Abstract
Peroxisome proliferator-activated receptor α (PPARα) is involved in the regulation of fatty acid and cholesterol metabolism. A high-cholesterol (HC) diet increases the risk of developing cardiovascular diseases (CVD); however, it is unclear whether the toxic effects of cholesterol involve changes in thrombotic factor expression, and whether PPARα is necessary for such effects. To investigate this possibility, we fed a HC diet to wild-type (WT) and Ppara-null mice and measured cholesterol and triglyceride contents, liver histology, serum/plasma levels of coagulation factors, hepatic expression of the coagulation factors, liver/serum sulfatide levels, hepatic sulfatide metabolism, hepatic expression of lipid transporters, and hepatic oxidative stress and its relating enzymes. In Ppara-null mice, the HC diet caused triglyceride accumulation and exacerbated inflammation and oxidative stress in liver, increased levels of coagulation factors, including tissue factor, plasminogen activator inhibitor-1 and carboxypeptidase B2 in blood and liver, and decreased levels of anti-thrombotic sulfatides in serum and liver. These changes were much less marked in WT mice. These findings imply that cholesterol overload exerts its toxic effects at least in part by enhancing thrombosis, secondary to abnormal hepatic lipid metabolism, inflammation, and oxidative stress. Moreover, we reveal for the first time that PPARα can attenuate these toxic effects by transcriptional regulation of coagulation factors and sulfatides, in addition to its known effects of controlling lipid homeostasis and suppressing inflammation and oxidative stress. Therapies aimed at activating PPARα might prevent HC diet-induced CVD through modulating various pro- and anti-thrombotic factors.
Keywords: High-cholesterol diet, Peroxisome proliferator-activated receptor α (PPARα), Coagulation factors, Cardiovascular diseases, Sulfatides
Introduction
Peroxisome proliferator-activated receptor α (PPARα) is a nuclear receptor involved in the control of fatty acid (FA) metabolism mainly in liver, heart, kidney, and skeletal muscle (Aoyama et al. 1998; Kersten et al. 2000; Kamijo et al. 2002). PPARα is a master regulator of the hepatic response of food deprivation or fasting where it mobilizes mitochondrial and peroxisomal FA β-oxidation (Aoyama et al. 1998; Kersten et al. 2000). Because FAs are PPARα ligands, a FA-enriched high-fat diet activates hepatic PPARα resulting in expression of FA-metabolizing enzymes leading to FA catabolism (Aoyama et al. 1998; Kersten et al. 2000). It was reported that PPARα activation suppresses oxidative stress and pro-inflammatory responses caused by a high-fat diet through up-regulation of reactive oxygen species (ROS)-eliminating enzymes and the inhibitor IκBα (Tanaka et al. 2011; Zúñiga et al. 2011; Valenzuela et al. 2012; Hu et al. 2017).
In addition to these roles, hepatic PPARα is known to influence cholesterol metabolism. PPARα modulates the metabolic pathway generating cholic acid synthesis from cholesterol via transcriptional regulation of 12α-hydroxylase to generate 7α-hydroxy cholesterol (Hunt et al. 2000), and regulation of intracellular cholesterol and cholic acid transporters (Chakravarthy et al. 2005; Badman et al. 2007). Therefore, it is possible that PPARα may ameliorate the toxic effects of a high-cholesterol (HC) diet. However, almost all the previous studies investigating the relationship between PPARα and lipids have used a FA-enriched high-fat diet (Ye et al. 2001; Patsouris et al. 2006), and these studies were not capable of demonstrating a direct relationship between PPARα function and cholesterol toxicity. A study using a HC diet, containing FA and TG that are similar levels to a normal diet, is required to clarify this relationship.
Excessive cholesterol intake and the resulting hypercholesterolemia increase the risk of cardiovascular diseases (CVD) through increases in vascular plaque formation and thrombosis following plaque rupture (Abela et al. 1995). However, there may still be additional mechanisms underlying the development of CVD that have not been identified. Various pro-thrombotic molecules are thought to play an important role in the development of CVD (Toschi et al. 1997; Kohler and Grant 2000; Steffel et al. 2006). Many clinical and experimental studies reported that high levels of representative coagulation factors, including tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1), indicate a high risk of CVD development (Hamsten et al. 1987; Toschi et al. 1997; Kohler and Grant 2000; Steffel et al. 2006), and that feeding a HC diet increases the levels of these coagulation factors and enhances their pro-thrombotic effects (Kato et al. 1996; Ichino et al. 1997). However, the molecular mechanisms underlying the enhanced pro-thrombotic effects of cholesterol overload, as well as the influence of other thrombotic factors, remain to be identified.
Carboxypeptidase B2 (CPB2) is a liver enzyme that suppresses fibrinolysis (Mosnier and Bouma 2006). Sulfatides are major mammalian serum sphingoglycolipids which are synthesized and secreted mainly from the liver as a component of lipoproteins (Kimura et al. 2012). Our previous experimental and clinical studies have demonstrated that serum sulfatides exert anti-coagulative and anti-platelet functions, and that lower levels of serum sulfatides might be associated with the development of CVD (Kamijo et al. 2012; Kimura et al. 2012; Yuzhe et al. 2015). It was reported that expression of these important molecules (TF, PAI-1, CPB2, and sulfatides) is regulated by PPARα and oxidative stress (Marx et al. 2001; Kimura et al. 2012; Masuda et al. 2012; Kanbe et al. 2014; Gonsalves et al. 2015; Wojewodzka-Zelezniakowicz et al. 2017). We hypothesized that a HC diet exerts its toxic effects through changes in lipid metabolism, oxidative stress, and production of these thrombosis-related factors, and that PPARα might strongly influence such effects, thereby modulating development of CVD. To test this hypothesis, we fed a HC diet containing normal amounts of FA and TG to wild-type (WT) and Ppara-null mice and compared the effects on serum and hepatic contents of TG and cholesterol, the expression of TF, PAI-1, CPB2, sulfatides, and intracellular transporter of sulfatides, and oxidative stress between these genotypes.
Materials and methods
Mice and experimental design
Male WT and Ppara-null mice on a SV/129 genetic background (age 14–18 weeks; body mass 24–30 g) were used, as previously described (Lee et al. 1995). These mice were fed with a cholesterol-free diet (control group) or a 1.5% (w/w) cholesterol diet, both of which contained normal amounts of FA and TG, for 8 weeks (n = 6 per group). The detailed composition of each diet is given in Supplementary Table 1. The experimental conditions for the current study were determined through preliminary experiments, which are described in the Supplementary methods. The mice were maintained in a specific pathogen-free facility and housed in a light- and temperature-controlled environment (12-h light/dark cycle; 25 °C), and were provided with tap water ad libitum. All animal experiments and procedures were conducted in accordance with the guidelines approved by the Shinshu University, the National Institutes of Health, and the Association for Assessment and Accreditation of Laboratory Animal Care.
Histopathologic analysis
Mouse livers were fixed in 10% formaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. The deparaffinized sections were then stained with hematoxylin and eosin (H&E).
