Abstract
Background:
Mucosal melanomas are rare and aggressive neoplasms, with little published population-based data on predictors of survival.
Objective:
To assess the influences of race/ethnicity, sex, tumor stage, tumor thickness, and anatomic site on mucosal melanoma survival estimates.
Methods:
We analyzed 132,751 cases of melanoma, including 1,824 mucosal melanomas, diagnosed between 1994 and 2015 and reported to the California Cancer Registry. Kaplan-Meier survival analysis and Cox proportional hazards regression assessed the prognostic variables.
Results:
The 5-year relative survival for mucosal melanomas (27.64%, 95% confidence interval [CI] 25.42 – 29.91) was significantly lower than for cutaneous melanomas (76.28%, 95% CI 76.03 – 76.53). Stage independently influenced survival, and thickness did not predict survival for neoplasms of known depth. Less common anatomic sites conferred worse prognoses (hazard ratio [HR] 1.93, 95% CI: 1.41 – 2.64).
Limitations:
Lack of a standardized staging system may have resulted in misclassification of stage for some neoplasms. The influence of genetics is unknown because our database did not contain genetic characteristics.
Conclusions:
Stage and anatomic site, but not thickness (i.e. Breslow depth), race, or ethnicity, determine prognosis of mucosal melanomas. Considering the poor prognosis for all stages of mucosal melanoma, dermatologists should incorporate examination of the oropharynx and genitalia in the full body skin exam.
Keywords: mucosal melanoma, survival, treatment, extracutaneous melanoma, epidemiology, population-based, California Cancer Registry
CAPSULE SUMMARY
This population-based study underscores that extracutaneous melanomas are rare and aggressive neoplasms. Poorer survival in patients with mucosal melanoma was observed in relation to stage and anatomic site, but not tumor thickness (i.e., Breslow depth) or patient race/ethnicity.
Due to the poor prognosis for all stages of mucosal melanoma, dermatologists should consider incorporating examination of the oropharynx and genitalia in the full body skin exam.
INTRODUCTION
Accounting for approximately 1% of all melanomas,1–4 mucosal melanomas (i.e., extracutaneous melanomas) are rare and poorly characterized neoplasms that can be found on any mucosal surface. Due to their occult anatomical locations and lack of early presenting signs and symptoms, they are difficult to diagnose at an early stage, resulting in a poorer prognosis than cutaneous melanomas.3–6 Little evidence exists regarding predictors of survival from population-based survival data (i.e., data obtained from geographic or national registry databases), which are necessary for development of screening recommendations and targeted treatments.
Mucosal melanomas are pertinent to dermatologists because these neoplasms are often on the differential for pigmented lesions in sites that dermatologists examine on full body skin exams, including the oral mucosa and genitalia. The poor prognosis and lack of disease-specific treatment guidelines or randomized controlled trials for mucosal melanomas requires extra attention.
Treatments for mucosal melanomas are often extrapolated from data based on therapies for metastatic cutaneous melanoma. However, while Breslow depth is a well-known predictor of survival in cutaneous melanomas, population-based multivariate analyses have yet to examine the effect of thickness on survival in mucosal melanomas. Although population-based studies have shown that the five-year relative survival proportions of mucosal melanoma are poor compared with cutaneous melanoma4,7, of particular interest is how survival predictors differ between mucosal melanoma and cutaneous melanoma.
This study’s objective was to assess the independent influences of race/ethnicity, stage at diagnosis, and tumor thickness on mucosal melanoma survival, using a population-based database. While more detailed survival statistics on cutaneous melanoma exist elsewhere,8 we also included information on cutaneous melanomas for comparison with mucosal melanomas, as appropriate.
MATERIALS AND METHODS
Data source
Population-based melanoma incidence data were obtained from the California Cancer Registry (CCR: www.ccrcal.org), which comprises 10 regional registries. Patients selected for analysis were residents of California diagnosed with invasive melanoma between January 1, 1994 and December 31, 2015 and reported to the CCR as of August 2016. Information regarding sex, race/ethnicity, tumor thickness, and other information were abstracted from the medical record within months after diagnosis.
