Abstract
It is urgent that the means to improve liver regeneration (LR) be found, while mitigating the concurrent risk of hepatocarcinogenesis (HCG). Nuclear receptor corepressor 1 (NCoR1) is a co-repressor of nuclear receptors, which regulates the expression level of metabolic genes; however, little is known about its potential contribution for LR and HCG. Here, we found that liver-specific NCoR1 knockout in mice (NCoR1Δhep) dramatically enhances LR after partial hepatectomy and, surprisingly, blocks the process of diethylnitrosamine (DEN)-induced HCG. Both RNA-sequencing and metabolic assay results revealed improved expression of Fasn and Acc2 in NCoR1Δhep mice, suggesting the critical role of de novo fatty acid synthesis (FAS) in LR. Continual enhanced de novo FAS in NCoR1Δhep mice resulted in overwhelmed adenosine triphosphate ATP and nicotinamide adenine dinucleotide phosphate (NADPH) consumption and increased mitochondrial reactive oxygen species production, which subsequently attenuated HCG through inducing apoptosis of hepatocytes at an early stage after DEN administration. Conclusion: NCoR1 functions as a negative modulator for hepatic de novo FAS and mitochondria energy adaptation, playing distinct roles in regeneration or carcinogenesis.
The liver has a remarkable capacity for regeneration after injury.(1,2) Sustained liver reparation and regeneration triggered by chronic liver injury can bring about genetic mutations and induce hepatocarcinogenesis (HCG).(3,4) However, the signals that initiate or terminate hepatocyte proliferation are still incompletely defined.(5,6)
Nuclear receptor corepressor 1 (NCoR1) serves as a cofactor that facilitates the interaction of several nuclear proteins that regulate the transcription rates of metabolic stress-induced genes.(7,8) Interactions between NCoR1, nuclear receptors and deacetylases, and transducing beta-like 1 has been studied, and it has been found that they can form a co-repressor complex for the down-regulation of target genes.(9–11) Expression of their target genes is increased in the absence of NCoR1, which confirms the important role that NCoR1 has in controlling the amplitude of nuclear receptors.(10) Interestingly, steatosis was also observed in NCoR1 hepatic knockout (NCoR1Δhep mice.(9) Thus, disrupting Ncor1 expression could up-regulate the expression of metabolic genes, such as lipogenesis genes and lipid oxidation genes.(12)
Lipid metabolism was identified as playing a major role in the regulation of liver regeneration (LR) and HCG.(13) Peripheral lipid mobilization was determined to be indispensable for the initiation of LR.(2) Improved lipid metabolism in chronic liver diseases, such as nonalcoholic steatohepatitis (NASH), was found to have a promoting role in hepatocellular carcinoma (HCC).(14) Taken together, these observations suggest that enhanced lipid metabolism induced by liver injury is involved in the compensatory proliferation and development of HCC. However, hampering lipogenesis could also promote tumor growth, as evidenced by the results of knockdown of acetyl-CoA carboxylases (ACCs) ACC1 or ACC2, a key enzyme of lipogenesis that facilitates cancer cell survival and tumor formation.(15)
Based on the intriguing relationship between NCoR1 and lipid metabolism, as revealed in previous studies, the current work was undertaken to assess the influence of liver-specific disruption of NCoR1 on compensatory proliferation after partial hepatectomy (PH) and HCG.
Materials and Methods
The full materials and methods are available in the Supporting Information.
GENERATION OF NCoR1Δhep MICE
NCoR1 floxed (NCoR1fl/fl) and albumin (Alb)-Cre mice (Alb-creTg/0) were generated as described using the Cre-loxP system.(16) Briefly, offspring that transmitted the mutated allele, in which the selection marker was excised, and that lost the Flp transgene (NCoR1L2/WT mice) were selected, mated with mouse Alb-Cre mice, and then further intercrossed to generate mutant (Alb)-creTg/0/-NCoR1L2/L2 mice, which were termed NCoR1Δhep mice. NCoR1Δhep mice, back-crossed for over 10 generations to C57BL/6J, were used in experiments with NCoR1fl/fl used as controls (Supporting Fig. S1B,C).
ANIMALS AND EXPERIMENTAL PROCEDURES
All animal experiments were performed in accord with the National Institutes of Health guidelines and approved by the Animal Care and Use Committee of the Second Military Medical University (Shanghai, China). PH surgeries were performed in 8- to 12-week-old mice by removal of two thirds or one third of the liver as described.(17) For diethylnitros-amine (DEN)-induced HCG, mice at day 15 of age were injected intraperitoneally with DEN (10 mg/kg, intraperitoneally) diluted in saline buffer. GW6471 (peroxisome proliferator-activated receptor [PPAR]α antagonist) was injected intraperitoneally (1 mg/kg, intraperitoneally). Adeno-associated virus (AAV) was diluted in phosphate-buffered saline (PBS) and injected through the caudal vein (virus titer, 1011/mL, 200 μL/mouse). For more information, please see the Supporting Information.
STATISTICAL ANALYSIS
All data in this study represent at least three experiments and are expressed as the mean ± SEM. Differences between groups were compared using the Student t test or two-way analysis of variance (as indicated in each figure/table). Statistical significance was determined as P < 0.05. Analysis was performed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
Results
ENHANCED CELL PROLIFERATION IN NCoR1Δhep MICE AFTER PH
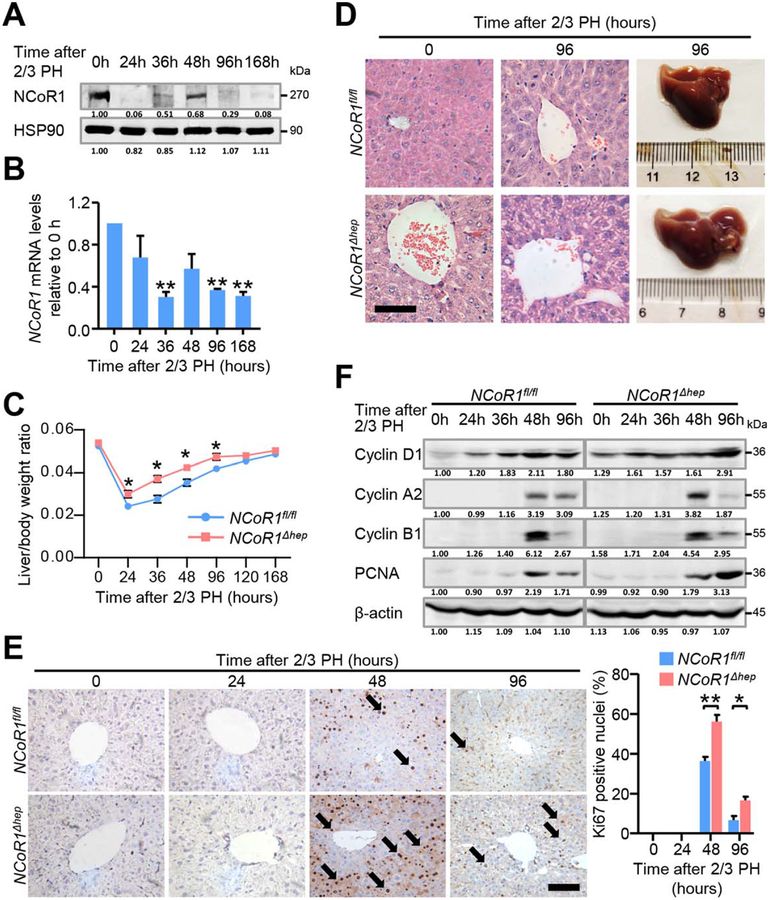
We performed two-thirds PH on NCoR1fl/fl mice and NCoR1Δhep mice. NCoR1 protein levels rapidly reduced at 24 hours post-two-thirds PH, but rebounded at 48 hours after two-thirds PH (Fig. 1A). NCoR1 mRNA expression was also down-regulated after two-thirds PH (Fig. 1B). The change of liver/body weight ratio in NCoR1fl/fl and NCoR1Δhep mice during the process of LR revealed that restoration of liver/body weight ratios in NCoR1Δhep mice occurred much earlier and faster than in NCoR1fl/fl mice. The same result was observed in one-third PH models. However, liver/body weight ratios of NCoR1fl/fl mice were similar to that of NCoR1Δhep mice at 5–7 days post-two-thirds PH (Fig. 1C). In the process of sample collection, liver mass of NCoR1Δhep mice was larger than NCoR1fl/fl mice at 96 hours post-two-thirds PH (Fig. 1D). More vacuolated and swelling hepatocytes were observed in livers of NCoR1Δhep mice, even at 96 hours after two-thirds PH, as revealed by hematoxylin-eosin (H&E) staining (Fig. 1D). In the next step, immunohistochemical (IHC) analysis of Ki67, a marker of cell-cycle entry, was detected. The result revealed that more Ki67-positive cells were observed at 48 hours post-two-thirds PH in NCoR1Δhep mice compared to NCoR1fl/fl mice. At 96 hours post-two-thirds PH, some Ki67-positive cells were still observed in livers of NCoR1Δhep mice, coincident with higher and prolonged cell mitosis (Fig. 1E). Pathway analysis of cell-cycle-related proteins revealed an earlier induction of Cyclin D1 (marker of G1/S) at the initial time and even higher expression at 96 hours post-two-thirds PH (Fig. 1F). In agreement with morphology results, enhanced expression of Cyclin A2 (marker of G2/M or mitosis) at 48 hours post-two-thirds PH and prolonged expression of Cyclin B1 (marker of G2/M) and proliferating cell nuclear antigen (PCNA) was observed (Fig. 1F). A similar tendency was noted on mRNA levels (Supporting Fig. S1A). Because cell-cycle inhibitors P21 and P27 are known to inhibit CCND1-CDK4 complex activity, we further examined the expression change of P21 and P27 proteins during LR and found that the protein level of P27 was dramatically decreased in NCoR1Δhep mice (Supporting Fig. S1F). These results indicate that hepatic NCoR1 deficiency contributed to enhanced LR after two-thirds PH, at least partly through modulating cell-cycle entry.
