Abstract
Background:
Two objectives of the NIMH Research Domain Criteria (RDoC) initiative are to identify (a) mechanisms that are common to multiple psychiatric disorders, and (b) mechanisms that are unique to specific psychiatric symptoms, and that reflect markers of differential risk for these symptoms. With respect to these objectives, a brain-behavior dimension that has received considerable attention and that is directly relevant to the Positive Valence Systems domain of the RDoC initiative involves reward processing.
Methods:
The present review paper first examines the relationship between reward processing and mood-related symptoms from an RDoC perspective. We then place this work in a larger context by examining the relationship between reward processing abnormalities and psychiatric symptoms defined broadly, including mood-related symptoms, schizophrenia, and addiction.
Results:
Our review suggests that reward hyposensitivity relates to a subtype of anhedonia characterized by motivational deficits in unipolar depression, and reward hypersensitivity relates to a cluster of hypo/manic symptoms characterized by excessive approach motivation in the context of bipolar disorder. Integrating this perspective with research on reward processing abnormalities in schizophrenia and addiction, we further argue that the principles of equifinality and multifinality may be preferable to a transdiagnostic perspective for conceptualizing the relationship between reward processing and psychiatric symptoms defined broadly.
Conclusion:
We propose that vulnerability to either motivational anhedonia or approach-related hypo/manic symptoms involve extreme and opposite profiles of reward processing. We further propose that an equifinality and multifinality perspective may serve as a useful framework for future research on reward processing abnormalities and psychiatric symptoms.
Keywords: Anhedonia, hypo/mania, RDoC, reward processing, approach-motivation, dopamine
Introduction
A tectonic shift occurred in 1980 with the publication of the third edition of the Diagnostic and Statistical Manuel of Mental Disorders (DSM-3rd ed; American Psychiatric Association, 1980). DSM-III moved away from broadly defined terms like neurosis, and instead focused its taxonomy on clinical consensus and specifically defined syndromes with the goal of increasing the reliability of psychiatric diagnosis, which was lacking in the first two editions of the manual. Although DSM’s continued focus on clinical consensus has facilitated reliable clinical diagnosis, many have questioned the validity of these diagnoses. This questioning stems from the fact that the development of DSM predates important breakthroughs in psychology, neuroscience, and genetics, as well as multiple problems that have been documented over the past several years (see Insel et al., 2010; Insel & Cuthbert, 2015). Specifically, diagnostic categories based on clinical consensus and self-reported symptoms (a) may fail to align with current findings from psychological science, neuroscience, and genetics, (b) are not predictive of treatment response, and (c) do not appear to capture the fundamental underlying mechanisms of dysfunction. That is, DSM is not carving nature at its joints.
To help address this issue, the National Institute of Mental Health (NIMH) recently launched the Research Domain Criteria (RDoC) initiative. The RDoC initiative reflects a second tectonic shift in the field of psychiatry and psychology, arguing for the development of new ways of classifying psychiatric illness based on core brain-behavior dimensions (Insel et al., 2010; Insel & Cuthbert, 2015). Rather than start with an illness definition based on clinical observation and seek its mechanistic underpinnings, RDoC begins with our current understanding of brain-behavior dimensions and aims to link these dimensions to specific symptoms. The intention of RDoC is to eventually generate a classification system for psychiatric disorders that is grounded in contemporary science. The argument is that this precision medicine perspective will facilitate more accurate and timely psychiatric diagnosis and the development of targeted treatments that are informed by up-to-date research on psychology, neuroscience, and genetics.
In its present form, the RDoC framework involves five domains or dimensions reflecting contemporary knowledge about major systems of cognition, motivation, and behavior. These domains are Negative Valence Systems, Positive Valence Systems, Cognitive Systems, Systems for Social Processes, and Arousal/Regulatory Systems. RDoC specifies multiple units of analysis that can be used to examine these domains, including, genes, molecules, cells, circuits, physiology, behaviors, self-reports, and paradigms. One stated goal of RDoC is to identify pathophysiological mechanisms that cut across, or are common to, multiple psychiatric disorders. Identifying pathophysiological mechanisms underlying transdiagnostic symptom clusters can help break down potentially arbitrary distinctions between categorically defined psychiatric disorders and account for comorbidity among current DSM diagnostic categories. As an example, deficits in threat-related processes (Negative Valence Systems), executive control (Arousal/Regulatory Systems), and working memory (Cognitive Systems) are observed across multiple psychiatric disorders, including unipolar depression (Hamilton et al., 2012; Wagner et al., 2006), bipolar disorder (Phillips & Vieta, 2007; Almeida, Versace, Hassel, Kupfer, & Phillips, 2010), and anxiety disorders (Etkin & Wager, 2007; Pacheco-Unguetti, Acosta, Marqués, & Lupiáñez, 2011). Thus, deficits in threat processing, executive control, and working memory may reflect risk factors for transdiagnostic symptoms that are common to multiple psychiatric conditions.
Another stated goal of RDoC, however, is to identify mechanisms that are unique to specific psychiatric symptoms, and that reflect signatures of differential risk for these distinct symptom profiles. Throughout medicine, disorders once considered unitary based on clinical presentation often turn out to be heterogeneous and characterized by clinically and scientifically meaningful subtypes. For example, under the DSM-5 (2013) definition of a Major Depressive Episode, which requires the presence of 5 out of 9 possible symptoms, two individuals may both be diagnosed with major depression while only sharing a single symptom in common. This heterogeneity may mask important associations that are related to specific symptoms, rather than the whole diagnostic category. Relevant to this topic is evidence that certain psychiatric disorders are characterized by distinct and opposite profiles of reward processing and approach motivation within the Positive Valence Systems (Alloy, Olino, Freed, & Nusslock, 2016; Whitton, Treadway, & Pizzagalli, 2015). Reward processing relates to the value an individual places on potential rewards, the perceived probability of reward receipt, and the mechanisms by which an individual processes rewards or goal-relevant cues. These cues can be either external (presence of a desired reward) or internal (expectancies of reward attainment). Approach motivation involves mechanisms/processes that regulate the pursuit of desired rewards and goals in the environment.
Whereas unipolar depression (without a history of hypomania or mania; hereafter referred to as hypo/mania) has been associated with abnormally reduced positive emotion, reward processing, and approach motivation (e.g., Forbes, 2009; Pizzagalli, Iosifescu, Hallet, Ratner, & Fava, 2008; Thibodeau, Jorgensen, & Kim, 2006; Treadway, in press; Treadway & Zald, 2011), bipolar disorder has been associated with abnormally elevated reward processing and approach motivation (e.g., Alloy & Abramson, 2010; Alloy, Nusslock, & Boland, 2015; Johnson, 2005; Johnson et al., 2012b; Nusslock, Young, & Damme, 2014). Furthermore, and relevant to the RDoC initiative, is growing evidence that abnormal reward processing in mood disorders is particularly related to a subgroup of symptoms characterized by motivational deficits and abnormalities. Thus, if one were to look for mechanisms of differential risk for specific mood-related psychiatric symptoms or subtypes, we argue that the Positive Valence Systems may be an important target.
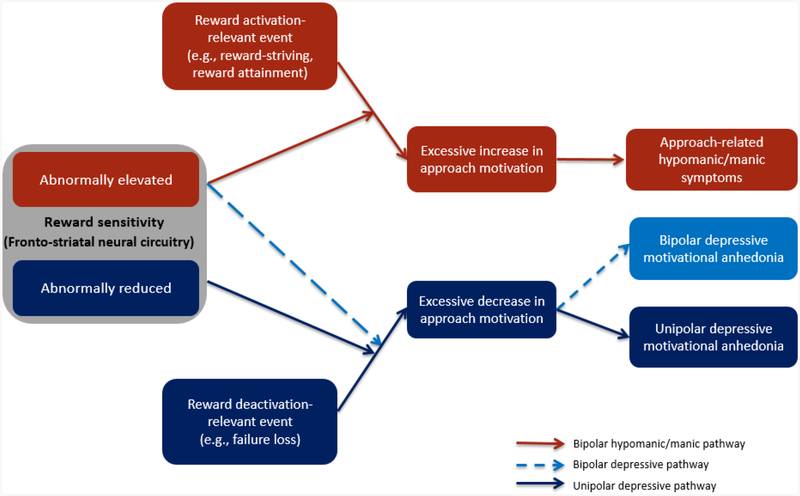
Covering evidence from self-report, behavioral, neurophysiological, and neural levels of analysis, the present review paper examines the relationship between reward processing and mood-related symptoms from an RDoC perspective. We first review evidence that unipolar depression (without a history of hypo/mania) and bipolar disorder are characterized by differential profiles of reward processing and reward-related neural activation. Next, we move beyond considering unipolar depression and bipolar disorder as unitary constructs or homogenous disorders and instead discuss the relationship between specific profiles of abnormal reward processing and specific symptoms. This aim is directly in line with one of the stated goals of the RDoC initiative, which is to identify mechanisms that are uniquely related to specific psychiatric symptoms and that reflect signatures of differential risk for these distinct symptom profiles (Insel et al., 2010; Insel & Cuthbert, 2015). In particular, we summarize literature suggesting that reward hyposensitivity and decreased approach motivation is related to anhedonia in the context of unipolar depression, and that reward hypersensitivity and elevated approach motivation is related to a subgroup of hypo/manic symptoms characterized by excessive approach motivation and psychomotor hyperactivation in the context of bipolar disorder (elevated energy, increased goal-directed activity, decreased need for sleep, increased confidence, irritability) (Figure 1). As discussed, future research is needed to better understand the relationship between reward sensitivity and bipolar depression.
Figure 1.
Reward sensitivity vulnerability-stress model of motivational anhedonia and approach-related hypo/manic symptoms (adapted from Alloy, Olino, Freed, & Nusslock, 2016).
We also summarize literature arguing that in addition to RDoC’s focus on unpacking heterogeneity within diagnostic categories, it is equally important to address heterogeneity within specific symptoms, as distinct pathophysiological processes may have a unique relationship to specific sub-components of a symptom. We address this issue as it pertains to anhedonia, where Treadway and colleagues (2011; in press) have argued that reward hyposensitivity is uniquely associated with a sub-component of anhedonia characterized by motivational, as opposed to hedonic, deficits. Collectively, we propose that vulnerability to motivational anhedonia in the context of unipolar depression versus approach-related hypo/manic symptoms in the context of bipolar disorder involve extreme and opposite profiles along a brain-behavior dimension of reward sensitivity and approach motivation.
Finally, we integrate this perspective with research on reward processing abnormalities and psychiatric symptoms defined broadly, with a particular focus on schizophrenia (i.e., non-affective psychosis) and addiction. We extend the argument first put forth by Whitton and colleagues (2015) that the principles of equifinality (a given outcome can be reached by different means or mechanisms) and multifinality (similar means or mechanisms can lead to dissimilar outcomes) may be preferable to a transdiagnostic perspective for contextualizing future research on reward processing abnormalities and psychiatric symptoms defined broadly.
The Reward System
Although many regions in the brain respond to reward, the fronto-striatal neural circuit is at the heart of the reward system (Berridge, Robinson, & Aldridge, 2009; Haber & Knutson, 2010; Kringelbach & Berridge, 2009, Schultz, 2000; Schultz, Tremblay, & Hollerman, 2000). This circuit involves dopaminergic projections from midbrain nuclei (e.g., the ventral tegmental area) to subcortical regions that are central to processing the rewarding properties of stimuli (e.g., the ventral striatum, including the nucleus accumbens) to cortical target regions (e.g., the orbitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex). Both animal and human research highlights the central role that this circuit plays in reward-responsivity, incentive-based learning, assessing probability of reward receipt, prediction error, and goal directed behavior. Down-regulation or deactivation of the reward system leads to decreased motivation and goal-related cognitions, and increased withdrawal, as well as emotions such as sadness and anhedonia.