Tumor Characteristics and Patient Demographics
We categorized melanomas into cutaneous and mucosal melanoma according to the methods of McLaughlin et al,1 excluding ocular melanoma for this analysis. Invasive tumors with histology codes 8720-8790 (International Classification of Diseases for Oncology, 3rd edition [ICD-O-3]) were selected for analysis (N=144,904).
Selected tumors were classified by their Breslow depth, anatomic site, and histologic type. Thickness was categorized in the same groups typically found in survival analyses (<1mm, 1mm to <2mm, 2mm to <4mm, and 4mm or greater).
Anatomic site was identified from ICD-O-2 codes and classified according to the scheme used by McLaughlin et al.1 In the ICD-O-2 classification scheme, the skin of the anus and perianal skin are considered to be cutaneous. Cutaneous melanomas (ICD-O-2 code C44.0-C44.9) excluded the skin of the vulva, penis, and scrotum, which are instead included with mucosal melanomas. Melanomas with unknown primary site were considered to be cutaneous.
We adopted the clinical and pathologic TNM staging system of the Surveillance, Epidemiology and End Results (SEER) program, distinguishing between localized (to the tumor boundary), regional (with lymph node or direct extension involvement only), and distant involvement.
Race/ethnicity was grouped into the mutually exclusive categories of Non-Hispanic Whites, Non-Hispanic Blacks, Hispanics, and Asian/Pacific Islanders, according to the race/ethnicity reported to the CCR. As done in other population-based studies on extracutaneous melanoma, Hispanic ethnicity was established using the North American Association of Central Cancer Registries Hispanic Identification Algorithm.9 Patients of any other race or unknown race and patients with unknown diagnosis or follow-up dates were removed from the study population, leaving 132,751 cases for analysis.
Statistical methods:
SAS Software 9.4 (SAS Institute Inc., Cary, NC) was used to perform survival analysis and multivariate regression. Survival time was measured as the number of months between diagnosis and death. Patients still alive at last follow-up contributed their known survival time, but were censored from the analysis at the date of their last follow-up without an event (death). Survival curves and univariate descriptive statistics for survival by demographic and tumor characteristics were calculated using the Kaplan-Meier method. Because tumor thickness was unknown for over 70% of mucosal melanomas and tumor stage was missing for over 7% of mucosal melanomas, we included an “Unknown” group in all analyses involving tumor thickness or stage.
Cox proportional hazards regression was used to perform a multivariate analysis modeling survival on age, race/ethnicity, tumor thickness and stage at diagnosis, and primary tumor site for mucosal melanomas. For categorical variables including race, stage, thickness, and primary site, the selected reference groups were Non-Hispanic White, localized disease, <1 mm thickness, and oral cavity, respectively. Associations with death from disease are presented in the results as hazard ratios with corresponding p-values.
RESULTS
Of the 132,751 diagnoses of melanoma registered in California between 1994 - 2015 and which included race/ethnicity data, 130,927 melanomas (98.6%) were cutaneous, and 1,824 (1.4%) were mucosal. The five-year survival estimate for mucosal melanoma (27.64%, 95% confidence interval [CI] 25.42 – 29.91) was significantly lower than for cutaneous melanomas (76.28%, 95% CI 76.03 – 76.53).
