Abstract
Objective:
Anxiety disorders (ADs) are common, can result in life-long suffering, and frequently begin prior to adolescence. Evidence from adults suggests that altered prefrontal-limbic connectivity is a pathophysiological feature of ADs. More specifically, in adults with ADs decreased fractional anisotropy (FA), a measure of white matter integrity, has been observed in the uncinate fasciculus (UF), the major tract that connects limbic and prefrontal regions. Due to the early onset of ADs and the increased incidence in ADs in females during their reproductive years, it is important to understand whether the reduction in UF FA exists in children with ADs and the extent to which this alteration is sex related. To address these issues, we assessed FA in the UF, in unmedicated boys and girls with ADs.
Method:
FA measures were derived from diffusion tensor images that were acquired from 98 unmedicated children (age 8–12); 52 met criteria for generalized anxiety disorder, separation anxiety disorder, social anxiety disorder or anxiety disorder not otherwise specified, and 46 were matched controls.
Results:
Tract-based results demonstrated that AD children have significant reductions in UF FA. A significant sex-by-group interaction and post-hoc testing revealed that this effect was only evident in boys. No other main effects or sex-by-group interactions were found for other white matter tracts.
Conclusions:
These findings provide evidence of UF white matter alterations in boys with ADs. The data demonstrate that AD-related alterations in prefrontal-limbic structural connectivity are present early in life, are not related to psychotropic medication exposure, and are sex specific. Building on these findings, future research has the potential to provide insights into the genesis and sexual dimorphism of the pathophysiology that leads to ADs, as well as the identification of sex specific, early life treatment targets.
Introduction
Anxiety disorders (ADs) are among the most common psychiatric disorders and can become chronic1,2. ADs affect more than 1 -in-4 individuals at some point in their life and are generally more prevalent in females than in males3,4. Individuals suffering from ADs have impairments in psychosocial functioning and quality of life5, and when severe, ADs can be disabling. Children are especially affected by pathological anxiety, as ADs are among the earliest psychiatric illnesses to develop2, affecting up to 20% of youth6−8. Additionally, anxiety symptoms in children are often comorbid with other conditions, and are strong predictors of the subsequent development of other anxiety disorders, affective disorders, and comorbid substance abuse2. While early and effective treatments have the potential to prevent the life long suffering and psychosocial dysfunction associated with these disorders, current treatments for childhood ADs, such as cognitive behavioral therapy and selective serotonin reuptake inhibitors, are suboptimal. Many children fail to respond to these treatments and those that do respond have relatively high rates of relapse9. Establishing a better understanding of the pathophysiology of childhood ADs will facilitate the development of novel, more effective treatments10.
Despite the prevalence and importance of childhood ADs, few studies have focused on characterizing the neural alterations that underlie the early manifestations of these disorders11. Current knowledge of the pathophysiology of ADs is mostly derived from studies of adults, with a few studies examining adolescent and preadolescent AD patients11. Previous work revealed that ADs are associated with hyperactivation of the amygdala and insula in response to negative emotional stimuli12−14. While recent work from our group has shown that amygdala activation is significantly higher in preadolescent children with ADs when confronted with uncertain conditions15. We, as well as others, also report decreased anxiety-related functional coupling between the amygdala and regulatory prefrontal cortical (PFC) regions such as anterior cingulate cortex, orbital frontal cortex (OFC), dorsolateral prefrontal cortex and medial prefrontal cortex16−19. Decreased anxiety-related functional coupling between the amygdala and PFC is evolutionarily conserved, as we demonstrated similar findings in a rhesus monkey model of early life anxiety18.
Complementing these findings, diffusion tensor imaging (DTI) studies in patients with ADs report altered structural connectivity between temporal lobe and PFC regions, revealing that fractional anisotropy (FA) in the uncinate fasciculus (UF) is reduced in AD patients20−25. These studies focused predominantly on AD adults, with only 1 study that included adolescent AD patients25. The UF is highly relevant to anxiety and emotion regulation as it connects structures that are crucial to affective processing such as the amygdala, entorhinal/perirhinal cortices, and parahippocampal gyrus to frontal regions including the anterior PFC, OFC, ventromedial PFC, anterior cingulate cortex and insula26,27.
While these structural alterations may reflect the pathophysiology associated with ADs, it is also possible that these alterations result from illness chronicity, medication exposure, and/or other non-pathophysiological factors. Studies in medication-naive children have the advantage of examining white matter pathways early in the illness and in the absence of many of the influences that may indirectly affect white matter. Additionally, these studies may inform mechanisms underlying the childhood risk to develop ADs and may also point to new early life treatment and prevention strategies.
