Abstract
Background.
Resection has been the standard of care for patients with solitary hepatocellular carcinoma (HCC). Transarterial embolization and percutaneous ablation are alternative therapies often reserved for suboptimal surgical candidates. Here we compare long-term outcomes of patients with solitary HCC treated with resection versus combined embo-ablation.
Methods.
We previously reported a retrospective comparison of resection and embo-ablation in 73 patients with solitary HCC <7 cm after a median follow-up of 23 months. This study represents long-term updated follow-up over a median of 134 months.
Results.
There was no difference in survival among Okuda I patients who underwent resection versus embo-ablation (66 vs 58 months, p = .39). There was no difference between the groups in the rate of distant intrahepatic (p = .35) or metastatic progression (p = .48). Surgical patients experienced more complications (p = .004), longer hospitalizations (p < .001), and were more likely to require hospital readmission within 30 days of discharge (p = .03).
Conclusion.
Over a median follow up of more than 10 years, we found no significant difference in overall survival of Okuda 1 patients with solitary HCC <7 cm who underwent surgical resection versus embo-ablation. Our data suggest that there may be a greater role for primary embo-ablation in the treatment of potentially resectable solitary HCC.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. Its prevalence and high ratio of mortality to incidence (0.93) make HCC the third most common global cause of cancer death, second only to lung and gastric cancer.1 Hepatitis C virus (HCV) is the primary risk factor in Europe, Japan, and North America. The epidemic history of HCV in the United States is linked to intravenous opiate use in the 1960s coupled with contaminated blood transfusions resulting in an exponential rise in HCV infection that peaked at 3.6 million in 2001.2 Between 25 and 35 % of chronically infected HCV patients will develop cirrhosis, and among those the risk of hepatocellular carcinoma has been estimated at 3–5 % per year.3 This figure is expected to rise because of the increasing age of the HCV-infected cohort and increasing prevalence of diabetes and nonalcoholic steatohepatitis (NASH), each representing an independent risk factor for HCC.4–6 The incidence of HCC is projected to peak between 2020 and 2030, and contemporary demographic trends anticipate an older patient population with increased comorbidity.7–9
While surgical resection has long been the standard of care for patients with solitary HCC, unfavorable tumor location, insufficient hepatic reserve, or other comorbidities preclude surgery in many patients.10 Hepatic artery embolization and percutaneous tumor ablation have been used alone or in combination for unresectable disease.11–15 Here we compare long-term outcomes between patients with solitary HCC up to 7 cm in diameter who were treated with surgical resection or combined embolization and ablation (embo-ablation).
MATERIALS AND METHODS
Patients
A preliminary study of this cohort was published in 2005.16 Institutional Review Board waiver was obtained for this retrospective study. A query of a HIPAA-compliant Memorial Sloan-Kettering surgical database from January 1996 to August 2002 retrieved 372 new HCC patient consultations. Of these, 73 had solitary lesions measuring up to 7 cm in diameter. Based on Disease Management Team (DMT) consensus, 40 patients were selected for surgical resection and 33 were referred to Interventional Radiology (IR) for embo-ablation. Of the 33 referred for embo-ablation, 16 were considered poor surgical candidates because of advanced cirrhosis diagnosed either preoperatively (13 patients) or at laparoscopy (3 patients). Three elderly patients (ages 79–91) with coronary vascular disease and 3 patients with poor pulmonary reserve (1 post-pneumonectomy, 1 with severe emphysema, and 1 patient with synchronous NSCLC) were considered high risk for surgery. In 3 patients tumor resection would have required excessive functional parenchymal loss. A single patient who was a surgical candidate opted for less-invasive therapy and 1 patient with a 2.5-cm HCC was referred to IR with the goal of minimizing parenchymal loss in consideration of his young age (48 years) and likelihood of recurrence. Three patients were referred directly from a medical oncologist, and no documentation is available for 3 patients. Patient demographic information is presented in Table 1.
TABLE 1.