FIG. 1.
Liver regeneration after 2/3 partial hepatectomy is enhanced in NCoR1Δhep mice. (A) Protein and (B) mRNA expression change of NCoR1 in the process of liver regeneration were detected by western blot and qPCR, Data are presented as mean ± SEM (n=4). *P<0.05; **P<0.01. (C) Liver weight to body weight ratio analysis in NCoR1fl/fl and NCoR1Δhep mice over a time course from 0 to 168 hours after 2/3 PH. Data are presented as mean ± SEM (n=5). *P<0.05; **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice at the indicated times. (D) Morphological changes of livers of NCoR1fl/fl mice and NCoR1Δhep mice at the 96 hours after 2/3 PH as determined by H&E staining. scale bar: 50 μm. (E) Immunohistochemical analysis of Ki67 in paraffin tissues from livers of NCoR1fl/fl and NCoR1Δhep mice at the indicated times after 2/3 PH (Left). Quantification of the percentage of Ki67 labeled nuclei (Right). Data are presented as mean ± SEM (n=4). The different degrees of significance were indicated as follows in the graphs: *P<0.05, **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl at indicated time. (F) Western blot analysis of CyclinD1, CyclinA2, CyclinB1, PCNA using RIPA extracts of NCoR1fl/fl and NCoR1Δhep livers obtained at the indicated times after 2/3 PH. (Two Way ANOVA plus Student’s t test for B, C; Student’s t test for E).
BIOINFORMATICS ANALYSIS OF NCoR1 TARGET GENES ACCOUNTED FOR THE ENHANCEMENT OF COMPENSATORY PROLIFERATION IN NCoR1Δhep MICE
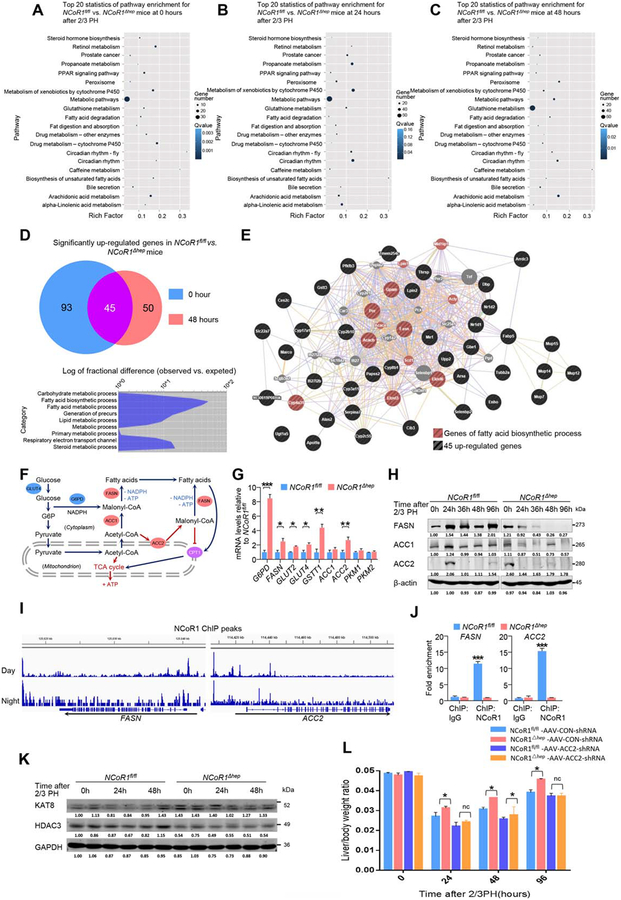
In order to find the specific target genes of NCoR1 that play a key role in regulating the enhancement of compensatory proliferation in NCoR1Δhep mice at an early stage, liver tissues were collected from NCoR1fl/fl and NCoR1Δhep mice at 0, 24, and 48 hours after two-thirds PH and an RNA-sequencing was carried out. Obvious differences in cellular morphology and molecular study were discovered at indicated time points. Analysis of differences existing in the expression of genes at the three indicated times between two groups showed that metabolism-related genes may have had a dominant role in regulating regenerative capacity of hepatocytes (Fig. 2A,C). At 0 and 48 hours after two-thirds PH, 45 genes in NCoR1Δhep were observed to be exclusively up-regulated compared to NCoR1fl/fl mice by intergroup comparison (Fig. 2D). Furthermore, these genes were mostly fatty acid synthesis (FAS)-related genes (Fig. 2D). Gene coexpression network analysis showed that the genes in the central node of the network have a large number of connections, such as acetyl-CoA carboxylase 2 (Acacb, Acc2) and fatty acid synthase (Fasn; Fig. 2E). ACC2 is a rate-limiting enzyme in de novo FAS, but is normally expressed in myocytes and not hepatocytes (Fig. 2F). Furthermore, FASN plays a pivotal role in many process of de novo FAS (Fig. 2F). qPCR assay further confirmed elevation of glucose transport type 4 (Glut4), glucose-6-phosphate dehydrogenase (G6pd), Fasn, and Acc2 genes in NCoR1Δhep mice compared to NCoR1fl/fl mice (Fig. 2G).
FIG. 2.
Bioinformatic analysis of specific genes for NCoR1 regulating compensatory proliferation. (A-C) A transcriptome re-sequencing of RNA from NCoR1fl/fl and NCoR1Δhep mouse liver samples at 0 hours, 24 hours and 48 hours. Pathway analysis of differentially-expressed genes between NCoR1fl/fl and NCoR1Δhep mice are displayed at the indicated times. (D) Functional analysis of genes upregulated at 0 hours and 48 hours. (E) Gene co-expression network of the 45 up-regulated genes are displayed. (F) Schematic diagram of de novo FAS: fatty acids could be produced in liver from glucose or acetyl-CoA. The rate-limiting enzyme are FASN, ACC1 and ACC2. Fatty acids could also be used to produce ATP through CPT1, which could be effectively inhibited by ACC2 regulated malonyl-CoA. The production of fatty acids is ATP consumption and NADPH consumption, which is produced from PPP pathway. (ACC, acetyl-CoA carboxylase; FASN, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; CPT1, carnitine palmitoyl transterase-1). (G) qPCR analysis of mRNAs of the glucose metabolism genes Glut2, Glut4, Pkm1, Pkm2 and G6pd; lipid synthesis genes Fasn, Acc1, Acc2, redox state-related gene Gstt1 at the 0 time point in NCoR1fl/fl and NCoR1Δhep mice are presented. Data are presented as mean ± SEM (n=4). *P<0.05, **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice. (H) Western blot analysis of expression change of FASN, ACC1 and ACC2 protein levels at the indicated time points after 2/3 PH. (I) ChIP-seq results of NCoR1 binding sites in Fasn and Acc2 genes during the day (10 am) and night (10 pm). (J) ChIP-qPCR results using anti-NCoR1 antibody and anti-IgG antibody for Fasn and Acc2. Data are presented as mean ± SEM (n=3). ***P<0.001, NCoR1Δhep mice compared to NCoR1fl/fl mice. (K) Western blot analysis of expression change of KAT8 and HDAC3 protein levels at the indicated time points after 2/3 PH. (L) Liver weight to body weight ratio analysis in NCoR1fl/fl and NCoR1Δhep mice over a time course from 0 to 96 hours after 2/3 PH. Data are presented as mean ± SEM (n=5). *P<0.05; **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice at the indicated times. (Student’s t test for G/J/L).
