Abstract
The gut microbiome is increasingly implicated in the regulation of social behavior across model organisms. In this issue of Neuron, Sgritta et al. (2018) examine the role of the gut microbiome in social reward circuits and sociability in three mouse models of autism spectrum disorder.
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by impaired social communication and repetitive behavior. Alterations in the gut microbiome have been increasingly implicated in ASD. Subsets of individuals with ASD exhibit comorbid medical conditions, including immune dysregulation and gastrointestinal issues, and the gut microbiota plays a fundamental role in regulating immune homeostasis and intestinal function (Vuong and Hsiao, 2017). In addition, evidence suggests that the gut microbiome responds to host genetics and mediates the effects of various environmental exposures, including stress, immune activation, and altered diet, on host brain and behavior; some of these environmental factors have been associated with increased risk for ASD. Finally, animal studies report that germ-free rearing, antibiotic depletion, or gnotobiotic modification of the gut microbiome alters social behavior across mice, rats, zebrafish, and flies. Altogether, these findings raise the question of whether alterations in the gut microbiome may contribute to the manifestation of ASD-associated symptoms and whether targeted manipulation of the gut microbiome can be used as a tractable strategy to ameliorate ASD-associated behavioral and physiological abnormalities.
In this issue of Neuron, Sgritta et al. (2018) from the laboratory of Dr. Mauro Costa-Mattioli extend their previous work reporting that the indigenous gut bacterium Lactobacillus reuteri improves abnormalities in sociability and preference for social novelty in offspring born to dams fed a high-fat diet (Buffington et al., 2016). Sgritta et al. now test whether L. reuteri similarly promotes social behavior in three etiologically distinct mouse models for ASD: (1) Shank3B−/− mice, which model genetic polymorphisms in SHANK3B that have been linked to Phelan-McDermid syndrome, a syndromic form of ASD (Peça et al., 2011); (2) maternal valproic acid (VPA) injection, which models epidemiological findings that maternal exposure to the antiepileptic drug VPA predisposes offspring to features of ASD (Nicolini and Fahnestock, 2018); and (3) BTBR mice, a naturally occurring strain known to exhibit reduced sociability compared to mice from other genetic backgrounds. Sgritta and co-authors validate that all three mouse models display reduced sociability. The BTBR model also exhibits altered preference for social novelty compared to controls, but the Shank3B−/− and VPA models do not. In addition, each mouse model displays alterations in the composition of the gut microbiota compared to littermate controls. However, the specific bacterial taxonomic changes in the gut microbiota vary across animal models. This is consistent with the lack of a common microbial signature across several studies of human ASD individuals reporting changes in the gut microbiota in cohorts of ASD compared to neurotypical controls (Vuong and Hsiao, 2017). Overall, these characterizations reveal that the three mouse models are phenotypically distinct, which supports the researchers’ aim to interrogate the effects of L. reuteri treatment across ASD models with differing levels of face and construct validity.
Notably, Sgritta and colleagues find that treating mice for 4 weeks with live L. reuteri ameliorates abnormalities in sociability across all three mouse models. This occurs despite the finding that levels of L. reuteri are normally reduced in the Shank3B−/− and BTBR mice, but not in offspring of mothers treated with VPA. Interestingly, monocolonizing germ-free mice with L. reuteri in the absence of any other bacterial colonization sufficiently promotes social behavior, suggesting that the effect is conferred by L. reuteri itself rather than its interactions with other members of the gut microbiota. Consistent with this, L. reuteri treatment has no overt impact on the composition of the endogenous gut microbiota, and the authors’ previous work (Buffington et al., 2016) reported that only live, not heat-killed, L. reuteri alters social behavior. This study tested the effects of L. reuteri relative to vehicle-treated controls on social behavior, leaving open the question of whether any other bacterial species or communities can similarly promote social behavior in mice. Notably, a separate study revealed that the colonization of pregnant dams with the murine gut bacterium segmented filamentous bacterium sufficiently induced abnormal social, communicative, and repetitive behavior in offspring of the maternal immune activation (MIA) model for ASD (Kim et al., 2017). Another study demonstrated that the bacterium Bacteroides fragilis ameliorates communicative, sensori-motor, and stress-induced behavior in the MIA model, but has no effect on social behavior (Hsiao et al., 2013). Whether L. reuteri specifically modifies social behavior or whether it also alters other behavioral abnormalities associated with ASD remains unclear.
