Abstract
Purpose of Review:
We review recent evidence for circadian rhythm dysregulation in bipolar spectrum disorders (BSDs). We examine evidence for endogenous abnormalities in the biological clock and disruptions in the external entrainment of circadian rhythms in BSDs. We also address whether circadian dysregulation provides vulnerability to onset of BSD and evidence for a new integration of reward and circadian dysregulation in BSD.
Recent Findings:
Relative circadian phase delay (e.g., later melatonin peak, evening chronotype) is associated with BSD, particularly in the depressive phase. More consistent evidence supports irregularity of social rhythms, sleep/wake and activity patterns, and disruptions of social rhythms by life events, as stable trait markers of BSD and potential vulnerabilities for BSD onset. Growing research supports an integrative reward/circadian model.
Summary:
Both endogenous abnormalities in the biological clock pacemaking function and disruptions in the external entrainment of circadian rhythms by physical and social cues are involved in BSDs. Circadian dysregulation may provide vulnerability to BSD onset.
Keywords: bipolar spectrum disorders, circadian rhythms, chronotype, social rhythms, zeitgeber, melatonin
Introduction
Bipolar disorder involves dysregulation of mood and behavior, with extreme highs and lows in mood, motivation, cognition, and behavior occurring within the same individual. Within the bipolar category, a group of disorders form a spectrum of severity from the milder cyclothymic disorder (involving multiple periods of hypomania and depression that do not meet diagnostic criteria for manic or major depressive episodes), to bipolar II disorder (characterized by hypomanic and major depressive episodes), to bipolar I disorder (involving full-blown manic episodes) at the most severe end of the continuum [1,2]. Moreover, milder bipolar disorders often progress to more severe forms [1–4], supporting the spectrum concept. Approximately 4.4% of the US population exhibits a disorder in the bipolar spectrum [5] and these disorders are often associated with severe personal, social, and economic costs, including high rates of divorce, substance abuse, suicide, and academic and occupational impairment [6,7]. Given their prevalence and associated impairment, understanding the basis of bipolar spectrum disorders (BSDs) is of great public health importance.
Circadian rhythms, biological processes including sleep/wake, body temperature regulation, and neurotransmitter and hormone secretion that repeat cyclically, are regulated by a central pacemaker or biological clock located in the suprachiasmatic nucleus (SCN) within the anterior hypothalamus [8]. Although the SCN can function autonomously, normally, it is entrained by external cues or zeitgebers (German for “time givers”), yielding a period of slightly more than 24 hours in humans [9]. The most reliable and powerful zeitgeber is light, but non-photic cues such as auditory stimuli, exercise, and social rhythms or daily activity schedules also can entrain circadian rhythms [10]. Melatonin is considered to be the most accurate physiological indicator of circadian function [11].
Dysregulation of circadian rhythms has been hypothesized to be a central mechanism in the pathophysiology of BSDs [12–14]. Circadian rhythm dysregulation may arise either from stable, trait-like abnormalities of the SCN itself and its pacemaking control over bodily functions, or be the result of dysfunction in the entrainment of the SCN by external zeitgebers [10]. According to the social zeitgeber theory of BSDs [10,15], when individuals with or vulnerable to BSDs experience life events that disrupt their daily activity schedules or social rhythms (e.g., bedtime, wake time, mealtimes, start of work), these schedule changes disturb their circadian rhythms and precipitate somatic symptoms, eventually culminating in bipolar mood episodes. Indeed, naturally occurring or experimentally induced changes in daily activity schedules have been found to be associated with changes in circadian rhythms in healthy individuals [16]. Social rhythm disrupting (SRD) events (e.g., causing a change in bedtime) may disrupt circadian rhythms by influencing individuals’ exposure to light or via other nonphotic zeitgebers [17], which can phase shift melatonin and other circadian rhythms [9,16].
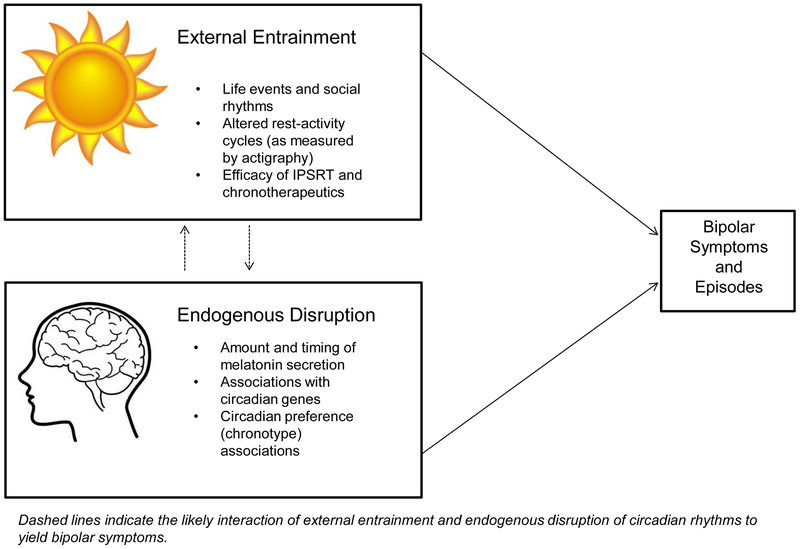
In this article, we review recent evidence for circadian rhythm dysregulation in BSDs. We organize our review in terms of evidence for potential stable abnormalities in the biological clock itself versus evidence supporting disruptions in the external entrainment of circadian rhythms in BSDs. However, throughout, we note that the circadian rhythm abnormalities observed among individuals with or vulnerable to BSDs are likely the result of both endogenous dysfunction within the circadian system and external perturbations of circadian rhythms (see Figure 1). In our view, if circadian dysregulation is a central mechanism in BSDs, it should be present all along the bipolar spectrum and precede onset of BSD. Thus, throughout our review, we also discuss whether the extant evidence is consistent with a role for circadian rhythm dysregulation in vulnerability to onset of BSDs. Finally, we end with a brief review of evidence for an exciting integration of circadian function and reward system hypersensitivity that may provide a more complete understanding of mechanisms involved in the onset and course of BSDs.
Figure 1.
Dual pathways to circadian dysregulation in symptoms of bipolar spectrum disorders.