Here, we use DTI to assess white matter integrity in unmedicated preadolescent children with ADs compared to healthy controls, to test the hypothesis that childhood ADs are associated with alterations in the UF FA. Because of known sex differences in the prevalence of ADs3,4, and the interest in sexual dimorphism in the relation to brain development28,29, we also examine sex differences in this effect. Additional analyses aimed to further characterize these microstructural alterations across the brain by investigating three supplementary diffusivity measures, as well as investigate interactions with symptoms measures and steroid hormones. As such tract-based analyses of six additional structures for which the relations to ADs have been less consistent20−25‘30‘31, as well as whole brain voxel-based analyses were performed.
Methods
Participants
For a detailed description see eMethods. Briefly, diffusion-weighted magnetic resonance imaging (MRI) scans were obtained from a preadolescent sample across two research sites; University of Wisconsin (UW) and National Institute of Mental Health (NIMH). The final sample consisted of 98 children (age 8−12; 46 controls, 50 girls; Table 1) across the two sites. Control subjects were age and sex matched at the group level to the AD sample with no current or past psychiatric illness. Neither the AD patients nor controls were currently on any psychotropic medication, and neither reported any psychotropic medication usage at any point during their life. Informed assent and consent was obtained from all participants and their parents, in accordance with the institutional review board of UW and NIMH. Individuals were compensated for their time and effort.
Table 1.
Demographic, symptom and hormone levels for children with anxiety disorders (ADs) and controls.
| Demographics | Control | AD | p-values | Control Girls | AD Girls | p-values | Control Boys | AD Boys | p-values |
|---|---|---|---|---|---|---|---|---|---|
| Total; number (% female) | 46 (50%) | 52 (51%) | 0.854 | 23 (100%) | 27 (100%) | 23 (0%) | 25 (0%) | ||
| Age (years); mean (SD) | 10.58 (1.29) | 10.42 (1.33) | 0.701 | 10.74 (1.32) | 10.50 (1.23) | 0.633 | 10.38 (1.30) | 10.39 (1.43) | 0.987 |
| IQ (WASI); mean (SD) | 119.52 (13.44) | 117.42 (13.52) | 0.377 | 119.00 (16.33) | 116.12(12.81) | 0.313 | 120.04(10.11) | 118.72(14.33) | 0.696 |
| Physical development (Tanner); mean (SD) | 3.93 (2.06) | 4.04 (1.87) | 0.14 | 4.20 (2.09) | 4.65 (2.00) | 0.095 | 3.36 (1.71) | 3.67 (1.86) | 0.607 |
| Symptom measure scores | |||||||||
| Anxiety (parent SCARED); mean (SD) | 7.21 (8.83) | 28.59 (12.71) | <0.001* | 6.01 (6.13) | 29.56(12.00) | <0.001* | 6.10 (8.26) | 27.78(13.15) | <0.001* |
| Anxiety (child SCARED); mean (SD) | 13.41 (11.26) | 25.93 (13.71) | <0.001* | 12.44 (9.93) | 31.20(13.73) | <0.001* | 11.92 (9.23) | 21.36(12.52) | 0.005* |
| Depression (CDI); mean (SD) | 28.68 (18.87) | 38.52 (21.21) | <0.001* | 27.18(18.96) | 44.81(21.26) | <0.001* | 27.52(17.73) | 33.32(19.80) | 0.010* |
| ADHD (CPRS-R); mean (SD) | 45.67 (6.91) | 61.06 (12.74) | <0.001* | 46.05 (4.68) | 65.40(14.24) | <0.001* | 42.74 (2.68) | 57.25 (9.34) | <0.001* |
| Endocrines Measures | |||||||||
| Cortisol (Saliva); mean (SD) | 0.100 (0.038) | 0.102 (0.039) | 0.894 | 0.095(0.042) | 0.107(0.041) | 0.738 | 0.104(0.033) | 0.095(0.036) | 0.505 |
| Testosterone (Saliva); mean (SD) | 24.113(9.322) | 22.628(12.330) | 0.216 | 28.538(8.449) | 25.389(14.719) | 0.155 | 18.883(7.659) | 19.176(7.579) | 0.469 |
| Estradiol (Saliva); mean (SD) | 0.778 (0.313) | 0.648 (0.350) | 0.037* | 0.788(0.377) | 0.650(0.415) | 0.184 | 0.766(0.233) | 0.645(0.261) | 0.205 |
indicates two-tailed significance
All participants underwent a Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL)32 that was administered by a trained clinical psychologist (PhD) or psychiatrist (MD), see eMethods for details on cross-rater agreement. Beyond diagnosis, children’s symptoms were rated by both the child and a parent using multiple symptom questionnaires (see eMethods).
