Abstract
Type 1 diabetes is a chronic autoimmune disease characterised by insulin deficiency and resultant hyperglycaemia. Knowledge of type 1 diabetes has rapidly increased over the past 25 years, resulting in a broad understanding about many aspects of the disease, including its genetics, epidemiology, immune and β-cell phenotypes, and disease burden. Interventions to preserve β cells have been tested, and several methods to improve clinical disease management have been assessed. However, wide gaps still exist in our understanding of type 1 diabetes and our ability to standardise clinical care and decrease disease-associated complications and burden. This Seminar gives an overview of the current understanding of the disease and potential future directions for research and care.
Introduction
At first consideration, type 1 diabetes pathophysiology and management might seem straightforward; however, the more that is learnt about the disease, the less it seems is truly known. Improved understanding of the disease’s pathogenesis has not led to a single unifying Koch’s postulate for all cases. What once seemed like a single autoimmune disorder, with roots in T-cell mediated attack of insulin-producing β cells, is now recognised to result from a complex interplay between environmental factors and microbiome, genome, metabolism, and immune systems that vary between individual cases.
Despite known genetic underpinnings, most people who are diagnosed with type 1 diabetes do not have a relative with the disease or even the highest risk combination of HLA alleles, making attempts at primary disease prevention difficult. Although survival and patient health have improved considerably, particularly in the past 25 years, a cure for type 1 diabetes remains elusive.1,2 Additionally, despite advances in technology, glycaemic control for most people with type 1 diabetes is not optimised, and many cannot access modern therapies because of the high costs of even basic care.
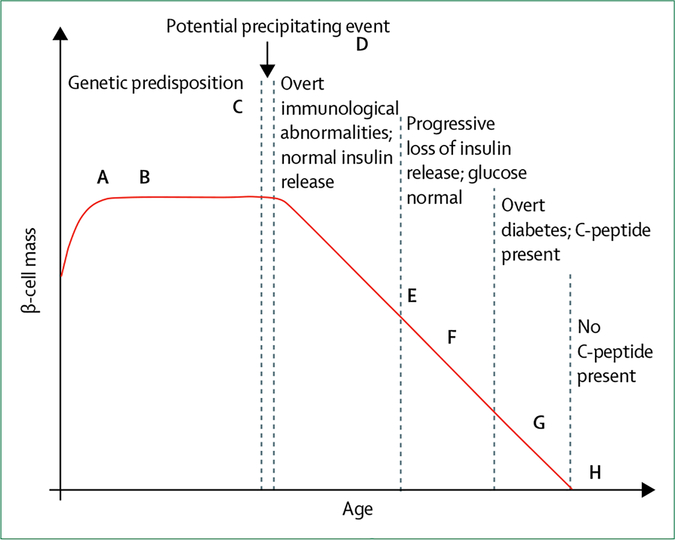
In 1984, George Eisenbarth developed a conceptual model for the pathogenesis of type 1 diabetes that is still used nowadays (figure 1).3 The model plots β-cell mass against age, highlighting an event sequence starting with predisposing genetic risk, then a precipitating environmental trigger that causes islet-specific auto-immunity, followed by β-cell loss, dysglycaemia, clinical diabetes, and rapid progression to complete β-cell loss. Although useful, this model does not address the increasingly apparent complexity of type 1 diabetes pathogenesis. Additionally, the disease pathogenesis is shown by a single line of disease course over time; however, at all stages of the disease heterogeneity exists that is not well understood.
Figure 1: Challenges to the Eisenbarth model of the natural history of type 1 diabetes.
Key events of the Eisenbarth model3 over the course of the disease (measured in years) are shown by dotted lines at different time points. Challenges to this model, taking into account the increasing complexity of type 1 diabetes, include the following: precipitating immune events that might occur prenatally (A); large variation in starting β-cell mass and function, defects in one or both could be developmentally programmed (B); initiation of autoimmunity is measured by autoantibodies, but other immunological abnormalities probably precede the presence of detectable pancreatic antibodies (C); the patient’s environment could affect their entire disease course (D); β-cell loss could relapse or remit (E); dysglycaemia occurs before clinical diagnosis (F); decline in β-cell function might not mirror decline in β-cell mass—methods to measure β-cell mass have not been established (G); and residual C-peptide is detectable in many people who have long duration type 1 diabetes (H). Furthermore, progression through stages A–C is heterogeneous, and will be affected by immune, genetic, environment, and key demographic features (ie, age, body-mass index). Adapted from Atkinson et al.4
This Seminar provides a review of type 1 diabetes and the status of research in the field. We focus on developments from the past 5 years that highlight the heterogeneity and complexity of the disease.
Diagnosis
A diagnosis of diabetes is based on a fasting blood glucose concentration above 7·0 mmol/L (126 mg/dL), a random blood glucose concentration above 11·1 mmol/L (200 mg/dL) with symptoms, or an abnormal result from an oral glucose tolerance test.5 In the absence of symptoms, abnormal glycaemia must be present on two different occasions. A diagnosis of diabetes can also be made on the basis of a glycated haemoglobin (HbA1c) concentration above 48 mmol/mol (6·5%). However, since dysglycaemia progression can be rapid in patients with type 1 diabetes, HbA1c is less sensitive for diagnosis than fasting or stimulated blood glucose measurements.5
Children with type 1 diabetes commonly present with symptoms of polyuria, polydipsia, and weight loss; approximately a third present with diabetic ketoacidosis.6 The onset of type 1 diabetes can be more variable in adults, who might not present with the classic symptoms seen in children. Although traditional definitions classified type 1 diabetes as juvenile onset, the disease can occur at any age, with up to 50% of cases occurring in adulthood.7 As many as 50% of adults with type 1 diabetes might be initially misclassified as having type 2 diabetes.8 Similarly, in conjunction with the epidemic of childhood obesity, type 2 diabetes is increasingly common in adolescents (particularly in non-white individuals), and monogenic diabetes (eg, maturity diabetes onset of the young) accounts for 1–6% of childhood diabetes cases.9–11
Although low C-peptide concentration as a marker of severe endogenous insulin deficiency is useful to guide both classification and treatment in cases of diabetes assessed over 3 years after clinical diagnosis,12 no single clinical feature can perfectly distinguish type 1 from non-type 1 diabetes at diagnosis. Classification depends on an appreciation of other risk factors for type 1 versus other subtypes and the integration of clinical features (eg, age of diagnosis and body-mass index) with biomarkers (eg, pancreatic autoantibodies).13
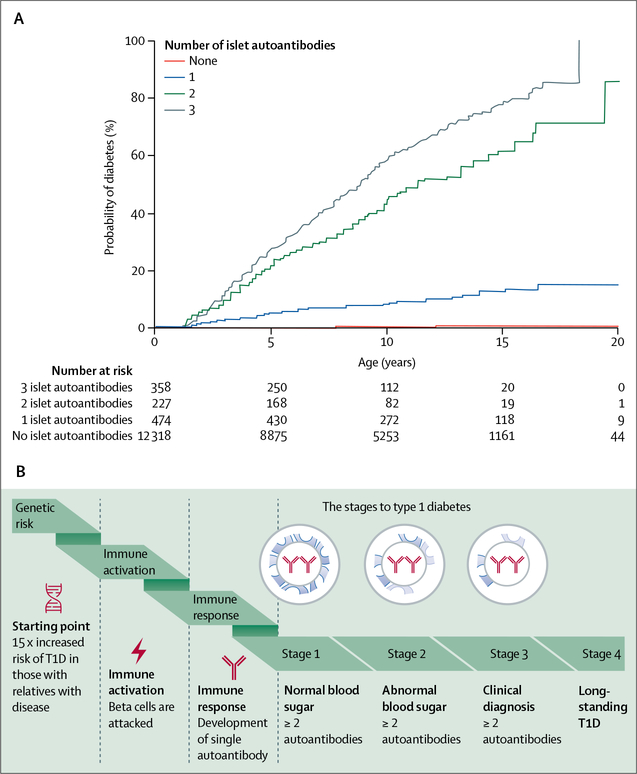
Over 90% of people with newly diagnosed type 1 diabetes have measurable antibodies against specific β-cell proteins, including insulin, glutamate decarboxylase, islet antigen 2, zinc transporter 8, and tetraspanin-7.14 Birth cohort studies15,16 of individuals with a high genetic risk for diabetes have shown a peak incidence of first autoantibody development before age 2 years. Most people with a single autoantibody do not progress to type 1 diabetes, but seroconversion to the presence of two or more serum autoantibodies in children is associated with an 84% risk of clinical type 1 diabetes by the age of 18 years (figure 2A).16 The high risk of progression in the presence of multiple autoantibodies has led to a redefining of type 1 diabetes stages. In this new paradigm, a preclinical stage 1 case of type 1 diabetes is defined as the presence of two or more autoantibodies, while stages 2 and 3 are defined as the progression of metabolic abnormalities from abnormal glycaemia to overt diabetes, diagnosed by standard criteria (figure 2B).18 Since the progression from islet autoantibody positivity to clinical diabetes could take months or years, defining multiple auto-antibody positivity as stage 1 allows targeting of immune interventions to a realistic primary outcome and facilitates early life intervention studies.19
Figure 2: Factors contributing and disease progression to type 1 diabetes.