Endogenous Abnormalities of the Biological Clock in BSDs
Melatonin studies.
Melatonin, a hormone produced by the pineal gland, plays an important role in the synchronization and regulation of circadian rhythms. There is mounting evidence that people with BSD exhibit melatonin secretion abnormalities. One line of evidence suggests that differences in the amount and timing of melatonin secretion may reflect different mood states of the disorder. For example, increased melatonin levels during the daytime and advanced night-time melatonin peak have been found in mania [18,19], whereas lower levels and later onset of melatonin secretion have been documented in bipolar depression compared with unipolar depression [20] and the euthymic state of BSDs compared with matched controls [21,22]. However, other studies suggest that melatonin secretion is consistently low across mood states of BSDs and may be a trait, rather than a state, marker of BSD [23]. Hypersensitivity to melatonin suppression by light has been found in people at risk for [24] or diagnosed with BSD [25,26], indicating that dysfunctional external entrainment of the SCN may be a risk factor for BSD. However, several studies obtained no evidence for such an effect in people with BSDs [22,27,28]. More research is needed to better understand whether at-risk individuals also exhibit alterations in the amount and timing of melatonin secretion.
Genetic studies.
Evidence supporting a genetic role in circadian rhythm dysregulation in BSD has emerged over the past few decades. The circadian locomotor output cycles kaput (CLOCK) gene [29,30] as well as GSK3-β [31], PER3 [32], ARNTL [32], TIMELESS [33], and more recently NR1D1 ROR [34] genes all have demonstrated modest associations with BSDs, supporting a polygenetic heritability through which multiple genes contribute to risk for BSD in an additive fashion [14].
There is some evidence that expression of circadian genes differs across phases of BSDs, which further implicates circadian dysregulation in the pathogenesis of the disorder. Nováková and colleagues [19] examined daily profiles of Per1 and NR1D1 clock genes in a sample of individuals with BSD in the manic phase, depressive phase and healthy controls. Relative to depressed individuals, gene profiles were advanced in mania, although this effect only reached trend level significance compared to controls. Additionally, the amplitude of the NR1D1 expression profile was higher in individuals in a manic phase relative to those in a depressive phase. This finding is consistent with prior evidence demonstrating that pharmacological treatments for BSD (e.g., lithium, valproaic acid, SSRIs) affect circadian rhythmicity [21].
As stated earlier, it is likely that circadian rhythm abnormalities in BSD occur as a result of both endogenous abnormalities and external disruption of entrainment. Although the genetic evidence presented thus far supports an endogenous pathway, other evidence suggests that circadian rhythm dysregulation may occur as the result of gene/environment interactions. A recent study by Benedetti and colleagues [35] suggests that the course of BSD, particularly suicidality, depends on both genetic vulnerability and exposure to stressful life events. Among a sample of depressed patients with BSD, those who carried the Rs1801260*C CLOCK gene variant reported worse Hamilton Depression Rating Scale scores for suicidality relative to non-carriers, and the likelihood of attempting suicide was highest among *C carriers who experienced greater numbers of stressful life events in early life relative to those *C carriers who did not.
Chronotype studies.
Chronotype is a dimension of between-person variation in circadian rhythmicity, representing an individual’s self-reported, trait-like preference for activity/wakefulness in the morning versus evening. An individual with ‘evening chronotype’ endorses preference for later wake-times and later bedtimes, coupled with greater alertness/activity during the evening hours compared to the morning hours. Conversely, individuals endorsing ‘morning chronotype’ report high levels of alertness and strong mental performance during the earlier part of the day, in addition to preference for going to bed early and waking up early. Approximately 40% of the population is classified as either the morning or evening chronotype, whereas 60% are classified as neither chronotype (i.e., endorsing a mixture of morning and evening chronotype traits) [37]. Evening chronotype (e.g., eveningness) has been linked to physiological measures of circadian phase delay [36], including later circadian temperature phase and later melatonin onset time as compared to morning chronotype [37]. Eveningness has been consistently linked to mood disorders [37], including BSDs (see 38 for comprehensive review).
Several recent studies compared levels of eveningness in BSD individuals to healthy controls (HCs) and found that BSD individuals exhibit greater eveningness [39–44]; however, in contrast, another study found no difference in eveningness between BSD and HC groups [45]. Several studies have compared eveningness in BSDs to various psychiatric control groups. One study found no difference in morningness-eveningness score between BSD and MDD patients [46]. In a study comparing eveningness in patients with bipolar I disorder and schizophrenia, bipolar I patients exhibited more eveningness than patients with schizophrenia [47]. However, Ahn and colleagues [39] did not find a chronotype difference between BSD patients and schizophrenia patients.
The relationship between chronotype and the course of BSDs also has been examined. Several studies explored the association between chronotype and mood state in BSDs; Wood and colleagues [44] found an association between higher depressive symptoms and eveningness in BSDs. Another study found that, specifically in patients with rapid-cycling BSD, those in a depressive episode showed phase delay of morning activities compared to those in euthymic or manic states [48]. However, two other studies suggested no difference between eveningness scores and mood state in BSD [44,49], suggesting that chronotype is stable across mood state and may be a trait marker of the disorder. Several studies also compared eveningness in bipolar I versus bipolar II, and one found no difference [44], whereas two studies found that bipolar II patients had higher eveningness scores than bipolar I patients [40,46]. Functional impairment and outcome also have been studied in relation to eveningness in BSD samples; eveningness was related to poor sleep quality, more unhealthy dietary habits, and more impaired interpersonal relationships [50,51].
Few studies to date have examined chronotype as a vulnerability for developing BSDs, but several genetic studies link the eveningness trait to BSDs [52–54], suggesting that eveningness may be a pre-existing risk factor for developing BSDs rather than a mere consequence of the illness. Bullock and colleagues [55] found that eveningness was a weak, but significant, predictor of the bipolar vulnerability trait. Another study compared healthy controls to a mixed psychiatric “high-risk group” composed of individuals at risk for developing psychosis or bipolar disorder [56], and no group differences were identified. Although the extant research appears to suggest a link between BSDs and evening chronotype, particularly the depressive aspects of BSDs, future research should seek to clarify whether eveningness is a risk factor or consequence of BSDs by employing high-risk and prospective study designs.