Analysis of mRNA expression
Total liver RNA was extracted using the RNeasy Mini Kit (QIAGEN, Tokyo, Japan). Total RNA extracted from whole livers was reverse-transcribed using oligo(dT) primers and SuperScript III reverse transcriptase (Invitrogen Co., Carlsbad, CA, USA). Subsequently, the cDNAs were quantified by real-time polymerase chain reactions (PCR) using a SYBR Premix Ex Taq II (Takara Bio, Otsu, Japan) on a Thermal Cycler Dice TP800 system (Takara Bio, Otsu, Japan). The sequences of specific primers are shown in Supplementary Table 2. The mRNA encoding glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal control, and the relative expression of each target mRNA was calculated using the comparative threshold cycle (Ct) method for PCR amplification (Livak and Schmittgen 2001).
Immunoblot analysis
Whole liver lysates were prepared as previously described (Aoyama et al. 1989). The nuclear and cytoplasmic fractions of whole liver extracts were prepared for each mouse using NE-PER Nuclear and Cytoplasmic Extraction Reagent (Thermo Fisher Scientific, Rockford, IL, USA), and the protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Nuclear and cytoplasmic proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. The membranes were incubated with the respective primary antibodies, followed by incubation with an alkaline phosphatase-conjugated secondary antibody (Tanaka et al. 2008). Immunoblotting was performed using antibodies targeting tissue factor (TF), carboxypeptidase B2 (CPB2), plasminogen activator inhibitor 1 (PAI-1), cerebroside sulfotransferase (CST), arylsulfatase A (ARSA), ceramide galactosyltransferase (CGT), galactosylceramidase (GALC), fatty acid binding protein 1 (FABP1), glycolipid transfer protein (GLTP), sterol carrier protein 2 (SCP2), microsomal triglyceride transfer protein (MTTP), and 4-hydroxy-2-nonenal (4-HNE). β-Actin was used as the internal control for protein loading. Primary antibodies targeting TF, CPB, PAI-1, FABP, and β-actin were purchased from Abcam (Cambridge, MA, USA), those targeting CGT, GALC, SCP2, and MTTP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and those targeting GLTP, CST, ARSA, and 4-HNE were purchased from Proteintech (Chicago, IL, USA), Abnova corporation (Jhouzih St., Taiwan), Everest Biotech (Upper Heyford, Oxfordshire, UK, USA), and Alexis Biochemicals (Farmingdale, NY, USA), respectively. Specific protein band intensity was quantified by densitometry using NIH Image J software (Bethesda, MD, USA).
Lipid analysis
Lipids were extracted from liver using the hexane/isopropanol method, as previously described (Hara and Radin 1978). Serum and liver concentrations of total cholesterol (TC) and liver TG were measured using enzymatic assay kits (Wako Pure Chemical Industries. Ltd, Osaka, Japan).
Measurement of serum levels of alanine aminotransferase (ALT) and TF, and plasma levels of CPB2 and PAI-1
Serum levels of ALT and TF were measured using enzymatic assay kits (ALT, Wako Pure Chemical Industries, Ltd., Osaka, Japan and TF, Assay Pro, Anaheim, CA, USA, respectively). Plasma levels of CPB2 and PAI-1 were also measured using enzymatic assay kits (CPB2, SAB, Baltimore, MD, USA and PAI-1, CUSABIO, Baltimore, MD, USA, respectively).
Analysis of oxidative stress markers
Tissue content of malondialdehyde (MDA), a marker of oxidation, was measured using a lipid peroxidation colorimetric assay kit (Oxis International, Beverly Hills, CA, USA). The abundance of 4-HNE-modified proteins, another marker of oxidation, was measured by immunoblot analysis.
Extraction and measurement of hepatic and serum sulfatides
Sulfatides were extracted from serum and liver tissue. Samples were microsonicated in six volumes of cold water, and lipids were extracted using the hexane/isopropanol method, as previously described (Hara and Radin 1978). Lipid extracts were then treated with methanolic sodium hydroxide to convert sulfatides to their corresponding lysosulfatides. After purification, lysosulfatide preparations were assayed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Seven species of lysosulfatides were detected, and the total amount of sulfatides in each sample was calculated as the sum of each of these species. Detailed methods of extraction and measurement of sulfatides have been previously described (Li et al. 2007).
Other experiments
The DNA-binding activities of PPARα, δ, and γ were measured in liver nuclear protein fractions using PPARα, δ, and γ Transcription Factor Assay kits (Cayman Chemical, Ann Arbor, MI, USA). These assay kits are enzyme-linked immunosorbent assays that use specific DNA binding sequence-immobilized microplates and specific antibodies for each target transcription factor. This assay system is non-radioactive and sensitive, and has been recently used as an alternative method to the radioactive electrophoretic mobility shift assay system (Harada et al. 2016). The results of each DNA-binding assay are shown as a fold difference from the WT control group.
Statistical analysis
All values are expressed as mean ± standard deviation (SD). Significant interactions between two factors were identified using two-way ANOVA. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (version 22.0J; SPSS, Inc., Chicago, IL, USA).
Results
PPARα attenuates HC diet-induced TG accumulation and inflammation in liver
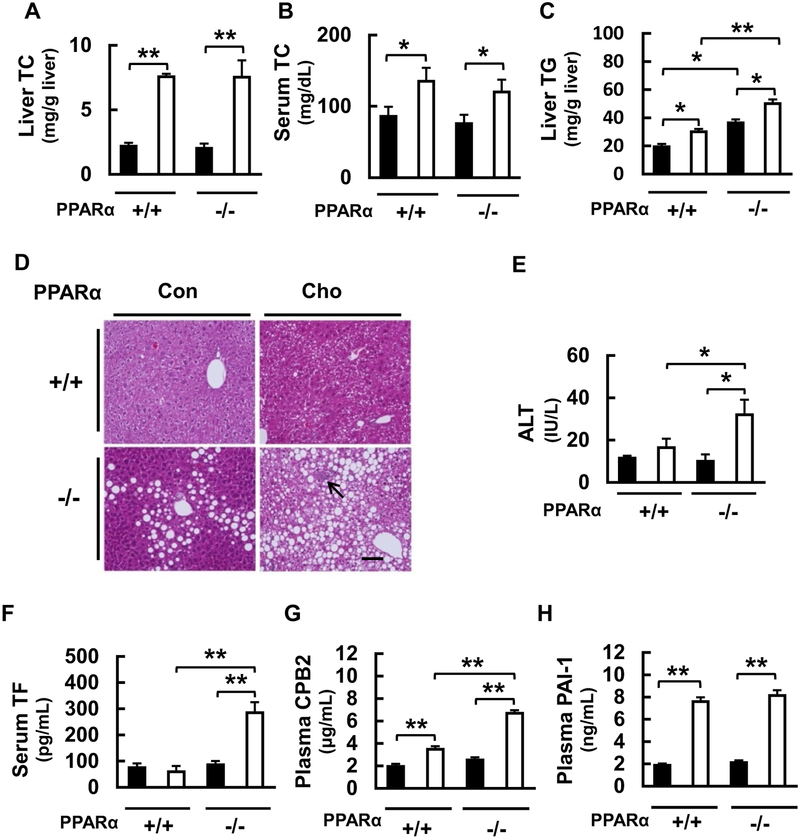
We investigated the effects of feeding a HC diet for 8 weeks on hepatic and serum TC levels. Hepatic and serum TC levels were identical between WT and Ppara-null mice fed the control diet, but were significantly higher in both mouse lines fed with the HC diet (Fig. 1a, b). Obvious liver swelling was not detected, but white coloration of the liver was recorded in the HC diet-fed Ppara-null mice, indicating the possibility of fatty liver. To evaluate this further, we measured hepatic TG content and histologically assessed hepatic lipid content by H&E staining. Hepatic TG was significantly higher in Ppara-null mice than in WT mice in the control diet-fed groups, but was significantly higher in the HC diet-fed mice of both genotypes, in particular in Ppara-null mice (Fig. 1c). Histological analysis demonstrated no hepatic steatosis in the WT groups, whereas mild hepatic steatosis was observed in the Ppara-null group fed with the control diet, and this was compounded by the presence of inflammatory cell infiltration in the HC diet-fed Ppara- null group, suggestive of the development of steatohepatitis (Fig. 1d). To investigate the hepatocyte damage further, we measured serum levels of ALT and AST. Serum ALT levels were identical between WT and Ppara-null mice on the control diet, but the level was significantly higher in Ppara-null group fed the HC diet (Fig. 1e). No significant differences in AST levels were detected among the groups (data not shown). These findings suggest that the HC diet can induce TG accumulation and inflammation in the liver, and that PPARα suppresses these effects, and are consistent with previous reports demonstrating that PPARα controls TG and FA metabolism, and has anti-inflammatory effects (Aoyama et al. 1998; Kersten et al. 2000; Kamijo et al. 2007; Hashimoto et al. 2012).