Because NCoR1 binds to nuclear receptors to repress the transcriptional activity of target genes, several published hepatic NCoR1 chromatin immunoprecipitation sequencing (ChIP-seq) data sets were retrieved in order to find the specific NCoR1 target genes (Gene Expression Omnibus, GSM647027 and GSM647028). In addition, genes were defined that they have peaks within 3,000 base pairs around the transcriptional start site, and this may represent NCoR1 target genes. Therefore, these 45 genes near NCoR1-binding sites were checked from two ChIP-seq results in liver (day and night, 10:00 am and 10:00 pm). ChIP-seq results showed that Fasn and Acc2 were potential NCoR1 target genes in normal mice without surgery (Fig. 2I). This result was also verified by ChIP-qPCR with antibody of NCoR1 and Control, only NCoR1 antibody could directly combined with sequence of promoter region of Fasn and Acc2 (Fig. 2J).
ACC1, ACC2, and FASN are three rate-limiting enzymes of de novo FAS, but unlike ACC1, ACC2 usually expresses in heart and muscle, which regulates a bypass of de novo FAS and could inhibit fatty acid β-oxidation (FAO) by indirectly regulating carnitine palmitoyltransferase 1 (CPT1; Fig. 2F). Thus, ACC2 could facilitate lipid accumulation more effectively. Interestingly, the western blotting results showed that the expression peak of ACC2 came earlier in NCoR1Δhep mice than NCoR1fl/fl mice and disappeared rapidly in both groups (Fig. 2H). Expression of FASN in NCoR1Δhep mice was up-regulated at 0 hours and decreased quickly, whereas it hit its expression peak at 24 hours and maintained a stable level in NCoR1fl/fl mice (Fig. 2H). A recent article documented that acetylation of FASN is positively correlated with degradation of FASN through the ubiquitin-proteasome pathway.(18) The effect of acetylation of FASN is may be occurred in deficiency of NCoR1, as NCoR1 plays a role of deacetylation by cooperation with HDAC3.(10) We thus examined the expression level of the specific acetylase, lysine acetyltransferase 8 (KAT8), and the deacetylase, histone deacetylase 3 (HDAC3), targeting FASN. Interestingly, Fig. 2K shows that KAT8 is comprehensively up-regulated in NCoR1Δhep mice and HDAC3 is down-regulated in NCoR1Δhep mice, which implied that the intracellular balance of KAT8 and HDAC3 is necessary for protein level of FASN.
To further verify the direct contribution of ACC2 for LR, adeno-associated virus (AAV-ACC2-shRNA [small hairpin RNA]) targeting expression of ACC2 in liver was applied before PH in NCoR1fl/fl or NCoR1Δhep mice by caudal vein injection (knockdown efficiency was verified by the real-time PCR method, as shown in Supporting Fig. S5A). Figure 2L shows that knockdown expression of ACC2 significantly retarded the proliferative rate of hepatocytes in NCoR1Δhep mice, and a similar regeneration curve was found in both NCoR1Δhep and NCoR1fl/fl mice after PH. It should be noted that administration of AAV-ACC2-shRNA could not completely block hepatocyte proliferation, and a slight increase in regeneration rate in NCoR1Δhep mice was observed in the absence of ACC2 (24 and 48 hours post-PH in Fig. 2L). These results indicate that deficiency of NCoR1 may facilitate de novo FAS in the early stage of LR by up-regulating the expression of specific genes involved in lipid metabolism at the 0 time point, such as Fasn and Acc2.
ENHANCED DE NOVO FAS, GLUCOSE FLUX, MITOCHONDRIAL FUNCTION FOLLOWING PH IN NCoR1Δhep MICE
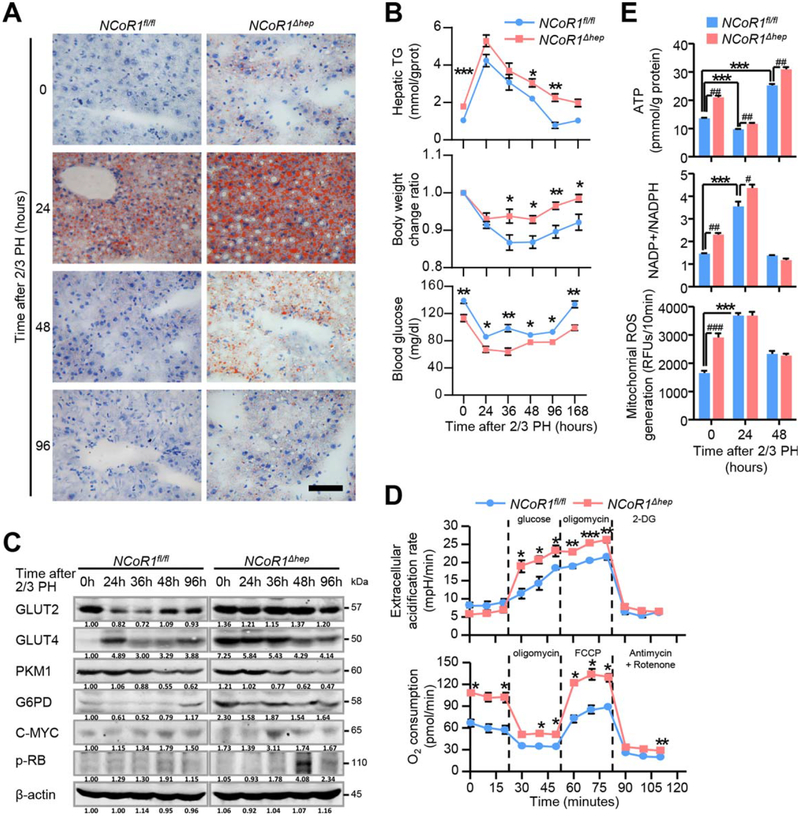
In order to verify whether enhanced de novo FAS, predicted by bioinformatics analysis, does exist in the two-thirds PH model of NCoR1Δhep mice, Oil Red O staining for these tissue sections was detected and the result showed increased accumulation of lipid droplets, which was correlated with transient elevated triglyceride (TG) levels in livers of NCoR1Δhep mice compared to NCoR1fl/fl mice (Fig. 3A,B). The accumulation of lipid drops may come from two different sources: (1) from glucose by de novo FAS and (2) from fatty acids by peripheral fat mobilization. In order to determine which process was influenced by NCoR1 deficiency, we detected the glucose level in serum and the change ratio of body weight in the process of LR. NCoR1Δhep mice showed a lower serum glucose level in each time point, but with less weight loss, compared to NCoR1fl/fl mice (Fig. 3B; Supporting Table S1). In addition, because it was determined that one-third PH induced less stimulus and peripheral lipid mobilization in the process of LR,(28) we performed a one-third PH in NCoR1fl/fl and NCoR1Δhep mice. More lipid accumulation in 36 hours after one-third PH was observed in NCoR1Δhep mice (Fig. 4E,G). These results indicate that increased lipid drops at 24 hours after two-thirds PH in NCoR1Δhep mice may be regulated by enhanced de novo FAS, which is correlated with advanced expression of FASN and ACC2 in NCoR1Δhep at the initial time.
FIG. 3.
Enhanced glucose flux, mitochondrial function and de novo FAS genesis in NCoR1Δhep mice at the early stages of liver regeneration after 2/3 PH. (A) Analysis of the lipids by Oil Red O staining performed with frozen liver sections. scale bar: 50 μm (B) Analysis of liver triglyceride (TG) contents, body weight change ratios and serum glucose in NCoR1fl/fl and NCoR1Δhep mice at the indicated time points after 2/3 PH. Data are presented as mean ± SEM (n=5). The different degrees of significance were indicated as follows in the graphs: *P<0.05; **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice. (C) Western blot analysis of expression change of protein level of GLUT2, GLUT4, PKM1, G6PD, C-MYC and p-RB at the indicated time points after PH. (D) Upper panel: Extracellular acidification rate (ECAR) of NCoR1fl/fl and NCoR1Δhep primary hepatocytes, as measured by the Seahorse Analyzer (n = 4 each). Addition of glucose induces glycolysis-dependent lactic acid production and ECAR. Oligomycin then induces maximal ECAR, and 2-DG partially inhibits glycolysis-dependent ECAR. Lower panel: Oxygen consumption rate (OCR) of NCoR1fl/fl and NCoR1Δhep primary hepatocytes, as measured by the Seahorse Analyzer (n = 4 each). Oligomycin treatment inhibits ATP synthase dependent OCR. The proton gradient uncoupler FCCP then induces maximal OCR, and antimycin/rotenone finally inhibits all OxPhos-dependent OCR. *P<0.05; **P<0.01, ***P<0.001, NCoR1 KO hepatocyte compared to NCoR1fl/fl hepatocyte. (E) Biochemical detection of intracellular ATP levels, NADP+/NADPH ratios and mitochondrial ROS in NCoR1fl/fl and NCoR1Δhep mice at the indicated times after 2/3 PH. Data are presented as mean ± SEM (n=4). The different degrees of significance were indicated as follows in the graphs: *P<0.05; **P<0.01; ***P<0.001, NCoR1fl/fl mice at 0 hours compared with NCoR1fl/fl mice at indicated times after 2/3 PH. #P<0.05; ##P<0.01, ###P<0.001, NCoR1Δhep mice compared to NCoR1fl/fl mice at indicated times after 2/3 PH. (Two Way ANOVA plus Student’s t test for B, D; Student’s t test for E).