(A) The probability of developing diabetes in childhood stratified by the number of islet antibodies. In a study by Zeigler and colleagues,16 13 377 children were identified as at risk in the newborn or infant period on the basis of high-risk HLA genotypes or having a relative with type 1 diabetes, or both, and were followed-up regularly. The numbers at risk are the number of children receiving follow-up at ages 0, 5, 10, 15, and 20 years. Adapted from Ziegler et al16 with permission of the American Medical Association. (B) Type 1 diabetes progression and stages of type 1 diabetes. Stage 1 is the start of type 1 diabetes, marked by individuals having two or more diabetes-related autoantibodies and normal blood sugar concentrations. In stage 2, individuals have dysglycaemia without symptoms. Stage 3 is the time of clinical diagnosis. Reproduced from Greenbaum et al,17 with permission from the American Diabetes Association. T1D=type 1 diabetes.
Genetics
Type 1 diabetes is a heritable polygenic disease with identical twin concordance of 30–70%,20 sibling risk of 6–7%, and a risk of 1–9% for children who have a parent with diabetes.21 The overall lifetime risk varies greatly by country and geographical region but overall is around one in 250 people.22 The disease is slightly more common in men and boys than in women and girls.23 Two HLA class 2 haplotypes involved in anti gen presentation, HLA DRB1*0301-DQA1*0501-DQ*B10201 (DR3) and HLA DRB1*0401-DQA1*0301-DQB1*0301 (DR4-DQ8), are linked to approximately 50% of disease heritability and are prevalent in white people.24 Other haplotypes are known to reduce type 1 diabetes risk, including DRB1*1501-DQA1*0102-DQB1–0602 (DR15-DQ6).24 The mechanisms by which these HLA haplotypes interact and alter risk are not completely understood. Different HLA associations in other racial groups are recognised but remain poorly characterised.24 Genome-wide association studies have identified over 60 additional non-HLA loci associated with the risk of type 1 diabetes. These variants have been predominantly associated with the immune system and highlight pathways that are important in disease development—eg, insulin gene expression in the thymus, regulation of T-cell activation, and viral responses.24 These HLA and non-HLA genetic associations could identify potential targets for future disease-modifying therapies or subgroups of patients who could benefit from specific immune interventions.
Historically, people at high risk of type 1 diabetes have been identified for research by HLA risk or familial risk, or both.25 By contrast, individual non-HLA loci cannot be used to predict type 1 diabetes or discriminate it from other types of diabetes. Combined measurement of HLA and non-HLA loci into genetic risk scores could offer improved prediction of the risk of developing type 1 diabetes and discrimination of type 1 from type 2 diabetes.26,27 Furthermore, the continuing fall of genotyping costs could facilitate future population-level disease prediction by use of genetic risk scores.19,28
Epidemiology
Globally, type 1 diabetes is increasing both in incidence and prevalence, with overall annual increases in incidence of about 2–3% per year.29,30 US data31 suggest an overall annualised incidence from 2001 to 2015 of about 22·9 cases per 100 000 people among those younger than 65 years; data from other regions suggest similar incidences.32 The greatest observed increases in incidence of type 1 diabetes are among children younger than 15 years, particularly in those younger than 5 years.33 These increases cannot be explained by genetic changes, implicating environmental or behavioural factors, or both. Many environmental exposures are associated with type 1 diabetes, including infant and adult diet, vitamin D sufficiency, early-life exposure to viruses associated with islet inflammation (eg, enteroviruses), and decreased gut-microbiome diversity.34 Obesity is associated with increasing presentation of type 1 diabetes, with β-cell stress potentially providing a mechanistic underpinning.34,35 The large differences in the incidence of type 1 diabetes in genetically similar populations that are separated by socioeconomic borders36 and the increasing incidence of type 1 diabetes in genetically low-risk individuals37 highlight the importance of environmental risk factors regardless of genetic background risk. Further work is being done to understand the role of gene–environment interactions in the pathogenesis of type 1 diabetes, the role of different loci and pathways at different stages of the disease, and whether loci that are independent of disease risk could have a role in disease progression after development of autoimmunity.38–40 Some data31,41 suggest that the observed incidence could be declining in adults or potentially even levelling off across all age ranges; worldwide registry data will eventually reveal if this pattern is indeed true.42
The incidence of type 1 diabetes varies by country and by region within countries.31 At northern latitudes, people born in the spring are more likely to develop the disease than those born in the other seasons.43 The peak incidence of diagnosis is seen in children aged 10–14 years.31,32 Although many people present with type 1 diabetes in adulthood,44 the higher incidence of type 2 diabetes in adulthood compared with type 1 diabetes and the flawed criteria for distinguishing these forms of disease make assessment of the incidence of type 1 diabetes in adults very difficult.23,45 Most people living with type 1 diabetes are adults.46
The immune phenotype of type 1 diabetes
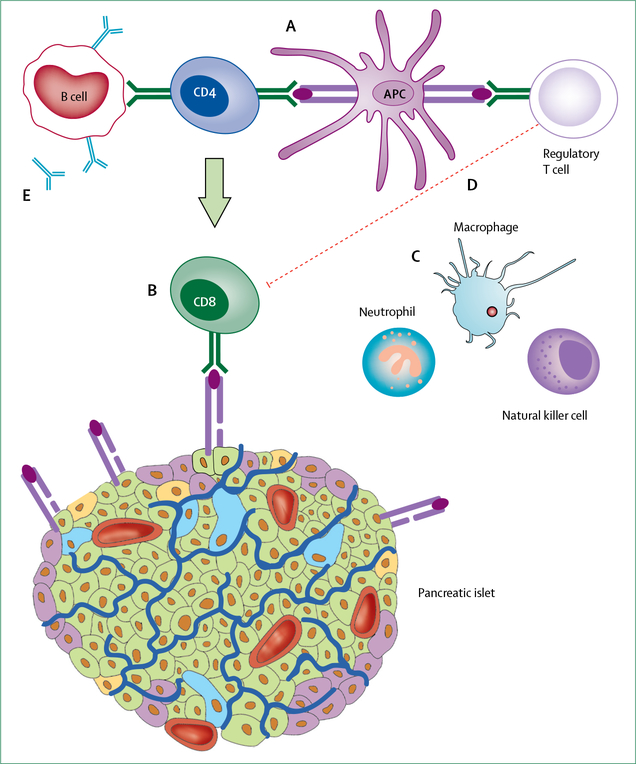
The pathogenesis of type 1 diabetes results from a complex interaction between the pancreatic β-cell and innate and adaptive immune systems (figure 3).47 The question of whether a trigger for the immune response against β cells exists or whether the immune response is a random stochastic event has been a subject of considerable speculation and controversy. Several viral infections are associated with type 1 diabetes, with enterovirus being one of the most commonly associated infections. Enteroviral major capsid protein VP1 and RNA have been detected in islets from people with recent-onset type 1 diabetes,48 along with hyper-expression of the class 1 major histo compatibility complex49 and other indices of viral infection. One possibility is that some people with type 1 diabetes have an atypical, chronic viral infection of β cells, leading to chronic inflammation and the development of autoimmunity. The viral hypothesis has been difficult to test, although both antiviral therapy and the development of vaccines targeting enteroviruses are being pursued for this purpose.