Fig. 1.
Effects of a high-cholesterol (HC) diet in wild-type (WT) and Ppara-null mice. a Effects of HC diet on total cholesterol (TC) content in the liver and b in serum induced in WT (+/+) and Ppara-null (−/−) mice. c Effect on triglyceride (TG) content in liver. d Photo-micrographs of liver sections stained with hematoxylin and eosin. Arrow indicates inflammatory cell infiltration, suggesting steatohepatitis. Scale bar = 100 μm. e Effect on serum level of ALT. f Effect on serum tissue factor (TF) concentration, g effects on plasma carboxypeptidase B2 (CPB2), and h plasminogen activator inhibitor-1 (PAI-1). Black bars indicate control groups and white bars indicate HC diet-fed groups. Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
PPARα partially suppresses the HC diet-induced increase of pro-coagulatory factors
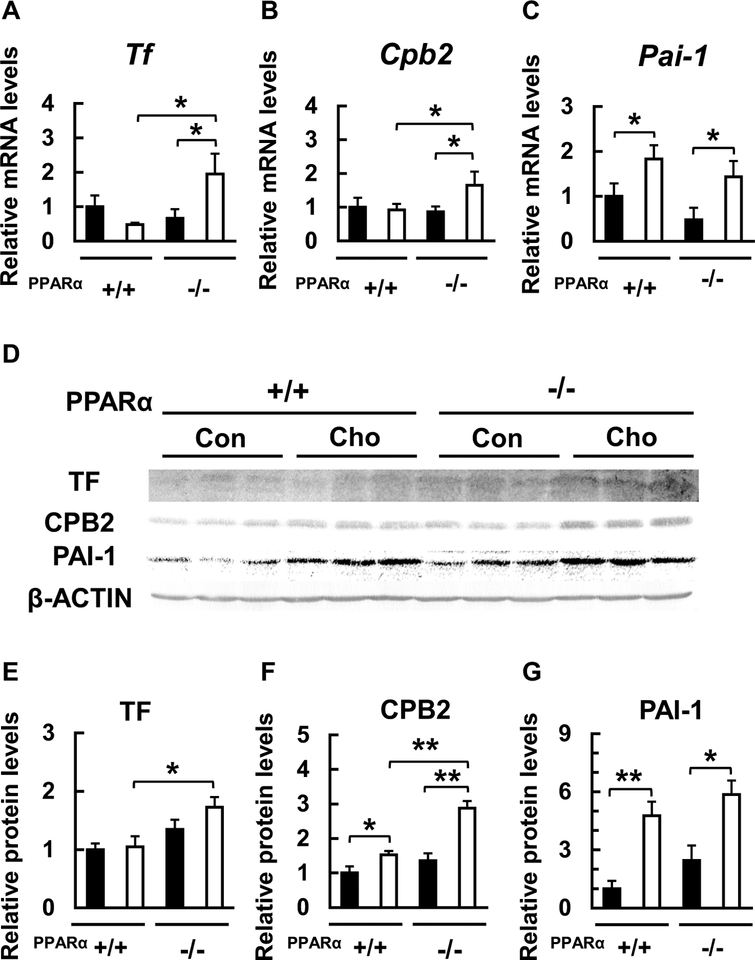
To elucidate the effects of the diet and the genotype on representative coagulation factors, we measured the blood protein content and liver mRNA expression of TF, CPB2, and PAI-1. The blood and liver levels of these three molecules did not differ between the groups fed control diet. While the HC diet did not affect or slightly increased the blood content and liver expression of TF and CPB2 in WT mice, HC diet dramatically increased the levels of proteins in Ppara-null mice (Figs. 1f, g, 2e, f). In contrast, the blood level and liver expression of PAI-1 were significantly and similarly increased by the HC diet in both WT and Ppara-null mice (Figs. 1h, 2g). The changes in hepatic mRNAs encoding these factors were similar to the protein levels (Fig. 2a–c, e–g), suggesting that the effects of feeding with the HC diet influence gene expression. These results indicate that the feeding with the HC diet can dramatically increase the expression of two coagulation factors, TF and CPB2, in the absence of PPARα expression. It is known that HC diet can increase blood and hepatic PAI-1 levels (Ichino et al. 1997), but our study is the first to demonstrate that these changes in PAI-1 are not significantly influenced by the absence of PPARα. Taken together, these findings suggest that the HC diet can increase blood and hepatic levels of at least three species of coagulation factors and that PPARα can ameliorate these effects partially.
Fig. 2.