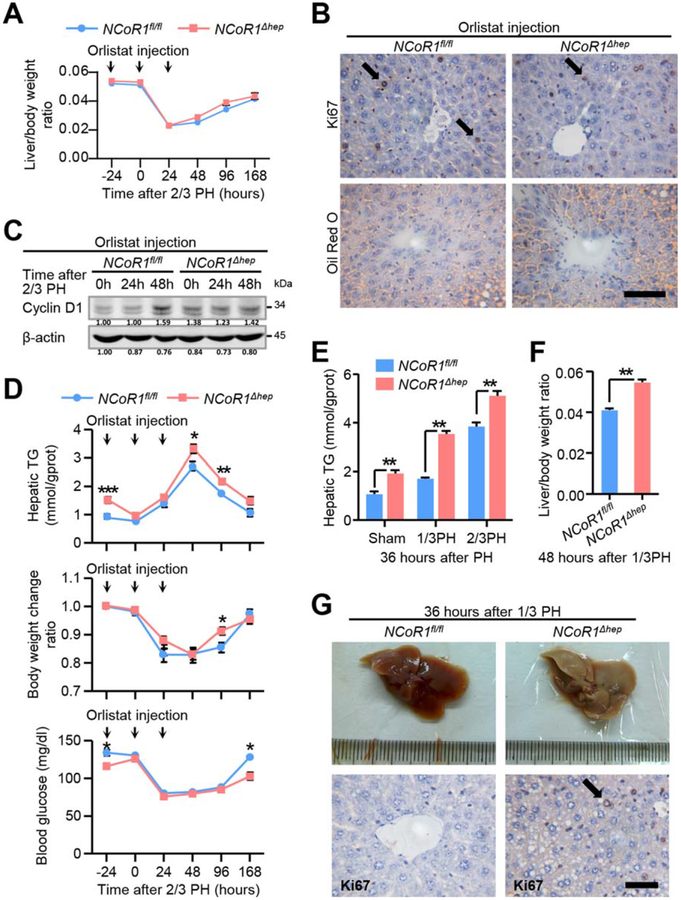
FIG. 4.
Enhanced de novo FAS facilitate compensatory proliferation after 2/3 PH and alters the metabolic state in liver. (A) Liver to body weight ratio analysis in NCoR1fl/fl and NCoR1Δhep mice over a time course from 0 to 168 hours after 2/3 PH and pretreated with orlistat from 24 hours before to 24 hours after 2/3 PH in a daily pattern, Data in are presented as mean ± SEM (n=5). (B) Immunohistochemical analysis of Ki67 in paraffin tissues from liver of NCoR1fl/fl and NCoR1Δhep mice at 48 hours after 2/3 PH and Oil Red O staining of frozen sections from livers of NCoR1fl/fl and NCoR1Δhep mice at 24 hours after 2/3 PH, which were pretreated with orlistat. scale bar: 50 μm. (C) Western blot analysis of CyclinD1 using RIPA extracts of NCoR1fl/fl and NCoR1Δhep livers obtained at the early stage after 2/3 PH. (D) Analysis of liver triglyceride (TG) content, body weight change ratio and serum glucose at indicated time points in NCoR1fl/fl and NCoR1Δhep mice pretreated with orlistat from 24 hours before to 24 hours after 2/3 PH, Data in are presented as mean ± SEM (n = 5). The different degrees of significance were indicated as follows in the graphs: *P<0.05; **P<0.01, NCoR1fl/fl mice compared to WT mice. (E) Biochemical detection of liver triglyceride content in NCoR1fl/fl and NCoR1Δhep mice at 36 hours after sham surgery, 1/3 PH or 2/3 PH. Data in are presented as mean ± SEM (n=5). **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice. (F) Analysis of the liver/body weight ratio of NCoR1fl/fl and NCoR1Δhep mice at 48 hours after 1/3 PH. Data in are presented as mean ± SEM (n=4). The different degrees of significance are indicated as follows in the graphs: **P<0.01, NCoR1Δhep mice compared to NCoR1fl/fl mice. (G) Morphological change of livers of NCoR1fl/fl mice and NCoR1Δhep mice at the 36 hours after 1/3 PH; Immunohistochemical analysis of Ki67 in paraffin tissues from liver of NCoR1fl/fl and NCoR1Δhep mice at the same time. scale bar: 50 μm. (Two Way ANOVA plus Student’s t test for A, D; Student’s t test for E, F).
Because enhanced de novo FAS is associated with glucose flux, expression of proteins regulating glucose metabolism was measured during the process of LR. Up-regulation of GLUT4 and G6PD was observed during LR, which was correlated with a lower serum glucose level in NCoR1Δhep mice (Fig. 3B,C). Enhanced glucose flux and mitochondrial activity could be induced by NCoR1 deficiency because more glucose and adenosine triphosphate (ATP) consumption are required for de novo FAS (Fig. 2F). To confirm this, glucose metabolism was measured by using the Seahorse analyzer to detect the O2 consumption rates and H+ production of primary hepatocytes from NCoR1Δhep and NCoR1fl/fl mice. Deficiency of NCoR1 increased both the basal and maximal oxidative phosphorylation capacity, as indicated by rising O2 consumption (Fig. 3D). However, even though the glycolytic capacity of primary hepatocytes from NCoR1Δhep mice was similar to NCoR1fl/fl mice, elevated glycolytic capacity was noted with glucose and oligomycin (a mitochondrial inhibitor) treatment (Fig. 3D). The result indicated that more glucose and energy is required for NCoR1Δhep hepatocytes. Then, the protein levels of C-MYC and retinoblastoma protein (p-RB) were found to be up-regulated, which is closely related to metabolism and mitosis in the process of LR (Fig. 3C).
Enhancement of de novo FAS could induce more ATP consumption, but more lipids could provide more ATP through FAO (Fig. 2F). To confirm the energy state during the regeneration process, intracellular ATP levels in NCoR1fl/fl and NCoR1Δhep mice at the early stages of LR were examined. At the 0 point, deficiency of NCoR1 was associated with increased intracellular ATP levels (Fig. 3E). Although ATP levels were decreased 24 hours after PH, which might result from the rapid production of lipids consuming more ATP transiently, a faster and stronger increase of ATP level was observed in NCoR1Δhep mice 48 hours after PH (Fig. 3E). These data suggest that deficiency of NCoR1 could enhance glucose flux and de novo FAS effectively in the short term and consequently provide more energy during the process of LR.
G6PD, a key enzyme in the pentose phosphate pathway (PPP), was up-regulated in NCoR1Δhep mice during LR (Fig. 3C). The PPP is the major production source of NADP+ reduced (NADPH), which is an indispensable reducing substance for consumption in de novo FAS (Fig. 2F). Enhancement of de novo FAS in deficiency of NCoR1 may also influence the NADPH level and redox state. We thus examined the nicotinamide adenine dinucleotide phosphate (NADP+)/NADPH ratios and mitochondrial reactive oxygen species (ROS) in NCoR1fl/fl and NCoR1Δhep mice after two-thirds PH. Interestingly, higher levels of NADP+/NADPH ratio and mitochondrial ROS were observed in NCoR1Δhep mice, but not NCoR1fl/fl mice (Fig. 3E). These data imply that enhancement of de novo FAS in the absence of NCoR1 may not only lead to consumption of NADPH (Fig. 2F) and mitochondrial reactive oxygen species (ROS) enrichment, but also induces compensatory reprogramming of the metabolic state, such as enhancing the G6PD-dependent PPP process to generate NADPH.
ENHANCEMENT OF DE NOVO FAS FACILITATES COMPENSATORY PROLIFERATION IN LIVER OF NCoR1Δhep MICE
In order to determine whether enhancement of de novo FAS facilitated compensatory proliferation in liver, we treated NCoR1fl/fl and NCoR1Δhep mice with a selective FASN inhibitor (orlistat) intraperitoneally at the indicated time point (Fig. 4A). As expected, no significant differences of liver/body weight ratio, Ki67, and lipid in oil staining, together with Cyclin D1 expression, were observed between NCoR1fl/fl and NCoR1Δhep mice after orlistat treatment (Fig. 4A–C). We also detected the effect of orlistat on hepatic TG concentration, body weight change ratio, and serum glucose level at indicated times after two-thirds PH (Fig. 4D), and found that orlistat attenuated the accumulation of TG at an early stage in both wild-type and knockout mice. The effects of NCoR1 depletion causing differences in body weight change ratio and serum glucose between NCoR1fl/fl and NCoR1Δhep mice were eliminated upon orlistat treatment. These data suggested that orlistat could effectively inhibit de novo FAS in liver, which plays a pivotal role in enhanced LR in NCoR1Δhep mice.