Figure 3: The immunopathogenesis of type 1 diabetes.
The development of type 1 diabetes is thought to be initiated by the presentation of β-cell peptides by antigen-presenting cell (APCs). APCs bearing these autoantigens migrate to the pancreatic lymph nodes where they interact with autoreactive CD4+ T lymphocytes, which in turn mediate the activation of autoreactive CD8+T cells (A). These activated CD8+ T cells return to the islet and lyse β cells expressing immunogenic self-antigens on major histocompatibility complex class I surface molecules (B). β-cell destruction is further exacerbated by the release of proinflammatory cytokines and reactive oxygen species from innate immune cells (macrophages, natural killer cells, and neutrophils; C). This entire process is amplified by defects in regulatory T lymphocytes, which do not effectively suppress autoimmunity (D). Activated T cells within the pancreatic lymph node also stimulate B lymphocytes to produce autoantibodies against β-cell proteins. These autoantibodies can be measured in circulation and are considered a defining biomarker of type 1 diabetes (E).
In the field, much effort has been given to the study of the adaptive immune system in type 1 diabetes by use of assays of peripheral lymphocytes selected for autoreactivity to islet antigens. Increased frequency of islet-specific autoreactive CD8+ T lymphocytes and decreased regulatory immune function have been associated with type 1 diabetes.50 Experiments, such as the transfer of type 1 diabetes following non-T-cell depleted allogeneic bone-marrow transplantation,51 development of type 1 diabetes in an individual with B-lymphocyte and antibody deficiency,52 and inherited genetic defects of T-lymphocyte function causing type 1 diabetes53 highlight the crucial role of T cells in the pathophysiology of type 1 diabetes.54 Almost all studies of peripheral autoimmunity in people with type 1 diabetes show overlap of phenotypes seen in the general population, and the proportion of islet autoreactive cells present in the periphery is often tiny (only a few cells among millions of non-autoreactive cells). As a result, connecting the population of autoreactive immune cells that is detectable in blood to the disease process in islets has been difficult. A key development has been the isolation of T lymphocytes that are reactive to β-cell antigen peptides from islets of organ donors with type 1 diabetes.55–57
Histopathologically, these processes are observed as insulitis or immune-infiltrated (insulitic) islets.58 CD8+ T lymphocytes are the most common immune cells within insulitic lesions, with CD4+ T cells present in lower numbers. Distinct patterns of insulitis that stratify with the aggressiveness of β-cell loss and age of diagnosis have been identified in insulitic islets.59 Although insulitis is common and intense in animal models of type 1 diabetes, it is much rarer and more variable in human beings (figure 3).60
The β-cell phenotype of type 1 diabetes
At diagnosis, people with type 1 diabetes have reduced β-cell function compared with healthy controls.61 With amelioration of hyperglycaemia, these β cells can have a partial recovery of insulin secretory function, leading to a so-called honeymoon period after diagnosis with minimal or no exogenous insulin needed. Over time, many of these residual cells are lost. However, analysis of pancreatic sections from individuals with long-term type 1 diabetes show the presence of residual β cells decades after diagnosis.62,63 When sensitive C-peptide measure ments are performed, 30–80% of people with long-term type 1 diabetes are found to be insulin microsecretors.64–67 So, although endogenous β-cell quantity and function decline with longer disease duration, this decline does not progress to a complete loss of all β cells.64–67 This finding is noteworthy because in the Diabetes Control and Complications Trial68,69 persistent C-peptide secretion was associated with reduced development of retinopathy, nephropathy, and hypoglycaemia. Additionally, the persistence of C-peptide secretion in people with long-term type 1 diabetes could improve glucagon responses to hypoglycaemia.70 Moreover, the presence of residual C-peptide secretion after the diagnosis of disease could also increase the possibility of an improved effect of interventions targeted at rescuing or augmenting the survival of this residual pool of β cells. Analyses of pancreatic specimens from the Network of Pancreatic Organ Donors repository have not found evidence of either increased neogenesis or proliferation in pancreatic cells from donors with type 1 diabetes.63 Thus, the mechanisms underlying the persistence of residual β cells in people with long-term type 1 diabetes remain unclear. Identifying pathways that have allowed these cells to escape the autoimmune attack could yield insight into new therapeutic approaches.
β-cell abnormalities might also contribute to type 1 diabetes pathogenesis, leading to the notion of so-called β-cell suicide. β-cell HLA class I overexpression is common in pancreatic sections from cadaveric donors with type 1 diabetes. This overexpression serves as a homing signal for cytotoxic T lymphocytes.49 However, whether this signal is a primary β-cell defect or a response to a stimulus (eg, a viral infection) is not yet known. Additionally, evidence also exists for increased β-cell endoplasmic reticulum stress linked with accelerated β-cell death.71,72 Endoplasmic reticulum stress in β cells has also been associated with alterations in mRNA splicing and errors in protein translation and folding; the resultant protein products have been proposed as potential immunogenic neoantigens.73
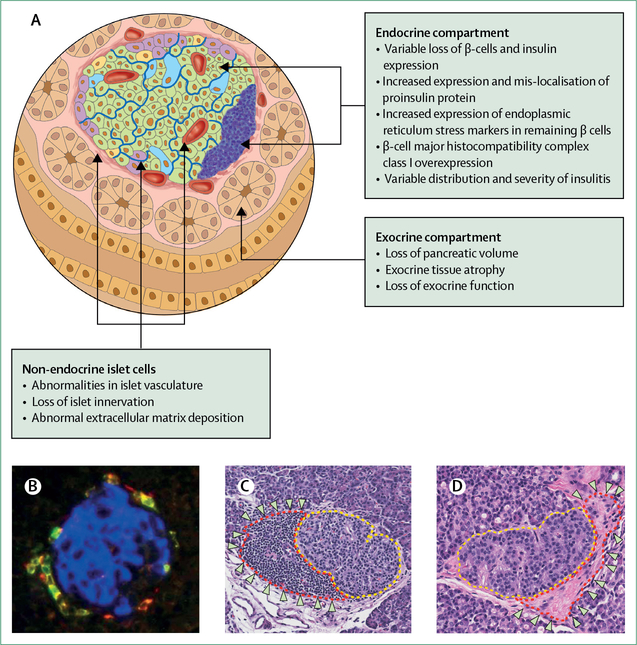
In addition to these defects in the β-cell compartment, alterations in non-endocrine islet cells and the exocrine pancreas have also been described (figure 4). These defects include abnormalities in the islet extracellular matrix74,75 and in islet innervation and vascularity.76–78 Data have also placed a renewed emphasis on the role of exocrine pancreatic pathology in type 1 diabetes. Compared with healthy individuals, people with type 1 diabetes have a decreased pancreatic weight and volume that continues to decrease with disease duration.79,80 This finding could be explained by developmental defects, or pancreatic atrophy in response to loss of the paracrine and pro-growth effects of insulin or chronic inflammation, or even autoimmune-mediated exocrine destruction. These possibilities are all topics of active investigation.
Figure 4: Pancreatic and islet abnormalities in type 1 diabetes.
(A) Type 1 diabetes is characterised by a variety of abnormalities that involve both the islet and the exocrine pancreas. The hallmark of type 1 diabetes is loss of insulin-producing β cells and immune infiltration of islets. However, the presence of insulitis, even within an individual pancreas, can be highly variable. (B) Immunofluorescent image of an insulitic islet from a cadaveric donor with long-term type 1 diabetes. Insulin is shown in blue and CD8+ T cells surrounding the islet are shown in yellow. (C) Haematoxylin and eosin staining of an islet from a cadaveric donor that exhibits a classic pattern of insulitis. The islet is circled with a yellow dotted line. The infiltrating immune cells are circled in red and indicated by arrows. (D) Haematoxylin and eosin staining of an islet, circled in yellow dotted line, from a cadaveric donor with long-term type 1 diabetes without any discernible immune infiltrate. By contrast with the islet in (C), this islet has evidence of peri-islet fibrosis as shown circled in red and indicated by arrows. Images B–D courtesy of M Campbell-Thompson, University of Florida, Gainesville, FL, USA.