Data Acquisition & Analyses
DTI acquisition:
For detailed description see eMethods. Briefly, brain images at both sites were collected on MRI scanners of the same make and model, with comparable parameters, optimized for diffusion-weighted imaging with 48 directions.
Steroid hormone collection:
See eMethods.
DTI analyses:
For detailed description see eMethods. Briefly, images were corrected for field inhomogeneity and eddy currents, after which the tensors were calculated using a robust tensor estimation. Tensor images of all subjects were coregistered iteratively using non-linear tensor-based normalization tools and then registered to MNI 152-space. In this template space diffusion measures were extracted to quantify local white matter microstructure. Deterministic fiber tractography was performed to delineate tracts of interest, that besides the UF20−25 included cortico-limbic association pathways previously implicated in ADs, including the cingulum bundle (CING)20‘30, superior longitudinal fasciculus (SLF)20,24,25, fornix (FX)20, and inferior frontal occipital fasciculus (IFO)20,25. Tracts shown to have anxiety-related changes in other publications were also extracted, including thalamocortical projection fibers in the internal capsule (IC)20,25, and interhemispheric commissural fibers of the corpus callosum (CC)31.
Next, in order to quantify the microstructure of an entire white matter structure a weighted mean was calculated per tract for each diffusion measure per subject. Tract-based analyses were performed with average tract measures predicting AD patient status. To characterize microstructural changes in these white matter pathways beyond FA, analyses assessed mean diffusivity (MD), axial diffusivity (XD) and radial diffusivity (RD) as well. We also examined the possibility of sexually dimorphic effects relating white matter microstructure to anxiety in AD children. To that effect, the interaction between patient status (AD, control) and sex (boys, girls) was tested in the same model. Age, sex and site were included in this model as covariates. Since we had no a priori hypothesis about left versus right tract differences, the bilateral components of each tract were combined into one average.
Analyses were run using standard linear regression techniques in R-studio (Version 1.0.136)33. To control for familywise error a Sidak correction for the number of tracts was applied where m = 7, for each white matter tract tested, resulting in a corrected two-tailed significance threshold of α < 0.0073 (αSID = 1 – (1 – a)1/m = 1 – (1–0.05)1/7 = 0.0073). The effect sizes of significant tract-based effects were reported using Cohen’s d34–36, to aid in interpretation. Cohen’s d values greater than 0.8 are considered a large effect size, while 0.5 is a medium effect36. Voxel-based analyses of FA across the brain were performed to test regions within and beyond the a priori determined tracts.
Additional analyses, described in detail in the results, included age, sex and site as covariates where applicable, and used standard linear regression techniques in R-studio (Version 1.0.136)33 and Python.
Steroid hormone analyses:
See eMethods.
Results
Demographics, Symptoms & Hormonal Measurements
Ninety-eight children (age 8–12; 50 girls, 46 controls) were included in the final analyses. AD and control children did not significantly differ in age (t(94) = 0.385, p = 0.701), sex (t(94) = 0.184, p = 0.854) or IQ (t (91) = 0.888, p = 0.377; Table 1). Furthermore, there was no significant group difference in physical development as assessed by the Tanner stages (t (86) = −1.499, p = 0.140; Table 1). Age and sex each significantly predicted Tanner scores (age; t (86) = 8.510, p < 0.001, sex; t (86) = −2.006, p = 0.048), with older children and females having significantly higher Tanner scores, but the group by sex interaction was non-significant (p = 0.406).
AD subjects had significantly higher symptom scores on all clinical scales (anxiety, depression and ADHD) compared to controls (ps < 0.001; Table 1). No group by sex interactions were found for depression and ADHD symptoms. A group by sex interaction approached significance for self-reported anxiety (t(89) = 1.82, p = 0.072; eFigure 1). Post hoc tests indicated that self-reported anxiety scores were significantly lower for AD boys compared to AD girls (t(48) = 2.49, p = 0.016).
Testosterone was significantly higher in girls compared to boys (z(55) = −2.57, p = 0.006; for mean values by group for each sex see Table 1), an effect that has previously been observed in pre-adolescent children37. Neither estradiol nor cortisol differed by sex (ps > 0.4). Age was positively related to testosterone (z(55) = 3.286, p = 0.006), and estradiol (z(55) = 2.538, p = 0.011).