To further confirm that inhibiting de novo FAS could hamper the energy production and disturb redox homeostasis during LR, intracellular ATP levels in NCoR1fl/fl and NCoR1Δhep mice pretreated with orlistat at 24 hours before and after two-thirds PH were measured. Deficiency of NCoR1 increased intracellular ATP levels at 24 hours after orlistat injection (Supporting Fig. S2A). However, at 48 hours after two-thirds PH, there was little difference of ATP level between the two mouse lines (Supporting Fig. S2A). This phenomenon indicates that the extent of lipid accumulation is associated with initiation of mitosis. Thus, inhibiting de novo FAS may delay LR because of less ATP supply. We also detected the NADP+/NADPH ratios and mitochondrial ROS in NCoR1fl/fl and NCoR1Δhep mice pretreated with orlistat at indicated times after two-thirds PH. The results showed that even though NADP+/NADPH ratios and mitochondrial ROS levels were up-regulated in NCoR1Δhep mice compared to NCoR1fl/fl mice, they were down-regulated after orlistat treatment. At 48 hours after two-thirds PH, NADP+/NADPH ratios and mitochondrial ROS levels were still higher than normal condition in both mouse lines (Supporting Fig. S2B, C). These results indicated that enhanced de novo FAS might result in a short-term oxidative stress in liver after two-thirds PH, and inhibiting de novo FAS not only delayed the hepatocyte mitosis, but also the recovery of redox state.
To exclude the possible effect of exogenous lipid on hepatocyte proliferation in our study, we performed a one-third PH in NCoR1fl/fl and NCoR1Δhep mice, which is a typical model with less stimulus and peripheral lipid mobilization in the process of LR (Fig. 4F).(28) We found earlier restoration of liver size and appearance of Ki67 staining-positive cells 36 hours after one-third PH in NCoR1Δhep mice (Fig. 4G). Additionally, survival and growth rate of NCoR1 knockdown cells were faster than control cells upon exogenous fatty acid deprivation (Dulbecco’s modified Eagle’s medium [DMEM] with 1% fetal bovine serum [FBS]). However, similar results were not found in normal culture medium (DMEM with 5% FBS; Supporting Fig. S3C–E). These data indicate that NCoR1 deficiency could provoke hepatocyte survival and proliferation through enhancing de novo FAS, but not mobilizing peripheral lipid.
DEFICIENCY OF NCoR1 ATTENUATED HCG IN DEN-INDUCED HCC MODEL
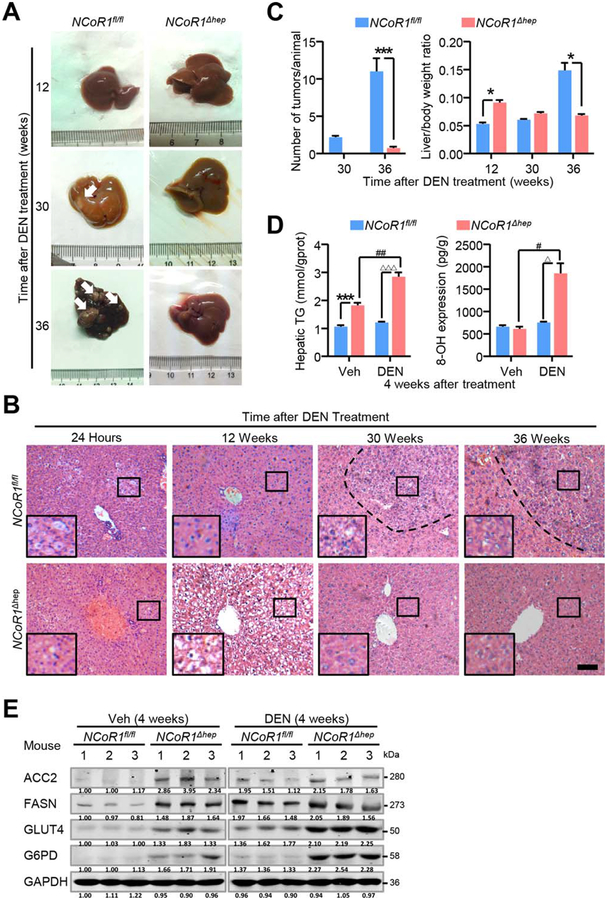
Higher metabolic rate and regenerative capacity in NCoR1 knockout hepatocytes than NCoR1fl/fl hepatocytes prompted us to explore the propensity of carcinogenesis of NCoR1 deficiency. Twenty-month-old NCoR1Δhep mice showed no lesion of tumor without other intervention (data not shown). When we submitted 15-day-old NCoR1fl/fl and NCoR1Δhep mice to an acute treatment of DEN, a larger number of vacuolated lesions were observed in livers of NCoR1Δhep mice in H&E staining, which indicated a more-severe response to DEN (Fig. 5B). However, 12 weeks after DEN treatment, the liver/body weight ratio increased far more in NCoR1Δhep mice than NCoR1fl/fl mice and with more swelling hepatocytes in H&E staining (Fig. 5A,B). Interestingly, NCoR1fl/fl mice developed mac-roscopically visible tumors 30 weeks after DEN treatment, which were barely observed in NCoR1Δhep mice (Fig. 5A). At 36 weeks after DEN treatment, numerous tumor nodules were observed in whole liver of NCoRfl/fl mice, whereas few lesions of tumor were discovered in NCoR1Δhep mice with decreased liver/body weight ratio (Fig. 5A,C). The obvious morphology change of swelling observed at the early stage after DEN treatment in NCoR1Δhep mice may be associated with lipid accumulation and DNA damage. Hence, we detected TG and 8-hydroxy-2′-deoxyguanosine (8-OHdG; 8-OH) level in livers 4 weeks after DEN treatment. A higher level of TG was detected in NCoR1Δhep mice than in NCoR1fl/fl mice (Fig. 5D). DEN increased 8-OHdG (an indicator of ROS-induced DNA damage) level in both NCoR1Δhep mice and NCoR1fl/fl mice, and we found that expression level of 8-OHdG was significantly improved in NCoR1Δhep mice (Fig. 5D). Furthermore, we detected the expression change of key proteins of de novo FAS (Fig. 2F) with or without DEN treatment 4 weeks after DEN treatment in NCoR1fl/fl and NCoR1Δhep mice. The result showed that the level of ACC2, FASN, GLUT4, and G6PD were higher in NCoR1Δhep mice than NCoR1fl/fl mice, even though DEN treatment could elevate their expression slightly (Fig. 5E). These results indicate that a chronic injury induced by DEN treatment could cause a higher level of de novo FAS and that NCoR1 deficiency might have attenuated the propensity of carcinogenesis after DEN treatment.
FIG. 5.
Attenuated formation of DEN-induced tumors in NCoR1Δhep mice. (A) NCoR1fl/fl and NCoR1Δhep mice were intraperitoneally injected with PBS or DEN at day 15 after born and livers collected at 24 hours after injection and 4, 12, 30, 36 weeks after injection. Representative images and (B) representative H&E histological features of livers from DEN-treated mice at the indicated times after killing were shown. scale bar: 100 μm. (C) Quantification of the number of macroscopic tumors per animal and analysis of the liver to body weight ratio of NCoR1fl/fl and NCoR1Δhep mice. Data are presented as mean ± SEM (n=5). The different degrees of significance are indicated as follows in the graphs: *P<0.05; ***P<0.001, NCoR1Δhep mice compared to NCoR1fl/fl mice. (D) Biochemical detection of liver triglyceride content and 8-OhDG (8-OH) in NCoR1fl/fl and NCoR1Δhep mice treated with PBS or DEN at four weeks after injection. Data are presented as mean ± SEM (n=4). ***P<0.001, NCoR1Δhep mice compared to NCoR1fl/fl mice treated with Veh. #P<0.05; ##P<0.01 NCoR1Δhep mice treated with Veh compared to NCoR1Δhep mice treated with DEN. ΔΔΔP<0.001, NCoR1fl/fl mice treated with DEN compared to NCoR1Δhep mice treated with DEN. (E) Western blot analysis of expression of protein level of ACC2, GLUT4, FASN, G6PD in NCoR1fl/fl and NCoR1Δhep mice treated with PBS or DEN at four weeks after DEN treatment. (Student’s t test for B, D).