Management of clinical disease
Methods of managing type 1 diabetes continue to improve, and although progress is generally slow and incremental, occasionally it is punctuated by rapid change. One such moment of change happened in 1993 with the publication of the Diabetes Control and Complication Trial.81 This trial and the follow-up observational Epidemiology of Diabetes Interventions and Complications trial convincingly showed that achieving and maintaining glucose concentrations as close to those seen in people without diabetes as possible leads to a reduction in microvascular and cardiovascular type 1 diabetes complications.82
Although insulin remains the mainstay of therapy, new insulin analogues with varying onsets and durations of action are widely available. Optimal glycaemic control requires multiple-dose insulin regimens that mimic physiological insulin release, with basal insulin for overnight and between-meal control, plus bolus doses of rapid-acting insulin analogues to cover ingested carbohydrate loads and treat hyperglycaemia. Insulin can be taken by injection (with an insulin pen if available) or, preferably for many people, with an insulin pump.83 Ultra-rapid inhaled insulin is also available, but little enthusiasm for this preparation exists because of its fixed dosing (four or eight unit increments only), issues with consistent delivery, cost, and the need for pulmonary function testing.84 A faster-acting subcutaneously-administered insulin (via injection or infusion) has also recently become available for clinical use. Appropriate insulin use requires frequent dosing adjustments for ingested carbohydrates, physical activity, and illness or stress.
While pramlintide is the only non-insulin medication approved for improved glycaemic control in patients with type 1 diabetes, metformin, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium-glucose co-transporter-2 (SGLT2) inhibitors have also been used of-label; however, fewer than 5% of patients use these medications.85 Metformin, an insulin sensitiser, is the most commonly prescribed drug for people with type 1 diabetes who have insulin resistance but it has not been shown to be effective in people younger than 18 years who are overweight or obese and have type 1 diabetes.86 Use of SGLT2 inhibitors is restricted in part because of early reports of euglycaemic diabetic ketoacidosis in people with type 1 diabetes treated with these compounds. A 2018 meta-analysis of these inhibitors suggests they are safe,87 but more data are needed.
Glucagon therapy is also poised to undergo a resurgence in management of type 1 diabetes. Although only an emergency kit has been commercially available up until now for cases of severe hypoglycaemia leading to seizure or loss of consciousness, nasal and stable liquid formulations are being developed. The nasal formulation will be available as a rapid rescue therapy only,88 whereas the stable liquid formulation could also be used in small doses for exercise and in dual hormone (ie, insulin and glucagon) closed-loop systems.89,90
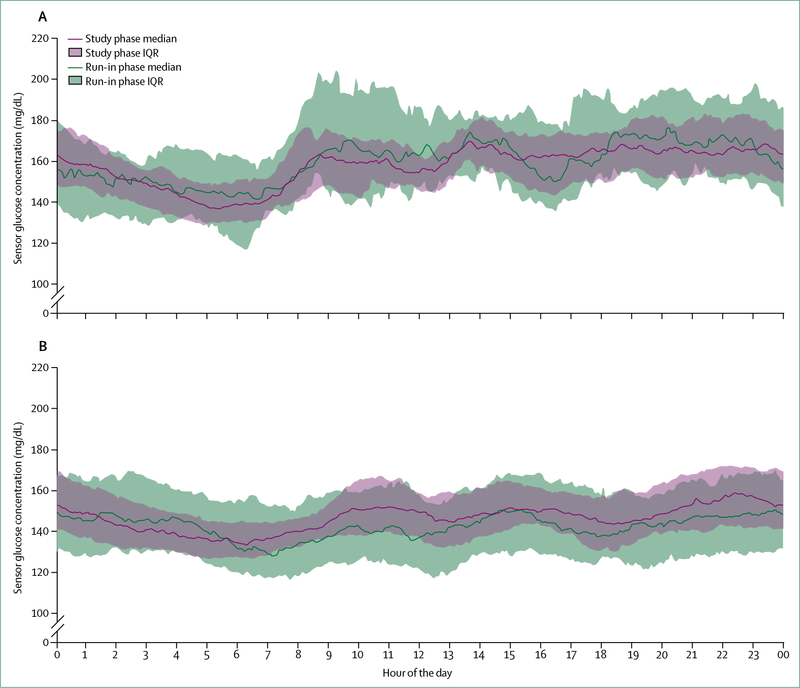
In the past 13 years, continuous glucose monitoring (CGM) and intermittently viewed CGM devices for at-home patient use with minimally invasive devices have become available, which have similar accuracy to capillary blood glucose monitors.91 Both CGM and intermittently viewed CGM allow examination of glucose concentration patterns over time and, although CGM devices still need periodic calibration, they obviate the need for frequent capillary blood glucose measurements. CGM is more sophisticated than intermittently viewed CGM because it can give the user a warning on the basis of absolute or projected glucose values. When CGM is incorporated into hybrid closed-loop insulin-pump systems that automatically regulate basal infusion rates, but that require manual delivery of meal boluses by trained wearers to cover estimated carbohydrate intakes, substantial improvements in glucose variability and overall glycaemic control are seen (figure 5).93 Combined use of automated insulin delivery and CGM offers the prospect of an artificial pancreas with little input from the user. The substantial advances that have been made in pump and sensor technology and the increase in the number of trials to test their efficacy show that partially or fully automated systems could become a reality.
Figure 5: Glucose concentrations in patients with type 1 diabetes with a hybrid closed-loop system, before and after use, over 24 h.
Sensor glucose profiles from 124 people with type 1 diabetes, of which 30 were adolescents (14–21 years; A) and 94 were adults (22–75 years; B), before (during run-in phase) and during the study phase using the Medtronic MiniMed 670 g hybrid closed-loop system (Medtronic, Northridge CA, USA) under clinical trial conditions. Median and IQR of sensor glucose values are given as a green line and band for the run-in phase, and a pink line and band for the study phase, respectively. In the run-in phase, the hybrid closed-loop system was in manual mode, with participants making all treatment decisions except for the pump automatically suspending before senor glucose concentrations became too low. In the study phase, the hybrid closed-loop system was in auto mode. Participants had less variability in their blood glucose concentration during auto mode. Reproduced from Garg et al,92 with permission from Mary Ann Liebert.
Guidelines from the American Diabetes Association, International Society for Pediatric and Adolescent Diabetes, and Candian Diabetes Association suggest a HbA1c target of less than 53 mmol/mol (7·0%) for adults and less than 58 mmol/mol (7·5%) in paediatric patients with type 1 diabetes;94–96 however, most individuals do not achieve these targets. Although setting more aggressive targets is associated with achieving lower HbA1c,97 these targets should be individualised on the basis of many factors including comorbidities, patient capability and attitude, and available care resources98—eg, even lower targets are often prescribed for pregnant women and women anticipating pregnancy than those prescribed to other patients.99 Higher targets might be appropriate for people with hypoglycaemia unawareness, history of severe hypoglycaemia, advanced complications, and short life expectancy. For optimal outcomes, people with diabetes should be cared for by a multidisciplinary care team, including diabetes educators, nurse practitioners, nurses, nutritionists, physician assistants, exercise physiologists, social workers, and psychologists. To optimise glycaemic control, clinical care with skilled and structured patient education and training sessions should be provided—including information on insulin adjustments, carbohydrate counting, and optimal use of available technology.100
People with type 1 diabetes also risk developing other autoimmune diseases, sometimes as part of a poly-glandular autoimmune syndrome. A study101 from the Type 1 Diabetes Exchange clinic registry noted the prevalence of autoimmune disease was 27% in a population of over 25 000 people with type 1 diabetes with a mean age of 23 years. The most common autoimmune disease is autoimmune thyroiditis (ie, Hashimoto thyroid itis and Graves’ disease) followed by coeliac disease. Other associated conditions include collagen-vascular diseases (eg, rheumatoid arthritis and lupus), autoimmune gastritis or pernicious anaemia, vitiligo, and Addison’s disease. Guidelines for the care of people with diabetes include periodic screening for these diseases, particularly thyroid and coeliac diseases.102
Complications of type 1 diabetes
The discovery of insulin in 1922 transformed type 1 diabetes from a terminal to a treatable disease. Despite the advances in care discussed previously, the disease continues to be associated with substantial medical, psychological, and financial burden. Hypoglycaemia and ketoacidosis are persistent potentially life-threatening complications. Severe hypoglycaemic events requiring treatment assistance from another person occur at rates of 16–20 per 100 person-years; hypoglycaemic events leading to loss of consciousness or seizure occur at a rate of 2–8 per 100 person-years.103–105 Recurrent hypoglycaemia results in an increased likelihood of hypoglycaemia unawareness and subsequent severe hypoglycaemic events, since recurrent hypoglycaemia reduces the glucose concentration that triggers the counter-regulatory responses to return to euglycaemia.106 Hypoglycaemia unawareness can improve with edu cation, support, and glucose targets that are aimed at avoiding biochemical hypoglycaemia, while maintaining overall metabolic control.107
Hypoglycaemic events are associated with adverse effects on cognitive function,108,109 and are associated with 4–10% of type 1 diabetes-related deaths.110–112 Observational studies suggest poor diabetes control does not reduce the risk of severe hypoglycaemia.113 Notably, rates of severe hypoglycaemic events have been decreasing over time104 and with CGM and other advanced diabetes technologies HbA1c can be lowered into the target range without increasing the risk of severe hypoglycaemia.114 Treatment in hospital for diabetic ketoacidosis occurs at a rate of 1–10 per 100 patient-years in paediatric populations with established type 1 diabetes, and accounts for 13–19% of type 1 diabetes-related mortality.105,110,111 Incidence of diabetic ketoacidosis is higher among women than among men, and among people with higher HbA1c levels than other people with type 1 diabetes.