While there were no group differences in cortisol or testosterone (p > 0.22), an unpredicted significant group effect was observed for estradiol levels, in which AD children had lower levels of estradiol compared to controls (z(55) =2.085, p = 0.037, Table 1, eFigure 2). None of the endocrine measures displayed a significant group by sex interaction (ps > 0.5).
Tract-based DTI Analyses
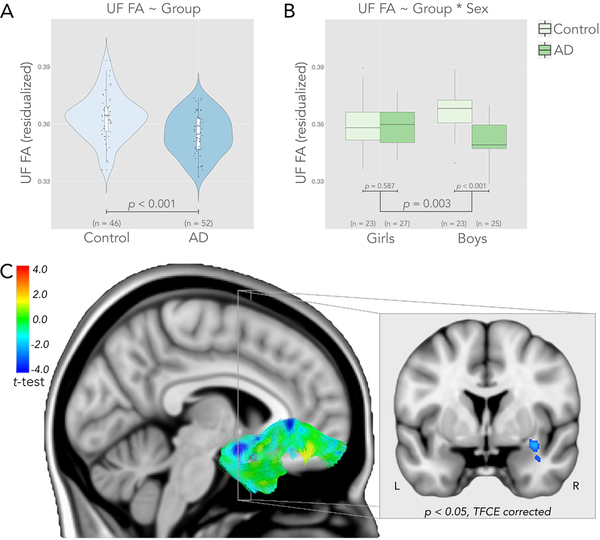
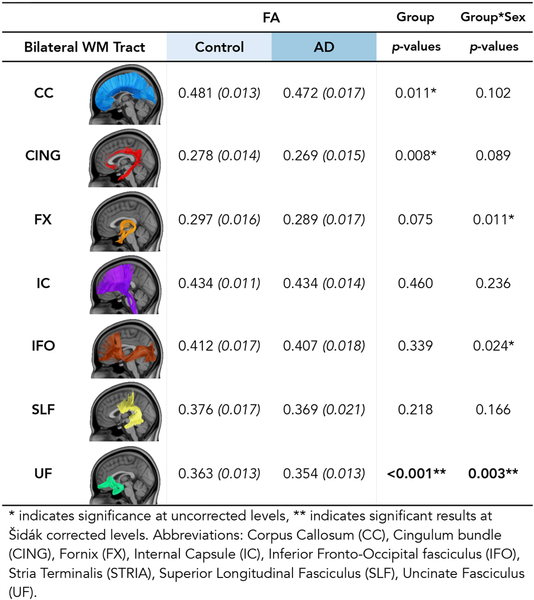
Tract-based analyses were performed on seven white matter pathways, leading to a multiple comparisons threshold after Sidak correction of p ≤ 0.0073, and were covaried for age, sex and site. Results indicated that only the UF tract significantly differed between groups (Figure 1 & Figure 2). Specifically, AD children had reduced UF FA compared to control children (t(92) = 3.650, p < 0.001, effect size: Cohen’s d = 0.73. See also Figure 1 & Figure 2). In addition, the interaction between group and sex was significant for UF FA (t(92) = 3.058, p = 0.003). Post hoc tests indicated that AD boys displayed significantly reduced UF FA compared to control boys (t(44) = 4.750, p < 0.001, Figure 1), whereas AD girls did not differ from control girls (t(46) = 0.547, p = 0.587, Figure 1). To further investigate which of these 4 groups were the different from the rest (AD girls, AD boys, control girls, control boys), we ran a contrast analysis. Results indicated that AD boys were different from all three other groups (t(92) = 3.937, p< 0.001).
Figure 1.
Children with anxiety disorders (ADs) have reduced fractional anisotropy (FA) in the uncinate fasciculus (UF). A) Children with ADs have significantly lower FA in the UF compared to controls. Violin plot of UF FA data residualized for sex, age and site. B) Box plot of significant interaction between group and sex. Post hoc analyses indicate no significant difference between healthy girls and AD girls, but are different between healthy boys and AD boys. UF FA is residualized for age and site. C) Whole-brain voxel-based analyses confirm significant differences in UF FA between controls and AD children, test results are shown on the right UF fiber tract. The coronal view shows the extent of significant differences after TFCE correction. Cooler colors indicate lower FA values in AD patients compared to healthy controls.
Figure 2.
Group differences in tract fractional anisotropy (FA). Weighted mean FA values by tract for control and anxiety disorder (AD) subjects, with standard deviations in parentheses. Significance of the regression statistics for the main effect of group, as well as the interaction of group by sex are noted. All analyses include age, sex and site as covariates.