Survival analysis by sex and race/ethnicity
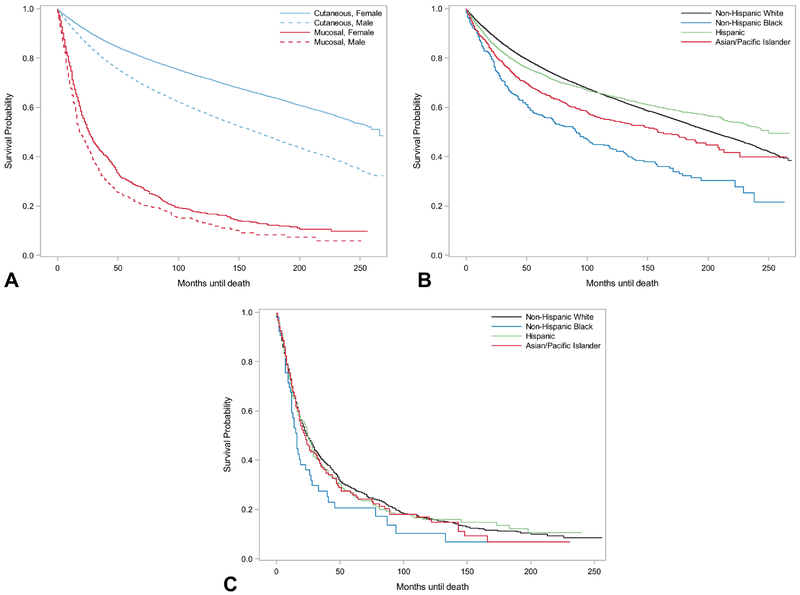
Patients diagnosed with mucosal melanomas experienced lower five-year survival estimates, regardless of race/ethnicity or sex, than individuals diagnosed with cutaneous melanoma (Table 1, Figure 1). For cutaneous melanoma, females had statistically significantly better survival rates than men. Similarly, for mucosal melanoma, there was a survival advantage for females, with only slightly overlapping CIs.
Table 1.
Cutaneous and mucosal melanomas by sex and race/ethnicity in California. California Cancer Registry, 1994-2015
| Cutaneous | Mucosal | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | 5-year Survival Estimate | Cases | 5-year Survival Estimate | |||||
| N | % | Survival Estimate (%) | 95% CI (%) | N | % | Survival Estimate (%) | 95% CI | |
| Sex | ||||||||
| Male | 77,921 | 59.51% | 72.20 | 71.86, 72.55 | 580 | 31.80% | 23.20 | 19.53, 27.06 |
| Female | 53,006 | 40.49% | 82.28 | 81.92, 82.63 | 1,244 | 68.20% | 29.71 | 26.96, 32.50 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 122,379 | 93.47% | 76.57 | 76.31, 76.83 | 1,261 | 69.13% | 28.48 | 25.82, 31.19 |
| Non-Hispanic Black | 470 | 0.36% | 56.97 | 51.99, 61.64 | 53 | 2.91% | 20.54 | 10.41, 33.05 |
| Hispanic | 6,924 | 5.29% | 73.96 | 72.79, 75.10 | 310 | 17.00% | 26.55 | 21.20, 32.18 |
| Asian/Pacific Islander | 1,154 | 0.88% | 66.53 | 63.39, 69.47 | 200 | 10.96% | 25.82 | 19.23, 32.90 |
| Total Cases | 130,927 | 1,824 | ||||||
Figure 1.
Survival for cutaneous and mucosal melanoma by sex and race/ethnicity in California. California Cancer Registry, 1994-2015. (a) Cutaneous versus mucosal melanoma survival by sex. (b) Cutaneous melanoma survival by race/ethnicity. (c) Mucosal melanoma survival by race/ethnicity.
While non-Hispanic whites had the best survival of all racial/ethnic groups for mucosal melanomas, the difference in rates was not statistically significant. For cutaneous melanomas, the five-year survival estimates for non-Hispanic whites, Hispanics, Asian/Pacific Islanders, and non-Hispanic blacks were 76.57% (95% CI: 76.3 – 76.8), 73.96% (95% CI: 72.79 – 75.10), 66.53% (95% CI: 63.39 – 69.47), and 56.97% (95% CI: 51.99 – 61.64), respectively.