Within the fronto-striatal circuit, the ventral striatum is a central hub of reward processing. Anatomical definitions of the ventral striatum vary across animal and human research; however, in human neuroimaging, it frequently includes the nucleus accumbens, the ventral medial caudate, and the rostroventral putamen (Haber & Knutson, 2010). Both metabolic positron emission tomography (PET) and fMRI studies indicate that exposure to both primary (e.g., pleasant tastes, sounds and sights) and secondary rewards (e.g., monetary rewards) increase striatal activity in humans (Blood & Zatorre, 2001; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Haber & Knutson, 2010; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001). The observed elevation in striatal activity to both primary and secondary rewards is consistent with the notion that striatal activation does not depend on sensory modality. A number of factors modulate striatal activity to reward cues, including the magnitude of the reward, the probability of reward receipt, the amount of time until the anticipated reward can be obtained (i.e., delay), and the effort required to pursue the reward (see Haber & Knutson, 2010 for review). Furthermore, elevated ventral striatal activity during reward anticipation is associated with elevated self-reported behavioral approach system (BAS)/reward sensitivity (Caseras, Lawrence, Murphy, Wise, & Phillips., 2013; Hahn et al., 2009).
The region of the cortex most often associated with reward is the orbitofrontal cortex (OFC; Haber & Knutson, 2010; Kringelbach & Rolls, 2004; Schultz et al., 2000). There is variability in how the OFC is anatomically defined, particularly across animal and human studies. Drawing from research on reward-related neural activation in bipolar disorder (Bermpohl et al., 2010; Nusslock et al., 2012a), we define the OFC as Brodmann Area (BA) 10, 11, and 47 for the present paper. Several neuroimaging studies indicate that sensory and abstract rewards recruit the OFC (Blood & Zatorre, 2001; Knutson, Westdorp, Kaiser, & Hommer, 2000; Small et al., 2001). A meta-analysis of these findings (Kringelbach & Rolls, 2004) suggests a potentially important distinction between medial and lateral regions of the OFC. This analysis indicates that the medial OFC (BA 10, 11) is clearly sensitive to the rewarding properties of stimuli and the generation of positive or approach-related affect, but the lateral OFC (e.g., BA 47) appears to be sensitive to both positive and negative (i.e., punishment cues) cues. Accordingly, activation of the lateral OFC has been interpreted in terms of arousal (Schmidt et al., 2009) and salience (Lewis, Critchley, Rothstein, & Dolan, 2007) as opposed to positive hedonic evaluation.
Both animal and human research highlights the central role of dopamine neurotransmission in the fronto-striatal reward circuit (Haber & Knutson, 2010; Schultz, 2002; Wise, 2002). Relative to placebo injection, ligand-based PET research indicates that amphetamine injection robustly increases striatal dopamine, and these increases correlate with positive and arousing affective experiences (Drevets et al., 2001; Volkow et al., 1999). Alcohol, cocaine, and secondary rewards such as gambling all increase dopamine release in the striatum (Boileau et al., 2003; Cox et al., 2009). As discussed below, however, dopamine appears to be more involved in reward anticipation and ‘wanting’, and less involved in reward outcome and ‘liking’ (see Berridge, 2007; Berridge et al., 2009 for review).
Reward Hyposensitivity and Major Depressive Disorder
Decreased approach motivation and reduced positive affect has long been considered a core feature of unipolar depression (Meehl, 1975; Lewinsohn & Graf, 1973). Indeed, anhedonia, characterized by a markedly diminished interest or pleasure in activities (American Psychiatric Association, 2013), is a cardinal symptom of depression. Individuals with unipolar depression self-report decreased behavioral approach system (BAS) sensitivity (Kasch, Rottenberg, Arnow, & Gotlib, 2002), report reduced extraversion and pleasure sensitivity (Kazdin, 1989; Kotov, Gamez, Schmidt, & Watson, 2010), and engage less frequently in goal-directed behavior (Forbes, 2009). During gambling or monetary-reward tasks, adults with depression make decisions that are more conservative (Corwin, Peselow, Feenan, & Rotrosen, 1990), slower (Kaplan et al., 2006), and less flexible in the face of shifting contingencies (Cella, Dymond, & Cooper, 2010), and expend less effort for rewards when compared with controls (Treadway et al., 2012a; Yang et al., 2014). Depression – and anhedonia in particular – is associated with a failure to exhibit a response bias toward rewarded stimuli in signal detection tasks, in which one set of stimuli is subtly rewarded more frequently than another (Pizzagalli, Jahn, & O’Shea, 2005; Pizzagalli et al., 2008). Moreover, reduced approach motivation and blunted positive affect have been concurrently and prospectively linked to depression onset in adult samples (Clark, Watson, & Mineka, 1994). In children, reduced positive affect at age 3 predicted depressogenic cognitive styles at age 7 (Hayden, Klein, Durbin, & Olino, 2006) and was associated with a maternal history of depressive disorders (Durbin, Klein, Hayden, Buckley, & Moerk, 2005).
At the neurophysiological unit of analysis, close to thirty years of research suggests that relative left versus right frontal electroencephalographic (EEG) activity reflects a neurophysiological index of approach motivation and reward-related affect (see Coan & Allen, 2004; Nusslock, Walden, & Harmon-Jones, 2015 for reviews). Increased relative left-frontal EEG activity indicates a propensity to approach or engage a stimulus, whereas decreased relative left-frontal activity is associated with decreased approach-motivation and blunted reward processing. Consistent with the reward hyposensitivity perspective of unipolar depression, individuals with unipolar depression typically show decreased relative left frontal EEG activity during both depressive (Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1991) and remitted states (Henriques & Davidson, 1990), suggesting that reduced left frontal EEG activity may be a state independent marker of unipolar depression (see Thibodeau et al., 2006 for meta-analytic review; although see Reid, Duke, & Allen, 1998; Nitschke, Heller, Palmieri, & Miller, 1999; and Thibodeau et al., 2006 for studies reporting no relationship between frontal EEG asymmetry and depression). Finally, unipolar depression is characterized by blunted reward responsiveness, as indexed by the feedback negativity (FN; Foti & Hajcak, 2009), an event-related potential (ERP) elicited by stimuli that indicate monetary gain versus loss. Moreover, a blunted FN prospectively predicts onset of a first major depressive episode (Bress, Foti, Kotov, Klein, & Hajcak, 2013).
With respect to functional MRI (fMRI), investigators have developed a number of tasks to assess reward neural activation in the fronto-striatal circuit (Richards, Plate, & Ernst, 2013), including simple guessing for rewards (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Forbes et al., 2009), behavioral performance for rewards (Knutson, Adams, Fong, & Hommer, 2001), and decision-making reward tasks (Ernst et al., 2004). These studies document reduced ventral striatal activation in major depressive disorder (MDD) to reward anticipation cues (Forbes et al., 2009; Smoski et al., 2009), reward receipt (McCabe, Mishor, Cowen, & Harmer, 2009; Pizzagalli et al., 2009; Wacker, Dillon, & Pizzagalli, 2009), reward prediction errors (i.e., the difference between experienced versus predicted rewards; Kumar et al., 2008; Steele, Kumar, & Ebmeier, 2007), and other positive stimuli (e.g., positive IAPS pictures, positive words) (although see Knutson et al., 2008 for a report of no reduction in ventral striatal activity to reward cues). Reduced striatal activation is present among individuals with MDD during remission (Dichter, Kozink, McClernon, & Smoski, 2012; Schiller, Minkel, Smoski, & Dichter, 2013; Takahashi et al., 2009), suggesting that blunted reward responsiveness is state-independent, and observed among offspring of depressed individuals who have yet to develop a depressive episode (Gotlib et al., 2010; McCabe, Woffindale, Harmer, & Cowen, 2012; Monk et al., 2008; Olino et al., 2014; Olino, Silk, Osterritter, & Forbes, 2015; Sharp et al., 2014).
Finally, reward-relevant life events also are related to the course of depression. According to the reward hyposensitivity model of major depression, life events that deactivate the reward system (i.e., certain loss or failure) should precipitate depressive symptoms and episodes (see dark blue pathway in Figure 1). Multiple conceptual frameworks similarly emphasize the role of life events in depression (Hammen, 2005; Harkness & Monroe, 2016; Monroe & Harkness, 2005), and empirical studies agree that stressful life events predict depression onset in early childhood (Bufferd et al., 2014), adolescence (Monroe, Rohde, Seeley, & Lewinsohn, 1999), and adulthood (Kendler, Hettema, Butera, Gardner, & Prescott, 2003). Consistent with the reward hyposensitivity model, reward-deactivating events involving irreconcilable failures and losses have been shown to predict first onset and recurrences of depression (see Alloy et al., 2005; Alloy, Abramson, Urošević, Bender, & Wagner, 2009a for review).
Reward Hyposensitivity and Anhedonia: An RDoC Perspective
Thus far, our review of reward hyposensitivity in unipolar depression has focused on individuals with DSM diagnoses. This is because most of the research on this topic has been conducted on depressive disorder samples. As stated, however, a goal of RDoC is to move beyond considering psychiatric disorders as unitary constructs and to instead examine the relationship between core brain-behavior dimensions and specific symptom profiles (Insel et al., 2010; Insel & Cuthbert, 2015). In line with this perspective, here we summarize literature arguing that reward hyposensitivity is uniquely related to the unipolar depressive symptom of anhedonia. Next, we discuss the argument put forth by Treadway and colleagues (Treadway & Zald, 2011; Treadway, in press) that even the term anhedonia is underspecified, and that reward hyposensitivity likely relates to a specific variant of anhedonia characterized by motivational, as opposed to hedonic, deficits.
Anhedonia involves diminished interest or pleasure in response to stimuli that were previously perceived as rewarding, and is one of two required symptoms for the DSM diagnosis of MDD (American Psychiatric Association, 2013). Recent reports estimate that approximately 37% of individuals diagnosed with MDD experience clinically significant anhedonia (Pelizza & Ferrari, 2009). Growing evidence from self-report (McFarland & Klein, 2009; Treadway & Zald, 2011), behavioral (Pizzagalli et al., 2005: Treadway et al., 2012a; Yang et al., 2014), and neurophysiological (i.e., feedback negativity; Liu et al., 2014) units of analysis suggests that reward hyposensitivity and reduced approach motivation reflect anhedonia. Neuroimaging studies indicate that anhedonia (but not general depression severity) is associated with reduced ventral striatal activation to positive and rewarding stimuli (Wacker et al., 2009; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005), as well as reduced ventral striatal volume (Wacker et al., 2009). Epstein et al. (2006) reported that depressed participants were characterized by reduced ventral striatal responses to positive pictures, and the strength of these responses was negatively correlated with self-reported anhedonia. Finally, we recently reported that anhedonia, but not general distress, was associated with deficits in functional connectivity between the ventromedial prefrontal cortex and nucleus accumbens during reward processing among individuals with MDD (Young et al., 2016).
Reward hyposensitivity associated with motivational deficits in anhedonia.