Microvascular complications of the disease manifest primarily as retinopathy, neuropathy, and nephropathy, but also can affect cognitive function, the heart, and other organs. Hyperglycaemia is the primary risk factor for microvascular disease, and reducing HbA1c through intensive diabetes management, particularly early during disease, is associated with striking (about 70%) reductions in incidence and slower progression of microvascular disease. However, differences in HbA1c do not fully explain the variation in the incidence of complications and the severity of disease between individuals. Variability in glucose concentrations (both during the day and longer term) and glycosylation rates also probably have a role in interindividual differences.115,116 Type 1 diabetes during puberty also appears to accelerate the development of complications.117
Macrovascular complications of type 1 diabetes include atherosclerosis and thrombosis in the heart, peripheral arteries, and brain. By contrast with microvascular complications, the risk of cardiovascular complications does not appear to be as attenuated by intensive blood sugar control. Diabetic nephropathy, whether manifesting as microalbuminuria, macroalbuminuria, or a reduced glomerular filtration rate progressively augments the overall risk of macrovascular complications.118 Cardiovascular disease remains the major cause of premature morbidity and mortality, with data119,120 suggesting an 8–13-year shorter life expectancy for people with type 1 diabetes than for healthy individuals.
People with diabetes might also have both chronic and acute neurocognitive changes that include decline in cognitive function with detrimental effects on psychomotor speed, cognitive flexibility, attention, and visual perception.121,122 Although the pathophysiology of neurocognitive changes is poorly understood, their development has been linked with both microvascular and macrovascular changes and changes in brain structure, neuronal loss, and cerebral atrophy.123,124 Risk factors include developing diabetes early in life, chronic hyperglycaemia, and repeated hypoglycaemia.
In the past 25 years, among people with type 1 diabetes the risks of microvascular and macrovascular compli-cations have substantially decreased and outcomes have improved.125,126 These improvements have been largely driven by better glycaemic control and improved management of associated risk factors—eg, hypertension and hyperlipidaemia. Several studies127–130 have identified additional non-glycaemic risk factors for the development of complications. Genetic studies have not yielded strong associations between specific gene variants and complication status. Low levels of education and income have been associated with high risks of both micro-vascular and macrovascular complications.127 Sex also appears to modify risk, since women with type 1 diabetes have been shown to have higher rates of all-cause premature mortality and vascular events than do men with type 1 diabetes.128 In the past 5 years, new technologies have been designed to attempt to better predict future risk and complications by combining risk factors into probability models. Two examples are the QDiabetes129 and QRISK3130 web calculators that were developed with a prospective general practice dataset of 803 044 people with diabetes (44 440 with type 1 diabetes). These calculators can be used to predict 10-year risk for microvascular and macrovascular complications. However, continued work is needed in this area to combine prediction models with disease-specific bio- markers and disease-modifying therapies that can prevent sequelae.
An additional noteworthy complication of type 1 diabetes is the patient-reported burden of adverse also their family, friends, and caregivers.131 Fear of hypoglycaemia is a prevalent issue, particularly for the families of very young children with type 1 diabetes.132 Furthermore, poor quality of life is predictive of subsequent poor glycaemic control.133
Disease-modifying therapies
For over 30 years, most efforts to cure type 1 diabetes have focused on altering the immune system’s attack on β cells. This approach began with trials of ciclosporin, an immunosuppressant that was given to inhibit T-cell activation. Although ciclosporin was unable to induce a durable disease remission, insulin requirements of patients decreased during active treatment, generating enthusiasm that immune modulation could treat type 1 diabetes.134–136 Subsequently, other strategies have been tested in both primary and secondary prevention paradigms. Most efforts have focused broadly on tolerance induction by use of antigens or modulation of T-lymphocyte, B-lymphocyte, and cytokine responses. Some primary prevention studies have also used dietary approaches.137,138
Antigen-based trials have used various forms of glutamate decarboxylase (GAD) protein, which have shown mixed but mostly negative results.139–141 The Diabetes Prevention Trial—Type 1, tested whether oral or parental insulin prevented the development of type 1 diabetes in people who were autoantibody positive. Neither approach reduced diabetes development, but subgroup analyses suggested a benefit of oral insulin in individuals with the highest titres of insulin auto-antibodies.142,143 Based on this finding, the Type 1 Diabetes TrialNet Network completed a trial144 of low-dose oral insulin in a second cohort of individuals who were autoantibody positive with similar insulin autoantibody profiles, but this trial was also negative. Negative results were also observed in another trial investigating intranasal insulin.145
Personalised strategies for tolerance induction are now also being pursued. One study tested repeated intradermal doses of a specific proinsulin peptide fragment in people with the HLA DRB1*0401 genotype,146 for whom this peptide was identified to be specifically immunogenic. Clinical trials at diagnosis have also tested approaches aimed at modulating T-cell and B-cell responses. Despite many attempts at immune intervention, only four categories of drugs have shown efficacy in preserving C-peptide secretion in recent onset type 1 diabetes in randomised placebocontrolled trials. These drugs include a monoclonal antibody against the B-cell CD20 receptor (rituximab),147 monoclonal antibodies against the T-cell CD3 receptor (teplizumab148,149 and otelixizumab150), cytotoxic T-lymphocyte protein 4 (CTLA4)-immunoglobulin-mediated co-stimulatory blockade with abatacept,151 and alefacept,152 which is a fusion protein that binds CD2 and targets CD4+ and CD8+ effector memory T cells. Although the phase 2 trials of these drugs met their primary or secondary endpoints, defined as an improvement in the C-peptide area-under-the-curve response during a mixed meal tolerance test, no drug has yet been able to induce insulin independence or progressed to a positive phase 3 trial that was translatable into clinical care. This gap in translating results from trials into clinical practice could highlight the need for alternative strategies. Combinatorial approaches that modulate multiple aspects of the immune response could result in better efficacy. For example, low-dose anti-thymocyte globulin in combination with granulocyte colony-stimulating factor has shown early and sustained efficacy in pilot studies153,154 and is being tested in a phase 2 study () in recent-onset type 1 diabetes. Another approach is to intervene earlier in the disease process, at a time when greater β-cell mass remains. To this end, abatacept () and teplizumab () are being tested in stage 1 and stage 2 type 1 diabetes through the TrialNet Network. Even modest preservation of β-cell function could have long-term benefits, and better glycaemic control early in the disease course could mitigate the likelihood of complications.155–157
One potential future therapy for type 1 diabetes is with replacement of β cells from an external source. Pancreas transplants have been performed for over 50 years and have become a standard-of-care treatment in individuals who have developed end-stage renal failure and require kidney transplantation.158 Simultaneous kidney and pancreas transplantation in experienced centres can offer an up to 80% chance of insulin independence for over 5 years, but there is substantial surgical risk, and the requirement of immunosuppression.159 Islet transplantation is a low-risk procedure, with donor islets infused into the liver via the portal vein. Shapiro and colleagues’ landmark work, by use of a steroid free Edmonton Protocol,160 showed that islet transplantation could achieve insulin independence and offered an example of a successful and low-risk cell-based therapy. However, only a minority of islet transplant recipients achieve durable insulin independence. Moreover, morbidity associated with immunosuppression and limitations in the supply of donor islets restricts the number of people who can benefit from islet transplantation.161 Currently, islet transplantation is used in a small subset of patients who have extremely severe hypoglycaemic unawareness. Even if insulin independence is not achieved, severe life-threatening hypoglycaemia can be prevented with minimal islet transplant function.162,163
Cell therapy as a potential cure for type 1 diabetes remains a field of great interest.2 Considerable effort has been focused on protocols to generate functional and glucose-responsive β cells from human embryonic stem cells or induced pluripotent stem cells from living donors. This approach offers the possibility of a limitless source of β cells that could be delivered in a semipermeable device that would permit functional insulin secretion but avoid the need for immuno-suppression.164 Several small molecules, growth factors, hormones, and nutrients have been shown to promote modest β-cell neogenesis and proliferation. However, most positive results come from animal models and have been difficult to replicate in human studies. While stem-cell-based therapies and neogenesis are a source of hope for potential cures, they are not realistic treatments in the immediate future.2
Other novel approaches include autologous haemopoietic stem-cell transplantation165,166 and autologous T-regulatory cell administration.167–169 In response to growing evidence highlighting an active role for the β cell in disease pathogenesis, several ongoing trials are testing drugs that have successfully targeted β-cell stress responses in mouse models of diabetes.170
Conclusions
Over the past 50 years, people with type 1 diabetes and their medical-care providers have been tantalised with optimism and subsequently disappointed at the seemingly unobtainable cure on the horizon. However, this long journey has been punctuated by several pivotal successes, including the discovery of insulin in 1922, the first pancreatic transplantation in 1966,171 the first insulin-pump studies, the first immunomodulatory trial in 1986,136 and the first definitive evidence linking glycaemic control with complication status in 1993.81 The past 25 years has brought an upsurge of technological advances, including designer insulin analogues, smart insulin pumps, continuous glucose sensors, and closed-loop insulin delivery systems.