Patient demographics
| Characteristic | Surgery (N = 40) |
Embolization (N = 33) |
p value* |
|---|---|---|---|
| Age, years, median (range) | 65.5 (26–78) | 71 (31–91) | .04 |
| Sex | .55 | ||
| Male | 6 (15 %) | 7 (21 %) | |
| Female | 34 (85 %) | 26 (79 %) | |
| Okuda stage | .0004 | ||
| 1 | 40 (100 %) | 24 (73 %) | |
| 2 | 0 (0 %) | 9 (27 %) | |
| Modified BCLC stage** | .43 | ||
| 0 | 1 (2 %) | 1 (3 %) | |
| A1 | 16 (40 %) | 16 (48 %) | |
| A2 | 2 (5 %) | 4 (12 %) | |
| B | 21 (53 %) | 12 (36 %) | |
| Childs–Pugh class | .04 | ||
| A | 39 (100 %) | 29 (88 %) | |
| B | 0 (0 %) | 4 (12 %) | |
| NA | 1 | ||
| Tumor size, median (range) | 5 (1.8–6.8) | 3.8 (1.7–7) | .05 |
| ≤3 cm | 7 (18 %) | 11 (33 %) | |
| 3.1–4.2 cm | 9 (22 %) | 9 (27 %) | |
| 4.3–5.5 cm | 12 (30 %) | 7 (21 %) | |
| 5.6–7 cm | 12 (30 %) | 6 (18 %) |
Fisher exact or Wilcoxon rank sum test
Modified BCLC stage incorporates the data available retrospectively, including Child Pugh score, serum bilirubin and presence/absence of portal hypertension. Functional status, a key component of BCLC stage was not available for all patients and was not included
Treatment and Follow-up
Surgical resections included 6 hemi-hepatectomies and 34 partial hepatectomies/segmentectomies. Our technical approach to HCC resection and embolization has been described.16 Percutaneous tumor radiofrequency ablation (RFA) or ethanol injection (PEI) was performed on the day following embolization. The ablative modality (RFA or PEI) was selected at the operator’s discretion; 11 patients underwent RFA and 22 underwent PEI.
Patients were followed at intervals of 3–6 months with multiphase CT or MRI for evidence of disease residual or progression. “Residual” was defined as nodular enhancement within or at the margin of the treated tumor. “Progression” was defined as new intrahepatic disease or extrahepatic metastasis. The presence of residual disease or disease progression was determined by consensus of 2 ABR-certified radiologists (EE, AC) who reviewed all follow-up imaging independently. One patient in the surgery group for whom no imaging was available was excluded from analysis. In total, 558 follow-up studies were reviewed.
Statistical Analyses
The distribution of baseline variables between the surgery and the embo-ablation groups was compared using Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
Overall survival was defined as the time from the initial procedure (either surgical resection or embo-ablation) until the date of death or the date of last follow-up. Median survival was estimated by the Kaplan–Meier method, and median survival was calculated using the reverse Kaplan–Meier. Survival distributions were compared between groups using the log-rank test. Cox regression was used to assess the association between continuous variables and overall survival. To analyze the association between overall survival and factors that were not yet known at the time of procedure such as length of hospital stay, 30-day readmission and major complications within 30 days, estimates of survival was landmarked from 30 days after the procedure. No patient had less than 30 days of follow-up.
Several disease endpoints were compared: time to recurrence, intrahepatic and extrahepatic progression, and time to first salvage therapy. Follow-up was calculated from the date of initial treatment until the date of detection of the event of interest (i.e., either recurrence or progression) or the date of last follow-up. Death without the recurrence or progression was treated as a competing event. The rate of the event of interest was estimated by the cumulative incidence function, and differences between groups were compared by Gray’s test.17 The proportion of extrahepatic progression events by 18 months were estimated, where those who did not progress but were not followed for at least that length of time were excluded and those who progressed after 18 months were considered to have no progression. The proportions between groups were compared by Fisher exact test. We also compared the 2 groups on the number of salvage treatments among those who received them using Wilcoxon rank sum test.
Statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). Competing risk analysis was performed in R version 2.11.1 (www.R-project.org) using library “cmprsk.”18
RESULTS
Survival
Median follow-up was 134 months (95 % CI 105–142 months). Median survival was 66 months for the surgery group and 54 months for the embo-ablation group (p = .10; Fig. 1). To control for Okuda stage we compared survival between Okuda I patients in the surgical (40 of 40) and embo-ablation (24 of 33) groups. Survival of Okuda I embo-ablation patients was superior to that of surgical patients until 4 years following treatment, at which point the trend was reversed. Overall survival between Okuda I patients undergoing primary resection or embo-ablation was similar (66 vs 58 months, p = .39).