Disruptions in External Entrainment of Circadian Rhythms in BSDs
Social rhythm and social rhythm disrupting event studies.
As noted above, social rhythms or daily activity schedules help to entrain circadian rhythms; thus, irregular social rhythms or social rhythm disrupting (SRD) life events should contribute to circadian rhythm dysregulation. Social rhythm regularity has been assessed with the self-report Social Rhythm Metric (SRM) [57] and the interviewer administered Biological Rhythms Interview of Assessment in Neuropsychiatry (BRIAN) [58]. Several studies have found that individuals all along the bipolar spectrum exhibit greater social rhythm irregularity than healthy controls as assessed by the SRM [59,60] or the BRIAN [61,62], even in a euthymic state [60,61]. Social rhythm irregularity also was associated with the severity of depressive symptoms and degree of functional impairment in BSD individuals [61,62] and SRM-assessed social rhythm irregularity in BSD individuals was found to be relatively stable over one year of follow-up [63]. Moreover, in longitudinal studies, controlling for baseline hypomanic and depressive symptoms and family history of BSD, lower levels of social rhythm regularity on the SRM at baseline predicted increases in depressive symptoms over four-month follow-up [63] and both a greater likelihood and shorter time to recurrence of major depressive and hypomanic or manic episodes over three-year follow-up [60].
As predicted by the social zeitgeber model, life events rated by interviewers as causing disruption of social rhythms (e.g., by changing bedtime or wake time) have been found to predict onsets of mood episodes in longitudinal studies of individuals with BSDs. Malkoff-Schwartz and colleagues [64,65] found that bipolar I patients experienced more SRD events in the 8- and 20-week periods prior to onset of manic, but not depressive, episodes than during a matched control period not preceding an episode onset. In a more recent study, Sylvia et al. [63] reported that bipolar II or cyclothymic participants experienced more SRD events in the 8-week periods prior to onsets of depressive, but not hypomanic, episodes than in matched 8-week control periods. In addition, in between-subjects analyses, BSD participants with a depressive episode onset experienced more SRD events in the 8 weeks before the episode than did demographically-matched BSD participants with no episode onset during the same 8-week period [63]. There was no effect for hypomanic episodes. Moreover, individuals with BSDs may exhibit hypersensitivity to life event-induced social rhythm disruption. Compared to healthy controls matched on age, sex, and race, individuals with BSDs exhibited significantly more interviewer-rated SRD and sleep loss following the occurrence of identical intensity (high or low) and valence (positive or negative) life events, controlling for trait social rhythm regularity on the SRM [66].
Studies of samples with no history of BSD, but at risk for developing BSDs, suggest that social rhythm irregularity may provide vulnerability to BSDs. For example, individuals with high General Behavior Inventory (GBI) [67] scores, a demonstrated measure of risk for BSD [68], exhibited less SRM social rhythm regularity [69,70] than those with low GBI scores, and low social rhythm regularity was associated with greater across-day mood symptom lability and higher depressive symptoms over a 2-week follow-up [70]. Similarly, Meyer and Maier [71] found that participants at risk for BSDs based on exhibiting hypomanic personality reported less regularity of their daily activities in a short-term diary study than did healthy controls or participants at risk for unipolar depression. The strongest evidence that social rhythm irregularity provides vulnerability to BSDs comes from a truly prospective study [72] in which low social rhythm regularity at baseline predicted first lifetime onset of a BSD over 3 years of follow-up among adolescents with no prior BSD, but previously shown to be at risk for developing a first onset of BSD [73]. Previously, Alloy et al. [73] demonstrated that adolescents with high self-reported and behavioral reward sensitivity were three times more likely to develop a first onset of BSD compared to adolescents with moderate reward sensitivity. Among these at-risk highly reward sensitive adolescents, those with low social rhythm regularity were even more likely to develop a first onset of BSD than those with high social rhythm regularity [72]. Further, Boland et al. [74] found that controlling for baseline mood symptoms, adolescents at risk for BSD based on exhibiting reward hypersensitivity experienced more SRD events over prospective follow-up than did adolescents at low risk for BSD, which, in turn, could make them vulnerable to experiencing bipolar mood episode onset. However, in contrast, Jones and colleagues [75] did not obtain evidence of social rhythm regularity differences between the offspring of parents with bipolar disorder and the age- and sex-matched offspring of controls. Moreover, a limitation of the social rhythm studies is that they did not directly examine whether the social rhythm irregularity or event-induced SRD actually led to circadian rhythm disruption.
Actigraphy studies.
Over the past decade, a growing number of studies have used actigraphy to investigate circadian and sleep-wake rhythm disruption in BSD, leading to the publication of several meta-analyses synthesizing their findings [76–78]. Taken as a whole, they report that individuals with BSDs have lower activity levels and earlier circadian acrophase (time of the peak of the fitted circadian rhythm). These individuals also sleep longer, take longer to fall asleep, spend more time in bed and awake in the middle of night, and show a more variable pattern of sleep-wake cycles. Data from several studies suggest that individuals with BSDs exhibit greater intra-daily variability and lower inter-daily stability and relative amplitude of the rest-activity cycle relative to controls [59,79,80]. In addition, even individuals at risk for BSD exhibit lower relative amplitude of the rest-activity cycle and more sleep irregularity compared with controls [78,81,82]. Notably, variability in the sleep-wake cycle has been shown to predict the onset of depressive episodes among people with interepisode bipolar disorder [83].
Circadian rhythm entrainment therapies.