Effects of HC diet on hepatic mRNA and protein expression of representative coagulation factors. a-c Effect of HC diet on liver Tf, Cpb2 and Pai-1 mRNA levels in WT (+/+) and Ppara-null (−/−) mice. d-g Effect of HC diet on liver protein levels of tissue factor (TF), carboxypeptidase B2 (CPB2), and plasminogen activator inhibitor-1 (PAI-1) in WT (+/+) and Ppara-null (−/−) mice. Black bars indicate control groups (Con) and white bars indicate HC diet-fed groups (Cho). Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
PPARα attenuates the HC diet-induced decrease of anti-thrombotic factors, sulfatides
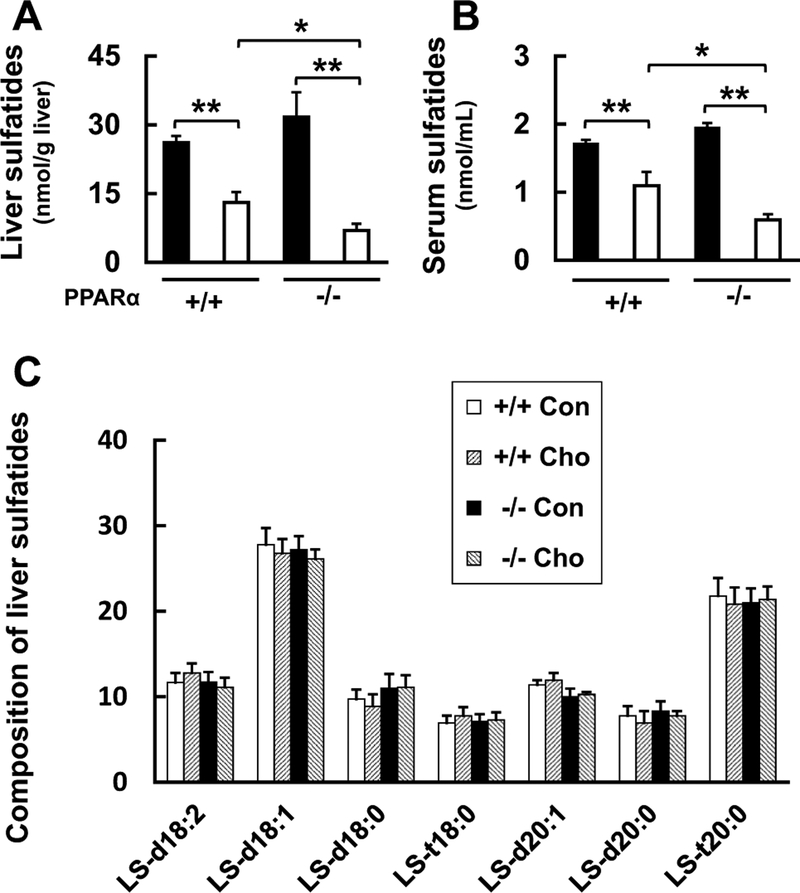
Next, we investigated the effect of the HC diet-feeding on serum and liver levels of the anti-coagulation and antiplatelet molecules, sulfatides, which are also regulated by PPARα, in WT and Ppara-null mice (Marx et al. 2001; Kimura et al. 2012; Masuda et al. 2012; Kanbe et al. 2014; Gonsalves et al. 2015; Wojewodzka-Zelezniakowicz et al. 2017). Liver and serum levels of sulfatides were similar between WT and Ppara-null mice fed with the control diet, and these levels became markedly lower in mice of either genotype fed the HC diet. However, the decrease of sulfatide level was significantly greater in Ppara-null mice than that in WT mice (Fig. 3a, b). Throughout these experiments, the composition of the sulfatides in the liver did not differ among the groups (Fig. 3c).
Fig. 3.
Effects of HC diet on sulfatide content of liver and serum. a Effect of HC diet on sulfatide levels in liver, and b serum of HC diet in WT (+/+) and Ppara-null (−/−) mice. Black bars indicate control groups (Con) and white bars indicate HC diet-fed groups (Cho). c Composition of sulfatides in the liver of each group. Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
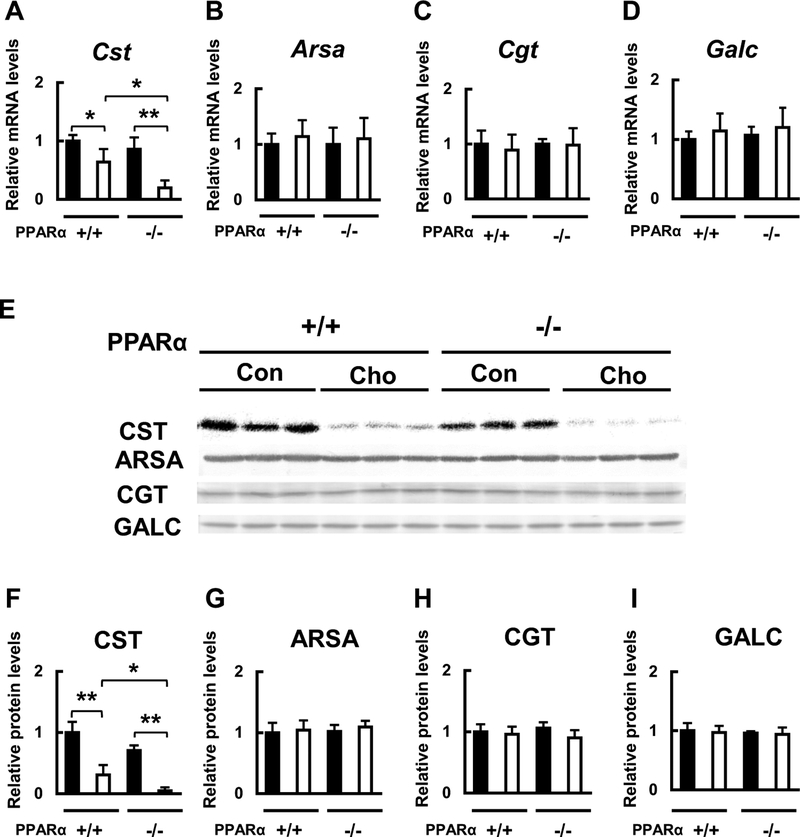
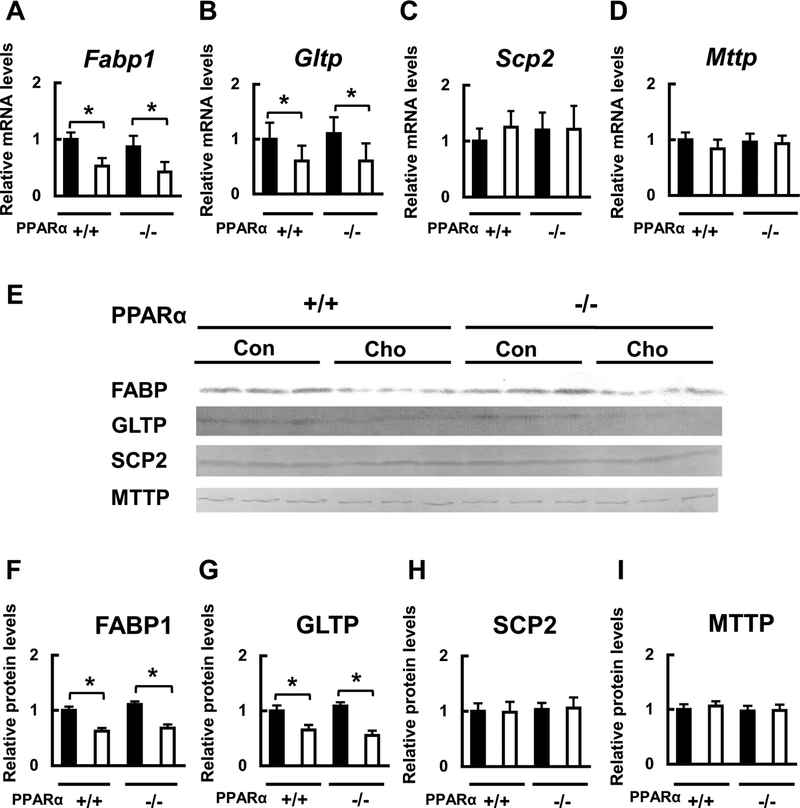
Because the HC diet significantly affected hepatic and serum sulfatide levels, we then measured the mRNA and protein expression of sulfatide-metabolizing enzymes in the liver. The mRNA and protein expression levels of the key sulfatide synthase, CST, which are known to be regulated by PPARα (Kimura et al. 2012), were similar between WT and Ppara-null mice fed the control diet. However, these levels were significantly lower in both WT and Ppara-null mice fed with the HC diet, and the decrease was significantly larger in Ppara-null mice than that in WT mice (Fig. 4a, e, f). The mRNA and protein expression levels of the other three sulfatide metabolizing enzymes, ARSA, CGT, and GALC, did not change among the groups (Fig. 4b–e, g–i). We also measured the mRNA and protein expression levels of intracellular lipid transport molecules. The mRNA and protein expression levels of FABP1 and GLTP, which, respectively, transport free FA and glycolipids such as sulfatides (Zou et al. 2011), were not different between WT and Ppara-null mice fed with the control diet, but both the levels were significantly lower in mice of both genotypes fed with the HC diet (Fig. 5a, b, e–g). The mRNA and protein levels of the other lipid transport proteins, SCP2 and MTTP, which, respectively, transport cholesterol and TG, did not differ among the groups (Fig. 5c–e, h, i). These findings suggest that the HC diet can reduce the serum sulfatides by reducing their synthesis and transport in liver, and that PPARα is involved in the regulation of these HC diet-induced effects.