NCoR1 DEFICIENCY ENHANCES THE OXIDATIVE STRESS LEVEL AND HEPATOCYTE APOPTOSIS AFTER DEN TREATMENT
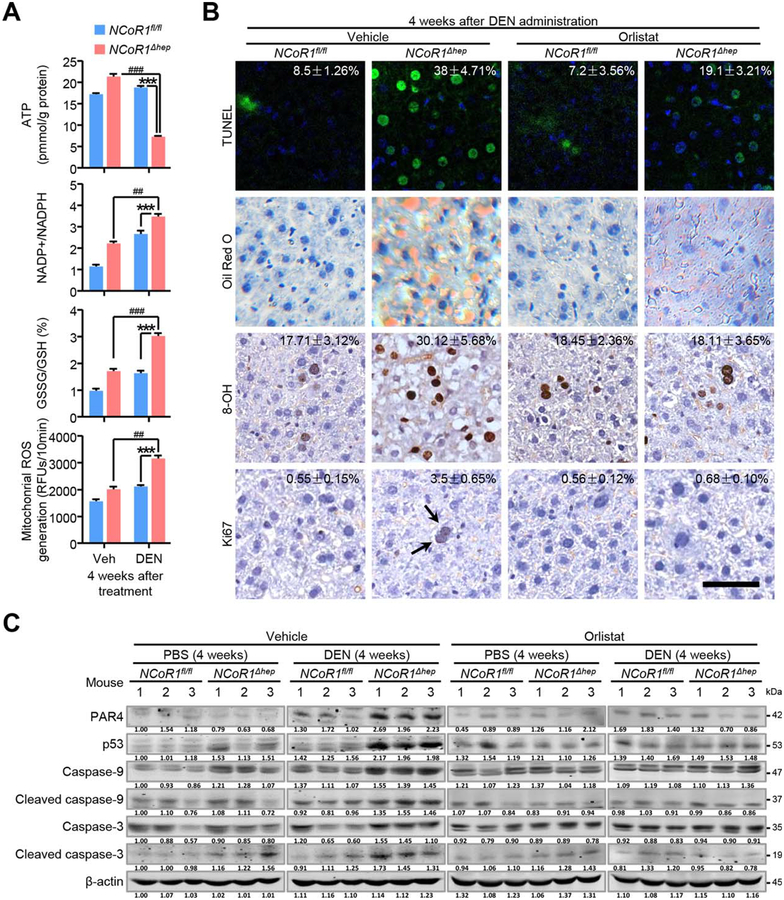
High expression levels of 8-OHdG (8-OH) and G6PD protein were observed in DEN-treated NCoR1Δhep mice at an early stage (Fig. 5E); we thus speculated that such prolonged de novo FAS in NCoR1Δhep mice may consume much more NADPH provided by the PPP pathway (Fig. 2F). Consistently, expression level of G6PD was still high 4 weeks after DEN treatment in NCoR1Δhep mice (Fig. 5E). These events may have resulted in an imbalance of the redox state in the liver given that NADPH was insufficient. In order to demonstrate this consequence of long-term FAS after DEN treatment, NADP+/NADPH ratio, glutathione disulfide (GSSG)/glutathione (GSH) ratio, and mitochondrial ROS were measured 4 weeks after DEN treatment. As expected, NADP+/NADPH and GSSG/GSH ratios and levels of mitochondrial ROS were increased in NCoR1Δhep mice compared to NCoR1fl/fl mice after DEN treatment (Fig. 6A). Meanwhile, ATP level was reduced in liver compared to NCoR1fl/fl mice 4 weeks after DEN treatment (Fig. 6A). These results indicate that DEN-treated NCoR1Δhep mice may suffer from more-severe oxidative stress and NADPH consumption than control mice.
FIG. 6.
Increased oxidative stress and apoptosis in early stage of DEN injury in NCoR1Δhep mice. (A) Biochemical detection of intracellular ATP levels, NADP+/NADPH ratios, GSSG/GSH ratios and mitochondrial ROS in NCoR1fl/fl and NCoR1Δhep mice treated with PBS or DEN at four weeks after DEN treatment. Data are presented as mean ± SEM (n=4). ***P<0.001, NCoR1Δhep mice treated with DEN compared to NCoR1fl/fl mice treated with DEN. ##P<0.01; ###P<0.001, NCoR1Δhep mice treated with DEN compared to NCoR1Δhep mice treated with control vehicle. (B) Four weeks after DEN administration, NCoR1fl/fl and NCoR1Δhep mice were treated with vehicle or orlistat for 5 days before sample collection. Representative Tunel staining, Oil Red O staining and immunohistochemical analysis of 8-OH and Ki67 were performed on mice liver samples. Quantification of the percentage of Tunel, 8-OH and Ki67 labeled nuclei were marked. Data are presented as meaňSEM of at least four mice per group. scale bar: 50 μm. (C) Four weeks after DEN administration, NCoR1fl/fl and NCoR1Δhep mice were treated with vehicle or orlistat for 5 days. Western blot analysis of expression of protein level of p53, PAR4, Caspase-3 and Caspase-9 (full length and their cleaved form) in mice liver samples were performed. (Student’s t test for A, B).
Generally, oxidative stress and DNA damage could induce apoptosis. This may explain the phenomenon that less tumor was formed in NCoR1Δhep mice after DEN treatment if apoptosis was enhanced. Indeed, morphological studies showed persistent cell apoptosis and DNA damage 4 weeks after DEN treatment in NCoR1Δhep mice, but not in NCoR1fl/fl mice, as revealed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) and 8-OHdG staining, respectively (Fig. 6B). In NCoR1Δhep mice without DEN treatment, TUNEL staining-positive cells were not observed (data not shown). In addition, higher levels of Oil Red O staining were observed 4 weeks after DEN treatment (Fig. 6B). Ki67 staining was weakly positive in a number of hepatocytes in NCoR1Δhep mice, but not in NCoR1fl/fl mice (Fig. 6B). In order to determine that the increased cell apoptosis was induced by over-reinforced de novo FAS in NCoR1Δhep mice, we administered orlistat, a selective inhibitor of de novo FAS, in two groups of mice after DEN treatment. Orlistat administration was provided 5 days before sample collection 4 weeks after DEN treatment. Notably, orlistat treatment clearly abrogated the distinct levels of TUNEL, 8-OHdG, Oil Red O, and Ki67 staining between the two groups of mice after DEN administration (Fig. 6B). In addition, expression of P53 and protease-activated receptor 4 (PAR4), genes related to apoptosis, was up-regulated in NCoR1-deficient livers 4 weeks after DEN treatment (Fig. 6C). To confirm the change of downstream apoptotic genes, classical proteins involved in apoptosis, such as Caspase-3 and Caspase-9, were measured 4 weeks after DEN treatment (Fig. 6C). NCoR1 deficiency slightly increased expression of Caspase-3 and Caspase-9 as well as their cleaved forms in PBS treatment, whereas DEN treatment significantly up-regulated their expression, especially the cleaved forms of Caspase-3 and Caspase-9, in NCoR1Δhep mice, whereas orlistat treatment significantly attenuated these differences between the two mouse groups (Fig. 6C). Together, these data indicate that there were more NADPH consumption, oxidative stress, and cell apoptosis induced after DEN treatment in NCoR1Δhep mice, compared to NCoR1fl/fl mice, which may have resulted from enhanced de novo FAS in the NCoR1 deficiency setting. Persistent cell apoptosis at the early stage of DEN treatment in NCoR1Δhep mice, but not in NCoR1fl/fl mice, may facilitate the clearance of proneoplastic lesions.
Discussion
This study characterized a distinct role of NCoR1 in the process of LR and carcinogenesis. Our results showed that significant differences exist in the restoration of liver/body weight ratio and the expression of cell-cycle-related proteins between NCoR1fl/fl and NCoR1Δhep mice following two-thirds PH. During the process of LR, 0–24 hours after two-thirds PH was identified as the metabolic phase with no significant hepatocyte proliferation, whereas 40–48 hours post-two-thirds PH(19) was nominated as the proliferation phase with much higher expression of Cyclin A2, a cell-cycle marker of mitosis, and Ki67 in NCoR1Δhep mice. In the metabolic phase, the significantly increased expression of Cyclin D1, a marker of the G1 phase, was observed in NCoR1Δhep mice, indicating that the abnormal expression of Cyclin D1 and material preparation of cell-cycle entry is indispensable for enhanced regenerative capacity. More hepatocytes will enter the G1 phase and undergo cell mitosis because the expression of Cyclin A2 was enhanced 48 hours after two-thirds PH in NCoR1Δhep mice. Some recent studies documented that Cyclin D1 takes its place in metabolic activation independent of the cell cycle.(20) Cyclin D1 could cooperate with cyclin-dependent kinase 4 and bind to the promoter of PPAR gamma, coactivator 1 α,(21) a metabolic activated factor. In our cytological study, proliferative rate was not influenced by NCoR1 knockdown, but reduced under the condition of lipid deprivation plus NCoR1 knockdown. These results suggest that faster recovery at the early stage of LR in NCoR1Δhep mice may be mainly associated with energy reserve for cell-cycle entry.