As posited in the social zeitgeber model of BSD, social rhythms are thought to entrain circadian rhythms, the stabilization of which is thought to improve affective symptoms and episodes. This theory forms the foundation of Interpersonal and Social Rhythm Therapy (IPSRT) [84], a psychosocial intervention for BSD that combines aspects of Interpersonal Psychotherapy with a social rhythm stabilization protocol that aims to stabilize daily routines, and thus, regularize circadian rhythms. The efficacy of IPSRT has been supported by RCTs that show significant improvement in mood [85,86] as well as longer time to relapse compared to medication management alone [85]. Other therapies aim to stabilize circadian rhythms via external manipulations of circadian cues (i.e., chronotherapeutics). These therapies primarily involve timed exposure to bright light, as well as manipulations of the sleep/wake cycle (e.g., total and/or partial sleep deprivation conducted either in a single night or over a course of administrations). Such therapies have shown to be effective in bipolar samples, with the addition of bright light therapy often sustaining the rapid antidepressant effect of sleep deprivation [87–89]. Some evidence suggests this effect is moderated by differences in objective versus self-reported severity of depression symptoms, however. Suzuki et al. [90] found that chronotherapeutics were less effective among individuals who demonstrated high discrepancy between clinician-assessed and self-reports of depression, suggesting that such baseline screening methodology may be an efficient and cost-effective way to improve treatment-matching practices. Crowe and colleagues [91] authored a comprehensive review of social rhythm interventions for BSD, and concluded that both IPSRT and sleep/light interventions are helpful in improving mood, although questions remain about whether IPSRT is superior to intensive supportive care, or if sleep/light interventions offer sustained improvement.
Integration of Reward and Circadian Systems in BSDs
An exciting development in circadian rhythm dysregulation research in BSDs is theoretical models and accompanying studies that integrate reward processing and circadian function [14,92–94]. This direction is significant because much evidence now supports hypersensitivity of the reward system as a vulnerability to BSDs, and increases and decreases in reward system activation as a central mechanism in mood episode onsets [73,92,95–98]. Growing evidence suggests that the SCN and dopamine-mediated corticostriatal neural circuitry involved in reward processing have multiple interactions and bidirectional associations with each other [14,99,100]. Indeed, clock genes are heavily expressed in many brain reward areas, including the ventral tegmental area, nucleus accumbens, and amygdala [101] and are associated with reward-related neural activity [102]. Thus, Alloy and colleagues [92] proposed an integrated Reward and Circadian Rhythm (RCR) dysregulation model to explain the symptoms, onset, and course of BSDs.
According to models of circadian-reward system integration [14,92,93,103], activation of the reward system is influenced by timing information from the SCN, such that appetitive motivation and reward sensitivity exhibit a circadian rhythm. Consequently, circadian rhythm disruption should also affect patterns of reward system activation, and thus, potentially bipolar symptoms [92]. Consistent with this hypothesis, circadian rhythm effects on reward motivation have been demonstrated in animal studies [104; see 103 for review]. Additionally, human studies have observed circadian rhythms in self-reported positive affect and subjective alertness (indicators of reward system activation) and performance on a reward task [103,105–107], with reward activation greatest in the afternoon. Similarly, in a pilot fMRI study of healthy young adults, Hasler, Forbes, and Franzen [108] found time of day (circadian) differences in the neural processing of monetary rewards, such that striatal responses to reward also were greater in the afternoon than morning. In addition, relative to morning chronotypes, adolescents with evening chronotype (suggesting relative circadian phase delay) exhibited less medial prefrontal cortex (mPFC) reactivity during reward anticipation and more striatal reactivity during reward outcomes on the fMRI monetary reward paradigm [109]. Moreover, among healthy adolescents, shifts in sleep timing during the weekends relative to weekdays (suggesting disruptions compared to the weekday rhythms) were associated with reduced mPFC and striatal reactivity to rewards in the fMRI monetary reward task [110].
Alloy and colleagues’ RCR model [92] is unique in also elucidating how reward hypersensitivity and reward-relevant life events may affect social and circadian rhythm disruption, and thus, bipolar symptoms. In individuals who are hypersensitive to rewards, reward system-activating (e.g., a job promotion) and –deactivating (e.g., loss of a loved one) events will lead to excessive reward system activation and deactivation, respectively (see 92 for a review of evidence), which, in turn, will disrupt social rhythms, and thus, circadian rhythms, and lead to hypomanic/manic or depressive symptoms, respectively. For example, upon encountering approach-activation events (e.g., a potential job promotion), a reward hypersensitive individual should experience excessive reward activation and exhibit excessively high goal striving, approach motivation, and response initiation [95,97,111], behaviors incongruent with maintaining regular social rhythms. Thus, he or she may work excessively long hours, neglect normal daily routines (e.g., bedtime, mealtimes), and consequently, disrupt their circadian rhythms, leading to hypomanic/manic symptoms. Similarly, excessive reward deactivation brought about by approach-deactivating events (e.g., irreversible job loss) also may lead to ignoring social routines and ultimately, depressive symptoms.
Consistent with the RCR model, Alloy et al. [72] found that reward hypersensitivity and social rhythm irregularity combined to predict first onset of BSDs in adolescents with no prior history of BSD followed for three years. In addition, Boland et al. [74] reported that high reward-sensitive adolescents experienced significantly more interviewer-rated SRD following the occurrence of approach-activating and –deactivating life events than did moderately reward-sensitive adolescents, as the RCR model predicts. Moreover, these approach-activating and -deactivating events predicted prospective increases in hypomanic and depressive symptoms, respectively, mediated by the increases in SRD, with these effects stronger for high than moderately trait reward-sensitive adolescents [74]. Thus, the evolving research on circadian-reward system interactions shows considerable promise for further elucidating the mechanisms involved in BSDs.
Conclusions
Considerable support has accumulated for the role of circadian dysregulation in BSDs; however, many gaps in the evidence base remain to be addressed. The extant literature suggests that both endogenous abnormalities in the SCN pacemaking function and disruptions in the external entrainment of circadian rhythms by light and social zeitgebers are involved in BSDs. Most of the existing research only has demonstrated that circadian dysfunction is associated with BSD. Much less evidence exists that directly tests whether circadian dysregulation provides vulnerability for the initial development of BSD or is a causal factor in the course of BSD.
Regarding endogenous circadian abnormalities, the evidence is mixed with respect to whether such dysregulation is a trait marker of BSD or mood state dependent. Some studies of melatonin, chronotype, and expression of circadian genes find differences in depressive versus manic phases of BSDs, suggesting relative circadian phase delay in depressive phases and phase advance in manic states. Alternatively, other studies report consistent abnormalities across mood state. Of these, most indicate relative circadian phase delay (e.g., later melatonin peak, evening chronotype) associated with BSD. Many fewer studies have examined endogenous circadian dysfunction in at-risk individuals, but those that do are mildly suggestive that evening chronotype and hypersensitivity to suppression of melatonin by light may provide vulnerability to BSD. No studies to date have provided a strong test of the vulnerability hypothesis by examining endogenous circadian abnormalities as prospective predictors of first onset of BSD.
