific culture conditions (medium composition, length of exposure, developmental stage treatment applied, and concentrations of oxygen) interact and impact the metabolism of the preimplantation embryo, resulting in altered embryo and fetal development (Ebner et al., 2003a; Leese et al., 2008; Gardner et al., 2015). The use of metabolic multi-sensors such as the device described herein are ideally suited for characterization studies of this nature, which may ultimately help to optimize embryo incubation conditions and enhance the success rate of assisted reproduction technologies.
3.3. Advantages of the Present Design over Other Metabolism Sensing Systems
A particular advantage of our design is its ability to measure respiration in the immediate vicinity of a single cell in the micro-chamber, eliminating the uncertainty of relative positioning in other methods such as SECM (Shiku et al., 2004). These instruments are bulky with inconsistent performance and high cost compared to the micro-chamber system described herein. Most SECM-based techniques involve movement of the sensor tip between the cell and bulk solution. The scanning system needs a precise positioner and motors to achieve an accurate control of the tip’s position, and multiple measurement sites are needed.
More recently, the most widely used instrument for assessment of cellular metabolism is the Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA), which is capable of monitoring rates of glycolysis (via extracellular acidification rate) and oxygen consumption in cell populations by fluorescence techniques in a multi-well format (Van der Windt et al., 2016; TeSlaa and Teitell, 2014). However, this analyzer is extremely expensive, requires separate assays to measure glycolysis and oxygen consumption, and generally requires hundreds to thousands of cells per assay. In this work, we describe an integrated metabolic multi-sensor capable of monitoring single embryo oxygen consumption, glucose uptake, and lactate production in real-time using amperometric methods, providing a simple and inexpensive method of monitoring single embryo metabolism during development in a small volume of medium.
4. Conclusions and Future Work
The present study describes a microfabricated multi-sensor system for simultaneous real-time measurement of glycolysis and mitochondrial respiration in single bovine embryos. The results demonstrate the ability of this system to sensitively monitor absolute and relative changes in these pathways as cellular metabolic demands shift during different stages of embryo development. Limitations of the current setup include challenges associated with handling and positioning of single embryos for analyses, which can be overcome by the addition of microscopy and microfluidic channels for sample visualization and manipulation that are currently under development in our laboratory. Our platform is also amenable to the inclusion of additional sensors, such as a pH electrode for monitoring the extracellular acidification rate (ECAR) during cell metabolism, which is also currently under development. Future applications of this technology could be extended to metabolic monitoring of single cells or small multi-cellular samples obtained from heterogeneous tissues such as tumors or granulomas relevant to the study of cancer and infectious disease, as well as other settings where sample paucity limits direct metabolic assessments by currently available methods.
Supplementary Material
Acknowledgements
The results presented in this paper are based upon collaborative work supported by a National Science Foundation NRT Grant No. 1450032. Additional funding was also provided by Colorado’s Advanced Industries program through an OEDIT grant. The authors would like to thank the sponsors for their generous financial support. The authors would also like to thank JoAnne Stokes from ERL for her assistance in some experiments.
Biography
Yusra Obeidat

Yusra received her Bachelor of Science degree from electrical and electronics engineering department at Yarmouk University in Jordan in 2010. Yusra received her MS from electrical engineering department at Colorado state university in fall 2014, her thesis was in modeling of optical waveguides with porous silica claddings and their use in LEAC sensors. Yusra is currently a PhD student at Colorado state university, her PhD project dissertation is to design integrated sensors including oxygen, glucose, lactate and pH for measuring cell metabolism using electrochemistry methods.
Giovana Di Donato Catandi

Giovana Di Donato Catandi received her D.V.M. at the University of Sao Paulo, Brazil, in 2016. She was a research assistant at the University of Minnesota in 2017, working in reproductive physiology in dairy cows. She is currently a Masters student in the Department of Biomedical Sciences at Colorado State University, and works as a graduate assistant at the Equine Reproduction Laboratory. Her thesis project involves mainly equine oocyte and embryo oxygen consumption and metabolism as well as the impacts of aging and diet supplementation on those.