FIG. 1.
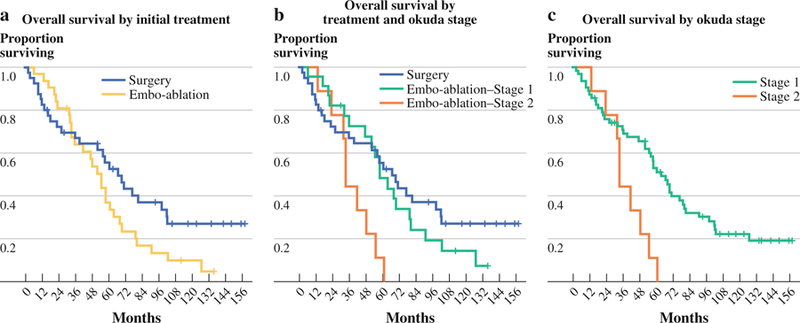
Survival. Median follow-up was 134 months. a Overall survival by initial treatment. b Overall survival by treatment and Okuda stage. c Overall survival by Okuda stage
The prognostic impact of Okuda stage was examined by comparing all Okuda I patients to the 9 Okuda II patients. Okuda II patients fared worse, with a median survival of 33 versus 63 months among Okuda I patients (p = .005). The prognostic impact of Okuda II stage was significant even within the embo-ablation group (median survival 33 vs 58 months, p = .007).
At 9 years (108 months) follow-up, 3 of the 24 Okuda I embo-ablation patients and 7 of the 40 Okuda I surgical patients were alive. No Okuda II patient survived longer than 60.5 months.
Disease Recurrence
Of 33 embo-ablation patients, 23 required retreatment for residual disease. As might be expected, residual tumor was rare following segmentectomy (3 of 40). The rate of distant intrahepatic progression was not different between the groups (p = .35; Fig. 2a).
FIG. 2.
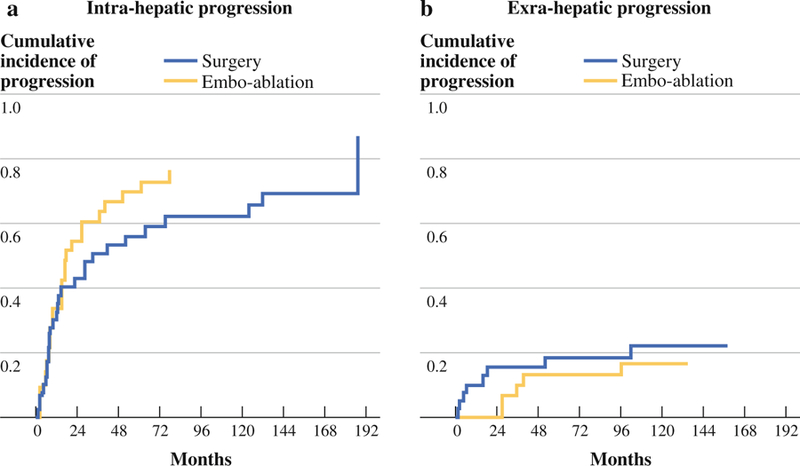
Disease recurrence. a Intrahepatic disease progression. b Extrahepatic disease progression
A total of 8 surgical patients and 5 embo-ablation patients developed extrahepatic metastases. Of the 8 surgical patients who developed extrahepatic metastases, 4 progressed within 6 months and died within 1 year and a total of 6 (75 %) progressed by 18 months. The first incident of metastatic disease among the embo-ablation patients occurred after 28 months. Metastatic progression was significantly more likely among surgical patients at 18 months (p = .03; Fig. 2b), but not across all time points (p = .48; Fig. 2b).