More consistent evidence indicates that disturbances in the external entrainment of circadian rhythms may be a mood state independent trait marker of BSDs, as well as a predictor of first onset and course of BSD. Irregularity of social rhythms/daily schedules and of sleep/wake and activity patterns, as well as disruptions of social rhythms by life events have been observed in the euthymic state and show some stability over time and across illness phases. Moreover, longitudinal studies indicate that social rhythm irregularity and SRD events predict subsequent mood episodes and a truly prospective study demonstrates that trait social rhythm irregularity predicts first onset of BSD in at-risk adolescents. Further, interventions designed to regularize external entrainment of circadian rhythms by social rhythms or light show some effectiveness in improving mood symptoms and functioning. However, a limitation of these studies is that they have not directly assessed the effects of social rhythm disruptions on circadian disruption.
Finally, growing evidence supports the bidirectional influence of the circadian and reward systems on each other. Reward system activation is influenced by circadian rhythms and, in turn, activation of the reward system by reward-relevant events leads to mood symptoms through their effect on social rhythm disruption. Further development of an integrative reward-circadian model of BSDs provides promise for a more complete understanding of the pathophysiology of BSDs and suggests exciting directions for new integrative studies. Ultimately, an integrated reward and circadian model should provide improved targets for intervention.
Acknowledgements:
Preparation of this article was supported by National Institute of Mental Health Grants MH77908 and MH102310 to Lauren B. Alloy. Tommy H. Ng was supported by the Sir Edward Youde Memorial Fellowship for Overseas Studies. Elaine M. Boland was supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Contributor Information
Lauren B. Alloy, Temple University, Philadelphia, PA
Tommy H. Ng, Temple University, Philadelphia, PA
Madison K. Titone, Temple University, Philadelphia, PA
Elaine M. Boland, Corporal Michael J. Crescenz Veterans Affairs Medical Center and University of Pennsylvania School of Medicine, Philadelphia, PA
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, et al. Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J. Abnorm. Psychol 2012b;121:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The course and outcome of bipolar youth (COBY) study. Am. J. Psychiatry 2009;166:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiskal HS, Djenderedjian AH, Rosenthal RH, Khani MK. Cyclothymic disorder: Validating criteria for inclusion in the bipolar affective group. Am J Psychiatry. 1977;134:1227–33. [DOI] [PubMed] [Google Scholar]
- 4.Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, Akiskal HS. Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. J. Affec. Disord 2005;85:181–9. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2007;64:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boland EM, Alloy LB. Sleep disturbance and cognitive deficits in bipolar disorder: Toward an integrated examination of disorder maintenance and functional impairment. Clin. Psychol. Rev 2013;33:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller S, Dell’Osso B, Ketter TA. The prevalence and burden of bipolar depression. J. Affec. Disord 2014;169:S3–11. [DOI] [PubMed] [Google Scholar]
- 8.Reppert SM, Wever DR. Molecular analysis of mammalian circadian rhythms. Ann. Rev. Physiol 2001;63:647–76. [DOI] [PubMed] [Google Scholar]
- 9.Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harbor Symp. Quant. Biol 2007;72:293–9. [DOI] [PubMed] [Google Scholar]
- 10.Grandin LD, Alloy LB, Abramson LY. The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clin. Psychol. Rev 2006;26:679–94. [DOI] [PubMed] [Google Scholar]
- 11.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int 1989;6:93–102. [DOI] [PubMed] [Google Scholar]
- 12.Abreu T, Braganca M. The bipolarity of light and dark: A review on bipolar disorder and circadian cycles. J. Affec. Disord 2015;185: 219–29. [DOI] [PubMed] [Google Scholar]
- 13.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol. & Ther 2007;114:222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**.Murray G, Harvey A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010;12:459–72. [DOI] [PubMed] [Google Scholar]; This theoretical review of biological rhythm pathways to bipolar disorder emphasizes the importance of person-environment feedback loops to circadian adaptation.
- 15.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Arch. Gen. Psychiatry 1988;45:948–52. [DOI] [PubMed] [Google Scholar]
- 16.Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinol. 2004;29:1250–59. [DOI] [PubMed] [Google Scholar]
- 17.Goel N Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am. J. Physiol. – Reg. Integrat. Compar. Physiol 2005;289:R209–16. [DOI] [PubMed] [Google Scholar]
- 18.Lewy AJ. Circadian misalignment in mood disturbances. Curr. Psychiat. Rep 2009;11:459–65. [DOI] [PubMed] [Google Scholar]
- 19.**.Nováková M, Praško J, Látalová K, Sládek M, Sumová A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. 2015;17:303–14. [DOI] [PubMed] [Google Scholar]; This recent study demonstrates differences in the circadian system in different mood states of bipolar disorder.
- 20.Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TKC, Hermens DF, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: Comparison of unipolar and bipolar phenotypes. Eur. Psychiat 2013;28:412–6. [DOI] [PubMed] [Google Scholar]
- 21.*.Dallaspezia S, Benedetti F. Melatonin, circadian rhythms, and the clock genes in bipolar disorder. Curr. Psychiatry Rep. 2009;11:488–93. [DOI] [PubMed] [Google Scholar]; This article reviews circadian rhythm disruption and chronotherapeutic strategies in bipolar disorder.