Fig. 4.
Effects of HC diet on hepatic mRNA and protein expression of sulfatide-metabolizing enzymes. a-d Effect of HC diet on hepatic Cst, Arsa, Cgt and Galc mRNA expression in WT (+/+) and Ppara-null (−/−) mice. e-i Effect of HC diet on CST, ARSA, CGT, and GALC protein expression in WT (+/+) and Ppara-null (−/−) mice. Black bars indicate control groups (Con) and white bars indicate HC diet-fed groups (Cho). Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
Fig. 5.
Effects of HC diet on hepatic mRNA and protein expression of sulfatide transport enzymes. a-d Effect of HC diet on Fabp1, Gltp, Scp2, and Mttp mRNA expression in WT (+/+) and Ppara-null (−/−) mice. e-i Effect of HC diet on FABP1, GLTP, SCP2, and MTTP protein expression in WT (+/+) and Ppara-null (−/−) mice. Black bars indicate control groups (Con) and white bars indicate HC diet-fed groups (Cho). Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
PPARα suppresses the HC diet-induced oxidative stress
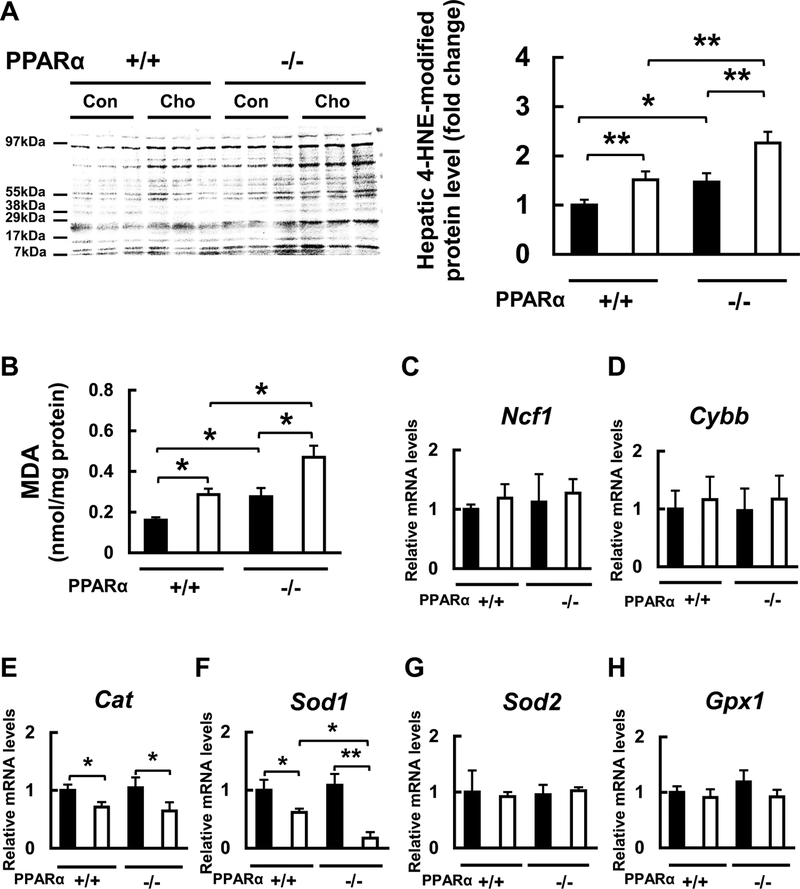
It was reported that the HC diet increases oxidative stress (Naziroglu et al. 2014). Indeed, levels of the thrombotic factors TF, PAI-1, and sulfatides are influenced by oxidative stress (Marx et al. 2001; Kimura et al. 2012; Masuda et al. 2012; Kanbe et al. 2014; Gonsalves et al. 2015; Wojewodzka-Zelezniakowicz et al. 2017), and that PPARα exerts anti-oxidative effects (Kamijo et al. 2007). Therefore, we investigated the effects of feeding the HC diet on hepatic oxidative stress and the related factors in WT and Ppara-null mice. To evaluate overall hepatic oxidative stress level, we measured the liver content of the lipid peroxidation markers, 4-HNE-modified protein and MDA. Both contents of these markers were higher in Ppara-null mice than in WT mice fed with the control diet (Fig. 6a, b). Both contents became significantly higher in both genotypes fed the HC diet, although the increases were larger in Ppara-null mice than those in WT mice (Fig. 6a, b). Next, we examined the effects of the HC diet on mRNA expression levels of molecules responsible for generating or eliminating reactive oxygen species (ROS). The hepatic expression of Ncf1 and Cybb mRNAs, encoding the ROS-generating neutrophil cytosolic factor 1 (NCF1) and NADPH oxidase 2 (Nox2), did not differ among the groups (Fig. 6c, d). PPARα is known to regulate the ROS-eliminating molecules such as catalase and Cu/Zn-superoxide dismutase (SOD) (Kamijo et al. 2007). The liver of Cat and Sodl mRNAs encoding catalase and Cu/Zn-SOD, respectively, were similar between WT and Ppara-null mice fed with the control diet, but the expression levels were significantly lower in the HC diet-fed mice of both genotypes (Fig. 6e, f). The decrease of Sod1 mRNA expression was much larger in Ppara-null mice than that in WT mice (Fig. 6f). The expression of hepatic Sod2 and Gpx1 mRNAs encoding the ROS-eliminating molecules Mn-SOD and glutathione peroxidase 1, respectively, did not differ among the groups (Fig. 6g, h). These new findings suggest that the HC diet can increase hepatic oxidative stress and that PPARα represents anti-oxidative effects through maintaining expression of Cu/Zn SOD.