In addition to RDoC’s focus on unpacking heterogeneity within diagnostic categories, Treadway and colleagues (Treadway, in press; Treadway & Zald, 2011) recently argued that it is equally important to address heterogeneity within the symptom of anhedonia. Their stance is consistent with a number of other reviews that have called for a critical reexamination of the anhedonia construct (Foussias & Remington 2008; Barch & Dowd 2010; Strauss & Gold, 2012; Pizzagalli, 2014). This perspective stems from animal and human research documenting distinct neural circuits underlying motivational (anticipation, “wanting”) versus hedonic (consumption, “liking”) reward-related states. Treadway and others (Treadway, in press; Treadway & Zald, 2011; Pizzagalli, 2014) argue that reward hyposensitivity in unipolar depression will be most strongly associated with a state of anhedonia characterized by motivational, versus hedonic, deficits for two reasons. First, preclinical research indicates that the dopaminergic fronto-striatal reward circuit is primarily involved in the motivational pursuit, anticipation, or “wanting” of a reward, as opposed to the hedonic consumption of reward (see Treadway, in press; Treadway & Zald, 2011 for review). Initially, dopaminergic activity in this circuit was thought to mediate an organism’s experience of pleasure, or “yumminess”, in response to rewarding stimuli (Wise, 1980). This perspective has been largely abandoned over the past thirty years, and dopamine signaling within the fronto-striatal circuit is now viewed as the engine that facilitates approach or goal-directed behavior to obtain rewards, as opposed to the mechanism by which an organism hedonically enjoys, savors or consumes a reward [the primary neurochemicals involved in pleasurable hedonic experiences appear to be endogenous opioids (see Treadway & Zald, 2011 for review)]. For example, lesions to dopamine synapses in the ventral striatum do not impair hedonic liking expressions in rats (Berridge & Robinson, 1998). Furthermore, dopamine depleted mice still favor sucrose-water over regular water and demonstrate a morphine-induced conditioned place preference (Cannon & Palmiter, 2003), and increasing dopamine shows no effect on liking or pleasure related behavior (Peciña, Berridge, & Parker, 1997). By contrast, altering dopaminergic functioning has a robust effect on an organism’s motivation to pursue and work for rewarding stimuli (Salamone, Correa, Farrar, & Mingote, 2007), highlighting the role of dopamine signaling in the pursuit of reward, as opposed to the pleasure of consuming the reward.
Second, and perhaps more controversial, is the proposal that anhedonia may not necessarily involve a reduction in the capacity to experience pleasure, but rather primarily a deficit in ability or willingness to recruit motivational resources to pursue pleasurable rewards (Treadway & Zald, 2011). Take for example the “sweet taste test,” which assesses hedonic capacity by having individuals rate the pleasantness of different sucrose concentrations. In four separate studies, individuals with depression and matched controls reported no differences in their ratings of the sucrose, suggesting that there is no deficit in the hedonic capacity to experience a natural reinforcer in depression (Amsterdam, Settle, Doty, Abelman, & Winokur, 1987; Berlin, Givry-Steiner, Lecrubier, & Puech, 1998; Dichter, Smoski, Kampov-Polevoy, Gallop, & Garbutt, 2010; Kazes et al., 1994). Contrary to these data, however, are findings from Hajcak and colleagues showing attenuated neurophysiological responses to reward versus loss outcome among individuals with unipolar depression (Foti & Hajcak, 2009; Bress et al., 2013). Thus, future research is needed to determine the extent to which anhedonia in the context of depressive symptoms is primarily driven by motivational deficits, or both motivational and hedonic deficits. Regardless of the outcome of this research, however, we agree with the argument first put forth by Treadway and colleagues (Treadway & Zald, 2011) that reduced signaling in the fronto-striatal reward circuit will be most strongly associated with motivational, as opposed to hedonic, components of anhedonia.
Taken together, the above studies highlight the need for clinical research to distinguish motivational from hedonic components of anhedonia. In fact, Treadway and colleagues (Treadway, in press; Treadway & Zald, 2011) have argued that mood-related symptom heterogeneity may be as problematic as diagnostic heterogeneity, as both preclinical and clinical research highlight dissociable neural circuits underlying motivational versus hedonic deficits in anhedonia. Viewing anhedonia as a homogenous construct not only impedes scientific progress into its pathophysiology, but also reduces the precision with which anhedonia can be targeted in treatment. Unfortunately, the majority of clinical assessment and research to date does not distinguish motivation from hedonics, and if anything, gives primacy to hedonic or pleasure deficits in anhedonia. For example, DSM-5 defines anhedonia as “markedly diminished interest or pleasure in all or almost all activities”, and says nothing about whether this diminished pleasure is motivationally versus hedonically driven. In keeping with DSM-5, the Structured Clinical Interview for DSM Disorders (SCID) simply asks patients whether they “have lost interest or pleasure in things they usually enjoy”. Finally, a content review by Treadway and Zald (2011) of items used in the most common anhedonia measures revealed that they unanimously emphasize the experience of pleasure in response to positive stimuli with little or no attention to diminished drive or motivation.
Treadway and colleagues have recently begun to address this issue in a very sophisticated manner with the development of their effort expenditure for reward task (EEfRT), which examines neural substrates of effort mobilization in humans (Treadway et al., 2012a; Treadway et al., 2012b; Wardle, Treadway, Mayo, Zald, & de Wit, 2011). During this task, participants perform a series of trials in which they are asked to choose between completing a “High Effort” and a “Low Effort” task in exchange for monetary compensation, where the required effort is in the form of speeded button presses. Mirroring the effects of dopamine potentiation in rats, administration of a dopamine agonist (d-amphetamine) produces a dose-dependent increase in the willingness to work for rewards in the EEfRT (Wardle et al, 2011), and the magnitude of dopamine release in dorsomedial and ventral components of the striatum positively predicts the proportion of High-Effort choices participants made during low-probability trials (Treadway et al., 2012b). Furthermore, and in line with the perspective that depression is characterized by fundamental motivational deficits, patients with MDD expend less effort for reward when compared with controls (Treadway et al., 2012a; Yang et al., 2014), and the longer the depressive episode, the more impaired the decision-making (Treadway et al., 2012a). Future research with the EEfRT task and related paradigms examining motivational deficits in anhedonia promises to have important scientific and treatment implications.
Developmental pathways to motivational deficits in anhedonia.
An important question for future research is to better understand the developmental mechanisms leading to the eventual onset of reward hyposensitivity and motivational deficits in anhedonia. Gene-environment models propose an interaction and/or correlation between polygenic risk factors modulating dopamine signaling and both early adversity (e.g., maternal separation, childhood maltreatment) and chronic life stress (see Pizzagalli, 2014 for review). In line with this perspective are genetic studies identifying several polymorphisms related to dopaminergic function that increase one’s risk for developing depression and anhedonia (Lopez Leon et al., 2005; Chiaroni et al., 2000). Additionally, early and chronic life adversity downregulate mesolimbic dopamine signaling, reward-related brain function, and reward responsiveness in both animals and humans (see Nusslock & Miller, 2016; Pizzagalli, 2014 for reviews), all of which have been associated with anhedonia (Dillon et al., 2009; Guyer et al., 2006). Further research is needed, however, to better model the nature of the relationship between genetic and environmental factors in the onset and course of reward hyposensitivity and motivational deficits in anhedonia.
Complimenting traditional gene-environment models, we argue that peripheral inflammation may reflect a second developmental mechanism facilitating the initial onset of reward hyposensitivity and motivational deficits in anhedonia (Nusslock & Miller, 2016). Considerable preclinical research indicates that dopamine signaling in the fronto-striatal reward circuit is a primary target of peripheral inflammation, which can spread to the brain through multiple mechanisms (see Miller, Haroon, Raison, & Felger, 2013 for review). This blunted reward sensitivity, mediated by inflammatory cytokines, is part of a generalized set of adaptations to infection (Miller, Maletic, & Raison, 2009; Maier & Watkins, 1998). These adaptions are collectively referred to as sickness behaviors and, along with anhedonia, include dysphoria, fatigue, psychomotor slowing, and behavioral disengagement (Dantzer, O’Conner, Freund, Johnson, & Kelley, 2008), all of which resemble the motivational anhedonia associated with reward hyposensitivity discussed in the present paper. Human imaging studies indicate that inflammatory agonists, such as lipopolysaccharide (LPS), typhoid vaccine, and chronic hepatitis C, all result in significant reductions in reward-related neural activation in the ventral striatum (Eisenberger et al., 2010; Harrison et al., 2009; Capuron et al., 2012). Importantly, this reduction in reward-related brain function is secondary to blunted dopamine transmission in both animals (Miller et al., 2013) and humans (Capuron et al., 2012).
This inflammatory mediated reduction in reward sensitivity and reward-related brain function is highly adaptive when it occurs in moderation and reflects a time-limited response to pathogen exposure. However, considerable evidence suggests that early life adversity (e.g., childhood maltreatment; low socioeconomic status) and chronic stress are associated with a proinflammatory phenotype characterized by chronically larger volumes of inflammatory cytokines (see Nusslock & Miller, 2016 for review). Given that inflammation attenuates reward sensitivity, reduces dopamine mediated reward-related brain function, and induces motivational deficits, we argue that chronic inflammation, secondary to early life adversity and/or chronic stress, may reflect a second developmental mechanism underlying reward hyposensitivity and motivational deficits in anhedonia. Future research is needed to test this prediction.
Reward Hypersensitivity and Bipolar Disorder
Whereas unipolar depression is characterized by blunted reward sensitivity, growing evidence suggests that risk for bipolar disorder is associated with a hypersensitivity to reward-relevant cues. In this section, we first review evidence relevant to the Reward Hypersensitivity Model of bipolar disorder. Next, we move beyond considering bipolar disorder as a homogenous construct and propose that reward hypersensitivity uniquely relates to a cluster of hypo/manic symptoms characterized by psychomotor hyperactivation and excessive approach motivation (referred to as approach-related hypo/manic symptoms).
The DSM defines bipolar spectrum disorders as encompassing three diagnoses: cyclothymia, bipolar II disorder, and bipolar I disorder. All three diagnoses involve extreme highs (hypomania or mania) and lows (depression) of mood, motivation, cognition, and behavior, but differ in severity, with bipolar I disorder being the most severe and cyclothymia the least severe. Moreover, having a milder form of bipolar disorder (cyclothymia, bipolar II) increases the risk for developing full-blown bipolar I disorder in both children/adolescents (Birmaher et al., 2009; Kochman et al., 2005) and adults (Alloy et al., 2012b), supporting the concept that bipolar disorder involves a spectrum of severity.
Contrary to unipolar depression, evidence suggests that bipolar disorder is characterized by elevated reward sensitivity and increased approach motivation. These data have been conceptualized in the context of the Reward Hypersensitivity Model of bipolar disorder (Alloy & Abramson, 2010; Alloy et al., 2009a; Alloy, Nusslock, & Boland, 2015; Johnson, 2005; Johnson, Edge, Holmes, & Carver, 2012b; Nusslock et al., 2014; Urošević, Abramson, Harmon-Jones, & Alloy, 2008). This model proposes that risk for bipolar disorder symptoms, and in particular hypo/manic symptoms, is characterized by a hypersensitivity to goal- and reward-relevant cues. This hypersensitivity can lead to an excessive increase in approach-related motivation (e.g., working excessively long hours) during life events involving rewards or goal striving and attainment (e.g., when striving for or receiving a job promotion). In the extreme, this excessive increase in approach motivation is reflected in hypo/manic symptoms, such as elevated or irritable mood, decreased need for sleep, increased psychomotor activation, extreme self-confidence, and pursuit of rewarding activities without attention to risks (see red pathway in Figure 1). Thus, from the perspective of the Reward Hypersensitivity Model, symptoms of hypo/mania involve extreme expressions along an underlying core brain-behavior dimension of reward-processing and approach motivation (see below for a detailed discussion of reward-processing and bipolar depression).1
There is evidence that reward hypersensitivity is a mood-independent trait associated with bipolar spectrum disorders, as well as a vulnerability factor for the onset and recurrence of mood episodes and a worse course of bipolar disorder. For example, controlling for bipolar mood symptoms, personality characteristics associated with high incentive motivation and reward drive (such as achievement motivation, ambitious goal-striving, perfectionism, and self-criticism) as well as self- or parent-reports of high BAS/reward sensitivity are greater in individuals with bipolar conditions all along the spectrum compared to healthy controls or individuals with unipolar depression (e.g., Alloy et al., 2008; 2009b; Fulford, Johnson, Llabre, & Carver, 2010; Gruber et al., 2013; Johnson, Carver, & Gotlib, 2012a; Lam, Wright, & Smith, 2004; Lozano & Johnson, 2001; Meyer, Johnson, & Winters, 2001; Quilty, Mackew, & Bagby, 2014; Salavert et al., 2007; Scott, Stanton, Garland, & Ferrier, 2000; but see Hayden et al., 2008 for an exception). And, the relationship between bipolarity and reward sensitivity appears to be state-independent in that it is not related to current levels of hypo/mania (Alloy et al., 2008; Lozano & Johnson, 2001; Salavert et al., 2007; Scott et al., 2000), and reward sensitivity continues to be elevated in remission relative to controls (Lam et al., 2004; Meyer et al., 2001). Further corroborating this questionnaire evidence, bipolar I patients exhibit less ability to delay responding for rewards (Swann, Lijffijt, Lane, Steinberg, & Moeller, 2009) and higher hypo/manic symptoms are associated with greater emotional and cognitive responsiveness to rewards (Johnson, Ruggero, & Carver, 2005) on behavioral tasks. Finally, self-reported reward hypersensitivity, as well as elevated goal-striving and hypo/manic symptoms, are each associated with greater odds of choosing the “high effort” option on the EEfRT task when reward probability is low (Boland et al., 2016a).