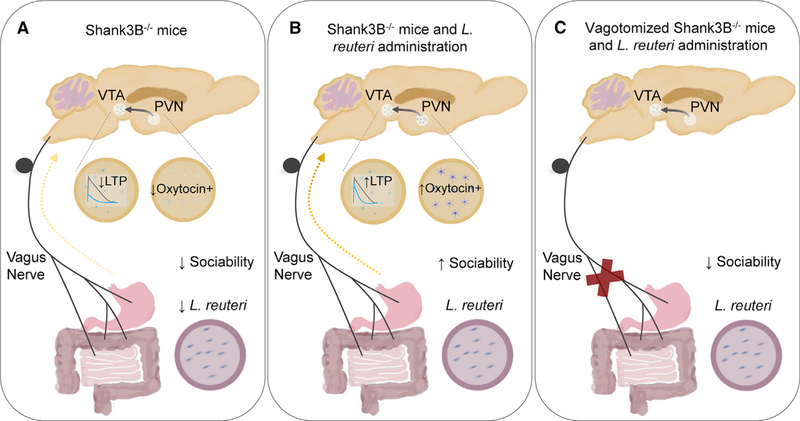
To further interrogate potential pathways for communication between the gut microbiome and brain, Sgratti et al. test the hypothesis that signaling through the vagus nerve may be involved. The authors find that bilateral subdiaphragmatic vagotomy abrogates the ability of L. reuteri to restore sociability and reciprocal social interaction in Shank3B−/− mice (Figure 1). This is consistent with recent findings that signaling through intestinal afferent sensory neurons mediates the effects of gut endocrine, immune, and dietary sensory signals on brain activity (Han et al., 2018; Kaelberer et al., 2018). Together, these results reveal that the vagus nerve is required for social behavioral modification by L. reuteri, and further suggest that future studies examining how select microbes and microbial factors modulate intestinal sensory neuronal activity may uncover novel molecular targets for altering social behavior.
Figure 1. L. reuteri Modulates Social Behavior in Autism Spectrum Disorder Mouse Models through the Vagus Nerve.
(A) Sgritta et al. (2018) show that select ASD mouse models exhibit reduced relative abundances of L. reuteri in the fecal microbiota, decreased oxytocin expression in the paraventricular nucleus (PVN), decreased long-term potentiation (LTP) in ventral tegmental area (VTA) dopamine (DA) neurons, and reduced social behavior.
(B) L. reuteri administration to Shank3B−/− mice increases oxytocin expression in the PVN, LTP in the VTA, and sociability.
(C) Subdiaphragmatic vagotomy of Shank3B−/− mice abrogates the effects of L. reuteri on sociability.
Sgratti and colleagues next move downstream of the vagus nerve to examine microbial effects on brain areas important for social behavior, including the paraventricular nucleus (PVN) of the hypothalamus and the ventral tegmental area (VTA) of the midbrain. During social interactions, the neuropeptide oxytocin is released from the PVN, which increases the activity of dopamine neurons in the VTA. Social reinforcement is then obtained by coordinated actions of dopamine, oxytocin, and serotonin on respective receptors in the nucleus accumbens (Hung et al., 2017). Interestingly, the researchers find that L. reuteri treatment promotes oxytocin expression in the PVN and long-term potentiation (LTP) in dopaminergic neurons in the VTA of Shank3B−/− mice (Figure 1). To determine whether oxytocin signaling mediates the effects of L. reuteri on sociability in Shank3B−/− mice, the authors performed gain- and loss-of-function experiments. Intranasal oxytocin administration sufficiently promotes sociability in Shank3B−/−, BTBR, VPA, and vagotomized Shank3B−/− mice. Moreover, either knocking out the oxytocin receptor in dopaminergic neurons or pharmacologically blocking the oxytocin receptor by intranasal injection of the antagonist L-371,257 prevents the ability of L. reuteri to modulate social behavior and LTP in the VTA. Overall, these results suggest that L. reuteri increases sociability in mice at least in part by promoting oxytocin expression and signaling and modulating synaptic plasticity of VTA DA neurons. These findings lay the groundwork for determining whether modulating LTP in the VTA is sufficient to enhance sociability in mice and whether L. reuteri treatment impacts any other components of social reward circuits. More broadly, these results motivate continued efforts to dissect how microbial factors modulate neuronal circuits underlying complex host behaviors.
The findings by Sgratti et al. provide pre-clinical evidence supporting a microbe-based treatment for social behavioral impairments in mouse models and further inspire investigation into the safety and efficacy of translating the work to human ASD. While the prospect of applying a live biotherapeutic approach to treating behavioral disorders is exciting, there are several open questions that require attention. Does L. reuteri influence other brain circuits, behaviors, or host physiologies in addition to social behavior? Would L. reuteri confer alternative outcomes in the context of other ASD-associated symptoms and co-morbidities, or when applied chronically versus acutely? Can particular microbial factors be identified from L. reuteri that mediate its effects on behavior, and would these offer novel molecular targets for pharmacological intervention? Continued exploration into the molecular basis for signaling across the microbiome-gut-brain axis could uncover fundamental principles for interactions across organ systems, with the potential to inform future strategies for modifying symptoms of disease.
REFERENCES
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, and Costa-Mattioli M (2016). Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, et al. (2018). A neural circuit for gut-induced reward. Cell 175, 665–678.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, and Malenka RC (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, and Bohórquez DV (2018). A gut-brain neural circuit for nutrient sensory transduction. Science 361, 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, and Huh JR (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolini C, and Fahnestock M (2018). The valproic acid-induced rodent model of autism. Exp. Neurol 299 (Pt A), 217–227. [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, and Feng G (2011). Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472, 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, and Costa-Mattioli M (2018). Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101, this issue, 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, and Hsiao EY (2017). Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 81, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]