Lipid metabolism was reported to be indispensable for the initiation of LR.(13) Many regulators of peripheral lipid mobilization and formation of lipid droplets were also reported to participate in LR, such as farnesoid X receptor, caveolin-1, and PPARs.(13,22,23) PPARs belong to the super nuclear receptor family and mainly perform their functions in lipid metabolism. As a comprehensive nuclear receptor repressor gene, NCoR1 might affect lipid metabolism through this pathway. As expected, in the NCoR1 deficient setting, the downstream β-oxidation-related genes and FAS-related genes of PPARα are significantly up-regulated (Supporting Fig. S4A). This result suggested that activation of PPARα might accelerate lipid metabolism in NCoR1Δhep mice. After administering GW6471, a specific PPARα antagonist (Supporting Fig. S4B), we performed a two-thirds PH in both groups. An obvious difference of liver/body ratio between NCoR1-WT and NCoR1-CKO mice was still observed after administration of GW6471 (Supporting Fig. S4C,D), and no obvious changes were found in NCoR1-WT mice after two-thirds PH in the presence or absence of GW6471 (data not shown). Similar results were observed by Oil Red O staining and IHC analysis of Ki67 (Supporting Fig. S4E,F). Bioinformatics analysis of the differentially expressed genes between NCoR1fl/fl and NCoR1Δhep mice at the early phase after two-thirds PH (Fig. 2D) revealed that the key enzymes of de novo FAS, such as FASN and ACC2, hit their expression peaks at the initial time before PH in NCoR1Δhep mice but 24 hours after PH in NCoR1fl/fl mice. NCoR1 was degraded quickly after two-thirds PH by the proteasome path-way.(24) Given that the phosphorylation of ACC1 and ACC2 induced by lipid-activated AMP-activated protein kinase (AMPK) signaling could be degraded by the proteasome pathway,(15) the down-regulation of these enzyme in NCoR1Δhep mice at 24 hours after PH may have resulted from feedback of fast lipid drop accumulation and AMPK activation. Furthermore, interfering with de novo FAS by pretreatment with orlistat hampered lipid accumulation and slowed down the regeneration rate of liver mass after two-thirds PH in both mice groups. The proliferative rate of HCC cell lines was not influenced by NCoR1 knockdown, but reduced under the condition of exogenous lipid deprivation plus NCoR1 knockdown. That means that NCoR1 knockdown enhanced endogenous FAS to provide lipids for cell proliferation.
We suggest that de novo FAS in the G1 phase after two-thirds PH is different from de novo FAS in physical conditions. FASN and ACC2 are rate-limiting enzymes for de novo FAS. Furthermore, FASN plays a key role in many processes of lipid synthesis and coordinates with ACC1 and ACC2.(25–27) Here, we used orlistat, a selective FASN inhibitor, as an intervention. Other selective inhibitors targeting de novo FAS may be used in our future work. We found that ACC2, a specific rate-limiting enzyme in de novo FAS, was up-regulated in livers of NCoR1fl/fl mice at 24 hours after two-thirds PH. ACC2 is normally expressed in myocytes and adipocytes, but not in hepatocytes, whereas ACC1 is mainly expressed in hepatocytes. ACC2 is located at the mitochondrial membrane, but ACC1 is located in the cytoplasm. ACC2 could inhibit CPT1 more efficiently than ACC1 given that malonyl-CoA generated by ACC2 at the surface of the mitochondria could directly inhibit CPT1 and shut down the delivery of fatty acids destined for oxidation.(26) In NCoR1Δhep mice, up-regulation of ACC2 was observed at time 0, suggesting that early preparation for enhanced de novo FAS was underway. Increased expression of ACC2 could be the main cause for lipid accumulation, which was reported to be crucial for initiation of mitosis. Interestingly, the peak of NCoR1 expression appears in Fasn and Acc2 genes both in day and night (10:00 am and 10:00 pm) according to the ChIP-seq results. Those data suggested that NCoR1 acts as a repressor in the expression of Fasn, Acc2, or other genes involved in lipid metabolism in quiescent hepatocytes. NCoR1 was normally expressed in liver and sharply down-regulated at 24 hours after two-thirds PH. This down-regulation of protein level is faster than mRNA change and probably is related to proteasome degradation.(24) NCoR1 was known to bind to the PPARs/CCAAT enhancer binding proteins complex for regulation of metabolism, and any changes in this complex have been shown to impact PPARs and their target genes.(9) The existence of NCoR1 and HDACs limits the stress-related or tissue-specific responses of nuclear receptors to certain ligands by histone deacetylation.(9,24) Deficiency of NCoR1 in hepatocytes may down-regulate the threshold of gene expression, so the G1 phase-specific genes could be expressed without stimuli.
Hypermetabolism and the enhanced capacity of glucose uptake are associated with malignant transformation. Attenuation of tumorigenic lesions in NCoR1Δhep mice was observed 36 weeks after DEN treatment. It seemed that the de novo FAS in the presence of NCoR1 prevents liver carcinogenesis, which is different from lipid accumulation during NASH. It is well known that NASH was demonstrated to be tumor promoting, but NASH is caused by a model with sustained supplying of a high-fat diet in a passive way.(27) Besides, the utilization of glucose and fatty acids in liver with NASH is also hampered, which is induced by oxidative stress and sustained inflammation. However, NCoR1 deficiency increases de novo FAS and glucose uptake in a positive way, and the mitochondrial function is enhanced. Collectively, exogenous lipid is important for LR, but it could bring about steatohepatitis and the hazard of oncogenesis. Although de novo FAS is also regenerative promoting, it is protective for carcinogenesis.
Considering that improved regenerative capacity and carcinogenesis are positively correlated, the question arises of how NCoR1 mediates the balance between compensatory proliferation and carcinogenesis by affecting de novo FAS. PH is acute with larger numbers of hepatocyte loss, whereas DEN injury is chronic with a prolonged DNA damage. Sustained de novo FAS could break redox state homeostasis, given that de novo FAS consumes glucose and NADPH, which is a reducing agent required for the production of reduced glutathione and glutathione peroxidase activity to eliminate intracellular ROS. Enhanced de novo FAS was correlated with the up-regulation of G6PD, a key enzyme of PPP (Fig. 2F). This indicates that a high level of G6PD in NCoR1Δhep mice could be compensatory for maintenance of redox homeostasis. In addition, DNA damage after DEN administration could induce mitochondrial-dependent apoptosis by increasing mitochondrial ROS. Failure to maintain NADPH levels in NCoR1Δhep mice could amplify apoptosis signaling after DNA damage as a result of insufficient reducing agent to eliminate ROS. However, two-thirds PH is an acute injury with short-term stimulus. The redox balance could be achieved quickly after the liver was fully restored. Thus, enhanced de novo FAS may facilitate cells to enter the cell cycle under the process of LR, but attenuate prolonged HCG (Fig. 7B). Consistent with another study, knockdown of ACC2 resulted in the increase of tumor formation.(15)
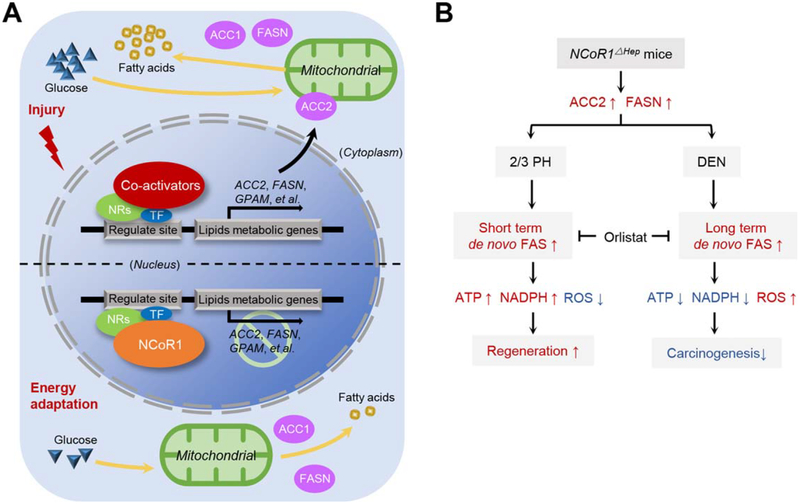
FIG. 7.