Growing evidence indicates that self-reported reward sensitivity has predictive validity for the onset and course of bipolar spectrum disorders. Elevated self-reported reward sensitivity is associated with a greater likelihood of having a lifetime bipolar spectrum diagnosis (Alloy et al., 2006), a greater likelihood of developing a first onset of a bipolar spectrum disorder (Alloy et al., 2012a), a shorter time to recurrences of hypo/manic episodes (Alloy et al., 2008), an increase in manic symptoms among recovered individuals with bipolar I disorder (Meyer et al., 2001), and a greater likelihood of progressing to a more severe bipolar diagnosis among those with milder bipolar spectrum diagnoses (Alloy et al., 2012b). Furthermore, hypo/manic episodes are triggered by both reward-striving (e.g., applying for a job; Nusslock, Abramson, Harmon-Jones, Alloy, & Hogan, 2007) and reward-attainment (e.g., receiving a job; Johnson et al., 2000) life events, and self-reported elevated reward sensitivity both predicts the greater occurrence of reward-relevant events, as well as interacts with these events to prospectively predict increases in hypo/manic symptoms (Alloy et al., 2009a; Boland et al., 2016b; Urošević et al., 2010).
At the neurophysiological unit of analysis, both individuals prone to hypo/manic symptoms (Harmon-Jones et al., 2002) and individuals with a bipolar spectrum disorder (Harmon-Jones et al., 2008) display elevated relative left frontal EEG activity – a neurophysiological index of approach motivation – during reward-related laboratory tasks compared to healthy controls (although see Allen, Iacono, Depue, & Arbisi, 1993, for a report of decreased relative left frontal activity among currently depressed bipolar participants). Among individuals with a bipolar spectrum diagnosis, elevated relative left-frontal activity was associated with a greater likelihood of converting from cyclothymia or bipolar II disorder to bipolar I disorder over a five-year follow-up period (Nusslock et al., 2012b). This is the first study to identify a neurophysiological risk factor for conversion to a more severe bipolar diagnosis and parallels the previously mentioned research indicating that elevated self-reported reward sensitivity is associated with a more severe bipolar course. In addition, individuals at temperamental risk for hypo/manic symptoms display elevated reward responsiveness, as indexed by the feedback negativity ERP component (Mason, O’Sullivan, Bentall, & El-Deredy, 2012).
In line with the BAS/reward hypersensitivity model, bipolar disorder is associated with an excessive increase in fronto-striatal reward-related neural activation to positive or approach-related stimuli. For example, bipolar individuals display elevated striatal (Hassel et al., 2008; Lawrence et al., 2004), OFC (Elliott et al., 2004), and amygdala (Bermpohl et al., 2009) activation to pictures of happy faces or pleasant stimuli compared to healthy controls. There is preliminary evidence that this effect is state-independent, as elevated reward-related neural activation to positive emotional stimuli has been observed in both remitted (Hassel et al., 2008) and manic (Bermpohl et al., 2009; Elliott et al., 2004) bipolar individuals [although see Liu et al. (2012) for evidence of decreased striatal, OFC, and ACC activation in bipolar individuals to happy versus neutral faces].
The small number of studies that have employed fMRI reward paradigms provide compelling, albeit nuanced, support for the Reward Hypersensitivity Model of bipolar disorder. Nusslock et al. (2012a) reported that euthymic bipolar I disorder participants displayed greater ventral striatal, medial OFC (BA 10), and left lateral OFC (BA 47) activation during the anticipation, but not the outcome, of monetary reward in a card-guessing paradigm relative to healthy controls. There were no differences in neural activation between bipolar I and healthy control participants during anticipation or receipt of monetary loss. That reward-related neural activation was abnormally elevated in bipolar I individuals during remission suggests that this profile of fronto-striatal activity may reflect a trait-like or endophenotypic risk factor for bipolar disorder. To establish a biological marker of a disorder, however, it is important to examine the marker across multiple phases of the illness. To date, two studies used an fMRI reward paradigm with bipolar I individuals during a manic episode, and two used such a paradigm with bipolar I individuals during a depressive episode. With respect to mania, bipolar I individuals in a manic episode displayed elevated left lateral OFC (BA 47) activation during reward anticipation using the monetary incentive delay task (Bermpohl et al., 2010), while healthy participants showed the inverse effect. In a second study, manic participants showed increased activation in the ventral striatum coupled with reward omission compared to healthy participants (Abler, Erk, & Walter, 2007), suggesting that bipolar individuals in a manic episode have a reduced capacity to discriminate between rewards on the basis of their actual value and relevance.
With respect to bipolar depression, two fMRI studies report decreased reward-related neural activation in both the anterior cingulate cortex (Chase et al., 2013) and ventral striatum (Redlich et al., 2015) among bipolar I individuals in a current major depressive episode relative to healthy controls, and one study reports that depressive severity among bipolar participants was associated with reduced ventral striatal activity to reward cues (Satterthwaite et al., 2015). These findings highlight the presence of state-dependent effects of depression on reward-related neural activation in the ACC and ventral striatum in individuals with bipolar disorder. However, Chase et al. (2013) further reported that bipolar depressed participants displayed elevated lateral OFC (BA 47) activation during anticipation, collapsing across reward and loss trials. Thus, even during depression, individuals with bipolar I disorder maintain heightened activation in regions of the fronto-striatal neural circuit.
Further evidence for elevated reward-related neural activation in bipolar disorder comes from research on individuals with a bipolar spectrum diagnosis (i.e., bipolar II disorder), and individuals at elevated risk for bipolar disorder who have not yet developed the illness. For example, euthymic bipolar II participants displayed greater ventral striatal and lateral OFC activation during reward anticipation compared to healthy controls (Caseras et al., 2013; contrary to prediction, this study did not find elevated ventral striatal activity during reward anticipation among bipolar I individuals). In a PET study, depressed bipolar II participants also displayed elevated metabolism in the ventral striatum, anteroventral putamen, and OFC (Mah et al., 2007). Finally, individuals with a hypomanic temperament who have not yet developed bipolar disorder exhibited elevated ventral striatal activation and lateral OFC activation during reward processing (Harada et al., 2013). This latter finding suggests that elevated functional reward-related neural activation may reflect a preexisting risk factor for bipolar disorder, as opposed to a consequence of the illness.
We and others (e.g., Johnson, 2005; Johnson et al., 2012b) propose that a propensity to experience an excessive increase in reward and approach-related neural activation is a central mechanism through which individuals with bipolar disorder are at risk for developing hypo/manic symptoms in the presence of reward-relevant life events. Specifically, it is proposed that individuals with bipolar disorder experience an excessive increase in reward/approach-related neural activation to reward-relevant life events, which is reflected in an excessive increase in approach motivation. In the extreme, this increase in approach motivation is reflected in hypo/manic symptoms (see Fig. 1).
Collectively, this work indicates that risk for unipolar depressive symptoms and hypo/manic symptoms are characterized by distinct and opposite profiles of reward sensitivity and approach motivation within the RDoC Positive Valence Systems domain. Specifically, risk for unipolar depression is characterized by reduced approach motivation and decreased reward-related neural activation, whereas risk for hypo/mania is associated with elevated approach motivation and increased reward-related neural activation. These findings have important implications for understanding the pathophysiology of unipolar depression and bipolar disorder. As indicated, both these disorders are characterized by comparable deficits in threat-related processes (Negative Valence Systems), executive control (Arousal/Regulatory Systems), and working memory (Cognitive Systems) (Hamilton et al., 2012; Phillips & Vieta, 2007; Almeida, et al., 2010; Wagner et al., 2006). We argue that deficits in these RDoC domains likely reflect risk factors for transdiagnostic symptoms that are common to depression and bipolar disorder. These mechanisms, however, may not be particularly informative in distinguishing what puts an individual at risk for symptoms of unipolar depression versus bipolar disorder. We further argue, however, that RDoC Positive Valence Systems are highly relevant for understanding differential risk for symptoms of unipolar depression versus bipolar disorder, and that reward-related neural activation may reflect an endophenotypic marker of this differential risk. Specifically, we propose that what differentiates risk for bipolar disorder versus unipolar depression is risk for mania, and one of the primary risk factors for mania involves a propensity to experience abnormally elevated approach motivation to rewarding cues in the environment. Thus, reward/approach-related processes are clearly important for understanding what distinguishes bipolar disorder from unipolar depression, whereas threat, executive control, and working memory processes may be more informative in understanding what is common or transdiagnostic across these illnesses. Finally, however, we suggest that this logic can only take us so far and, in line with the RDoC initiative, we argue that it is important to move beyond considering mood disorders as homogenous disorders or unitary constructs and instead examine the relationship between individual differences in reward processing and specific mood-related symptom clusters.
Reward Hypersensitivity and Approach-Related Hypo/Manic Symptoms: An RDoC Perspective
With respect to hypo/mania, we predict that reward hypersensitivity will be most strongly associated with a cluster of symptoms characterized by excessive approach motivation, specifically, elevated energy, increased goal-directed activity, decreased need for sleep, increased confidence, and irritability when goal-pursuit is thwarted. We base this prediction on the strong convergence between the clinical characteristics of these symptoms and elevated reward-related neural activation, which is characterized by increased approach motivation, increased reward sensitivity, and elevated goal pursuit. Reward processing and approach motivation have not been directly implicated in cognitive activity (Alloy, Nusslock, & Boland, 2015), and thus, hypo/manic symptoms of elation and expansiveness, as well as cognitive symptoms involving distractibility and flight of ideas, should be less related to reward hypersensitivity than the proposed cluster of approach-related hypo/manic symptoms. Decreased need for sleep is included in this cluster of approach-related hypo/manic symptoms, given the coupling of reward processing and approach motivation with sleep variables (Holm et al., 2009; Murray et al., 2009), circadian influences (Alloy, Boland, Ng, Whitehouse, & Abramson, 2015; Boland et al., 2016b; Murray et al., 2009; Hasler, Allen, Sbarra, Bootzin, & Bernett, 2010) and circadian genes (Forbes et al., 2011). Increased confidence is included in this cluster, given that elevated reward sensitivity, approach motivation, and bipolar spectrum disorders are linked with elevated confidence following goal-attainment (Eisner, Johnson, & Carver, 2008; Johnson & Jones, 2009; Meyer, Barton, Baur, & Jordan, 2010). Irritability is included because of the neurobiological overlap between anger and approach motivation (Harmon-Jones, 2003; Carver & Harmon-Jones, 2009) and the increase in approach-related neural activity if goal-pursuit is thwarted (Harmon-Jones, 2003). Finally, we propose that approach-related hypo/manic symptoms may be etiologically distinct from hyperactivity symptoms observed in attention deficit hyperactivity disorder (ADHD), given that ADHD has been associated with blunted reward processing and reward-related brain function (Volkow et al., 2009). However, ADHD is characterized by significant heterogeneity and there are high levels of comorbidity between ADHD and bipolar disorder (Wingo & Ghaemi, 2007). Thus, there may be symptom dimensions that cut across both ADHD and bipolar disorder that are characterized by enhanced approach motivation. Future research is needed to test these hypotheses.