Clinicians, investigators, and patients have gained a better appreciation of the true complexity of type 1 diabetes, and humility in the face of many unsuccessful trials aimed at inducing a durable disease remission. While scientists continue to untangle the complicated pathogenesis of the disease, patients and health-care providers should focus on advocating for improved access to modern advances in diabetes care, especially for affordable insulin analogues and technologies that can reduce the burden of managing this chronic disease. When insulin was discovered, the University of Toronto freely licensed the right to manufacture the drug; yet, people in resource-limited environments continue to die because they have no access to insulin.172
Additionally, crucial research must continue into strategies to prevent disease onset and preserve or restore β-cell function. These approaches offer the promise of ameliorating or eliminating disease complications, and greatly improving outcomes for those who have the disease. Continued development of new low-cost, low-burden, and highly effective therapies to improve glycaemic control is also needed. These approaches could include investigation into the effects of different dietary composition on glycaemic outcomes, and the safety and efficacy of open-source patient-designed artificial pancreas innovations. Given observed differences in care, health-care providers must be committed to initiatives for continuous quality improvement, with a focus on increasing uptake and implementation of best standards of care. A greater focus on patient-centred outcomes has been present in trials, and further exploration of these important endpoints is also crucial. If stakeholders in the field concentrate on the areas that are most likely to have a long-term effect, management of type 1 diabetes is poised to undergo further radical transformation.
Search strategy and selection criteria.
We searched MEDLINE for publications in English published between Jan 1, 2014, and March 1, 2018, using the term “type 1 diabetes” and MEDLINE subheadings and selected papers on the basis of our opinion of their scientific importance. Research published since the 2014 Lancet Seminar on this topic was given particular attention. We provide an overview of type 1 diabetes focusing on updating the reader on recent advances and controversies.
Acknowledgments
This work was partly supported by grants from the National Institutes of Health, JDRF, the Veteran’s Administration, Diabetes UK, the Leona M and Harry B Helmsley Charitable Trust, the BIRAX Regenerative Medicine Initiative, the Ball Brothers Foundation, George and Francis Ball Foundation, Sigma Beta Sorority, Cryptic Masons Medical Research Foundation, and the Luke Weise Research Fund. We thank W Tamborlane, C Matthews, and J Kushner for their review of a draft of this Seminar. We thank M Campbell-Thompson, F Syed, and T Weinzerl for assistance with figures. And we thank T Lewallen and M Wales for administrative support.
Declaration of interests
LAD reports personal fees from Eli Lilly, and grants from Medtronic, Sanofi, Xeris, Caladrius, Dexcom, and Janssen outside the submitted work. RAO holds a UK Medical Research Council institutional Confidence in Concept grant to develop a 10 SNP biochip type 1 diabetes genetic test in collaboration with Randox. CE-M declares no competing interests.
Contributor Information
Linda A DiMeglio, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA;.
Carmella Evans-Molina, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;.
Richard A Oram, Institute of Biomedical and Clinical Science, University of Exeter Medical School, and The Academic Kidney Unit, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.
References
- 1.Barnett R Type 1 diabetes. Lancet 2018; 391: 195. [DOI] [PubMed] [Google Scholar]
- 2.Skyler JS. Hope vs hype: where are we in type 1 diabetes? Diabetologia 2018; 61: 509–16. [DOI] [PubMed] [Google Scholar]
- 3.Eisenbarth GS. Autoimmune beta cell insufficiency—diabetes mellitus type 1. Triangle 1984; 23: 111–24. [Google Scholar]
- 4.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018; 41 (suppl 1): S13–27. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014; 133: e938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018; 6: 122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hope SV, Wienand-Barnett S, Shepherd M, et al. Practical classification guidelines for diabetes in patients treated with insulin: a cross-sectional study of the accuracy of diabetes diagnosis. Br J Gen Pract 2016; 66: e315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delvecchio M, Mozzillo E, Salzano G, et al. Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian pediatric diabetes centers during 2007 to 2012. J Clin Endocrinol Metab 2017; 102: 1826–34. [DOI] [PubMed] [Google Scholar]
- 10.Pihoker C, Gilliam LK, Ellard S, et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab 2013; 98: 4055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd M, Shields B, Hammersley S, et al. Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care 2016; 39: 1879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med 2013; 30: 803–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields BM, Peters JL, Cooper C, et al. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open 2015; 5: e009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin KA, Richardson CC, Ravishankar A, et al. Identification of tetraspanin-7 as a target of autoantibodies in type 1 diabetes. Diabetes 2016; 65: 1690–98. [DOI] [PubMed] [Google Scholar]
- 15.Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015; 58: 980–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013; 309: 2473–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenbaum CJ, Speake C, Krischer J, et al. Strength in numbers: opportunities for enhancing the development of effective treatments for type 1 diabetes—the TrialNet Experience. Diabetes 2018; published online May 16 DOI: 10.2337/db18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015; 38: 1964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler AG, Danne T, Dunger DB, et al. Primary prevention of beta-cell autoimmunity and type 1 diabetes—The Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) perspectives. Mol Metab 2016; 5: 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med 2008; 359: 2849–50. [DOI] [PubMed] [Google Scholar]
- 21.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet 2016; 387: 2331–39. [DOI] [PubMed] [Google Scholar]
- 22.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res 2001; 56: 69–89. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health 2015; 15: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun 2015; 64: 101–12. [DOI] [PubMed] [Google Scholar]
- 25.TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007; 8: 286–98. [DOI] [PubMed] [Google Scholar]
- 26.Oram RA, Patel K, Hill A, et al. A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care 2016; 39: 337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler C, Krumsiek J, Buettner F, et al. Feature ranking of type 1 diabetes susceptibility genes improves prediction of type 1 diabetes. Diabetologia 2014; 57: 2521–29. [DOI] [PubMed] [Google Scholar]
- 28.Bonifacio E, Beyerlein A, Hippich M, et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med 2018; 15: e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Eapidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39: 481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017; 376: 1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med 2017; 15: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forga Llenas L, Goñi Iriarte MJ, Cambra Contin K, Ibáñez Beroiz B, Chueca Guendulain M, Berrade Zubiri S. Incidence and temporal trends of childhood type 1 diabetes between 1975 and 2012 in Navarre (Spain). Gac Sanit 2015; 29: 51–54. [DOI] [PubMed] [Google Scholar]
- 33.Chobot A, Polanska J, Brandt A, et al. Updated 24-year trend of type 1 diabetes incidence in children in Poland reveals a sinusoidal pattern and sustained increase. Diabet Med 2017; 34: 1252–58. [DOI] [PubMed] [Google Scholar]
- 34.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet 2016; 387: 2340–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara CT, Geyer SM, Liu YF, et al. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care 2017; 40: 698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondrashova A, Reunanen A, Romanov A, et al. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Ann Med 2005; 37: 67–72. [DOI] [PubMed] [Google Scholar]