- 22.Nurnberger JI Jr, Adkins S, Lahiri DK, Mayeda A, Hu K, Lewy A, et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch. Gen. Psychiat 2000;57:572–9. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy SH, Kutcher SP, Ralevski E, Brown GM. Nocturnal melatonin and 24-hour 6-sulphatoxymelatonin levels in various phases of bipolar affective disorder. Psychiat. Res 1996;63:219–22. [DOI] [PubMed] [Google Scholar]
- 24.Nurnberger JI Jr, Berrettini W, Tamarkin L, Hamovit J, Norton J, Gershon E. Supersensitivity to melatonin suppression by light in young people at high risk for affective disorder. A preliminary report. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 1988;1:217–23. [DOI] [PubMed] [Google Scholar]
- 25.Lewy AJ, Nurnberger JI Jr, Wehr TA, Pack D, Becker LE, Powell RL, et al. Supersensitivity to light: possible trait marker for manic-depressive illness. Am. J. Psychiat 1985;142:725–7. [DOI] [PubMed] [Google Scholar]
- 26.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Rosenthal NE. Manic-depressive patients may be supersensitive to light. Lancet Lond. Engl 1981;1:383–4. [DOI] [PubMed] [Google Scholar]
- 27.Lam RW, Berkowitz AL, Berga SL, Clark CM, Kripke DF, Gillin JC. Melatonin suppression in bipolar and unipolar mood disorders. Psychiat. Res 1990;33:129–34. [DOI] [PubMed] [Google Scholar]
- 28.Whalley LJ, Perini T, Shering A, Bennie J. Melatonin response to bright light in recovered, drug-free, bipolar patients. Psychiat. Res 1991;38:13–9. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL,et al. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am. J. Med. Genet 2008;147B:1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J. Circad. Rhythms 2009;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am. J. Med. Genet 2003;123B:23–6. [DOI] [PubMed] [Google Scholar]
- 32.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am. J. Med. Genet 2006;141B:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansour HA, Monk TH, & Nimgaonkar VL. Circadian genes and bipolar disorder. Ann. Med 2005;37:196–205. [DOI] [PubMed] [Google Scholar]
- 34.Lai YC, Kao CF, Lu ML, Chen HC, Chen PY, Chen CH et al. Investigation of associations between NR1D1, RORA and RORB genes and bipolar disorder. PloS One, 2015;10(3):e0121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*.Benedetti F, Riccaboni R, Dallaspezia S, Locatelli C, Smeraldi E, Colombo C. Effects of CLOCK gene variants and early stress on hopelessness and suicide in bipolar depression. Chronobiol. Int 2015;32(8):1156–61. [DOI] [PubMed] [Google Scholar]; This study nicely illustrates the interaction between genetic vulnerability and environmental stress on the course of BSDs.
- 36.Bernert RA, Hasler BP, Cromer KR, Joiner TE. Diurnal preferences and circadian phase: A meta-analysis. Sleep 2006;29:A54–A55. [Google Scholar]
- 37.*.Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: A comprehensive review. Chronobiol. Internat 2012;29(9):1153–75. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review of the psychological correlates of chronotype.
- 38.*.Melo MCA, Abreu RLC, Linhares Neto VB., de Bruin PFC, de Bruin VMS. Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Med. Rev 2016; 10.1016/j.smrv.2016.06.007. [DOI] [PubMed] [Google Scholar]; This is the first comprehensive and systematic review of the role of morningness-eveningness in bipolar disorder.
- 39.Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008;10:271–5. [DOI] [PubMed] [Google Scholar]
- 40.Baek JH, Kim JS, Kim MJ, Ryu S, Lee K, Ha K, et al. Lifetime characteristics of evening-preference and irregular bed-rise time are associated with lifetime seasonal variation of mood and behavior: comparison between individuals with bipolar disorder and healthy controls. Behav. Sleep Med 2016;14(2):155–68. [DOI] [PubMed] [Google Scholar]
- 41.Boudebesse C, Lajnef M, Geoffroy PA, Bellivier I, Nieto S, Gard E, et al. Chronotypes of bipolar patients in remission: Validation of the French version of the Circadian Type Inventory in the FACE-BD sample. Chronobiol. Internat 2013;30:1042–49. [DOI] [PubMed] [Google Scholar]
- 42.Giglio LMF, Magalhaes PVS, Andersen ML, Walz JC, Jakobson, Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010;14:153–5. [DOI] [PubMed] [Google Scholar]
- 43.Kim KL, Weissman AB, Puzia ME, Cushman GK, Seymour KE, Wegbreit E, et al. Circadian phase preference in pediatric bipolar disorder. J Clin. Med. Res 2014;3(1):255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.*.Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiat. Res 2009;166:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This longitudinal study examines the relationship between eveningness and bipolar disorder using a large, well-defined sample with systemically recruited controls and evaluation of potential confounding factors.
- 45.Saunders EF, Novick DM, Fernandez-Mendoza J, Kamali M, Ryan KA, Langenecker SA, et al. Sleep quality during euthymia in bipolar disorder: the role of clinical features, personality traits, and stressful life events. Int. J. Bipolar Disord 2013;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung JK, Lee KY, Kim SH, Kim E, Jeong SH, et al. Circadian rhythm characteristics in mood disorders: Comparison among bipolar I disorder, bipolar II disorder, and recurrent major depressive disorder. Clin. Psychopharm. Neurosci 2012;10:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, et al. Circadian phase variation in bipolar I disorder. Chronobiol. Int 2005;22:571–84. [DOI] [PubMed] [Google Scholar]
- 48.Ashman SB, Monk TH, Kupfer JD, Clark CH, Myers FS, Frank E, et al. Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiat. Res 1999;86(1):1–8. [DOI] [PubMed] [Google Scholar]
- 49.Seleem MA, Merranko JA, Goldstein TR, Goldstein BI, Axelson DA, Brent DA, et al. The longitudinal course of sleep timing and circadian preferences in adults with bipolar disorder. Bipolar Disord. 2015;17:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brambilla C, Gavinelli C, Delmonte D, Fulgosi MC, Barbini B, Colombo C, et al. Seasonality and sleep: a clinical study on euthymic mood disorder patients. Depression Res. Treat 2012; 10.1155/2012/978962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng TH, Chung KF, Lee CT, Yeung WF, Ho FY. Eveningness and its associated impairments in remitted bipolar disorder. Behav. Sleep Med 2016;14(6):650–64. [DOI] [PubMed] [Google Scholar]
- 52.Etain B, Jamain S, Milhiet V, Lajnef M, Boudebesse C, Dumaine A, et al. Association between circadian genes, bipolar disorders and chronotypes. Chronobiol. Internat 2014;31(7):807–14. [DOI] [PubMed] [Google Scholar]
- 53.Kripke DF, Klimecki WT, Nievergelt CM, Rex KM, Murray SS, Shekhtman T, et al. Circadian polymorphisms in night owls, in bipolars, and in non-24-hour sleep cycles. Psychiat. Investig 2014;11:345–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee KY, Song JY, Kim SH, Kim SC, Joo EJ, Ahn YM, et al. Association between CLOCK 3111T/C and preferred circadian phase in Korean patients with bipolar disorder. Prog Neuropsychopharmacol. Biol Psychiat 2010;4:1196–1201. [DOI] [PubMed] [Google Scholar]
- 55.Bullock B, Corlass-Brown J, Murray G. Eveningness and seasonality are associated with the bipolar disorder vulnerability trait. J. Psychopathol. & Behav. Assess 2014;36:443–51. [Google Scholar]
- 56.Zanini MA, Castro J, Cunha GR, Asevedo E, Pan PM, Bittencourt L, et al. Abnormalities in sleep patterns in individuals at risk for psychosis and bipolar disorder. Schiz. Res 2015;169(3):262–7. [DOI] [PubMed] [Google Scholar]
- 57.Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ. The Social Rhythm Metric: An instrument to quantify the daily rhythms of life. J. Nerv. Ment. Dis 1990;178:120–6. [DOI] [PubMed] [Google Scholar]
- 58.Giglio LMF, Magalhaes PV, Andreazza AC, Walz JC, Jakobson L, et al. Development and use of a biological rhythm interview. J. Affec. Disord 2009;118:161–5. [DOI] [PubMed] [Google Scholar]
- 59.Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:1–11. [DOI] [PubMed] [Google Scholar]
- 60.*.Shen GC, Alloy LB, Abramson LY, Sylvia LG. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar Disord. 2008a;10:520–29. [DOI] [PMC free article] [PubMed] [Google Scholar]; This longitudinal study demonstrates the social rhythm irregularity prospectively predicts recurrences of major depressive and hypomanic/manic episodes in individuals with BSD.