Bipolar Depression: Reward Hyposensitivity or Hypersensitivity?
Collectively, we have proposed that reward hyposensitivity should be most strongly associated with the unipolar depressive symptom of motivational anhedonia, and reward hypersensitivity should be most strongly associated with a cluster of approach-related hypo/manic symptoms. This raises the obvious and important question of what mechanisms underlie bipolar depression, and in particular, anhedonia among individuals with bipolar disorder. In its original conceptualization, the Reward Hypersensitivity Model proposed that reward hypersensitivity underlies risk for both hypo/manic and bipolar depression symptoms (e.g., Depue & Collins, 1999; see also Alloy et al., 2015). The logic of this original conceptualization was that reward hypersensitivity should make individuals hypersensitive to both cues signaling the possible attainment and loss of reward, and that in the face of loss, individuals with reward hypersensitivity should be at increased risk for depression given the high value they place on rewards (see dashed light blue pathway in Figure 1). From this perspective, reward hypersensitivity is viewed as a risk factor for excessive lability in approach motivation, with excessive increases in approach motivation (i.e., hypo/mania) occurring in response to goal striving and reward attainment and excessive decreases in approach motivation (i.e., depression) occurring in response to irreconcilable reward loss (reward loss that is perceived to be remediable and merely a temporary thwarting of reward attainment should activate approach motivation and trigger anger/irritability symptoms of hypo/mania – e.g., Carver & Harmon-Jones, 2009).
To date, however, there is rather limited evidence related to this lability perspective (Alloy & Abramson, 2010; Alloy et al., 2015; 2016; Johnson, 2005; Johnson et al., 2012b; Nusslock et al., 2014), as the data indicate that reward hypersensitivity is more strongly related to risk for hypo/manic symptoms than bipolar depression symptoms. This suggests two possibilities. The first is that there is a relationship between reward hypersensitivity and bipolar depression that researchers have yet to identify. For example, by considering bipolar depression as a homogenous or unitary construct, researchers may have missed or masked the relationship between reward hypersensitivity and subtypes of anhedonia among bipolar individuals. The prediction from this perspective is that individuals with reward hypersensitivity (i.e., individuals at risk for bipolar disorder) are at particular risk for motivational deficits in anhedonia in the face of loss or irreconcilable failure to obtain a desired reward. The second possibility, however, is that reward hypersensitivity is not related to bipolar depression and different etiological mechanisms (e.g., threat processing) may underlie the symptom of anhedonia and affective lability among individuals with bipolar disorder compared to unipolar depression. Future research is needed to test these competing hypotheses.2
Beyond Mood Disorder Symptoms: An Equifinality and Multifinality Model of Reward Processing Abnormalities
Thus far, we have focused exclusively on the relationship between reward processing and mood disorder symptoms. However, abnormalities in reward processing and fronto-striatal neural circuitry have been implicated in other psychiatric symptoms, most notably, schizophrenia (i.e., non-affective psychosis) and addiction. We next briefly review this literature. Then, integrating this work with research on reward processing and mood-related symptoms summarized in the present paper, we discuss both an equifinality and multifinality perspective on reward processing abnormalities in psychiatric symptoms.
Reward processing in schizophrenia.
Abnormalities in fronto-striatal neural circuitry and dopamine transmission have long been considered a primary pathology in schizophrenia (Howes & Kapur, 2009; Howes & Kambeitz, 2012; Fusar-Poli & Meyer-Lindenberg, 2013). The Aberrant Salience or Dopamine Hypothesis of schizophrenia argues that negative and positive symptoms result from inappropriate (as opposed to chronically reduced or enhanced) dopamine release that fails to appropriately respond to meaningful reward cues (resulting in negative symptoms), while ascribing elevated or aberrant salience to irrelevant stimuli (resulting in positive symptoms).
Support for the Aberrant Salience Hypothesis is found in studies of negative symptoms in schizophrenia, which typically involve anhedonia, decreased affective expression, reduced motivation, and self-reported reductions in pleasurable experiences (see Strauss & Gold, 2012 for review). Phenomenologically, this clinical presentation is similar to the anhedonia and motivational deficits observed in unipolar depression. However, unlike unipolar depression, there is a growing consensus that negative symptoms in schizophrenia do not reflect a primary deficit in the capacity for hedonic experience or motivation, but rather difficulty in representing the value of rewarding experiences in cognition and working memory (Gold, Waltz, Prentice, Morris, & Heerey, 2008; Gold et al., 2013). For example, despite self-reporting low positive affect and pleasurable experiences on retrospective, prospective, and hypothetical (i.e., non-current) self-reports of positive emotion (Strauss & Gold, 2012; Horan, Kring, & Blanchard, 2006; Kring & Moran, 2008), individuals with schizophrenia typically show normative affective ratings when exposed to positive stimuli in the laboratory, including positive pictures, faces, sounds, words, and food (Cohen & Minor, 2010; Herbener, Song, Khine, & Sweeney, 2008; Kring, Kerr, Smith, & Neale, 1993). Results from naturalistic experience-sampling studies provide a similar picture, indicating that although individuals with schizophrenia have a lower frequency of positive events in their daily lives (Myin-Germeys, Delespaul, & deVries, 2000), they report experiencing increases in positive emotion that are comparable to those of healthy participants when engaged in pleasurable activities (Gard, Kring, Gard, Horan, & Green, 2007; Oorschot et al., 2013). Furthermore, studies using the EEfRT task developed by Treadway and colleagues (2012a; 2012b) report that individuals with schizophrenia do not exhibit an overall reduction in effort expenditure for reward (as demonstrated in individuals with MDD), but instead fail to select high effort options at times when it is most advantageous to do so (Gold et al., 2013; Fervaha, Foussias, Agid, & Remington, 2013; Barch, Treadway, & Schoen, 2014). Complimenting these findings is growing evidence of cognitive and working memory deficits in individuals with schizophrenia during non-current reward processing (e.g., retrospective, prospective, hypothetical self-reports of rewarding experiences; Gold et al., 2008; 2013), and compromises in orbital and dorsal prefrontal structures that play a critical role in the ability to represent the value of outcomes and plans (Barch & Ceaser, 2012; Barch & Dowd, 2010; Ursu et al., 2011). This suggests that negative symptoms (i.e., anhedonia) in schizophrenia may be driven more by deficits in the ability to cognitively represent past and future rewards, as opposed to hedonic deficits in responding to and/or savoring rewards in the moment.
Also supporting the Aberrant Salience Hypothesis is considerable evidence that dopamine signaling is substantially up-regulated in positive symptoms of schizophrenia (e.g., psychosis, hallucinations, delusions; see Fusar-Poli & Meyer-Lindenberg, 2013 for meta-analytic review), as well as fMRI studies highlighting associations between aberrant striatal responses and a propensity for psychotic symptoms (see Howes & Kapur, 2009 for review). Recent work has further demonstrated both a blunting of neural prediction errors to contextually relevant cues (Morris et al., 2011) and enhanced prediction error to contextually irrelevant stimuli (Morris, Griffiths, Le Pelley, & Weickert, 2013). Collectively, these findings suggest that the pathophysiology of schizophrenia does not involve abnormally elevated or attenuated dopamine transmission, but rather the misallocation of fronto-striatal reward signaling to task inappropriate cues.
Reward processing in Addiction.
Substance use and addiction are highly comorbid with mood-related psychopathology, often having destructive consequences for one’s personal and professional life (Conway, Compton, Stinson, & Grant, 2006; Grant et al., 2004). Recent integrative models of addiction suggest that abnormalities in reward processing and fronto-striatal neural circuitry, combined with poor impulse control, act in tandem to contribute to substance use pathology (Salloum & Thase, 2000). There is debate, however, about the specific profile of reward processing that puts an individual at greatest risk for developing an addiction. The Reward Deficiency Model of addiction postulates that persons with low reward sensitivity self-medicate negative emotions and/or attempt to elevate positive/rewarding emotions through high-risk addictive behaviors (Blum et al., 2000; Volkow, Fowler, & Wang, 2003; Bowirrat, & Oscar-Berman, 2005). Consistent with this perspective, preclinical research documents that blunted dopamine signaling in the striatum is centrally involved in many addictive behaviors, including drug and alcohol addiction, as well as food seeking and obesity (Volkow et al., 2003; Volkow, Fowler, Wang, Swanson, & Telang, 2007; Bowirrat, & Oscar-Berman, 2005). In humans, cause-and-effect relationships are less clear. However, preliminary findings from neurogenetic research indicate that reduced reward-related brain function in the striatum may reflect both a pre-existing vulnerability for, as well as a consequence of, engaging in high-risk, addictive behaviors (Stice, Spoor, & Bohon, 2008).
By contrast, a reward hypersensitivity perspective of addiction argues that abnormally elevated reward sensitivity should reflect a pre-existing vulnerability for addictive behaviors (Alloy et al., 2009c; Kambouropoulos & Staiger, 2004). Given that elevated reward sensitivity leads to approach behavior in situations involving potentially rewarding stimuli, and drugs of abuse have such rewarding properties, this perspective proposes that reward hypersensitivity should lead to greater substance use and prospectively put an individual at risk for addiction. In line with this logic, cross-sectional and retrospective studies report associations between elevated self-reported reward sensitivity and increased substance use and substance use disorders (Franken & Muris, 2006; Johnson, Turner, & Iwata, 2003; Knyazev, 2004). Behavioral measures of reward sensitivity also differentiate heavy or binge drinkers from light drinkers (Colder & O’Conner, 2002; Palfai & Ostafin, 2003) and drinking for enhancement reasons from drinking for coping or social reasons (Colder & O’Conner, 2002). Finally, elevated reward sensitivity, as measured by self-report or behavioral tasks, is also predictive of greater cravings, intention to drink, and positive affective responses in alcohol cue reactivity paradigms (Franken, 2002; Kambouropoulos & Staiger, 2001).
An Equifinality and Multifinality Perspective.
As noted earlier, there is a growing interest in identifying mechanisms that are transdiagnostic or common across psychiatric disorders and symptoms (Insel et al., 2010; Insel & Cuthbert, 2015). Given that reward processing has been implicated in everything from anhedonia, to hypo/mania, schizophrenia and addiction, a reasonable conclusion is that abnormalities in reward processing are a transdiagnostic risk factor for these diverse conditions, or at least symptom clusters within these conditions. We disagree with this perspective, and instead agree with Whitton and colleagues (2015) that an equifinality and/or multifinality perspective on reward processing abnormalities in psychiatric symptoms may be preferable. As noted, equifinality is the principle that a given end state can be reached by different means or mechanisms, whereas multifinality is basically the opposite, suggesting that similar conditions or mechanisms can lead to dissimilar outcomes.