Elaine Carnevale

Elaine Carnevale is a professor in the Department of Biomedical Sciences at Colorado State University. She received her D.V.M. at Colorado State University, as well as a M.S. in reproductive physiology before spending the next 2 years in clinical practice. Dr. Carnevale then completed a Ph.D. at the University of Wisconsin-Madison in reproductive physiology, studying the effects of equine maternal aging on fertility. She taught at Southern Illinois University before joining Colorado State University in 1998 to develop a program in equine assisted reproduction. Her research interests include assisted reproductive technologies and the impacts of maternal aging and metabolic status on oocyte developmental quality. Dr. Carnevale has leads the equine assisted reproduction program at Colorado State University’s Equine Reproduction Laboratory. She has merged clinical and research interest in translational research using the mare as an animal model.
Adam Chicco

Adam Chicco is an associate professor in the Department of Biomedical Sciences at Colorado State University and Fellow of the American Heart Association. He received his PhD at the University of Northern Colorado and developed an interest in the links between metabolism and cardiovascular physiology during his postdoctoral training at the University of Colorado. The current focus of his laboratory is to understand the mechanisms and (patho)physiological consequences of altered mitochondrial function and fatty acid metabolism in the context of physiological and pathological stress. His has expertise in high resolution respirometry enabling detailed studies of mitochondrial metabolism in a variety of sample preparations, and is a member of the Mitochondrial Physiology Network, an international group of scientists that strive to maintain and establish new standards of mitochondrial respirometry methodology, data reproducibility, and interpretation. His research has been continuously funded by the US National Institutes of Health, American Heart Association, US Department of Agriculture, and private foundations since 2005; and includes collaborative studies of mitochondrial metabolism in the contexts of environmental stress, developmental metabolic programming, and inflammation.
August DeMann

August DeMann is a graduate student in the Department of Physics at Colorado State University. He received his B.A. from St. Olaf College in 2012 and his M.S. from Colorado State University in 2014. His doctoral research focuses on the exploration of magnetic properties of superconducting materials by cryogenic scanning Hall-probe microscopy. His research interests include sensor design and fabrication, cryogenic apparatus and electronics, thin film deposition, and optical and electron-beam lithography techniques. He also currently serves as a photolithography research associate and manages the maskless lithography facility in the Department of Physics.
Stuart Field
Stuart Field is Associate Professor in the Department of Physics, Colorado State University. He received his B.S. degree from Stanford University, and his Ph.D. from the University of Chicago. He was on the faculty of the University of Michigan for seven years and has been at Colorado State since 1996. His research is in the area of superconductivity, specializing in the use of various imaging techniques to understand the physics of superconducting vortices and other superconducting phenomena. Dr. Field is an author on more than 40 refereed publications, as well as being a co-author of a leading introductory physics textbook.
Tom Chen

Tom Chen is a professor at the Department of Electrical and Computer Engineering (ECE) and School of Biomedical Engineering (SBME). He received his B.S. from Shanghai Jiaotong University and Ph.D. from the University of Edinburgh. He spent 4 years with Philips Semiconductors in Europe and 1 year with New Jersey Institute of Technology before joining Colorado State University. His research interests include all aspects of biosensors and bioelectronics and their applications to biology and biomedical sciences. Dr. Chen has over 180 refereed journal and conference publications in the areas of VLSI circuits and biosensor circuits and systems. Dr. Chen provided expert opinions for the Federal Trade Commission, and guest edited several IEEE and Elsvier journal issues on CMOS circuit designs and microelectronics. More recently, Dr. Chen is a member of the Steering Committee for IEEE Midwest Symposium on Circuits and Systems. He currently holds Business Challenge Endowment Professorship at Colorado State University.