Schematic representation of NCoR1Δhep mice in compensatory prolifaeration and DEN induced hepatocarcinogenesis. (A) Genes of specific lipid metabolism is up-regulated by the stimulation of injury in hepatocyte, such as Acc2 and Fasn. NCoR1 epigenetically controls the expression of lipid metabolic genes when energy adaptation but is degraded in acute injury. (B) Distinct mechanism of NCoR1Δhep mice in compensatory proliferation and DEN induced hepatocarcinogenesis. PH is an acute injury with large number of hepatocyte loss. Deficiency of NCoR1 could facilitate a short-term de novo FAS to provide more lipid for energy supplement. While the redox balance could be achieved quickly after the liver was fully restored. DEN injury is chronical with a prolonged DNA damage. Sustained de novo FAS without NCoR1 control could consume more NADPH and enhance oxidative stress, which induce the mitochondrial-dependent apoptosis. Thus, the early neoplasia are hampered.
In conclusion, NCoR1 represses genes involved in lipid synthesis in quiescent hepatocytes (Fig. 7A). In an acute liver injury situation, NCoR1 deficiency could enhance de novo FAS and provide more energy for LR. However, in a chronic liver injury circumstance, such as the DEN-induced HCC model, failure to suppress FAS-related genes could result in sustained de novo FAS in NCoR1Δhep mice, which subsequently increases cell apoptosis and attenuates the formation of proneoplastic lesions (Fig. 7B).
Supplementary Material
Acknowledgments:
We acknowledge the members of the International Co-operation Laboratory on Signal Transduction, especially Dong-Ping Hu, Shan-Hua Tang, Lin-Na Guo, Dan Cao, Dan-Dan Huang, Liang Tang, and Shan-Na Huang, for excellent technical assistance.
Supported by the State Key Project for Liver Cancer (2012ZX10002–009), the National Research Program of China (2012CB316503, 2012AA02A201), the National Natural Science Foundation of China (81422032, 81672860, 81300306, 81372674, and 91529303), and the Science Foundation of Shanghai (134119a3700).
Abbreviations:
- 8-OHdG
8-hydroxy-2’-deoxyguanosine
- AAV
adeno-associated virus
- ACC
acetyl-CoA carboxylase
- Acacb/Acc2
acetyl-CoA carboxylase 2
- Alb
albumin
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- ChlP-seq
chromatin immunoprecipitation sequencing
- CPT1
carnitine palmitoyltransferase 1
- DEN
diethylnitrosamine
- DMEM
Dulbecco’s modified Eagle’s medium
- FAO
fatty acid β-oxidation
- FAS
fatty acid synthesis
- FASN
fatty acid synthase
- FBS
fetal bovine serum
- G6PD
glucose-6-phosphate dehydrogenase
- GLUT
glucose transport type
- GSH
glutathione
- GSSG
glutathione disulfide
- HCC
hepatocellular carcinoma
- HCG
hepatocarcinogenesis
- HDAC3
histone deacetylase 3
- H&E
hematoxylin-eosin
- IHC
immunohistochemical
- KAT8
lysine acetyltransferase 8
- LR
liver regeneration
- NADP+
nicotinamide adenine dinucleotide phosphate
- NADPH
NADP+ reduced
- NASH
nonalcoholic steatohepatitis
- NCoR1
nuclear receptor corepressor 1
- NCoR1Δhep
NCoR1 liver-specific null mice
- NCoR1fl/fl
NCoR1-floxed mice
- PAR4
protease-activated receptor 4
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
- PH
partial hepatectomy
- PPAR
peroxisome proliferator-activated receptor
- PPP
pentose phosphate pathway
- p-RB
retinoblastoma protein
- ROS
reactive oxygen species
- shRNA
small hairpin RNA
- TG
triglyceride
- TUNEL
(terminal deoxynucleotidyl transferase dUTP nick end labeling)
Footnotes
Potential conflict of interest: Nothing to report.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29562/suppinfo.
REFERENCES
- 1.) Shyh-Chang N, Zhu H, de Soysa Yvanka T, Shinoda G, Seligson MT, Tsanov KM, et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 2013;155:778–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.) Pauta M, Rotllan N, Vales F, Fernandez-Hernando A, Allen RM, Ford DA, et al. Impaired liver regeneration in Ldlr−/− mice is associated with an altered hepatic profile of cytokines, growth factors, and lipids. J Hepatol 2013;59:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.) Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 2012;56:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.) Goldenberg D, Eferl R. p21Waf1/Cip1 revisited: oncogenic function in hepatocellular carcinoma. Gut 2014;63:1372–1373. [DOI] [PubMed] [Google Scholar]
- 5.) Malato Y, Ehedego H, Al-Masaoudi M, Cubero FJ, Bornemann J, Gassler N, et al. NF-kappaB essential modifier is required for hepatocyte proliferation and the oval cell reaction after partial hepa-tectomy in mice. Gastroenterology 2012;143:1597–1608.e.11. [DOI] [PubMed] [Google Scholar]
- 6.) Nouet Y, Dahan J, Labalette C, Levillayer F, Julien B, Jouvion G, et al. The four and a half LIM-only protein 2 regulates liver homeostasis and contributes to carcinogenesis. J Hepatol 2012; 57:1029–1036. [DOI] [PubMed] [Google Scholar]
- 7.) Zhang D, Cho E, Wong J. A critical role for the co-repressor N-CoR in erythroid differentiation and heme synthesis. Cell Res 2007;17:804–814. [DOI] [PubMed] [Google Scholar]
- 8.) Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, et al. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 2008;456: 997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.) Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell 2013;52:769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.) Mottis A, Mouchiroud L, Auwerx J. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev 2013; 27:819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.) Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet 2010;11:109–123. [DOI] [PubMed] [Google Scholar]
- 12.) Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan W, Downes M, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 2011;147:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.) Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, et al. Hepatocyte-specific deletion of farnesoid X receptor delays but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology 2012;56:2344–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.) Maehara Y, Fernandez-Checa JC. Augmenter of liver regeneration links mitochondrial function to steatohepatitis and hepatocellular carcinoma. Gastroenterology 2015;148:285–288. [DOI] [PubMed] [Google Scholar]
- 15.) Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 2012;485:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.) Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell 2011;147:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.) Freimuth J, Bangen JM, Lambertz D, Hu W, Nevzorova YA, Sonntag R, et al. Loss of caspase-8 in hepatocytes accelerates the onset of liver regeneration in mice through premature nuclear factor kappa B activation. HEPATOLOGY 2013;58:1779–1789. [DOI] [PubMed] [Google Scholar]
- 18.) Lin HP, Cheng ZL, He RY, Song L, Tian MX, Zhou LS, et al. Destabilization of fatty acid synthase by acetylation inhibits de novo lipogenesis and tumor cell growth. Cancer Res 2016;76: 6924–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.) Kohjima M, Tsai TH, Tackett BC, Thevananther S, Li L, Chang BH, Chan L. Delayed liver regeneration after partial hepatectomy in adipose differentiation related protein-null mice. J Hepatol 2013;59:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.) Shupe TD, Petersen BE. Liver regeneration: a consequence ofcom-plex, well-orchestrated signals. HEPATOLOGY 2015;62:644–645. [DOI] [PubMed] [Google Scholar]
- 21.) Bhalla K, Liu WJ, Thompson K, Anders L, Devarakonda S, Dewi R, et al. Cyclin D1 represses gluconeogenesis via inhibition of the transcriptional coactivator PGC1alpha. Diabetes 2014;63:3266–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.) Fernandez-Rojo MA, Restall C, Ferguson C, Martel N, Martin S, Bosch M, et al. Caveolin-1 orchestrates the balance between glucose and lipid-dependent energy metabolism: implications for liver regeneration. HEPATOLOGY 2012;55:1574–1584. [DOI] [PubMed] [Google Scholar]
- 23.) Zhang L, Wang YD, Chen WD, Wang X, Lou G, Liu N, et al. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. HEPATOLOGY 2012;56:2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.) Jo YS, Ryu D, Maida A, Wang X, Evans RM, Schoonjans K, Auwerx J. Phosphorylation of the nuclear receptor corepressor 1 by protein kinase B switches its corepressor targets in the liver in mice. Hepatology 2015;62:1606–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.) Marjanovic J, Chalupska D, Patenode C, Coster A, Arnold E, Ye A, et al. Recombinant yeast screen for new inhibitors of human acetyl-CoA carboxylase 2 identifies potential drugs to treat obesity. Proc Natl Acad Sci U S A 2010;107:9093–9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.) Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, Chirala SS, Wakil SJ. Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci U S A 2005;102:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.) Chan CB, Tse MC, Liu X, Zhang S, Schmidt R, Otten R, et al. Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem Biol 2015;22:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.) Gazit V, Weymann A, Hartman E, Finck BN, Hruz PW, Tzekov A, Rudnick DA. Liver regeneration is impaired in lipo-dystrophic fatty liver dystrophy mice. HEPATOLOGY 2010;52: 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.