Survival analysis by stage at diagnosis
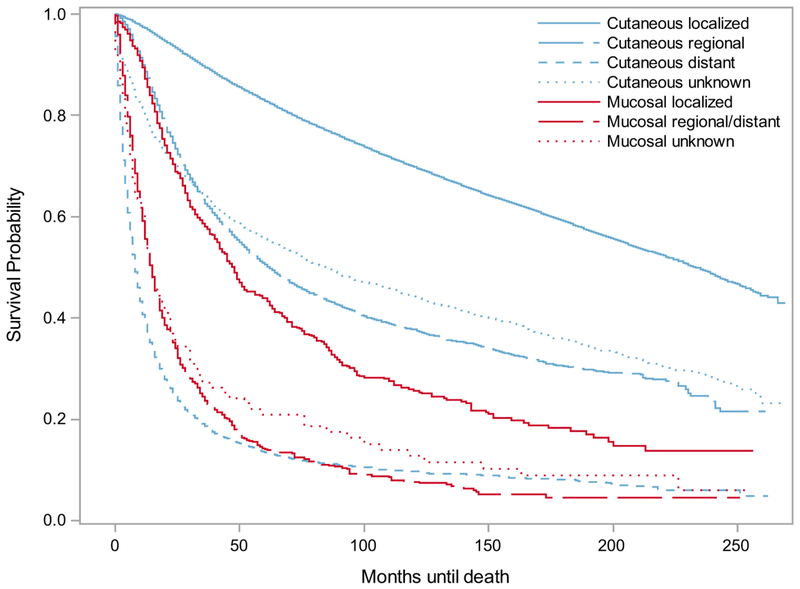
Regardless of stage, mucosal melanomas had worse survival rates than similarly staged cutaneous melanomas (Table 2). Localized mucosal melanomas had worse survival rates than even regionally metastasized cutaneous melanomas (Figure 2).
Table 2.
Survival for cutaneous and mucosal melanoma in California, by tumor stage at diagnosis. California Cancer Registry, 1994-2015
| Cutaneous | Mucosal | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | 5-year Survival Estimate | Cases | 5-year Survival Estimate | |||||
| N | % | Survival Estimate (%) | 95% CI (%) | N | % | Survival Estimate (%) | 95% CI | |
| Stage at Diagnosis | ||||||||
| Localized | 109,982 | 84.00% | 82.87 | 82.62, 83.12 | 790 | 43.31% | 43.93 | 40.13, 47.67 |
| Regional | 10,515 | 8.03% | 50.58 | 49.52, 51.64 | 518 | 28.40% | 18.76 | 15.20, 22.62 |
| Remote | 5,773 | 4.41% | 13.50 | 12.54, 14.50 | 384 | 21.05% | 7.84 | 5.13, 11.29 |
| Unknown | 4,657 | 3.56% | 55.65 | 54.09, 57.19 | 132 | 7.24% | 20.97 | 13.98, 28.93 |
| Total Cases | 130,927 | 1,824 | ||||||
Figure 2.
Survival for cutaneous and mucosal melanoma in California, by tumor stage at diagnosis. California Cancer Registry, 1994-2015
Survival analysis by melanoma thickness
Mucosal melanomas of any thickness had worse survival estimates than cutaneous melanomas of any thickness, and cutaneous melanomas were more frequently thinner tumors (Table 3). While survival decreased as cutaneous melanoma thickness increased, there was less prognostic value in tumor thickness for mucosal melanomas (Figure 3).
Table 3.
Survival for cutaneous and mucosal melanoma in California, by tumor thickness and primary site at diagnosis, California Cancer Registry, 1994-2015
| Cutaneous | Mucosal | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | 5-year Survival Estimate | Cases | 5-year Survival Estimate | |||||
| N | % | Survival Estimate (%) | 95% CI | N | % | Survival Estimate (%) | 95% CI | |
| Thickness at Diagnosis | ||||||||
| Less than 1mm | 74,885 | 57.20% | 87.70 | 87.44, 87.96 | 161 | 8.83% | 48.83 | 40.28, 56.82 |
| 1mm to <2mm | 20,413 | 15.59% | 77.38 | 76.74, 78.00 | 126 | 6.91% | 51.94 | 41.83, 61.12 |
| 2mm to <4mm | 11,777 | 9.00% | 61.10 | 60.12, 62.06 | 98 | 5.37% | 44.28 | 32.80, 55.14 |
| 4mm or greater | 8,642 | 6.60% | 43.10 | 41.94, 44.26 | 142 | 7.79% | 29.01 | 20.62, 37.91 |
| Unknown | 15,210 | 11.62% | 50.56 | 49.73, 51.40 | 1,297 | 71.11% | 21.29 | 18.92, 23.75 |
| Primary Site | ||||||||
| Oral Cavity | 174 | 9.54% | 27.85 | 20.58, 35.57 | ||||
| Anorectal | 358 | 19.63% | 16.68 | 12.58, 21.28 | ||||
| Nasal and Sinus | 452 | 24.78% | 23.77 | 19.65, 28.13 | ||||
| Genitourinary | 700 | 38.38% | 37.41 | 33.53, 41.29 | ||||
| Gastrointestinal | 68 | 3.73% | 20.94 | 11.82, 31.84 | ||||
| Other | 72 | 3.95% | 13.57 | 6.32, 23.58 | ||||
| Total Cases | 130,927 | 1,824 | ||||||
Figure 3.