References
- Beddows C, Gil M, Guthrie J, 1982. The immobilization of enzymes, bovine serum albumin, and phenylpropylamine to poly (acrylic acid)-polyethylene-based copolymers. Biotechnology and Bioengineering. 24, 6, 1371–1387. DOI: 10.1002/bit.260240610. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC, 2008. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 452, 230–233. DOI: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Ciobanu M, TaylorJr DE, Wilburn JP, Cliffel DE, 2008. Glucose and Lactate Biosensors for Scanning Electrochemical Microscopy Imaging of Single Live Cells. Ana. Chem 80(8), 2717–2727. DOI: 10.1021/ac7021184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RG, Laborda E, Ward KR, 2011. Understanding Voltammetry: simulation of electrode processes. 2nd Edition Imperial College Press; ISBN: 978–1–78326–323–3. [Google Scholar]
- Carnevale EM, 2016. Advances in Collection, Transport and Maturation of Equine Oocytes for Assisted Reproductive Techniques. Vet Clin North Am Equine Pract. 32, 379–399. DOI: 10.1016/j.cveq.2016.07.002 [DOI] [PubMed] [Google Scholar]
- De La Torre-Sanchez JF, Preis K, Seidel GE Jr 2006. Metabolic regulation of in-vitro-produced bovine embryos. I. Effects of metabolic regulators at different glucose concentrations with embryos produced by semen from different bulls. Reprod Fertil Dev. 18(5), 585–596. PMID:16836965. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB, 2008. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation”. Cell Metabolism. 7(1), 11–20. DOI: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Date Y, Takano S, Shiku H, Ino K, Ito-Sasaki T, Yokoo M, Abe H, Matsue T, 2011. Monitoring oxygen consumption of single mouse embryos using an integrated electrochemical microdevice. Biosens. Bioelectron 30, 100–106, DOI: 10.1016/j.bios.2011.08.037. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Rogers GW, Murphy AN, 2014. Measuring mitochondrial function in permeabilized cells using the Seahorse XF Analyzer of a Clark-type oxygen electrode. Current Protocols in Toxicolocy, 60, 25.2.1–25.2.16. DOI: 10.1002/0471140856.tx2502s60. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Sommergruber M, Tews G, 2003a. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development”, Hum Reprod Update, 9(3), 251–262. PMID: 12859046. [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Heesemann J, Rudel T, Goebel W, 2013. Metabolic host responses to infection by intracellular bacterial pathogens. Front. Cell. Infect. Microbiol 3, 24 DOI: 10.3389/fcimb.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ, 1986. Noninvasive measurement of nutrient uptake by single cultured preimplantation mouse embryos. Hum. Reprod 1, 25–27. PMID: 3455417. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ, 1990. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J. Reprod. Fertil 88, 361–368. PMID: 2313649. [DOI] [PubMed] [Google Scholar]
- Gott AL, Hardy K, Winston RML, Leese HJ, 1990. Non-invasive measurement of pyruvate and glucose uptake and lactate production by single human preimplantation embryos”. Hum Reprod. 5,104–108. DOI: 10.1093/oxfordjournals.humrep.a137028. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Batt P, 1993. Uptake and metabolism of pyruvate and glucose by individual sheep preattachment embryos developed in vivo”. Mol Reprod Dev. 36, 313–319. DOI: 10.1002/mrd.1080360305. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Phil D, Lane M, Stevens J, Schoolcraft WB, 2001. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential”. Fertil. Steril 76, 1175–1180. DOI: 10.1016/S0015-0282(01)02888-6. [DOI] [PubMed] [Google Scholar]