Fig. 6.
Effects of diet on oxidative markers and mRNA expression of oxidative stress-related enzymes. a Effect of HC diet on liver protein level of 4-hydroxy-nonenal (HNE)-modified protein in WT (+/+) and Ppara-null (−/−) mice. b Effect of HC diet on MDA content in the liver in WT (+/+) and Ppara-null (−/−) mice. c-h Effect of HC diet on oxidative stress-related Ncf1, Cybb, Cat, Sod1, Sod2, and Gpx1 mRNA expression in WT (+/+) and Ppara-null (−/−) mice. Black bars indicate control groups (Con) and white bars indicate HC diet-fed groups (Cho). Values are mean ± SD (n = 6 for each group). Data were compared between two groups using two-way ANOVA. Significant differences are indicated by *p < 0.05 and **p < 0.01
The changes in mRNA expression and DNA-binding ability of PPARs by the HC diet
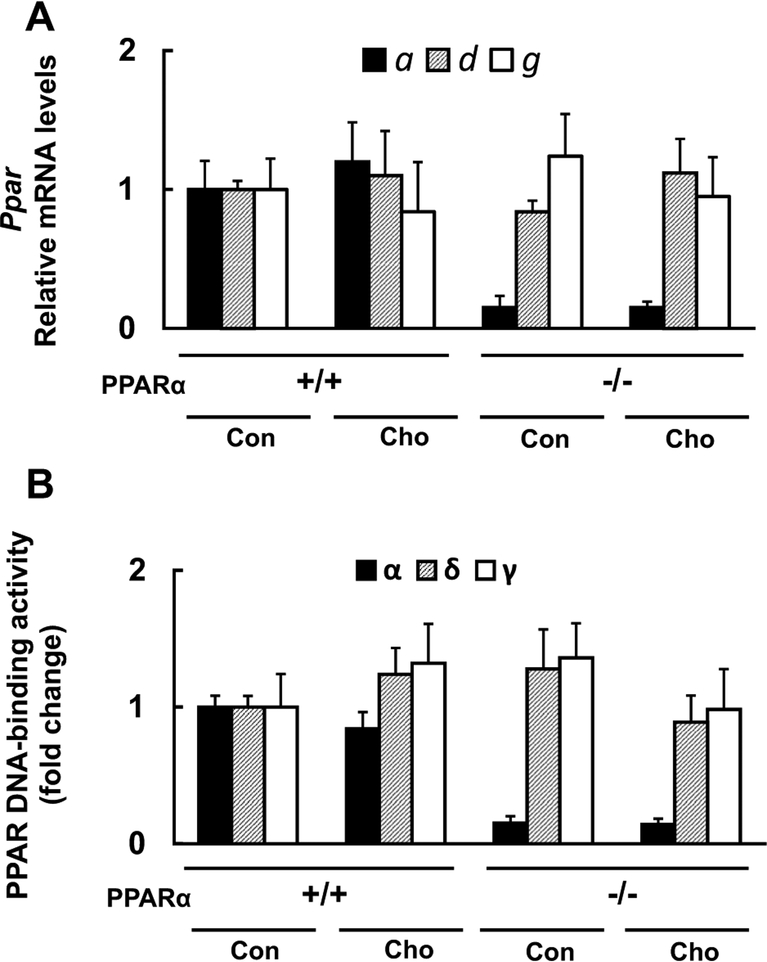
Lastly, we evaluated the mRNA expression and DNA-binding ability of PPARα, δ, and γ. The mRNA expression and DNA-binding ability of PPARα in WT mice were unchanged by the HC diet feeding, those of PPARδ and γ were not affected by both the HC diet and the mouse genotype (Fig. 7a, b).
Fig. 7.
Effects of diet on mRNA expression and DNA-binding activity of PPARs. a Effect of HC diet on Pparα (a), Ppard (d) and Pparg (g), mRNA expression in WT (+/+) and Ppara-null (−/−) mice. b Effect of HC diet on the DNA-binding activity of PPARα, PPARδ, and PPARγ in WT (+/+) and Ppara-null (−/−) mice. Values are mean ± SD (n = 6 for each group). Black, striped and white bars indicate PPARα, PPARδ, and PPARγ, respectively. Con and Cho indicates control and HC diet-fed groups, respectively
Discussion
In the current study, we fed the HC diet to WT and Ppara-null mice to investigate the influence of PPARα deficiency on the changes induced by dietary cholesterol overload. Compared with WT mice, the HC diet-fed Ppara-null mice exhibited a number of important differences: accumulation of TG and the induction of inflammation and oxidative stress in the liver, marked increases in liver expression and blood levels of the coagulation factors such as TF, PAI-1, and CPB2, and significant decreases in serum and liver levels of the anti-thrombotic sulfatides. These results suggest that cholesterol overload can induce toxic effects, including steatohepatitis, enhanced oxidative stress, and increased pro-thrombotic activity, and that PPARα deficiency can promote these effects. The HC diet used in this study contains normal levels of FA and TG; therefore, the findings are likely to reflect specific effects of cholesterol overload.
In addition to the known beneficial effects of PPARα such as maintenance of lipid homeostasis (Aoyama et al. 1998; Kersten et al. 2000; Kamijo et al. 2002), anti-inflammatory effects (Kamijo et al. 2002), and anti-oxidant effects (Kamijo et al. 2002), the present study is the first to show that PPARα might attenuate cholesterol-induced thrombotic effects by partial suppression of the increases in circulating coagulation factors and by antagonizing the decrease in levels of the anti-thrombotic sulfatides. Furthermore, the current study has also revealed that PPARδ and γ seem not to be involved in these protective effects, suggesting that PPARα is the predominant isoform responsible for protection against the deleterious effects of cholesterol overload in the liver.
The mechanism underlying the development of steatohepatitis and the higher levels of coagulation factors in Ppara-null mice
PPARα maintains TG and FA homeostasis, and exerts anti-inflammatory and anti-oxidative effects, by transcriptionally regulating FA-metabolizing enzymes, IκBα, catalase, and Cu/Zn-SOD (Aoyama et al. 1998; Kamijo et al. 2002, 2007; Hashimoto et al. 2012; Harada et al. 2016). It was reported that severe fatty liver is easily induced in Ppara-null mice by insults such as starvation and excess ethanol consumption through abnormal FA metabolism and/or oxidative stress (Lee et al. 1995; Nakajima et al. 2004; Okiyama et al. 2009; Kanbe et al. 2014). The HC diet may suppress de novo hepatic cholesterol synthesis and increase a key molecule for FA/TG synthesis, acetyl-CoA, resulting in the intrahepatic accumulation of TG. Moreover, the HC diet induces oxidative stress. Thus, the steatohepatitis induced in the HC diet-fed Ppara-null mice might be the result of their abnormal FA/TG metabolic ability and the decreases of anti-oxidative and anti-inflammatory effects, as well as noted with other cases of steatohepatitis (Matsuzawa et al. 2007; Komatsu et al. 2015; Tanaka et al. 2017; Hu et al. 2017).