Survival for cutaneous and mucosal melanomas in California, by tumor thickness and anatomic site. California Cancer Registry, 1994-2015. (a) Cutaneous versus mucosal melanoma survival by thickness. (b) Mucosal melanoma survival by thickness. (c) Mucosal melanoma survival by primary anatomic site.
Survival analysis by anatomic site
When mucosal melanomas of known primary sites were categorized by primary anatomic site, their five-year survival estimates were 37.41% for genitourinary, 27.85% for oral cavity, 23.77% for nasal and sinus, 20.94% for gastrointestinal, 16.68% for anorectal, and 13.57% for other sites (Table 3).
Multivariate analysis
After accounting for age, sex, race/ethnicity, and tumor stage at diagnosis, tumor thickness did not predict mucosal melanoma survival from tumors of known depth. The risk of death was increased for mucosal melanomas of unknown thickness (hazard ratio [HR] 1.47, 95% CI 1.15-1.89) (Table 4). Thickness was a statistically significant prognostic factor of death from cutaneous melanomas of all depths.
Table 4.
Multivariate models predicting cutaneous and mucosal melanoma death in the population of California. California Cancer Registry, 1994-2015
| Cutaneous (N=130,927) | Mucosal (N = 1,824) | |||
|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| Five-Year Age Group | 1.33** | 1.33, 1.34 | 1.12** | 1.09, 1.14 |
| Sex | ||||
| Male | reference | reference | ||
| Female | 0.75** | 0.74, 0.77 | 0.92 | 0.81, 1.04 |
| Race | ||||
| Non-Hispanic White | reference | reference | ||
| Non-Hispanic Black | 1.41** | 1.24, 1.60 | 1.06 | 0.77, 1.44 |
| Hispanic | 1.15** | 1.10, 1.20 | 1.03 | 0.89, 1.20 |
| Asian/Pacific Islander | 1.17* | 1.07, 1.29 | 0.93 | 0.78, 1.11 |
| Stage | ||||
| Localized | reference | reference | ||
| Regional | 1.96** | 1.90, 2.02 | 1.76** | 1.54, 2.02 |
| Distant | 7.64** | 7.37, 7.92 | 3.36** | 2.90, 3.92 |
| Unknown | 1.74** | 1.66, 1.82 | 1.71** | 1.38, 2.13 |
| Thickness | ||||
| <1mm | reference | reference | ||
| 1mm - <2mm | 1.47** | 1.43, 1.51 | 0.93 | 0.68, 1.26 |
| 2mm - <4mm | 1.95** | 1.89, 2.01 | 1.08 | 0.78, 1.51 |
| 4mm or more | 2.27** | 2.19, 2.35 | 1.18 | 0.88, 1.58 |
| Unknown | 1.92** | 1.85, 1.98 | 1.47* | 1.15, 1.89 |
| Primary Site | ||||
| Oral Cavity | reference | |||
| Anorectal | 1.37* | 1.10, 1.69 | ||
| Nasal and Sinus | 1.08 | 0.88, 1.33 | ||
| Genitourinary | 1.20 | 0.96, 1.50 | ||
| Gastrointestinal | 1.65* | 1.19, 2.27 | ||
| Other | 1.93** | 1.42, 2.64 | ||
Covariates include age, sex, race/ethnicity, tumor stage at diagnosis, tumor thickness at diagnosis, and mucosal primary site. Significance is indicated as follows:
p<0.01,
p<0.0001
After controlling for the above factors in addition to thickness, stage at diagnosis was a statistically significant predictor of survival for both mucosal and cutaneous melanomas. For cutaneous melanoma, risk of death for distantly metastasized disease was almost eight times as high as for localized disease (HR 7.64, 95% CI 7.37 – 7.92). The effect was mirrored, although not to the same degree, for mucosal melanomas, as distantly metastasized mucosal melanomas increased risk of death by more than three times (HR 3.36, CI 2.9 – 3.92).