Other variables that could potentially influence the results include: age, comorbid symptoms, and hormonal status. While there was a main effect of age on UF FA (t(92) = 2.786, p = 0.006), no significant sex by age or group by sex by age interactions were found (p > 0.28; eTable 1 & eFigure 3). In relation to anxiety, depression and ADHD scores, we performed analyses to test for interactions and found that these measures did not impact the findings, such that there were no significant group by sex by rating interactions (ps > 0.375, eTable 1). We also performed analyses that examined potential influences of the comorbid categorical diagnoses, ADHD (4 females, 2 males) and depression (3 females, 0 males). The results remained largely unchanged when excluding these patients from the analyses (Excluding ADHD; Group: t(86) = 3.592, p < 0.001, Group x Sex: t (86) = 2.502, p = 0.014. Excluding MDD; Group: t (89) = 3.749, p < 0.001, Group x Sex: t(89) = 2.872, p = 0.005). Finally, we tested hormonal status (cortisol, testosterone and estradiol) in relation to the findings, which demonstrated no significant interactions that could account for the effects (Group by Sex by Hormone level; ps > 0.175; eTable 1).
Other diffusivity measures explored besides FA, such as MD, XD and RD indicated that only UF RD was significantly increased in AD children (t (92) = −2.813, p = 0.006: Cohen’s d = 0.56, eFigure 4), and the Group by Sex interaction was nominally, uncorrected, significant (t (92) = −2.007, p = 0.048, eFigure 4). Several of the other six white matter tracts displayed some nominally, uncorrected, significant group effects and group by sex interactions for FA, MD, XD and RD (see Figure 2/eFigure 4). However, none passed multiple comparison corrections.
Voxel-based Analyses of FA
Whole-brain voxel-based analyses explored group differences beyond the a priori hypothesized tracts. The results confirmed the observations from the tract-based analyses, demonstrating significantly reduced FA in multiple locations along the UF tract (p < 0.05, TFCE corrected, Figure 1, Table 2, and https://neurovault.org/collections/161/). Areas particularly affected included white matter adjacent to the orbital gyrus, as well as white matter in the bend around the lateral fissure between the temporal and frontal lobes. We also detected group differences in the CC, IC and IFO pathways (Table 2 & https://neurovault.org/collections/161/). In the CC tract, alterations were mainly localized in the genu (p < 0.05, TFCE corrected, Table 2 & https://neurovault.org/collections/161/). In the left and right IC, significant clusters were found in the area of the postcentral gyrus in the somatosensory cortex (p < 0.05, TFCE corrected, Table 2 & https://neurovault.org/collections/161/). In the IFO tract significant clusters were mainly located in the anterior inferior portions of the right IFO (p < 0.05, TFCE corrected, Table 2 & https://neurovault.org/collections/161/).
Table 2.
Group differences (control vs. AD) in voxel-based analyses of whole-brain FA values. Overview of size and location of significant clusters after TFCE correction.
| Cluster # |
Volume (mm3) |
Location | Hemisphere | Peak t-value |
Peak MNI Coordinate |
|---|---|---|---|---|---|
| 1 | 7000 | CC/UF | L & R | 5.6 | (88 153 73) |
| 2 | 425 | IC | R | 4.3 | (111 89 140) |
| 3 | 290 | UF | R | 4.85 | (126 127 59) |
| 4 | 228 | CC/IFO | R | 4.53 | (118 74 103) |
| 5 | 148 | IC | L | 5.11 | (74 98 133) |
| 6 | 10 | IFO | R | 3.03 | (120 148 85) |
| 7 | 4 | UF | R | 3.47 | (129 124 45) |
| 8 | 3 | CC | R | 4.35 | (111 59 121) |
Based on results from the tract-based analyses, we also examined the group by sex interaction, which did not reveal any clusters passing TFCE correction at p = 0.05. However, separate analyses performed in girls and boys revealed significant FA reductions in AD boys compared to control boys in regions that overlap with the UF, CC and bed nucleus of the stria terminalis, and no such differences in girls (eFigure 5 & eTable 2 & https://neurovault.org/collections/161/).
Discussion
This study examined the microstructural integrity of the UF in unmedicated, preadolescent children with ADs and also examined potential sex differences. The findings revealed decreased UF FA in AD boys, and not girls. Tractography analyses of other white matter tracts demonstrated the relative specificity of this finding. The UF is a white matter tract critical for prefrontal-temporal lobe functional integration38, and the current data suggest that in boys this tract may be linked to the prefrontal-limbic dysregulation that is associated with ADs20–25. While UF FA differences have been reported in adolescents and adults with ADs, we are unaware of any reports that have addressed the issue of sexual dimorphism in AD patients. However, there have been inconsistent reports of sex related UF FA alterations in individuals with high levels of trait anxiety39–41.