- 61.Pinho M, Sehmbi M, Cudney E, Kauer-Sant’anna M, Magalhaes PV, et al. The association between biological rhythms, depression, and functioning in bipolar disorder: A large multi-center study. Acta Psychiat. Scand 2015;133:102–8. [DOI] [PubMed] [Google Scholar]
- 62.Rosa AR, Comes M, Torrent C, Sole B, Reinares M, et al. Biological rhythm disturbance in remitted bipolar patients. Internat. J. Bipolar Disord 2013;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sylvia LG, Alloy LB, Hafner JA, Gauger MC, Verdon K, Abramson LY. Life events and social rhythms in bipolar spectrum disroders: A prospective study. Behav. Ther 2009;40:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol. Med 2000;30:1005–10. [DOI] [PubMed] [Google Scholar]
- 65.Malkoff-Schwartz S, Frank E. Anderson BP, Sherrill JT, Siegel L, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: A preliminary investigation. Arch. Gen. Psychiatry 1998;55:702–7. [DOI] [PubMed] [Google Scholar]
- 66.*.Boland EM, Bender RE, Alloy LB, Conner BT, LaBelle, DR,Abramson LY. Life events and social rhythms in bipolar spectrum disorders: An examination of social rhythm sensitivity. J. Affec. Disord 2012;139:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study finds that individuals with BSDs are more sensitive to social rhythm disruption than matched controls following similar magnitude and valence life events.
- 67.Depue RA, Krauss S, Spoont MR, Arbisi P. General Behavior Inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. J. Abnorm. Psychol 1989;98:117–26. [DOI] [PubMed] [Google Scholar]
- 68.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, et al. Behavioral approach system and behavioral inhibition system sensitivities: Prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–22. [DOI] [PubMed] [Google Scholar]
- 69.Bullock B, Judd F, Murray G. Social rhythms and vulnerability to bipolar disorder. J. Affec. Disord 2011;135:384–8. [DOI] [PubMed] [Google Scholar]
- 70.Shen GC, Sylvia LG, Alloy LB, Barrett F, Kohner M, et al. Lifestyle regularity and cyclothymic symptomatology. J. Clin. Psychol 2008b;64:482–500. [DOI] [PubMed] [Google Scholar]
- 71.Meyer TD, Maier S. Is there evidence for social rhythm instability in people at risk for affective disorders? Psychiatr. Res 2006;141:103–14. [DOI] [PubMed] [Google Scholar]
- 72.**.Alloy LB, Boland EM, Ng T, Whitehouse WG, Abramson LY. Low social rhythm regularity predicts first onset of bipolar spectrum disorders among at risk individuals with reward hypersensitivity. J. Abnorm. Psychol 2015a;124:944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first truly prospective study to demonstrate that social rhythm irregularity predicts first onset of BSD in at-risk adolescents with reward hypersensitivity.
- 73.Alloy LB, Bender RE, Whitehouse WG. Wagner CA, Liu RT, et al. High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. J. Abnorm. Psychol 2012a;121:399–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.**.Boland EM, Stange JS, LaBelle DR, Shapero BG, Weiss RB, et al. Affective disruption from social rhythm and behavioral approach system (BAS) sensitivities: A test of the integration of the social zeitgeber and reward theories of bipolar disorder. Clin. Psychol. Sci 2016;4 418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to demonstrate that social rhythm disruption mediates the effects of reward-relevant life events in predicting prospective bipolar mood symptoms in individuals with BSDs.
- 75.Jones SH, Tai S, Evershed K, Knowles R, Bentall R. Early detection of bipolar disorder: A pilot familial high-risk study of parents with bipolar disorder and their adolescent children. Bipolar Disord. 2006;8:362–72. [DOI] [PubMed] [Google Scholar]
- 76.*.De Crescenzo F, Economou A, Sharpley AL, Gormez A, Quested DJ. Actigraphic features of bipolar disorder: A systematic review and meta-analysis. Sleep Med. Rev 2017. (in press). [DOI] [PubMed] [Google Scholar]; This is the most recent meta-analysis of studies using actigraphy to investigate differences in activity and sleep patterns in people with bipolar disorder versus healthy controls.
- 77.Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, Leboyer M, et al. Sleep in patients with remitted bipolar disorders: A meta-analysis of actigraphy studies. Acta Psychiatr. Scand 2015;131:89–99. [DOI] [PubMed] [Google Scholar]
- 78.**.Ng TH, Chung KF, Ho FYY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med. Rev 2015;20:46–58. [DOI] [PubMed] [Google Scholar]; This is a comprehensive meta-analysis of studies using actigraphy, polysomnography, questionnaires, and sleep diaries to examine sleep-wake and circadian rhythm disruption in individuals diagnosed with or at risk for bipolar disorder.