Salvage Therapy
Embo-ablation patients were more likely to require salvage therapy (p < .0001). In total, 39 patients underwent salvage embolization: 25 in the embo-ablation group and 13 in the surgical group. Also, 7 patients underwent surgical salvage therapy: 3 in the embo-ablation group and 4 in the surgical group. Regardless of initial therapy received, all patients who required salvage therapy underwent similar numbers of treatments: median number of salvage treatments was 2.0 (range 1–7) for the surgery group and 2.0 (range 1–8) in the embo-ablation group (p = .75)
Morbidity and Hospital Stay
Periprocedural complications are classified as per the Society for Interventional Radiology Standards of Practice.19 Surgical patients were significantly more likely to experience periprocedural complications (p = .004) as well as complications requiring hospital readmission within 30 days of discharge (p = .03; Table 2). There were 2 (6 %) major periprocedural complications encountered following embo-ablation; both were treated with IV antibiotics. There were 14 major perisurgical complications (35 %), most of which represented infections requiring IV antibiotics. Noninfectious complications included 1 minor CVA, a minor inferior wall intraprocedural myocardial infarction, 1 pulmonary embolus, and 1 episode of postoperative pulmonary insufficiency requiring extended mechanical ventilation. Of 6 surgical patients readmitted within 30 days of discharge, 1 presented with portal vein thrombosis, 4 had new ascites with or without wound infection, and 1 patient presented with UTI. Readmission within 30 days of discharge was a significant predictor of early mortality (p = .005; Table 3). Median survival for the 6 patients readmitted within 30 days of surgery was 13 months, compared with 59 months for patients who did not require readmission (p = .003; Table 2). No embo-ablation patient was readmitted within 30 days of discharge.
TABLE 2.
Hospital stay and periprocedural complications
| Surgery (N = 40) | Embolization (N = 33) | p value* | |
|---|---|---|---|
| Hospital stay (days), median (range) | 7 (4–22) | 3 (1–8) | <.0001 |
| 30-day hospital readmission | .03 | ||
| No | 34 (85 %) | 33 (100 %) | |
| Yes | 6 (15 %) | 0 (0 %) | |
| Periprocedural major complications | .004 | ||
| No | 26 (65 %) | 31 (94 %) | |
| Yes | 14 (35 %) | 2 (6 %) |
Fisher exact or Wilcoxon rank sum test
TABLE 3.
Univariate survival analysis
| Total | No. died | Median survival (months) | 95 % CI | p value* | |
|---|---|---|---|---|---|
| Age | 73 | 54 | .02 | ||
| Sex | .52 | ||||
| Female | 13 | 10 | 56 | 32–61 | |
| Male | 60 | 44 | 60 | 36–77 | |
| Okuda stage | .005 | ||||
| 1 | 64 | 45 | 63 | 52–79 | |
| 2 | 9 | 9 | 33 | 13–54 | |
| Child–Pugh class | .08 | ||||
| A | 68 | 49 | 60 | 47–71 | |
| B | 4 | 4 | 44 | 13–58 | |
| Tumor size | 73 | 54 | .05 | ||
| Length of hospital stay | 73 | 54 | .8 | ||
| 30-day hospital readmission | .005 | ||||
| No | 67 | 48 | 59 | 47–68 | |
| Yes | 6 | 6 | 13 | 2–70 | |
| Major complications within 30 days | .9 | ||||
| No | 57 | 40 | 56 | 40–67 | |
| Yes | 16 | 14 | 67 | 17–97 |
p value for log-rank test for categorical variables and Cox regression model for continuous variables
Postsurgical hospital stay (median 7 days; range 4–22 days) was significantly longer than that following embo-ablation (median 3 days; range 1–8 days; p < .0001).
DISCUSSION
The present study is the first to compare long-term outcomes of HCC patients treated surgically or with embo-ablation for curative intent. In 2006, Chen et al.12 reported equivalent survival and disease progression among patients randomized to surgery or ablation for small (<5 cm) solitary HCC. That study was limited by a short median follow-up interval of 26 months and strict inclusion criterion that would have excluded 22 of our 33 embo-ablation patients because of their age, tumor size, or Child–Pugh score.12 Studying RFA for small (<2 cm) HCC over a median follow-up of 31 months, Livraghi et al. 20 demonstrated 97.2 % complete response and 5-year survival of 68.5 %, arguing that RFA may be the treatment of choice in patients with potentially resectable <2 cm HCC. The same conclusion was drawn in a 2012 retrospective study of 145 patients with resectable <2 cm HCC, of whom 71 received RFA and 74 underwent resection.21 Each of these studies found significantly reduced morbidity and hospital stay for patients treated with ablation versus surgery. Such observations formed the basis for Forner et al’s 2012 revision22 to the BCLC schema, replacing surgical resection with ablation as first-line treatment for solitary lesions <2 cm.