While race/ethnicity had minimal effect on death from cutaneous melanomas, with HRs ranging from 1.15-1.41, this factor did not affect risk of death for mucosal melanomas (Table 4). There remained a small a survival advantage for females with cutaneous melanoma (HR 0.75, 95% CI 0.74-0.77) and mucosal melanoma.
Mucosal melanomas from less common anatomic sites (e.g., spine/CNS, lung and pleura, liver and pancreas) conferred the worst prognosis (HR 1.93, 95% CI: 1.41 – 2.64). Oral, anorectal and gastrointestinal sites also negatively affected survival, with HRs of 1.37 (95% CI: 1.10 – 1.70) and 1.65 (95% CI: 1.19 – 2.27), respectively.
DISCUSSION
Due to the rarity of mucosal melanomas, few population-based studies have reported predictors of survival from these neoplasms. Our population-based analysis of survival outcomes over a 21-year time period found that stage at diagnosis is an independent risk factor for survival from mucosal melanoma, while tumor thickness and race/ethnicity are not. Consistent with previous population-based reports,1–4 we found that mucosal melanomas comprised approximately 1% of all melanomas. Our aggregate five-year survival estimate of approximately 28% was similar to a previously reported U.S. population-based estimate,10 thereby underscoring the current knowledge that mucosal melanoma continues to be an aggressive disease that carries a poor prognosis.
Stage at diagnosis modified survival rates for cutaneous melanomas more than mucosal melanomas. This is likely because the risk of death from even a localized mucosal melanoma is even higher than for regionally metastatic cutaneous melanoma, possibly because these two types of melanomas are genetically distinct.11,12
While tumor thickness (i.e., Breslow depth) is the most important prognostic indicator for cutaneous melanomas,12 our results suggest that the same principle cannot be applied to mucosal melanomas. We found that tumor thickness conferred little prognostic value for survival from mucosal melanomas of known thicknesses, once stage was accounted for. Thickness likely had a smaller prognostic effect for mucosal melanomas because of their overall worse prognosis, compared to cutaneous melanomas. Notably, most (70%) of mucosal melanomas were of unknown depth, likely because they are difficult to detect and are likely to be metastatic at the time of diagnosis. Accordingly, the worst survival estimates were for patients with mucosal melanomas of unknown thickness. Thickness was not a predictor of survival for mucosal melanomas of known thickness and it could not be measured in a majority of mucosal melanomas, thus further reducing its utility as a basis for clinical decision-making for mucosal melanomas. The lack of difference in survival estimates between thin and thick mucosal melanomas suggests that even thin tumors should be aggressively treated with systemic therapy.
Consistent with previous population-based multivariate analyses,4 we found that race/ethnicity is not an independent risk factor for mucosal melanoma survival. Regardless of race/ethnicity, patients with mucosal melanoma had worse prognoses than individuals diagnosed with cutaneous melanoma.
We showed that anatomic site influences survival, as mucosal melanomas in the most occult anatomic sites (e.g., spine/CNS, lung and pleura, liver and pancreas) had the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Overall, our survival estimates based on anatomic site parallel the poor prognosis described in other population-based studies, which have provided five-year survival estimates ranging from 4% to 60%, depending on site.3,13 Although mucosal melanomas are infrequently encountered by dermatologists, they are often on the differential for pigmented mucosal lesions in sites that dermatologists can visualize, including the mouth and female genital tract. Currently, there are no mucosal melanoma consensus treatment guidelines or randomized controlled trials,14 and treatment regimens are often extrapolated from data based on therapies used to treat metastatic cutaneous melanoma.