- 37.Steck AK, Armstrong TK, Babu SR, Eisenbarth GS. Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes 2011; 60: 1045–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inshaw JRJ, Walker NM, Wallace C, Bottolo L, Todd JA. The chromosome 6q22.33 region is associated with age at diagnosis of type 1 diabetes and disease risk in those diagnosed under 5 years of age. Diabetologia 2018; 61: 147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krischer JP, Liu X, Lernmark A, et al. The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes 2017; 66: 3122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roshandel D, Gubitosi-Klug R, Bull SB, et al. Meta-genome-wide association studies identify a locus on chromosome 1 and multiple variants in the MHC region for serum C-peptide in type 1 diabetes. Diabetologia 2018; 61: 1098–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartee AK, Owens LA, Lahr BD, Yawn BP, Murray JA, Kudva YC. Incidence of type 1 diabetes is not increasing in a population-based cohort in Olmsted County, Minnesota, USA. Mayo Clin Proc 2016; 91: 1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G. Thirty years of prospective nationwide incidence of childhood type 1 diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes 2011; 60: 577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaiserman AM, Carstensen B, Voitenko VP, et al. Seasonality of birth in children and young adults (0–29 years) with type 1 diabetes in Ukraine. Diabetologia 2007; 50: 32–35. [DOI] [PubMed] [Google Scholar]
- 44.Thomas NJM, Jones S, Weedon M, Hattersley A, Oram R. Classifying diabetes by type 1 genetic risk shows autoimmune diabetes cases are evenly distributed above and below 30 years of age. Diabetologia 2016; 59 (suppl 1): S135. [Google Scholar]
- 45.Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia 2016; 59: 13–20. [DOI] [PubMed] [Google Scholar]
- 46.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014; 37: 2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia 2017; 60: 1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015; 64: 1682–87. [DOI] [PubMed] [Google Scholar]
- 49.Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 2016; 59: 2448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roep BO, Arden SD, de Vries RR, Hutton JC. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature 1990; 345: 632–34. [DOI] [PubMed] [Google Scholar]
- 51.Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet 1998; 351: 568–69. [DOI] [PubMed] [Google Scholar]
- 52.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-cell deficiency. N Engl J Med 2001; 345: 1036–40. [DOI] [PubMed] [Google Scholar]
- 53.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol 2012; 3: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglia M Experiments by nature: lessons on type 1 diabetes. Tissue Antigens 2014; 83: 1–9. [DOI] [PubMed] [Google Scholar]
- 55.Babon JA, DeNicola ME, Blodgett DM, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016; 22: 1482–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Culina S, Lalanne AI, Afonso G, et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 2018; 3: eaao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michels AW, Landry LG, McDaniel KA, et al. Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes 2017; 66: 722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 2013; 56: 2541–43. [DOI] [PubMed] [Google Scholar]
- 59.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016; 65: 1362–69. [DOI] [PubMed] [Google Scholar]
- 60.Campbell-Thompson M Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015; 16: 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenbaum CJ, Anderson AM, Dolan LM, et al. Preservation of β-cell function in autoantibody-positive youth with diabetes. Diabetes Care 2009; 32: 1839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010; 59: 2846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam CJ, Jacobson DR, Rankin MM, Cox AR, Kushner JA. β cells persist in T1D pancreata without evidence of ongoing β-cell turnover or neogenesis. J Clin Endocrinol Metab 2017; 102: 2647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015; 38: 476–81. [DOI] [PubMed] [Google Scholar]
- 65.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014; 57: 187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oram RA, McDonald TJ, Shields BM, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care 2015; 38: 323–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on β-cell function in long-standing type 1 diabetes. Diabetes Care 2009; 32: 2251–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lachin JM, McGee P, Palmer JP. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014; 63: 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003; 26: 832–36. [DOI] [PubMed] [Google Scholar]
- 70.Zenz S, Mader JK, Regittnig W, et al. Impact of C-peptide status on the response of glucagon and endogenous glucose production to induced hypoglycaemia in T1DM. J Clin Endocrinol Metab 2018; 103: 1408–17. [DOI] [PubMed] [Google Scholar]
- 71.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 2012; 55: 2417–20. [DOI] [PubMed] [Google Scholar]
- 72.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 2012; 61: 818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 2012; 8: e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bogdani M, Johnson PY, Potter-Perigo S, et al. Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis. Diabetes 2014; 63: 2727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagy N, Kaber G, Johnson PY, et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J Clin Invest 2015; 125: 3928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akirav EM, Baquero MT, Opare-Addo LW, et al. Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes 2011; 60: 876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lundberg M, Lindqvist A, Wierup N, Krogvold L, Dahl-Jørgensen K, Skog O. The density of parasympathetic axons is reduced in the exocrine pancreas of individuals recently diagnosed with type 1 diabetes. PLoS One 2017; 12: e0179911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taborsky GJ Jr, Mei Q, Hackney DJ, Mundinger TO. The search for the mechanism of early sympathetic islet neuropathy in autoimmune diabetes. Diabetes Obes Metab 2014;16 (suppl 1): 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016; 65: 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virostko J, Hilmes M, Eitel K, Moore DJ, Powers AC. Use of the electronic medical record to assess pancreas size in type 1 diabetes. PLoS One 2016; 11: e0158825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Control Diabetes and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–86. [DOI] [PubMed] [Google Scholar]
- 82.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 3922–37. [DOI] [PubMed] [Google Scholar]
- 84.Muchmore DB. The need for faster insulin. J Diabetes Sci Technol 2017; 11: 157–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lyons SK, Hermann JM, Miller KM, et al. Use of adjuvant pharmacotherapy in type 1 diabetes: international comparison of 49,996 individuals in the prospective diabetes follow-up and T1D exchange registries. Diabetes Care 2017; 40: e139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Libman IM, Miller KM, DiMeglio LA, et al. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA 2015; 314: 2241–50. [DOI] [PubMed] [Google Scholar]
- 87.El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2018; 137: 83–92. [DOI] [PubMed] [Google Scholar]
- 88.Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder:a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care 2016; 39: 555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017; 389: 369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haymond MW, Redondo MJ, McKay S, et al. Nonaqueous, mini-dose glucagon for treatment of mild hypoglycemia in adults with type 1 diabetes: a dose-seeking study. Diabetes Care 2016; 39: 465–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015; 9: 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017; 19: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kovatchev B The artificial pancreas in 2017: the year of transition from research to clinical practice. Nat Rev Endocrinol 2018; 14: 74–76. [DOI] [PubMed] [Google Scholar]
- 94.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care 2018; 41 (suppl 1): S55–64. [DOI] [PubMed] [Google Scholar]
- 95.Rewers MJ, Pillay K, de Beaufort C, et al. ISPAD clinical practice concensus guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric Diabetes 2014; 15 (suppl 20): 102–14. [DOI] [PubMed] [Google Scholar]
- 96.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can J Diabetes 2013; 37 (suppl 1): S1–3. [DOI] [PubMed] [Google Scholar]
- 97.Clements SA, Anger MD, Bishop FK, et al. Lower A1c among adolescents with lower perceived A1c goal: a cross-sectional survey. Int J Pediatr Endocrinol 2013; 2013: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–49. [DOI] [PubMed] [Google Scholar]
- 99.American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care 2018; 41 (suppl 1): S137–43. [DOI] [PubMed] [Google Scholar]
- 100.REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ 2017; 356: j1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hughes JW, Riddlesworth TD, DiMeglio LA, et al. Autoimmune diseases in children and adults with type 1 diabetes from the T1D exchange clinic registry. J Clin Endocrinol Metab 2016; 101: 4931–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.American Diabetes Association. 3. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2018. Diabetes Care 2018; 41 (suppl 1): S28–37. [DOI] [PubMed] [Google Scholar]
- 103.Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr Diabetes 2013; 14: 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW. Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000–2009. Diabetes Care 2011; 34: 2379–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karges B, Rosenbauer J, Holterhus PM, et al. Hospital admission for diabetic ketoacidosis or severe hypoglycemia in 31,330 young patients with type 1 diabetes. Eur J Endocrinol 2015; 173: 341–50. [DOI] [PubMed] [Google Scholar]