The inflammatory process can activate inflammatory cells in the liver, resulting in the production of large amounts of coagulation factors including TF, PAI-1, and CPB2, in the damaged cells. Furthermore, the expression of these coagulation factors are reported to be partially regulated by PPARα (Marx et al. 2001; Kimura et al. 2012; Masuda et al. 2012; Kanbe et al. 2014; Gonsalves et al. 2015). Therefore, the levels of these coagulation factors would increase in the liver of the HC diet-fed Ppara-null mice. In addition to these intrahepatic changes, feeding with the HC diet might lead to higher levels of circulating coagulation factors because of damage to vascular endothelial cells through the increased inflammation. In addition, PPARα is expressed in vascular endothelial cells of the systemic circulation (Lefebvre et al. 2006) and can suppress the release of coagulation factors from these cells, which might contribute to the enhanced levels of circulating coagulation factors in the Ppara-null mice.
Lower liver and serum levels of sulfatides in the HC diet-fed Ppara-null mice
The current study is the first to report that the HC diet greatly reduces serum levels of sulfatides by suppressing expression of the rate-limiting enzyme for sulfatide synthesis, CST, and that this effect is enhanced under PPARα deficiency. It was reported that oxidative stress decreases CST expression, and that PPARα regulates the CST expression at the transcriptional level (Kamijo et al. 2012; Kimura et al. 2012; Yuzhe et al. 2015). In Ppara-null mice, the increased HC diet-induced oxidative stress and the lower ability to maintain CST expression lead to markedly lower CST expression and consequent reduction in serum sulfatide levels. Moreover, this is the first study to show that the HC diet decreases the expression of an intracellular transporter of sulfatides, GLTP, which would further contribute to reduce serum sulfatides levels.
PPARα-specifìc function against cholesterol toxicity
In this study, the induction of TG accumulation, oxidative stress, and inflammation in the liver, as well as that of blood levels of coagulation factors, in mice fed with the HC diet, were more marked in Ppara-null mice than in WT individuals. Since the mRNA expression and functional activation of PPARα were not changed in the HC diet-fed WT mice, and those of PPARδ and γ were also unchanged in both the mouse strains, the presence of a certain level of PPARα seems to be important to suppress the toxicity by the HC diet described above.
Prior studies have reported that constitutive expression of PPARα is maintained by the presence of endogenous ligands such as FAs, which is important for the maintenance of basal FA metabolism in liver and heart (Aoyama et al. 1998; Watanabe et al. 2000). In the case of kidney dysfunction, PPARα expression becomes lower, which is insufficient to maintain its basic functions (Kamijo et al. 2007). As shown in this study, cholesterol toxicity is significantly enhanced in the absence of PPARα. Thus, maintenance of a certain level of PPARα and its function is important to protect against cholesterol toxicity.
Clinical applications of the present findings
High blood levels of TF and PAI-1 have been reported to indicate a high risk of CVD (Toschi et al. 1997; Steffel et al. 2006; Kohler and Grant 2000; Hamsten et al. 1987). The current study has shown for the first time that constitutive level of PPARα expression attenuates the pro-thrombotic changes induced by cholesterol overload. These data suggest that maintaining PPARα function using a specific agonist would ameliorate and prevent CVD. Indeed, the results of several large-scale randomized controlled trials using PPARα agonists, fibrates, have indicated the possibility that they could reduce the risk of CVD (Davis et al. 2011; Ansquer et al. 2005). In addition to its lipid-lowering effects, the beneficial preventive effect of PPARα agonism might be derived from the other effects described in the current study. Telmisartan and irbesartan, which are angiotensin II receptor blockers (ARBs), promote hepatic and renal PPARα expression (Harada et al. 2016; Clemenz et al. 2008), and many clinical studies using these ARBs have demonstrated protective effects against CVD (Yusuf et al. 2008; Brenner et al. 2001). This type of ARB may not only lower blood pressure or suppress the renin-angiotensin-aldosterone system but may also attenuate cholesterol toxicity by modulating PPARα function. The findings in this study suggest a novel molecular mechanism to explain the existing clinical evidence of CVD risk suppression by these agents.
Limitations of the study
We only examined TF, PAI-1, CPB2 and sulfatides in many types of pro- and anti-thrombotic factors and found the PPARα-specific regulation in these factors. Because we did not evaluate other factors or thrombotic activity directly, additional investigations will be necessary in the future. However, we believe that our findings, which demonstrate that cholesterol overload causes pro-thrombotic effects and that PPARα is important in preventing such effects should provoke further studies of PPARα agonism as a novel therapeutic/preventive strategy for CVD.
Supplementary Material
Acknowledgements
We thank Mark Cleasby, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding This study was supported by Grants-in-Aid for Scientific Research (KAKENHI) in Japan (nos. 25460329 and 18K08204).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00204-018-2335-4) contains supplementary material, which is available to authorized users.