- Gardner DK, 2007. In Methods in Molecular Medicine. Thornhill A, Ed.; Humana Press: Totowa, NJ, 132,1–9. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Wale PL, Collins R, Lane M, 2011. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Human Reproduction. 26, 1981–1986. DOI: 10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Wale PL, 2013. Analysis of metabolism to select viable human embryos for transfer. Fertil Steril. 99,1062–1072. DOI: 10.1016/j.fertnstert.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Guerif F, McKeegan P, Leese HJ, Sturmey RJ, 2013. A simple approach for consumption and release (CORE) analysis of metabolic activity in single mammalian embryos. PLoS One. 8, e67834 DOI: 10.1371/journal.pone.0067834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DK, Harvey AJ, 2015. Blastocyst metabolism. Reprod Fert Dev. 27(4), 638–654. DOI: 10.1071/RD14421. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Sparks LM, 2017. Metabolic Flexibility in Health and Disease. Cell Metab. 25, 1027–1036. DOI: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Hooper MA, Handyside AH, Rutherford AJ, Winston RM, Leese HJ, 1989. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum Reprod. 4,188–191. PMID: 2918073. [DOI] [PubMed] [Google Scholar]
- Leese HJ, 1991. Metabolism of the preimplantation mammalian embryo In: Milligan SR, editor. Oxford reviews of reproductive biology. London: Oxford University Press.13, 35–72. [PubMed] [Google Scholar]
- Leese HJ, 1992. Metabolism of the preimplantation mammalian embryo in: Oxford Reviews of Reproductive Biology. Oxford University Press, Oxford, U.K, 13, 35–72. [PubMed] [Google Scholar]
- Lane M, Gardner DK, 1996. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum Reprod. 11,1975–1978. PMID: 8921074. [DOI] [PubMed] [Google Scholar]
- Lane M, O’Donovan MK, Squires EL, Seidel GE Jr, Gardner DK, 2001. Assessment of metabolism of equine morulae and blastocysts. Mol Reprod Dev. 59, 33–37. DOI: 10.1002/mrd.1004. [DOI] [PubMed] [Google Scholar]
- Leese HJ, 2002. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability”. BioEssays. 24, 845–849. DOI: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK, 2005. Mitochondrial malate-aspartate shuttle regulates mouse embryo nutrient consumption. J Biol Chem. 280, 18361–18367. DOI: 10.1074/jbc.M500174200. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG, 2008. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 14, 667–672. DOI: 10.1093/molehr/gan065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Cho YM, Kwak SH, Lim S, Park KS, Shim EB, 2009. Mitochondrial dysfunction and metabolic syndrome-looking for environmental factors. Biochim Biophys Acta. March1800, 3, 282–289. DOI: 10.1016/j.bbagen.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Lowry OH, et al. , 1972. A Flexible System of Enzymatic Analysis. Academic Press: New York. [Google Scholar]
- Overstrom EW, Duby RT, Dobrinsky J, Roche JF, Boland MP, 1992. Viability and oxidative metabolism of the bovine blastocyst”. Theriogenology. 37, 269 DOI: 10.1016/0093-691X(92)90338-R. [DOI] [Google Scholar]
- Obre E, Rossignol R, 2015. Emerging concepts in bioenergetics and cancer research: Metabolic flexibility, coupling, symbiosis, switch, oxidative tumors, metabolic remodeling, signaling and bioenergetic therapy. Int J Biochem Cell Biol. 59C, 167181 DOI: 10.1016/j.biocel.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Obeidat Y, Chen T, 2016. Characterization of an O2 Sensor Using Microelectrodes. IEEE sensors. Orlando, FL, October 30 – Nov. 2. DOI: 10.1109/ICSENS.2016.7808460. [DOI] [Google Scholar]
- Obeidat Y, Evans A, Tedjo W, Chicco A, Carnevale E, Chen T 2018. Monitoring Oocyte/Embryo Respiration Using Electrochemical-Based Oxygen Sensors, Sensors and amp; Actuators: B. Chemical, 10.1016/j.snb.2018.07.157. [DOI] [Google Scholar]
- Pike Winer LS, Wu M, 2014. Rapid analysis of glycolytic and oxidative substrate flux of cancer cells in a microplate. PLoS One. 9(10): e109916 DOI: 10.1371/journal.pone.0109916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Loskutoff NM, Betteridge KJ, 1992a. Developmentally related changes in the metabolism of glucose and glutamine by cattle embryos produced and cocultured in vitro. J Reprod Fertil. 95(2), 558–595. PMID: 1518013. [DOI] [PubMed] [Google Scholar]
- Ryan D, Robards K, 2006. Metabolomics: The greatest omics of them all? Anal Chem. 78, 7954–7958. DOI: 10.1021/ac0614341. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Wapner RJ, Rebar RW, Tasca RJ, 2007. Infertility assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 109, 967–977. DOI: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- Shah S, Sharma A, Gupta M, 2006. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal Biochem, 351, 207–213. DOI: 10.1016/j.ab.2006.01.028 [DOI] [PubMed] [Google Scholar]