In light of these findings, we recommend that dermatologists examine the oropharynx and the genitalia when performing a full body skin examination. The examination of the oropharynx includes asking the patient to lift up their upper lip, pull down their lower lip, and open their mouth so that the clinician can examine the palate. If a suspicious lesion in the oral mucosa is identified, we recommend referral to a dentist for biopsy and further evaluation. We also discuss with our patients that examination of the genital areas is recommended for full body skin exams, but patients can defer if they choose, or if they have regular follow-up with a gynecologist or urologist. These added steps are easy to incorporate into the full body skin examination and do not significantly prolong the encounter. Using these methods, the authors have identified mucosal melanomas in the oral cavity and genital areas. Given the poor prognosis of mucosal melanomas, we recommend that dermatologists consider implementing a comprehensive method of performing full body skin exams.
Limitations include the lack of a standardized staging system for mucosal melanomas, which may have resulted in potential misclassification of the extent of spread of some mucosal melanomas. Because our database did not have data on the genetic characteristics of the cases or their tumors, it is difficult to determine whether these factors modify our observations. Despite the aforementioned limitations, this study has several strengths. Most importantly, we used a population-based dataset with a large enough sample size to explore relationships between multiple. Compared to data from a single clinical institution, in which patient participation could represent a biased selection of mucosal melanomas, our data from a population-based registry is more generalizable to the United States population.
In conclusion, this study of twenty-one years of population-based California Cancer Registry data demonstrated that mucosal melanoma continues to be an aggressive disease with a poor prognosis. We found that stage at diagnosis is an independent risk factor for survival from mucosal melanoma, but tumor thickness and race/ethnicity are not. Anatomical site affects survival from mucosal melanomas, with tumors of less common anatomic sites conferring the worst prognosis. Because of the poor prognosis for mucosal melanomas of all stages, clinicians should consider treating more aggressively at the time of diagnosis and advocating for development of treatments specific to mucosal melanoma.
Acknowledgments
Funding/Support: Dr. Cockburn was supported in part by the National Cancer Institute’s I Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This project was reviewed by the Institutional Review Board at the University of Southern California and determined to be exempt.
Conflict of interest disclosure: none declared.
REFERENCES
- 1.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103(5):1000–1007. [DOI] [PubMed] [Google Scholar]
- 2.Altieri L, Wong MK, Peng DH, Cockburn M. Mucosal melanomas in the racially diverse population of California. J Am Acad Dermatol. 2017;76(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoelke A, Willrodt S, Hauschild A, Schadendorf D. Primary extracutaneous malignant melanoma: a comprehensive review with emphasis on treatment. Onkologie. 2004;27(5):492–499. [DOI] [PubMed] [Google Scholar]
- 4.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int J Cancer. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5(8):739–753. [PMC free article] [PubMed] [Google Scholar]
- 6.Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71(2):366–375. [DOI] [PubMed] [Google Scholar]
- 7.Koomen ER, de Vries E, van Kempen LC, et al. Epidemiology of extracutaneous melanoma in the Netherlands. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1453–1459. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17-25.e11-13. [DOI] [PubMed] [Google Scholar]
- 9.NAACCR Race and Ethnicity Work Group. NAACCR guideline for enhancing Hispanic/Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2.2.1]. Springfield, IL: North American Association of Central Cancer Registries, 2011. [Google Scholar]
- 10.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. [DOI] [PubMed] [Google Scholar]
- 11.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. [DOI] [PubMed] [Google Scholar]
- 12.Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment In: Kaufman HL, Mehnert JM, eds. Cancer Treatment and Research. Vol 167 Springer International Publishing; 2015:295–320. [DOI] [PubMed] [Google Scholar]
- 13.Mallone S, De Vries E, Guzzo M, et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer. 2012;48(8):1167–1175. [DOI] [PubMed] [Google Scholar]
- 14.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56(5):828–834. [DOI] [PubMed] [Google Scholar]