Our study expands on these reports in key respects, including the benefit of a long median follow-up interval of 134 months. We treated larger lesions (<7 cm), making our results relevant to a greater proportion of HCC patients. Our selection criteria were also sufficiently broad that no patient with a solitary HCC <7 cm was excluded from undergoing embo-ablation. In fact the majority of patients treated with embo-ablation in our series were considered poor surgical candidates because of underlying comorbidity.
The decision to investigate intrahepatic and extrahepatic progression independently is rooted in our emerging awareness that surgery and ablation generate distinct regional or systemic immunomodulatory effects that may affect tumor progression. Goldberg and Rozenblum et al. 23,24 recently described regenerative changes in both treated and contralateral liver lobes in animal RFA models. Van der Bij et al.25 identified wound-healing factors essential to postoperative recovery and paradoxically, metastatic progression. Others have associated the risk of postoperative metastasis to the degree of intraprocedural trauma and blood loss.26,27 Observations like these offer some explanation as to why patients undergoing minimally invasive embo-ablation may be less likely to develop metastatic disease in the early postoperative period.
Our study limitations include all those inherent to a retrospective, nonrandomized investigation. Our conclusions must also be framed in the context of a relatively small sample size of 73 patients. In addition, the benefits of our long-term study come at the expense of studying outdated technique. This is particularly true with regard to our ablative protocol, and the high rate of marginal tumor recurrence following embo-ablation reflects this. In our current practice, thermal ablation would be delivered via multiple probes to treat lesions >5 cm and PEI is used sparingly. Furthermore, contemporary studies would typically establish prognostic staging according to BCLC rather than Okuda staging.
In conclusion, over a median follow-up of 134 months, we found no difference in survival or intrahepatic disease progression among patients who underwent surgical resection compared with embo-ablation for the primary treatment of solitary HCC <7 cm. Embo-ablation was associated with significantly shorter hospital admissions and fewer complications. As widespread screening programs detect earlier-stage HCC among an aging HCV-infected cohort, primary embo-ablation with curative intent may serve an increasingly important role.28–30
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Hepatitis C virus infection in the United States. J Hepatol 1999;31 Suppl 1:88–91. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35–50. [DOI] [PubMed] [Google Scholar]
- 4.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology 2004;127:1372–80. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369–80. [DOI] [PubMed] [Google Scholar]
- 7.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138:513–21, 521.e1–6. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817–23. [DOI] [PubMed] [Google Scholar]
- 9.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Unresectable hepatocellular carcinoma: meta-analysis of arterial embolization. Radiology 2004;230:300–1; author reply 301–2. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol 2006;16:661–9. [DOI] [PubMed] [Google Scholar]
- 14.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 2009;252:905–13. [DOI] [PubMed] [Google Scholar]
- 15.Takaki H, Yamakado K, Uraki J, Nakatsuka A, Fuke H, Yamamoto N, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol 2009;20:217–24. [DOI] [PubMed] [Google Scholar]
- 16.Maluccio M, Covey AM, Gandhi R, Gonen M, Getrajdman GI, Brody LA, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol 2005;16:955–61. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 18.R: a language and environment for statistical computing Available at: http://www.R-project.org. Accessed July 2012.
- 19.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199–202. [DOI] [PubMed] [Google Scholar]
- 20.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–9. [DOI] [PubMed] [Google Scholar]
- 21.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 2012;262:1022–33. [DOI] [PubMed] [Google Scholar]
- 22.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg SN. Science to practice: what do molecular biologic studies in rodent models add to our understanding of interventional oncologic procedures including percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists? Radiology 2012;262:737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozenblum N, Galun E, Zeira E, Nissenbaum I, Goldberg S. Abstract No. 16: The global effect of RF ablation throughout the liver. J Vasc Interv Radiol 2012;23:S10–S11. [Google Scholar]
- 25.van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 2009;249:727–34. [DOI] [PubMed] [Google Scholar]
- 26.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860–9; discussion 869–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 2008;248:1–7. [DOI] [PubMed] [Google Scholar]
- 28.Leykum LK, El-Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol 2007;5:508–12. [DOI] [PubMed] [Google Scholar]
- 29.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22. [DOI] [PubMed] [Google Scholar]
- 30.Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg 2008;247:666–73. [DOI] [PubMed] [Google Scholar]