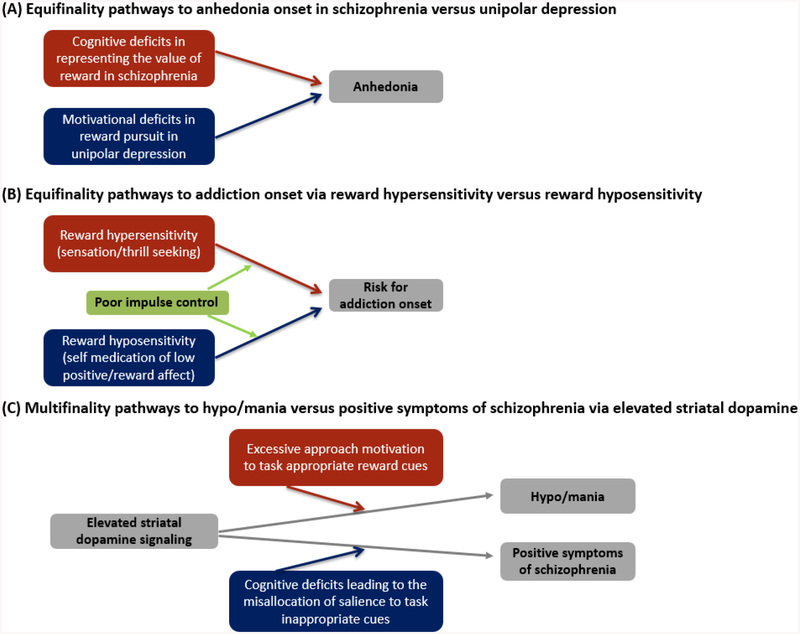
Whitton and colleagues (2015) were the first to highlight anhedonia as an example of equifinality in the context of unipolar depression and schizophrenia. They argue that although anhedonia has a similar clinical presentation in unipolar depression and schizophrenia, it is likely driven by distinct pathophysiological mechanisms across these two disorders. Anhedonia in unipolar depression is argued to be driven by a reduced capacity for hedonic experience, motivation, or decision making, whereas anhedonia in schizophrenia is argued to be a consequence of deficits in higher-order cognitive systems involved in working memory and value representation of past and future rewards (see also, Barch, Pagliaccio, & Luking, 2016) (see Figure 2A).
Figure 2.
An equifinality and multifinality model of reward processing abnormalities
We argue here that an equifinality perspective may also be relevant for understanding addiction (see Figure 2B). Instead of reflecting opposing models of addiction risk, the reward deficiency and reward hypersensitivity perspectives on addiction may instead represent different pathways to addiction onset. That is, whereas individuals with reward deficiency or hyposensitivity may initially be drawn to addictive substances to elevate deficient positive affect and/or attenuate negative affect, individuals with reward hypersensitivity may be drawn to these same substances for very different reasons, e.g., sensation and thrill seeking purposes. Once in contact with the addictive substance, the final common pathway to addiction onset (i.e., altered dopamine signaling secondary to chronic substance use; e,.g., Volkow et al., 2003) may look similar regardless of whether reward deficiency or hypersensitivity initially propelled the individual to the high-risk addictive substances. But the point here is that the end (addiction) can be reached by different means (reward hyposensitivity versus hypersensitivity). Furthermore, reward deficiency and hypersensitivity may reflect distinct mechanisms underlying elevated rates of comorbidity between addiction and both unipolar depression and bipolar disorder. Reward deficiency or hyposensitivity may reflect a common mechanism underlying elevated rates of comorbidity between addiction and unipolar depression, as individuals with reward hyposensitivity may be prone to self-medicate their low positive affect with addictive substances. By contrast, reward hypersensitivity may underlie elevated comorbidity between addiction and bipolar disorder, as individuals with reward hypersensitivity may be more likely to engage in high-risk, addictive behaviors during sensation/thrill seeking.
By contrast, we argue that the concept of multifinality is relevant for understanding the nature of the relationship between bipolar symptoms of hypo/mania and positive symptoms of schizophrenia (see Figure 2C). Both these conditions are characterized by elevated dopamine signaling in striatal circuitry (Berk et al., 2007; Fusar-Poli & Meyer-Lindenberg, 2013). In bipolar disorder, excessive striatal signaling is typically directed towards contextually appropriate reward cues in one’s environment. As discussed in the present paper, this reward hypersensitivity can then result in an excessive increase in approach- and reward-related affect, which, in the extreme, is reflected in hypo/manic symptoms (e.g., Alloy & Abramson, 2010; Johnson, 2005; Johnson et al., 2012b). By contrast, positive symptoms of schizophrenia appear to be associated with elevated reward or dopamine signaling to irrelevant or task inappropriate cues (e.g., Howes & Kapur, 2009; Morris et al., 2013). Thus, in line with the logic of multifinality, similar means (elevated striatal dopamine signaling) can lead to dissimilar outcomes (hypo/mania vs positive symptoms of schizophrenia). Furthermore, elevated striatal dopamine signaling in hypo/mania and schizophrenia may be driven, in part, by distinct pathophysiological mechanisms. Whereas elevated striatal signaling in risk for hypo/mania is associated with an abnormally elevated hedonic or motivational response to reward cues (e.g., Nusslock et al., 2014), elevated striatal signaling in schizophrenia may be driven more by cognitive deficits in the cortex that lead to the misallocation of salience to inappropriate or irrelevant stimuli (Barch, & Ceaser, 2012; Gold et al., 2008; 2013; Morris et al., 2013).
In summary, we agree with Whitton and colleagues (2015) that despite the fact that reward processing abnormalities have been observed across multiple disorders, an equifinality/mutifinality perspective on these abnormalities may be preferable than a transdiagnostic approach. Such a perspective does a better job of recognizing that reward processing is not a unitary construct, and acknowledging that a symptom observed across different disorders may be driven by distinct striatal abnormalities (equifinality), or that striatal abnormalities can lead to dissimilar outcomes across different disorders (multifinality). We, of course, acknowledge that other symptoms and systems may be better captured by a transdiagnostic perspective, but argue that in the context of reward processing, an equifinality/multifinality approach may lead to more precise models and interventions. Future research is needed to test these predictions.
Conclusion
A goal of the RDoC initiative is to identify pathophysiological mechanisms that are common across multiple psychiatric disorders, as well as mechanisms that are unique to specific psychiatric symptoms, and that reflect biosignatures of differential risk for these distinct symptom profiles (Insel et al., 2010). Here we summarize literature suggesting that the Positive Valence Systems domain of the RDoC initiative may be particularly relevant for identifying mechanisms of differential risk for specific psychiatric symptoms. In particular, we highlight research suggesting that reward hyposensitivity uniquely relates to a subtype of anhedonia characterized by motivational, as opposed to hedonic, deficits. By contrast, we propose that reward hypersensitivity is related to a cluster of hypo/manic symptoms characterized by excessive approach motivation and goal-directed activity. Future research is needed to test these predictions. Finally, we integrate this perspective with research on reward processing abnormalities and psychiatric symptoms defined broadly, with a particular focus on schizophrenia (i.e., non-affective psychosis) and addiction. We argue that the principles of equifinality (a given outcome can be reached by different means or mechanisms) and multifinality (similar means or mechanisms can lead to dissimilar outcomes) may be preferable to a transdiagnostic perspective for contextualizing future research on reward processing abnormalities and psychiatric symptoms defined broadly.
Acknowledgements
Preparation of this article was supported by National Institute of Mental Health grants MH100117 and MH077908 to Robin Nusslock and MH077908 and MH102310 to Lauren B. Alloy. We thank Virginia Hoch and Ajay Nadig for their help in preparing this manuscript.
Footnotes
Footnote
Future work is needed to examine the extent to which the BAS/reward hypersensitivity model and research on the fronto-striatal neural circuit in bipolar disorder can account for mixed episodes. Many individuals with bipolar disorder (up to 40% in some clinical samples; Swann et al., 2013) present with mixed symptoms, and these are even more common in individuals with early-onset bipolar disorder (Perlis et al., 2009). Understanding the pathophysiology of mixed states has important scientific, diagnostic, and treatment implications. We propose that mixed states may involve the co-activation of both the fronto-striatal neural circuit, as reflected in excessive approach-related affect, and the cortico-amygdala circuit, as reflected in excessive negative affect. Future research is needed to test this hypothesis.
Despite tentative evidence that unipolar depression is characterized by greater levels of anxiety and general distress, there does not appear to be clear distinctions in the symptom profiles of unipolar depression versus bipolar depression, or in how anhedonia is expressed across these two disorders (see Cuellar, Johnson, & Winters, 2010 for review).
Contributor Information
Robin Nusslock, Northwestern University.