Importantly, we demonstrate that these white matter alterations are present early in life and in children that have never been exposed to psychotropic medications. In contrast to studies in adult AD populations20, the results from this study can be interpreted without the potential confounds of prior medication exposure and/or illness chronicity. These findings provide developmental continuity in relation to the previously reported UF FA reductions in adolescents and adults, suggesting that the UF white matter alterations observed in these older populations may have their origins in childhood.
The UF FA differences that we found in AD boys are intriguing and raises the question as to what underlies this sexual dimorphism. We found no evidence that age or sex hormones could account for this finding. Previous studies in children and adults indicate either no, or a minimal, effect of sex on UF FA42–44. Likewise, studies in pubertal children examining sex steroids have found little association between hormonal levels and white matter volume45,46, however little is known about the specific relation between sex hormones and UF FA. In contrast, age is well known to be associated with FA42‘43,47,48, and even with the relatively constrained age that we studied, we found that UF FA increased with age. One possibility that could provide an explanation for the sexually dimorphic finding reported here, is that the developmental trajectory of UF FA could differ between boys and girls. However, we found no significant age by sex interactions, which is consistent with other work43,44, and importantly we found no age by sex by group interactions. We also assessed levels of the stress hormone cortisol and found no significant influences of cortisol on UF FA that could account for the UF FA reductions in boys with ADs, or interactions with sex. Because of the episodic nature of the secretion of steroid hormones, it is possible that more frequent sampling would reveal a relation between steroid hormone levels and UF FA.
Most, but not all DTI studies of AD patients report reductions in UF FA, as well as other white matter alterations20−25‘30‘31‘49‘50. Inconsistencies in the UF FA findings across studies could be due to a variety of factors, including heterogeneity in diagnostic composition of the sample, sample size, and DTI acquisition/processing. We also note that reduced UF FA is not specific to ADs, as similar white matter alterations have been observed in some studies of individuals with trait anxiety39−41,51−54, as well as in patients with affective and other psychiatric disorders55,56.
Our assessment of multiple diffusivity measures allows for a deeper understanding of the nature of the microstructural alterations found in AD children. In addition to reduced FA, AD boys exhibited increased RD. The combination of decreased FA and increased RD is thought to reflect reduced myelination and/or reduced axonal density57,58. Changes in myelination and axonal density are associated with alterations in the speed, timing and accuracy of information passing through white matter59. Because the UF is the major tract connecting medial temporal lobe structures, such as the amygdala and hippocampus, with prefrontal cortex, alterations in UF structure are likely to alter information flow relevant to emotion regulation60. Although lesions affecting the UF can alter the regulation of anxiety in primates61−63, the extent to which individual differences in UF myelination influence anxiety-related neuronal signaling is unclear. Of note, a recent study in humans demonstrates that individual differences in UF FA are related to individual differences in cognitive-emotional processing64.
Although axon myelination is most active early in development, the microstructural integrity of UF continues to increase into adulthood and is one of the last white matter pathways to reach peak FA43. Myelin producing oligodendrocytes are highly plastic throughout adulthood and oligodendrocyte precursor cells are the major proliferating cell type in adult brains65. Evidence is accumulating that myelination occurs dynamically in response to neural activity and can continue throughout adulthood59,66,67. This white matter plasticity provides a potential mechanism by which targeted therapies could be focused on restoring UF integrity. Interestingly, data in children suggest that aerobic exercise increases white matter integrity68, and it is well know that exercise has antianxiety effects69. Our data suggests that early life interventions targeted at increasing UF integrity could be particularly effective in boys in ameliorating the symptoms of anxiety, and also could have a protective impact on the development of white matter connectivity between brain regions critical for adaptive emotion regulation.
Taken together with other studies16−25, these data provide convergent support for PFC-temporal lobe dysfunction, that now extends to childhood anxiety. The findings demonstrate early life alterations in a key white matter tract involved in conveying information relevant to emotion and anxiety regulation. The findings also point to the importance of considering brain related sex differences prior to puberty. The data from this study, along with future studies, has the potential to guide the development of novel treatments focused on restoring the adaptive prefrontal regulation of anxiety. Such early interventions support the possibility of treating AD children with therapies that could reduce or even prevent long term chronic psychopathology.