References
- Abela GS, Picon PD, Friedl SE, Gebara OC, Miyamoto A, Federman M, Tofler GH, Muller JE (1995) Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation 91:776–784 [DOI] [PubMed] [Google Scholar]
- Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G, DAIS Investigators (2005) Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am J Kidney Dis 45:485–493 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV, Gonzalez FJ (1989) Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem 264:10388–10395 [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ (1998) Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem 273:5678–5684 [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E (2007) Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators (2001) Effects of losartan on renal and cardio-vascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345:861–869 [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF (2005) “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1:309–322 [DOI] [PubMed] [Google Scholar]
- Clemenz M, Frost N, Schupp M, Caron S, Foryst-Ludwig A, Böhm C, Hartge M, Gust R, Staels B, Unger T, Kintscher U (2008) Liver-specific peroxisome proliferator-activated receptor alpha target gene regulation by the angiotensin type 1 receptor blocker telmisartan. Diabetes 57:1405–1413 [DOI] [PubMed] [Google Scholar]
- Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O’Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC, Fenofibrate Intervention and Event Lowering in Diabetes Study investigators (2011) Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54:280–290 [DOI] [PubMed] [Google Scholar]
- Gonsalves CS, Li C, Malik P, Tahara SM, Kalra VK (2015) Peroxisome proliferator-activated receptor-α-mediated transcription of miR-301a and miR-454 and their host gene SKA2 regulates endothelin-1 and PAI-1 expression in sickle cell disease. Biosci Rep 35:e00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamsten A, de Faire U, Walldius G, Dahlén G, Szamosi A, Landou C, Blombäck M, Wiman B (1987) Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet 2:3–9 [DOI] [PubMed] [Google Scholar]
- Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90:420–426 [DOI] [PubMed] [Google Scholar]
- Harada M, Kamijo Y, Nakajima T, Hashimoto K, Yamada Y, Shimojo H, Gonzalez FJ, Aoyama T (2016) Peroxisome proliferator-activated receptor α-dependent renoprotection of murine kidney by irbesartan. Clin Sci (Lond) 130:1969–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kamijo Y, Nakajima T, Harada M, Higuchi M, Ehara T, Shigematsu H, Aoyama T (2012) PPARα activation protects against anti-Thy1 nephritis by suppressing glomerular NF-κB signaling. PPAR Res 2012:976089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Tanaka N, Guo R, Lu Y, Nakajima T, Gonzalez FJ, Aoyama T (2017) PPARα protects against trans-fatty-acid-containing diet-induced steatohepatitis. J Nutr Biochem 39:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gåfvels M, Einarsson C, Alexson SE (2000) The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem 275:28947–28953 [DOI] [PubMed] [Google Scholar]
- Ichino K, Okazaki M, Usami S, Oguchi K (1997) Involvement of enhanced coagulation and fibrinolysis system in induction of atherosclerosis in hyperlipidemic rabbits fed on a high cholesterol diet. In Vivo 11:115–123 [PubMed] [Google Scholar]
- Kamijo Y, Hora K, Tanaka N, Usuda N, Kiyosawa K, Nakajima T, Gonzalez FJ, Aoyama T (2002) Identification of functions of peroxisome proliferator-activated receptor alpha in proximal tubules. J Am Soc Nephrol 13:1691–1702 [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Hora K, Kono K, Takahashi K, Higuchi M, Ehara T, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T (2007) Pparalpha protects proximal tubular cells from acute fatty acid toxicity. J Am Soc Nephrol 18:3089–3100 [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Wang L, Matsumoto A, Nakajima T, Hashimoto K, Higuchi M, Kyogashima M, Aoyama T, Hara A (2012) Long-term improvement of oxidative stress via kidney transplantation ameliorates serum sulfatide levels. Clin Exp Nephrol 16:959–967 [DOI] [PubMed] [Google Scholar]
- Kanbe H, Kamijo Y, Nakajima T, Tanaka N, Sugiyama E, Wang L, Fang ZZ, Hara A, Gonzalez FJ, Aoyama T (2014) Chronic ethanol consumption decreases serum sulfatide levels by suppressing hepatic cerebroside sulfotransferase expression in mice. Arch Toxicol 88:367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Elsayed YA, Namoto M, Nakagawa K, Sueishi K (1996) Enhanced expression of tissue factor activity in the atherosclerotic aortas of cholesterol-fed rabbits. Thromb Res 82:335–347 [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405:421–424 [DOI] [PubMed] [Google Scholar]
- Kimura T, Nakajima T, Kamijo Y, Tanaka N, Wang L, Hara A, Sugiyama E, Tanaka E, Gonzalez FJ, Aoyama T (2012) Hepatic cerebroside sulfotransferase is induced by PPARα activation in mice. PPAR Res 2012:174932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler HP, Grant PJ (2000) Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med 342:1792–1801 [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kimura T, Yazaki M, Tanaka N, Yang Y, Nakajima T, Horiuchi A, Fang ZZ, Joshita S, Matsumoto A, Umemura T, Tanaka E, Gonzalez FJ, Ikeda S, Aoyama T (2015) Steatogenesis in adult-onset type II citrullinemia is associated with downregulation of PPARα. Biochim Biophys Acta 1852:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ (1995) Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15:3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Hu R, Kamijo Y, Nakajima T, Aoyama T, Inoue T, Node K, Kannagi R, Kyogashima M, Hara A (2007) Establishment of a quantitative, qualitative, and high-throughput analysis of sulfatides from small amounts of sera by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Anal Biochem 362:1–7 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Marx N, Mackman N, Schönbeck U, Yilmaz N, Hombach V, Libby P, Plutzky J (2001) Pparalpha activators inhibit tissue factor expression and activity in human monocytes. Circulation 103:213–219 [DOI] [PubMed] [Google Scholar]
- Masuda Y, Saotome D, Takada K, Sugimoto K, Sasaki T, Ishii H (2012) Peroxisome proliferator-activated receptor-alpha agonists repress expression of thrombin-activatable fibrinolysis inhibitor by decreasing transcript stability. Thromb Haemost 108:74–85 [DOI] [PubMed] [Google Scholar]
- Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, Miyamoto K, Kaneko S (2007) Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 46:1392–1403 [DOI] [PubMed] [Google Scholar]
- Mosnier LO, Bouma BN (2006) Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol 26:2445–2453 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, Fukushima Y, Peters JM, Gonzalez FJ, Aoyama T (2004) Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology 40:972–980 [DOI] [PubMed] [Google Scholar]
- Naziroglu M, Güler M, Özgül C, Saydam G, Küçükayaz M, Sözbir E (2014) Apple cider vinegar modulates serum lipid profile, erythrocyte, kidney, and liver membrane oxidative stress in ovariectomized mice fed high cholesterol. J Membr Biol 247:667–673 [DOI] [PubMed] [Google Scholar]
- Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T (2009) Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol 50:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Reddy JK, Müller M, Kersten S (2006) Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147:1508–1516 [DOI] [PubMed] [Google Scholar]
- Steffel J, Lüscher TF, Tanner FC (2006) Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation 113:722–731 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T (2008) Pparalpha activation is essential for HCV core proteininduced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest 118:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Sugimoto T, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T (2011) Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int 79:871–882 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Aoyama T, Kimura S, Gonzalez FJ (2017) Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther 179:142–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernández-Ortiz A, Chesebro JH, Badimon L, Nemerson Y, Fuster V, Badimon JJ (1997) Tissue factor modulates the thrombogenicity of human atherosclerotic plaques. Circulation 95:594–599 [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Espinosa A, González-Mañán D, D’Espessailles A, Fernández V, Videla LA, Tapia G (2012) N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS One 7:e46400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Fujii H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, Kamijo Y, Gonzalez FJ, Aoyama T (2000) Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem 275:22293–22299 [DOI] [PubMed] [Google Scholar]
- Wojewodzka-Zelezniakowicz M, Gromotowicz-Poplawska A, Kisiel W, Konarzewska E, Szemraj J, Ladny JR, Chabielska E (2017) Angiotensin-converting enzyme inhibitors attenuate propofol-induced pro-oxidative and antifibrinolytic effect in human endothelial cells. J Renin Angiotensin Aldosterone Syst 18:1470320316687197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW (2001) Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 50:411–417 [DOI] [PubMed] [Google Scholar]
- Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Telmisartan randomised assessment study in ACE intolerant subjects with cardiovascular disease (TRANSCEND) investigators (2008) Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 372:1174–1183 [DOI] [PubMed] [Google Scholar]
- Yuzhe H, Kamijo Y, Hashimoto K, Harada M, Kanno T, Sugiyama E, Kyogashima M, Oguchi T, Nakajima T, Kanno Y, Aoyama T (2015) Serum sulfatide abnormality is associated with increased oxidative stress in hemodialysis patients. Hemodial Int 19:429–438 [DOI] [PubMed] [Google Scholar]
- Zou X, Gao Y, Ruvolo VR, Gardner TL, Ruvolo PP, Brown RE (2011) Human glycolipid transfer protein gene (GLTP) expression is regulated by Sp1 and Sp3: involvement of the bioactive sphingolipid ceramide. J Biol Chem 286:1301–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, Videla LA, Fernández V (2011) N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS One 6:e28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.