Lauren B. Alloy, Temple University
References
- Abler B, Erk S, & Walter H (2007). Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology, 191, 823–833. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Iacono WG, Depue RA, & Arbisi P (1993). Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biological Psychiatry, 33, 642–646. [DOI] [PubMed] [Google Scholar]
- Alloy LB, & Abramson LY (2010). The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science, 19, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urošević S, Bender RE, & Wagner CA (2009a). Longitudinal predictors of bipolar spectrum disorders: A Behavioral Approach System (BAS) perspective. Clinical Psychology: Science and Practice, 16, 206–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Urošević S, Walshaw PD, Nusslock R, & Neeren AM (2005). The psychosocial context of bipolar disorder: Environmental, cognitive, and developmental risk factors. Clinical Psychology Review, 25, 1043–1075. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, … Hogan ME (2008). Behavioral approach system (BAS) and behavioral Inhibition system (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders, 10, 310–322. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, Hughes M, … Nusslock R (2006). Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation and Emotion, 30, 143–155. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, … Harmon-Jones E (2009b). Behavioral approach system (BAS) – relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. Journal of Abnormal Psychology, 118, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, … Abramson LY (2012a). High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology, 121, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, … Harmon-Jones E (2009c). Bipolar spectrum-substance use co-occurrence: behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. Journal of Personality and Social Psychology, 97, 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Boland EM, Ng TH, Whitehouse WG, & Abramson LY (2015). Low social rhythm regularity predicts first onset of bipolar spectrum disorders among at risk individuals with reward hypersensitivity. Journal of Abnormal Psychology, 124,944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Nusslock R, & Boland EM (2015). The development and course of bipolar spectrum disorders: An integrated reward and circadian rhythm dysregulation model. Annual Review of Clinical Psychology, 11, 213–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed, & Nusslock R (2016). Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behavior Therapy, 47(5), 600–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urošević S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, & Hogan M (2012b). Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121(1), 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Hassel S, Kupfer DJC, & Phillips ML (2010). Elevated amygdala activity sad facial expressions: A state marker of bipolar but not unipolar depression. Biological Psychiatry, 67, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd ed.). Arlington, VA: American Psychiatric Association Press. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Association Press. [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, & Winokur A (1987). Taste and smell perception in depression. Biological Psychiatry, 22, 1481–1485. [DOI] [PubMed] [Google Scholar]
- Barch DM, & Ceaser AE (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends in Cognitive Science, 16, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Dowd EC (2010). Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36, 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, & Luking K (2016). Mechanisms underlying motivational deficits in psychopathology: Similarities and differences in depression and schizophrenia. Current Topics in Behavioral Neurosciences, 27, 411–449. [DOI] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, & Schoen N (2014). Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. Journal of Abnormal Psychology, 123, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Saint’anna M, Malhi GS, Bourin M, Kapczinski F, & Norman T (2007). Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatrica Scandinavica, 116, 41–49. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, & Puech AJ (1998). Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. European Psychiatry, 13(6), 303–309. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Dalaney U, Kahnt T, Sajonz B, Heimann H, Ricken R, … Bauer M (2009). A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorders, 11, 70–75. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, … Heinz A (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping, 31, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology, 191, 391–431. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gil MK, Hunt J, … Keller M (2009). Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry, 166, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, & Zatorre RJ (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Science, 98, 11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, … Comings DE (2000). Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs, 32, 1–112. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M … Dagher A (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49, 226–231. [DOI] [PubMed] [Google Scholar]
- Boland EM, Smith RV, Burke TA, Bart C, Nusslock R, & Alloy LB (2016a). High Behavioral Approach System (BAS) sensitivity is associated with increased effortful pursuit of rewards at low, but not medium or high, levels of reward probability. Manuscript in preparation. [Google Scholar]
- Boland EM, Stange JP, LaBelle DR, Shapero BG, Weiss RB, Abramson LY, & Alloy LB (2016b). Affective disruption from social rhythm and behavioural approach system (BAS) sensitivities: A test of the integration of the social zeitgeber and reward theories of bipolar disorder. Clinical Psychological Science, 4, 418–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, & Oscar-Berman M (2005). Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 132, 29–37. [DOI] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50, 74–81. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Olino TM, Dyson MW, Laptook RS, Carlson GA, & Klein DN (2014). Predictors of the onset of depression in young children: A multi-method, multi-informant longitudinal study from ages 3 to 6. Journal of Child Psychology and Psychiatry, 55(11), 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CM, & Palmiter RD (2003). Reward without dopamine. Journal of Neuroscience, 23(34), 10827–10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ … Miller AH (2012): Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry, 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & Harmon-Jones E (2009). Anger is an approach-related affect: Evidence and implications. Psychological Bulletin, 135, 183–204. [DOI] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, & Phillips ML (2013). Ventral striatum activity in response to reward: Differences between bipolar I and bipolar II disorders. American Journal of Psychiatry, 170, 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Dymond S, & Cooper A (2010). Impaired flexible decision-making in major depressive disorder. Journal of Affective Disorders, 124, 207–210. [DOI] [PubMed] [Google Scholar]
- Chase H, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, & Phillips ML (2013). Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders, 15, 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaroni P, Azorin JM, Dassa D, Henry JM, Giudicelli S, Malthiery Y, & Planells R (2000). Possible involvement of the dopamine D3 receptor locus in subtypes of bipolar affective disorder. Psychiatric Genetics, 10, 43–49. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, & Mineka S (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103, 103–16. [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Cohen AS, & Minor KS (2010). Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia Bulletin, 36, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, & O’Connor R (2002). Attention biases and disinhibited behavior as predictors of alcohol use and enhancement reasons for drinking. Psychology of Addictive Behaviors, 16, 325–332. [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, & Grant BF (2006). Lifetime comorbidity of DSM–IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry, 67, 247–257. [DOI] [PubMed] [Google Scholar]
- Corwin J, Peselow E, Feenan K, Rotrosen J, & Fieve R (1990). Disorders of decision in affective disease: An effect of beta-adrenergic dysfunction? Biological Psychiatry, 27, 813–833. [DOI] [PubMed] [Google Scholar]
- Cox SM, Benkelfat C, Dagher A, Delaney JS, Durand F, McKenzie SA … Leyton M (2009). Striatal dopamine responses to intranasal cocaine self-administration in humans. Biological Psychiatry, 65, 846–850. [DOI] [PubMed] [Google Scholar]
- Cuellar AK, Johnson SL, & Winters R (2005). Distinctions between bipolar and unipolar depression. Clinical Psychology Review, 25, 307–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077. [DOI] [PubMed] [Google Scholar]
- Depue RA, & Collins PF (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–569. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon FJ, & Smoski MJ (2012). Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders, 136(3), 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, & Garbutt JC (2010). Unipolar depression does not moderate responses to the Sweet Taste Test. Depression and anxiety, 27(9), 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA (2009): Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry 66:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, … Mathis CA (2001). Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry, 49, 81–96. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, & Moerk KC (2005) Temperamental emotionality in preschoolers and parental mood disorders. Journal of Abnormal Psychology, 114, 28–37. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner L, Johnson SL, & Carver CS (2008). Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. Journal of Abnormal Psychology, 117, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, & Sahakian BJ (2004). Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biological Psychiatry, 55, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, … Silbersweig DA (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry, 163, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, … Towbin K (2004). Choice selection and reward anticipation: An fMRI study. Neuropsychologia, 42(12), 1585–1597. [DOI] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2013). Neural substrates underlying effort computation in schizophrenia. Neuroscience and Biobehavioral Reviews, 37, 649–65. [DOI] [PubMed] [Google Scholar]
- Forbes EE (2009). Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry, 66, 199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry, 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JRC, Ferrell RE, Nimgaonkar VL, Mansour H, … Phillips ML (2011). PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry, 71, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81, 1–8. [DOI] [PubMed] [Google Scholar]
- Foussias G, & Remington G (2008). Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophrenia Bulletin, 36(2), 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IHA (2002). Behavioral approach system (BAS) sensitivity predicts alcohol craving. Personality and Individual Differences, 32, 349–355. [Google Scholar]
- Franken IHA, & Muris P (2006). BIS/BAS personality characteristics and college students’ substance use. Personality and Individual Differences, 40, 1497–1503. [Google Scholar]
- Fulford D, Johnson SL, Llabre MM, & Carver CS (2010). Pushing and coasting in dynamic goal pursuit: Coasting is attenuated in bipolar disorder. Psychological Science, 21(7), 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, & Meyer-Lindenberg A (2013) Striatal presynaptic dopamine in schizophrenia, part II: 1109 meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophrenia Bulletin, 39, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinston FS, Dawson DA, Chou P, Dufour MC, Compton W, … Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry, 61, 807–816. [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry, 74, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, & Heerey EA (2008). Reward processing in schizophrenia: A deficit in the representation of value. Schizophrenia, 34, 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, & Rosenfeld JP (1998). Frontal EEG asymmetry, depression, and cognitive functioning. Cognition & Emotion, 12, 449–478. [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry, 67, 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Gilbert KE, Youngstrom E, Youngstrom JK, Feeny N, & Findling RJ (2013). Reward dysregulation and mood symptoms in an adolescent outpatient sample. Journal of Abnormal Child Psychology. 41, 1053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M (2006): Behavioral alterations in reward system function: The role of childhood maltreatment and psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, Polak T, … Fallgatter AJ (2009). Neural response to reward anticipation is modulated by Gray’s impulsivity. NeuroImage, 46, 1148–1153. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, & Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293–319. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman D, Lemus M, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry, 169, 693–703. [DOI] [PubMed] [Google Scholar]
- Harada M, Hoaki N, Tero T, Takeshi T, Hatano K, Kohno K, … Kochiyama T (2013). Hyperthymic temperament and brightness judgment in healthy subjects: Involvement of left inferior orbitofrontal cortex. Journal of Affective Disorders, 151, 143–148. [DOI] [PubMed] [Google Scholar]
- Harkness KL, & Monroe SM (2016). The Assessment and Measurement of Human Life Stress: Basic Premises, Operational Principles, and Design Requirements. Journal of Abnormal Psychology, 125, 727–745. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E (2003). Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology, 40, 838–848. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urošević S, Turonie L, … Fearn M (2008). Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry, 63, 693–698. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, & Harmon-Jones C (2002). Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology, 82, 610–618. [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, & Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, & Bernert RA (2010). Morningness-eveningness and depression: Preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Research, 176, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N Nau S, Ladouceur CD, Fissell K, … Phillips ML (2008). Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders, 10, 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Shekhar A, Nurnberger JI, O’Donnell BF, & Hetrick WP (2008). A multimethod investigation of the behavioral activation system in bipolar disorder. Journal of Abnormal Psychology, 117, 164–70. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, & Olino TM (2006). Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development & Psychopathology, 18, 409–23. [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1990). Regional brain electrical asymmetries discriminate between previously depressed and healthy controls. Journal of Abnormal Psychology, 99, 22–31. [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1991). Left frontal hypoactivation in depression. Journal of Abnormal Psychology, 100, 535–545. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Song W, Khine TT, & Sweeney JA (2008). What aspects of emotional functioning are impaired in schizophrenia? Schizophrenia Research, 98, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, & Dahl RE (2009). Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health, 45, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, & Blanchard JJ (2006). Anhedonia in schizophrenia: a review of assessment strategies. Schizophrenia Bulletin, 32, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, & Kapur S (2009). The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia Bulletin, 35, 549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, & Kapur S (2012). The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of General Psychiatry, 69, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, & Cuthbert B (2015). Brain disorder? Precisely: Precision medicine comes to psychiatry. Science, 348, 499–500. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang PW (2010). Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. American Journal of Psychiatry, 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Johnson SL (2005). Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review, 25, 241–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, & Gotlib IH (2012a). Elevated ambitions for fame among individuals diagnosed with bipolar I disorder. Journal of Abnormal Psychology. 121, 602–609. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, & Carver CS (2012b). The behavioral activation system and mania. The Annual Review of Clinical Psychology, 8, 243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, & Jones S (2009). Cognitive correlates of mania risk: Are responses to success, positive moods, and manic symptoms distinct or overlapping? Journal of Clinical Psychology, 65, 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero C, & Carver CS (2005). Cognitive, behavioral and affective responses to reward: links with hypomanic vulnerability. Journal of Social Clinical Psychology, 24, 894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, & Keitner G (2000). Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology, 109, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, & Iwata N (2003). BIS/BAS levels and psychiatric disorder: An epidemiological study. Journal of Psychopathology and Behavioral Assessment, 25, 25–36. [Google Scholar]