- 106.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014; 37: 2114–22. [DOI] [PubMed] [Google Scholar]
- 108.Cameron FJ, Wherrett DK. Care of diabetes in children and adolescents: controversies, changes, and consensus. Lancet 2015; 385: 2096–106. [DOI] [PubMed] [Google Scholar]
- 109.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007; 356: 1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. Diabetes Care 2008; 31: 922–26. [DOI] [PubMed] [Google Scholar]
- 111.Patterson CC, Dahlquist G, Harjutsalo V, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia 2007; 50: 2439–42. [DOI] [PubMed] [Google Scholar]
- 112.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006; 49: 298–305. [DOI] [PubMed] [Google Scholar]
- 113.Haynes A, Hermann JM, Miller KM, et al. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes 2017; 18: 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karges B, Kapellen T, Wagner VM, et al. Glycated hemoglobin A1c as a risk factor for severe hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes 2017; 18: 51–58. [DOI] [PubMed] [Google Scholar]
- 115.Bergenstal RM. Glycemic variability and diabetes complications: does it matter? Simply put, there are better glycemic markers! Diabetes Care 2015; 38: 1615–21. [DOI] [PubMed] [Google Scholar]
- 116.Virk SA, Donaghue KC, Cho YH, et al. Association between HbA1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab 2016; 101: 3257–63. [DOI] [PubMed] [Google Scholar]
- 117.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes 2014; 15: 18–26. [DOI] [PubMed] [Google Scholar]
- 118.Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009; 58: 1651–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huo L, Shaw JE, Wong E, Harding JL, Peeters A, Magliano DJ. Burden of diabetes in Australia: life expectancy and disability-free life expectancy in adults with diabetes. Diabetologia 2016;59: 1437–45. [DOI] [PubMed] [Google Scholar]
- 120.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA 2015; 313: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care 2008; 31: 1892–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tonoli C, Heyman E, Roelands B, et al. Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J Diabetes 2014; 6: 499–513. [DOI] [PubMed] [Google Scholar]
- 123.Nunley KA, Ryan CM, Orchard TJ, et al. White matter hyperintensities in middle-aged adults with childhood-onset type 1 diabetes. Neurology 2015; 84: 2062–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seaquist ER. The impact of diabetes on cerebral structure and function. Psychosom Med 2015; 77: 616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev 2014;14: CD009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Secrest AM, Costacou T, Gutelius B, Miller RG, Songer TJ, Orchard TJ. Associations between socioeconomic status and major complications in type 1 diabetes: the Pittsburgh epidemiology of diabetes complication (EDC) study.Ann Epidemiol 2011; 21: 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis.Lancet Diabetes Endocrinol 2015; 3: 198–206. [DOI] [PubMed] [Google Scholar]
- 129.Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate future risk of blindness and lower limb amputation in patients with diabetes: cohort study. BMJ 2015; 351: h5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017; 357: j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ 2012; 38: 562–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Van Name MA, Hilliard ME, Boyle CT, et al. Nighttime is the worst time: parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes 2018; 19: 114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hilliard ME, Mann KA, Peugh JL, Hood KK. How poorer quality of life in adolescence predicts subsequent type 1 diabetes management and control. Patient Educ Couns 2013; 91: 120–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention: association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988; 37: 1574–82. [PubMed] [Google Scholar]
- 135.De Filippo G, Carel JC, Boitard C, Bougnères PF. Long-term results of early cyclosporin therapy in juvenile IDDM. Diabetes 1996; 45: 101–04. [DOI] [PubMed] [Google Scholar]
- 136.Feutren G, Papoz L, Assan R, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet 1986;2: 119–24. [DOI] [PubMed] [Google Scholar]
- 137.Hummel S, Pflüger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 2011; 34: 1301–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Writing Group for the TRIGR Study Group. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. JAMA 2018; 319:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beam CA, MacCallum C, Herold KC, et al. GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: findings from a Bayesian meta-analysis. Diabetologia 2017; 60: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ludvigsson J, Chéramy M, Axelsson S, et al. GAD-treatment of children and adolescents with recent-onset type 1 diabetes preserves residual insulin secretion after 30 months. Diabetes Metab Res Rev 2014; 30: 405–14. [DOI] [PubMed] [Google Scholar]
- 141.Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011; 378: 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685–91. [DOI] [PubMed] [Google Scholar]
- 143.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial—type 1. Diabetes Care 2005; 28: 1068–76. [DOI] [PubMed] [Google Scholar]
- 144.Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017; 318: 1891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008; 372: 1746–55. [DOI] [PubMed] [Google Scholar]
- 146.Alhadj Ali M, Liu YF, Arif S, et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med 2017; 9: eaaf7779. [DOI] [PubMed] [Google Scholar]
- 147.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009; 361: 2143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herold KC, Gitelman S, Greenbaum C, et al. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009; 132: 166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002; 346: 1692–98. [DOI] [PubMed] [Google Scholar]
- 150.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005; 352: 2598–608. [DOI] [PubMed] [Google Scholar]
- 151.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 378: 412–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol 2013; 1: 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haller MJ, Gitelman SE, Gottlieb PA, et al. Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes 2016; 65: 3765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 2015; 125: 448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pinckney A, Rigby MR, Keyes-Elstein L, Soppe CL, Nepom GT, Ehlers MR. Correlation among hypoglycemia, glycemic variability, and C-peptide preservation after alefacept therapy in patients with type 1 diabetes mellitus: analysis of data from the immune tolerance network T1DAL trial. Clin Ther 2016;38: 1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sørensen JS, Johannesen J, Pociot F, et al. Residual β-cell function 3–6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care 2013; 36: 3454–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients 2017; 9: E437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lindahl JP, Hartmann A, Horneland R, et al. Improved patient survival with simultaneous pancreas and kidney transplantation in recipients with diabetic end-stage renal disease. Diabetologia 2013; 56: 1364–71. [DOI] [PubMed] [Google Scholar]
- 159.Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9: 555–62. [DOI] [PubMed] [Google Scholar]
- 160.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230–38. [DOI] [PubMed] [Google Scholar]
- 161.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355: 1318–30. [DOI] [PubMed] [Google Scholar]
- 162.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab 2012; 97: E2078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35: 1436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ellis C, Ramzy A, Kieffer TJ. Regenerative medicine and cell-based approaches to restore pancreatic function. Nat Rev Gastroenterol Hepatol 2017; 14: 612–28. [DOI] [PubMed] [Google Scholar]
- 165.Cantú-Rodríguez OG, Lavalle-González F, Herrera-Rojas MÁ, et al. Long-term insulin independence in type 1 diabetes mellitus using a simplified autologous stem cell transplant. J Clin Endocrinol Metab 2016; 101: 2141–48. [DOI] [PubMed] [Google Scholar]
- 166.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009; 301: 1573–79. [DOI] [PubMed] [Google Scholar]
- 167.Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 2015; 7: 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T ce prolongs survival of pancreatic islets—results of one year follow-up. Clin Immunol 2014; 153: 23–30. [DOI] [PubMed] [Google Scholar]
- 169.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Administration of CD4+CD25highCD127-regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012;35: 1817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bone RN, Evans-Molina C. Combination immunotherapy for type 1 diabetes. Curr Diab Rep 2017; 17: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61: 827–37. [PubMed] [Google Scholar]
- 172.Greene JA, Riggs KR. Why is there no generic insulin? Historical origins of a modern problem. N Engl J Med 2015; 372: 1171–75. [DOI] [PubMed] [Google Scholar]