Supplementary Material
Acknowledgements
We thank the HealthEmotions Research Institute, the Waisman Laboratory for Brain Imaging and Behavior, the Lane Neuroimaging Laboratory, Neuroscience Training Program.
This work has been supported by National Institutes of Health grants; R21 MH092581, & grant Z-002781. Do P.M. Tromp had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of interest:
Do P.M. Tromp reports no conflicts of interest.
Lisa E. Williams reports no conflicts of interest.
Andrew S. Fox reports no conflicts of interest.
Jonathan A. Oler reports no conflicts of interest.
Patrick H. Roseboom reports no conflicts of interest.
Gregory M. Rogers reports no conflicts of interest.
Brenda E. Benson reports no conflicts of interest.
Andrew L. Alexander reports no conflicts of interest.
Daniel S. Pine reports no conflicts of interest.
Ned H. Kalin’s Disclosure of Competing Interest and Financial Support:
Honoraria: CME Outfitters; Elsevier Inc.; Pritzker Consortium
Scientific Advisory Board: Pritzker Neuroscience Consortium, Neuronetics, Ketamine Clinics of America Editor: Psychoneuroendocrinology, Elsevier
Patents: Promoter sequences for corticotropin-releasing factor CRF2alpha and method of identifying agents that alter the activity of the promoter sequences: U.S. Patent issued on 07–04-06; #7071323, U.S. Patent issued on 05–12-09; #7,531,356. Promoter sequences for urocortin II and the use thereof: U.S. Patent issued on 08–08-06; #7087385. Promoter sequences for corticotropin-releasing factor binding protein and use thereof: U.S. Patent issued on 10–17-06; #7122650.
References
- 1.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2011; 18(1):23–33. doi: 10.1017/S1121189X00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Ruscio AM, Shear K, Wittchen H-U. Epidemiology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:21–35. [PubMed] [Google Scholar]
- 3.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelvemonth and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21 (3): 169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKinaw-Koons B, Vasey MW. Considering sex differences in anxiety and its disorders across the life span: A construct-validation approach. Appl Prev Psychol. 2000;9:191–209. doi: 10.1016/S0962-1849(05)80004-6. [DOI] [Google Scholar]
- 5.Mendlowicz MV, Stein MB. Quality of life in individuals with anxiety disorders. Am J Psychiatry. 2000; 157(1):669–682. doi: 10.1176/appi.ajp.157.5.669. [DOI] [PubMed] [Google Scholar]
- 6.Merikangas KR, Nakamura EF, Kessler RC. Epidemiology of mental disorders in children and adolescents. Dialogues Clin Neurosci. 2009;11:7–20. doi: 10.2964/jsik.kuni0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland W, Angold A, Shanahan J, Costello EJ. Longitudinal Patterns of Anxiety From Childhood to Adulthood: The Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2014;53(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merikangas K, Jian-ping H, Burstein M, et al. Lifetime Prevalence of Mental Disorders in US Adolescents: Results from the National Comorbidity Study-Adolescent Supplement. J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017.Lifetime. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry. 2010;71(7):839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- 11.Ayling E, Aghajani M, Fouche J-P, Wee N. Diffusion Tensor Imaging in Anxiety Disorders. Curr Psychiatry Rep. 2012; 14(3): 197–202. doi: 10.1007/s11920-012-0273-z. [DOI] [PubMed] [Google Scholar]
- 12.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(0ctober):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel K, Bandelow B, Gruber O, Wedekind D, Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci. 2011; 13(6):453–461. doi: 10.1007/S00702-008-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Martin, Ressler KJ, Binder E, Nemeroff CB. The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Clin Lab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams LE, Oler J a, Fox AS, et al. Fear of the Unknown: Uncertain Anticipation Reveals Amygdala Alterations in Childhood Anxiety Disorders. Neuropsychopharmacology. 2014:1–8. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010; 167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 18.Birn RM, Shackman AJ, Oler JA, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;19(January):915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–576. doi: 10.1016/S0084-3970(08)79329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tromp DPM, Grupe DW, Oathes DJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry. 2012;69(9):925–934. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan KL, Orlichenko A, Boyd E, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66(7):691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baur V, Brühl AB, Herwig U, et al. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: A quantitative fiber tractography study. Hum Brain Mapp. 2013;00(July):437–446. doi: 10.1002/hbm.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hettema JM, Kettenmann B, Ahluwalia V, et al. Pilot multimodal twin imaging study of generalized anxiety disorder. Depress Anxiety. 2012;29(3):202–209. doi: 10.1002/da.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baur V, Hànggi J, Rufer M, et al. White matter alterations in social anxiety disorder. J Psychiatr Res. 2011;45(10): 1366–1372. doi: 10.1016/j.jpsychires.