- Kambouropoulos N, & Staiger PK (2001). The influence of sensitivity to reward on reactivity to alcohol-related cues. Addiction, 96, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Kambouropoulos N, & Staiger PK (2004). Reactivity to alcohol-related cues: Relationship among cue type, motivational processes, and personality. Psychology of Addictive Behaviors, 18, 275–283. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Erickson K, Luckenbaugh DA, Weiland-Fiedler P, Geraci M, Sahakian BJ, … & Neumeister A (2006). Differential performance on tasks of affective processing and decision-making in patients with panic disorder and panic disorder with comorbid major depressive disorder. Journal of Affective Disorders, 95, 165–171. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, & Gotlib IH (2002). Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology, 111, 589–597. [DOI] [PubMed] [Google Scholar]
- Kazdin AE (1989). Evaluation of the pleasure scale in the assessment of anhedonia in children. Journal of American Academy of Child & Adolescent Psychiatry, 28(3), 364–372. [DOI] [PubMed] [Google Scholar]
- Kazes M, Danion JM, Grange D, Pradignac A, Simon C, Burrus-Mehl F, Schlienger JL, & Singer L (1994). Eating behaviour and depression before and after antidepressant treatment: a prospective, naturalistic study. Journal of Affective Disorders, 30(3), 193–207. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML (2005). The neural correlates of anhedonia in major depressive disorder. Biological. Psychiatry 58, 843–853. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, & Prescott CA (2003). Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry, 60(8), 789–796. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, & Gotlib IH (2008). Neural responsiveness to anticipated reward in major depression. Biological Psychiatry, 63, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12, 20–27. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G (2005). Distributed neural representation of expected value. Journal of Neuroscience, 25, 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG (2004). Behavioural activation as predictor of substance use: Mediating and moderating role of attitudes and social relationships. Drug and Alcohol Dependence, 75, 309–321. [DOI] [PubMed] [Google Scholar]
- Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, & Akiskal HS (2005). Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. Journal of Affective Disorders, 85, 181–189. [DOI] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, & Watson D (2010). Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychological Bulletin, 136(5), 768–821. [DOI] [PubMed] [Google Scholar]
- Kring AM, Kerr SL, Smith DA, & Neale JM (1993). Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. Journal of Abnormal Psychology, 102, 507–517 [DOI] [PubMed] [Google Scholar]
- Kring AM, & Moran EK (2008). Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin, 34, 819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M & Berridge KC (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Science, 13, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, & Rolls ET (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, & Steele JD (2008). Abnormal temporal difference reward-learning signals in major depression. Brain, 131, 2084–2093. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, & Smith N (2004). Dysfunctional assumptions in bipolar disorder. Journal of Affective Disorders, 79, 193–199. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, … Phillips ML (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguished patients with bipolar disorder and major depression. Biological Psychiatry, 55, 578–587. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, & Graf M (1973). Pleasant activities in depression. Journal of Consulting and Clinical Psychology, 42, 261–268. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rothstein P, & Dolan RJ (2007). Neural correlates of processing valence and arousal in affective words. Cerebral Cortex, 17, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, & Blumberg HP (2012). Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders, 14, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, & Chan RC (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220. [DOI] [PubMed] [Google Scholar]
- Lopez Leon S, Croes EA, Sayed-Tabatabaei FA, Claes S, Van Broeckhoven C, & van Duijin CM (2005). The dopamine D4 receptor gene 48-base-pair repeat polymorphism and mood disorders: A meta-analysis. Biological Psychiatry, 57, 999–1003. [DOI] [PubMed] [Google Scholar]
- Lozano BE, & Johnson SL (2001). Can personality traits predict increases in manic and depressive symptoms? Journal of Affective Disorders, 63, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Singh J, Duan Y, Luckenbaugh DA, Manji HK, & Drevets WC (2007). Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biological Psychiatry, 61, 765–775. [DOI] [PubMed] [Google Scholar]
- Maier SF, & Watkins LR (1998). Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review, 105, 83–107. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Bentall RP, & El-Deredy W (2012). Better than I thought: Positive evaluation bias in hypomania. PLOS One, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, & Cowen PJ (2012). Neural processing of reward and punishment in young people at increased familial risk of depression. Biological Psychiatry, 72(7), 588–594. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, & Harmer CJ (2009). Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry, 67, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, & Klein DN (2009). Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety, 26, 117–122. [DOI] [PubMed] [Google Scholar]
- Meehl PE (1975). Hedonic capacity: Some conjectures. Bulletin of the Menninger Clinic, 39, 295–307. [PubMed] [Google Scholar]
- Meyer B, Johnson SL, & Winters R (2001). Responsiveness to threat and incentive in bipolar disorder. Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment, 23, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Barton S, Baur M, & Jordan G (2010). Vulnerability factors for bipolar disorders as predictors of attributions in ability-based and chance-based tests. Journal of Individual Differences, 31, 29–37 [Google Scholar]
- Miller AH, Haroon E, Raison CL, & Felger JC (2013). Cytokine targets in the brain: Impact in neurotransmitters and neurocircuits. Depression and Anxiety, 30, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009): Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton Iii JL, … Fromm S (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry, 165(1), 90. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, & Lewinsohn PM (1999). Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. Journal of Abnormal Psychology, 108(4), 606–614. [DOI] [PubMed] [Google Scholar]
- Monroe SM, & Harkness KL (2005). Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review, 112(2), 417–445. [DOI] [PubMed] [Google Scholar]
- Morris R, Griffiths O, Le Pelley ME, & Weickert TW (2013). Attention to irrelevant cues is related to positive symptoms in schizophrenia. Schizophrenia Bulletin, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Vercammen A, Lenroot R, Moore L, Langton JM, Short B, … Weickert TW (2011). Disambiguating ventral striatum fMRI-related bold signal during reward prediction in schizophrenia. Molecular Psychiatry, 17, 280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, & Trinder J (2009). Nature’s clock and human mood: The circadian system modulates reward motivation. Emotion, 9, 705–716. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Delespaul PA, & deVries MW (2000). Schizophrenia patients are more emotionally active than is assumed based on their behavior. Schizophrenia Bulletin, 26, 847–854. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, & Miller GA (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology, 36, 628–637. [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, LaBarbara EJ, Klein C, & Phillips ML (2012a). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar adults. Bipolar Disorders, 14, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, & Hogan ME (2007). A goal-striving life event and the onset of hypomanic and depressive episodes and symptoms: perspective from the behavioral approach system (BAS) dysregulation theory. Journal of Abnormal Psychology, 116, 105–115. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urošević S, Goldstein KE, & Abramson LY (2012b). Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology, 121, 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuro-immune network hypothesis. Biological Psychiatry, 80, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Walden K, & Harmon-Jones E (2015). Asymmetrical frontal cortical activity a marker of differential risk for mood and anxiety disorder symptoms: An RDoC perspective. International Journal of Psychophysiology, 98, 249–261. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Young C, & Damme K (2014). Elevated reward-related neural activation as a unique biological marker of bipolar disorder. Behavioral Research and Therapy, 62, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, … Forbes EE (2014). Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience, 8, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Silk JS, Osterritter C, & Forbes EE (2015). Social Reward in Youth at Risk for Depression: A Preliminary Investigation of Subjective and Neural Differences. Journal of Child and Adolescent Psychopharmacology, 25(9), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, van Os J, Delespaul P, & Myin-Germeys I (2013). Emotional experience in negative symptoms of schizophrenia: no evidence for a generalized hedonic deficit. Schizophrenia Bulletin, 39, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Marqués E, & Lupiáñez J (2011). Alterations of the attentional networks in patients with anxiety disorders. Journal of Anxiety Disorders, 25, 888–895. [DOI] [PubMed] [Google Scholar]
- Palfai TP, & Ostafin BD (2003). Alcohol-related motivational tendencies in hazardous drinkers: Assessing implicit response tendencies using the modified-IAT. Behaviour Research and Therapy, 41, 1149–1162 [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC, & Parker LA (1997). Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacology Biochemistry and Behavior, 58(3), 801–811. [DOI] [PubMed] [Google Scholar]
- Pelizza L, & Ferrari A (2009). Anhedonia in schizophrenia and major depression: state or trait?. Annals of General Psychiatry, 8(1), 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Dennehy EB, Miklowitz DJ, DelBello MP, Ostacher M, Calabresem JR, … Sachs G (2009). Retrospective age at onset of bipolar disorder and outcome during two-year follow up: Results from the STEP-BD study. Bipolar Disorders, 11, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, & Vieta E (2007). Identifying Functional Neuroimaging Biomarkers of Bipolar Disorder: Toward DSM-V. Schizophrenia Bulletin, 33, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, & O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry, 57, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, … Fava M (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166, 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research, 43, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilty LC, Mackew L, & Bagby RM (2014). Distinct profiles of behavioral inhibition and activation system sensitivity in unipolar versus bipolar mood disorders. Psychiatry Research, 219, 228–31. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, & Allen JJB (1998). Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology, 35, 389–404. [PubMed] [Google Scholar]
- Reidlich R, Dohm K, Grotegerd D, Opel N, Zwitserhood P, Heindel W, Arolt V, Kugel H, & Dannlowski U (2015). Reward processing in unipolar and bipolar depression: A functional MRI Study. Neuropsychopharmacology, 40, 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, & Ernst M (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews, 37(5), 976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, & Mingote SM, (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology, 191, 461–482. [DOI] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, & San L (2007). The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences, 42, 1323–1331. [Google Scholar]
- Salloum IM, & Thase ME (2000). Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disorders, 2, 269–280. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, … Wolf DH (2015). Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology, 40, 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Minkel J, Smoski MJ, & Dichter GS (2013). Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. Journal of Affective Disorders, 151(2), 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Clery-Melin ML, Lafargue G, Valabrègue R, Fossati P, Dubois B, & Pessiglione M (2009). Get aroused and be stronger: emotional facilitation of physical effort in the human brain. Journal of Neuroscience, 29, 9450–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1,199–207. [DOI] [PubMed] [Google Scholar]
- Schultz W (2002). Getting formal with dopamine and reward. Neuron, 36, 241–263. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, & Hollerman JR (2000). Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex, 10, 272–284. [DOI] [PubMed] [Google Scholar]
- Scott J, Stanton B, Garland A, & Ferrier IN (2000). Cognitive vulnerability in patients with bipolar disorder. Psychological Medicine, 30, 467–472. [DOI] [PubMed] [Google Scholar]
- Sharp CR, Kim S, Herman L, Pane H, Reuter T, & Strathearn L (2014). Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. Journal of Abnormal Psychology, 123(2), 298–309. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, & Jones-Gotman M (2001). Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain, 124, 1720–1733. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, & Dichter GS (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Kumar P, & Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain, 130, 2367–2374. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, & Small DM (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322, 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, & Gold JM (2012), A new perspective on anhedonia in Schizophrenia. American Journal of Psychiatry, 169, 364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lafer B, Perugi G, Frye MA, Bauer M, Bahk WM, Scott J, Ha K, & Suppes T (2013). Bipolar mixed states: an international society for bipolar disorders task force report of symptom structure, course of illness, and diagnosis. American Journal of Psychiatry, 170, 31–42. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, & Moeller FG (2009). Severity of bipolar disorder is associated with impairment of response inhibition. Journal of Affective Disorders, 116, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Yucel M, Lorenzetti V, Nakamura K, Whittle S, Walterfang M, … Allen N (2009). Midline brain structures in patients with current and remitted major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(6), 1058–1063. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S (2006). Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology, 115, 715–729. [DOI] [PubMed] [Google Scholar]
- Treadway MT (in press). The Neurobiology of Motivational Deficits in Depression – An Update on Candidate Pathomechanisms. Current Topics in Behavioral Neuroscience. [DOI] [PubMed] [Google Scholar]
- Treadway MT & Zald DH (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35, 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, & Zald DH (2012a) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. Journal of Abnormal Psychology, 121(3), 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, & Zald DH (2012b). Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of Neuroscience, 32(18), 6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, & Alloy LB (2008). Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review, 28, 1188–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Alloy LB, Nusslock R, Harmon-Jones E, Bender R, & Hogan ME (2010). Increased rates of events that activate or deactivate the behavioral approach system, but not events related to goal attainment, in bipolar spectrum disorders. Journal of Abnormal Psychology, 119, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, Solomon M, & Carter CS (2011). Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. American Journal of Psychiatry, 168, 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, & Wang GJ (2003). The addicted human brain: Insights from imaging studies. Journal of Clinical Investigation, 111, 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, & Telang F (2007). Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Archives of Neurology, 64, 1575–1579. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C … Pappas NR (1999). Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. Journal of Pharmacology and Experimental Therapeutics, 291, 409–415. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, … & Swanson JM (2009). Evaluating dopamine reward pathway in ADHD, Journal of American Medical Association, 302, 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, & Pizzagalli DA (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage, 46, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H, Sauer H, & Schlösser RGM (2006). Cortical inefficiency in patients with unipolar depression: An event-related fMRI study with the stroop task. Biological Psychiatry, 59, 958–965. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, & de Wit H (2011) Amping up effort: effects of d-amphetamine on human effort-based decision-making. The Journal of Neuroscience, 31(46), 16597–16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, & Pizzagalli DA (2015). Reward Processing Dysfunction in Major Depression, Bipolar Disorder, and Schizophrenia. Current Opinion In Psychiatry, 28(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo A, & Ghaemi SN (2007). A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. Journal of Clinical Psychiatry, 11, 1776–1784. [DOI] [PubMed] [Google Scholar]
- Wise RA (1980). Action of drugs of abuse on brain reward systems. Pharmacology Biochemistry and Behavior, 13, 213–223. [DOI] [PubMed] [Google Scholar]
- Wise RA (2002). Brain reward circuitry: insights from unsensed incentives. Neuron,36, 229–240. [DOI] [PubMed] [Google Scholar]
- Yang XH, Huang J, Zhu CY, Wang YF, Cheung EFC, Chan RCK, & Xie GR (2014) Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Research, 220(3), 874–882. [DOI] [PubMed] [Google Scholar]
- Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, & Menon V (in press). Anhedonia and General Distress Show Dissociable Ventromedial Prefrontal Cortex Connectivity in Major Depressive Disorder. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]