- 79.Faedda GL, Ohashi K, Hernandez M, McGreenery CE, Grant MC, Baroni A, et al. Actigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls. J. Child Psychol. Psychiatry 2016;57:706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rock P, Goodwin G, Harmer C, Wulff K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiol. Int 2014;31:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bullock B, Murray G. Reduced amplitude of the 24-hr activity rhythm: a biomarker of vulnerability to bipolar disorder? Clin. Psychol. Sci 2014;2:86–96. [Google Scholar]
- 82.Castro J, Zanini M, Gonçalves B da SB, Coelho FMS, Bressan R, Bittencourt L, et al. Circadian rest–activity rhythm in individuals at risk for psychosis and bipolar disorder. Schizophr. Res 2015;168:50–5. [DOI] [PubMed] [Google Scholar]
- 83.Ng TH, Chung K-F, Ng T-K, Lee C-T, Chan M-S. Correlates and prognostic relevance of sleep irregularity in inter-episode bipolar disorder. Compr. Psychiatry 2016;69:155–62. [DOI] [PubMed] [Google Scholar]
- 84.Frank E, Swartz HA, Boland E. Interpersonal and social rhythm therapy: an intervention addressing rhythm dysregulation in bipolar disorder. Dialogues Clin. Neurosci 2007; 9(3):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch. Gen. Psychiat 2005;62(9):996–1004. [DOI] [PubMed] [Google Scholar]
- 86.Swartz HA, Frank E, Cheng Y. A randomized pilot study of psychotherapy and quetiapine for the acute treatment of bipolar II depression. Bipolar Disord. 2012; 14(2):211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.*.Benedetti F, Riccaboni R, Locatelli C, Poletti S, Dallaspezia S, Colombo C. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J. Clin. Psychiat 2014;75:133–40. [DOI] [PubMed] [Google Scholar]; Benedetti and his team have published extensively on the use of chronotherapeutics as adjunctive treatments for BSD. This article in particular highlights the effect of combined sleep deprivation and light therapy on suicidal symptoms, a particularly important treatment target in BSD
- 88.Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E. Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiat. Res 2000;95:43–53. [DOI] [PubMed] [Google Scholar]
- 89.Tseng PT, Chen YW, Tu KY, Chung W, Wang HY, Wu CK, Lin PY. Light therapy in the treatment of patients with bipolar depression: A meta-analytic study. Eur. Neuropsychopharmacol 2016;26:1037–47. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki M, Dallaspezia S, Locatelli C, Uchiyama M, Colombo C, Benedetti, F. Discrepancy between subjective and objective severity as a predictor of response to chronotherapeutics in bipolar depression. J. Affect. Disord 2016;204:48–53. [DOI] [PubMed] [Google Scholar]
- 91.**.Crowe M, Beaglehole B, Inder M. Social rhythm interventions for bipolar disorder: a systematic review and rationale for practice. J Psychiat. Ment. Health Nurs 2016; 23(1):3–11. [DOI] [PubMed] [Google Scholar]; This article is a comprehensive review of IPSRT and light therapies for the treatment of BSD, discussing the scientific underpinnings of the treatments as well as their clinical applicability.
- 92.**.Alloy LB, Nusslock R, Boland EM. The development and course of bipolar spectrum disorders: An integrated reward and circadian rhythm dysregulation model. Annual Rev. Clin. Psychol 2015b;11:213–50. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reviews evidence for the reward hypersensitivity and social/circadian dysregulation theories of BSD and provides evidence for an integrated Reward Circadian Dysregulation model.
- 93.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clin. Exper. Res 2013;37:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Coan JA. Psychosocial interventions for bipolar disorder: perspective from the behavioral approach system (BAS) dysregulation theory. Clin Psychol. Sci. Prac 2009;16:449–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alloy LB, Abramson LY. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Curr. Direc. Psych. Sci 2010;19:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alloy LB, Olino T, Freed R, Nusslock R. Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behav. Ther 2016;47:600–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson SL, Edge MD, Holmes MK, Carver CS. The behavioral activation system and mania. Ann. Rev. Clin. Psychol 2012;8:243–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clin. Psychol. Rev 2008;28:1188–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Res. 2007;1129:34–42. [DOI] [PubMed] [Google Scholar]
- 100.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. Proc. Nat. Acad. Sci 2006;103:6386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Albrecht U The circadian clock, reward, and memory. Front. Molec. Neurosci 2011;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Forbes EE, Dahl RE, Almeida JRC, Ferrell RE, Nimgaonkar VL, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol. Psychiatry 2012;71:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.**.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ. et al. Nature’s clock and human mood: The circadian system modulates reward motivation. Emotion. 2009;9:705–16. [DOI] [PubMed] [Google Scholar]; Three studies demonstrate that reward sensitivity exhibits a circadian rhythm in humans.
- 104.Baltazar RM, Coolen LM, Webb IC. Diurnal rhythms in neural activation in the mesolimbic reward system: Critical role of the medial prefrontal cortex. Europ. J. Neurosci 2013;38:2319–27. [DOI] [PubMed] [Google Scholar]
- 105.Hasler BP, Mehl MR, Bootzin RR, Vazire S. Preliminary evidence of diurnal rhythms in everyday behaviors associated with positive affect. J. Res. Personal 2008;42:1537–46. [Google Scholar]
- 106.Hasler BP, Allen JJB, Sbarra DA, Bootzi RR. Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatr. Res 2010;176:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murray G, Allen NB, Trinder J. Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiol. Int 2002;19:1151–69. [DOI] [PubMed] [Google Scholar]
- 108.*.Hasler BP, Forbes EE, Franzen PL. Time-of-day differences and short-term stability of the neural response to monetary reward: A pilot study. Psychiat. Res: Neuroimag 2014;224:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to demonstrate time of day differences in neural reward processing.
- 109.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiat. Res: Neuroimag. 2013;214:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol. Psychol 2012;91:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berridge KC, Robinson,TE. Parsing reward. Trends Neurosci. 2003;26:507–13. [DOI] [PubMed] [Google Scholar]