- Shiku H, Shraishi T, Aoyagi S, Utsumi Y, Matsudaira M, Abe H, Hoshi H, Kasai S, Ohya H, Matsue T, 2004. Respiration activity of single bovine embryos entrapped in a cone shaped microwell monitored by scanning electrochemical microscopy. Anal. Chim. Acta 522, 51–58. DOI: 10.1016/j.aca.2004.06.054. [DOI] [Google Scholar]
- Shiku H, Yasukawa T, Matsue T, Ito-Sasaki T, Yokoo M, Abe H, 2007. Oxygen consumption of mammalian embryos and oocytes monitored by scanning electrochemical microscopy. IEEE Sens conference, Atlanta, GA, USA, USA, 28–31. DOI: 10.1109/ICSENS.2007.4388676. [DOI] [Google Scholar]
- Smith D, Maggio E, Kenyon G, 1975. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry, 14, 766–771. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ, 2003. Energy metabolism in pig oocytes and early embryos. Reproduction. 126, 197–204. PMID: 12887276. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Gardner DK, 2005. Noninvasive methods to assess embryo quality. Curr Opin Obstet Gynecol. 17, 283–288. PMID: 15870563. [DOI] [PubMed] [Google Scholar]
- Satoh W, Hosono H, Yokomaku H, Morimoto K, Upadhyay, Suzuki H, 2008. Integrated Electrochemical Analysis System with Microfluidic and Sensing Functions” Sensors. 8(2), 1111–1127. DOI: 10.3390/s8021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura S, Matoba S, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Konishi K, Imai K, 2012. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J. Reprod. Develop 58(6), 636–641. DOI: 10.1262/jrd.2012-082. [DOI] [PubMed] [Google Scholar]
- Santos SC, Kowaltowski AJ, Bertotti M, 2017. Single Cell Oxygen Mapping (SCOM) by Scanning Electrochemical Microscopy Uncovers Heterogeneous Intracellular Oxygen Consumption. Sci. Rep 7, 11428 DOI: 10.1038/s41598-017-11956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ,1996. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. Journal of Reproduction and Fertility. 106(2), 299–306. PMID: 8699414. [DOI] [PubMed] [Google Scholar]
- TeSlaa T, Teitell MA, 2014. Techniques to Monitor Glycolysis. Methods in enzymology. 542, 91–114. DOI: 10.1016/B978-0-12-416618-9.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhove E, Ben-Amor S, Charlot S, Colin D, Devin A, Rigoulet M, Sojic N, Sékli Belaïdi F, Launay J, Temple-Boyer P, Arbault S, 2013. Development of Electrochemical Microsensors for the Monitoring of Mitochondrial Activities. IEEE, Transducers. Barcelona, SPAIN, DOI: 10.1109/Transducers.2013.6626972. [DOI] [Google Scholar]
- Van der Windt GJ, Chang CH, Pearce EL, 2016. Measuring Bioenergetics in T Cells Using a Seahorse Extracellular Flux Analyzer”. Curr. Protoc. Immunol 113:3.16B.1–3.16B.14. DOI: 10.1002/0471142735.im0316bs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales RG, Whittingham DG, Hardy K, Craft IL, 1987. Metabolism of glucose by human embryos. J. Reprod. Fertil 79, 289–297. [DOI] [PubMed] [Google Scholar]
- Wolf B, Kraus M, Brischwein M, Ehret R, Baumann W, Lehmann M, 1998. Biofunctional hybrid structures — cell-silicon hybrids for applications in biomedicine and bioinformatics”. Bioelectrochemistry and Bioenergetics. 46(2), 215–225. DOI: 10.1016/S0302-4598(98)00169-X. [DOI] [Google Scholar]
- Wu C, Saito T, Yasukawa T, Shiku H, Abe H, Hoshi H, Matsue T, 2007. Microfluidic chip integrated with amperometric detector array for in situ estimating oxygen consumption characteristics of single bovine embryos. Sens. Actuators B. 125, 680–687, DOI: 10.1016/j.snb.2007.03.017. [DOI] [Google Scholar]
- Wale PL, Gardner DK, 2012. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol. Reprod 24, 1–8. DOI: 10.1095/biolreprod.112.100552 [DOI] [PubMed] [Google Scholar]
- Yotter RA, Wilson DM, 2004. Sensor Technologies for Monitoring Metabolic Activity in Single Cells-Part II: Nonoptical Methods and Applications. IEEE sensors journal. 4(4), 412 – 429. DOI: 10.1109/JSEN.2004.830954. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.