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Liao M, Yang F, Zhang Y, He Z, Su L, Li L. White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry. 2014; 14(1):41. doi: 10.1186/1471-244X-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 27.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in Vivo Interactive Dissection of White Matter Fasciculi in the Human Brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 28.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Beilis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001; 11 (iii):552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Li L, Yu R, et al. White matter integrity alterations in first episode, treatment-naive generalized anxiety disorder. J Affect Disord. 2013;148(2–3):196–201. doi: 10.1016/j.jad.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 31.Liao W, Xu Q, Mantini D, et al. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–177. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Birmaher B, Ehmann M, Axelson D a., et al. Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children - A preliminary psychometric study. J Psychiatr Res. 2009;43(7):680–686. doi: 10.1016/j.jpsychires.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RStudio Team −. RStudio: Integrated Development for R. [Online] RStudio, Inc, Boston, MA: URL http://www.rstudio.com 2016:RStudio, Inc., Boston, MA. doi: 10.1007/978-81-322-2340-5. [DOI] [Google Scholar]
- 34.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.X. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan GM, Feinn R. Using Effect Size - or Why the P Value Is Not Enough. J Grad Med Educ. 2012;4(3):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci. 1988;2nd:567. doi: 10.1234/12345678. [DOI] [Google Scholar]
- 37.Dorn LD, Kolko DJ, Susman EJ, et al. Salivary gonadal and adrenal hormone differences in boys and girls with and without disruptive behavior disorders: Contextual variants. Biol Psychol. 2009;81(1):31–39. doi: 10.1016/j.biopsycho.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain.] 2009. doi: 10.1093/acprof:oso/9780195104233.001.0001. [DOI] [Google Scholar]
- 39.Montag C, Reuter M, Weber B, Markett S, Schoene-Bake JC. Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience. 2012;217:77–83. doi: 10.1016/j.neuroscience.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Kim MJ, Brown AC, Mattek AM, et al. The inverse relationship between the microstructural variability of amygdala-prefrontal pathways and trait anxiety is moderated by sex. 2013:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MJ, Avinun R, Knodt AR, Radtke SR, Hariri AR. Neurogenetic plasticity and sex influence the link between corticolimbic structural connectivity and trait anxiety. Sci Rep. 2017;7(1). doi: 10.1038/s41598-017-11497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan KM, Iftikhar A, Kamali A, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. [DOI] [PubMed] [Google Scholar]
- 44.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peper JS, Brouwer RM, Schnack HG, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Perrin JS, Hervé P-Y, Leonard G, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebel C, Beaulieu C. Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. J Neurosci. 2011. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Zhang Y, Li L, et al. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011; 133(1–2):294–299. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 50.Brambilla P, Como G, Isola M, et al. White-matter abnormalities in the right posterior hemisphere in generalized anxiety disorder: a diffusion imaging study. [DOI] [PubMed]
- 51.Westlye LT, Bjornebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry. 2011;68(4):369–377. doi: 10.1001/archgenpsychiatry.2011.24. [DOI] [PubMed] [Google Scholar]
- 52.Baur V, Hanggi J, Jancke L. Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neurosci. 2012; 13(1). doi: 10.1186/1471-2202-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37): 11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modi S, Trivedi R, Singh K, et al. Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behav Brain Res. 2013;238:188–192. doi: 10.1016/j.bbr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review of Diffusion Tensor Imaging Studies in Affective Disorders. Biol Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 56.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. 2013; 136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldman HM, Yeatman JD, Lee ES, Barde LHF, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr. 2010;31(4):346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of Cerebral White Matter Properties Using Quantitative Magnetic Resonance Imaging Stains. Brain Connect. 2011;1(6). doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampaio-Baptista C, Johansen-Berg H. White Matter Plasticity in the Adult Brain. Neuron. 2017. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 61.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray E a. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013; 16(8): 1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kazama A, Bachevalier J. Selective Aspiration or Neurotoxic Lesions of Orbital Frontal Areas 11 and 13 Spared Monkeys’ Performance on the Object Discrimination Reversal Task. J Neurosci. 2009;29(9):2794–2804. doi: 10.1523/JNEUROSCI.4655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lapate RC, Rokers B, Tromp DPM, et al. Awareness of Emotional Stimuli Determines the Behavioral Consequences of Amygdala Activation and Amygdala-Prefrontal Connectivity. Sei Rep. 2016;6. doi: 10.1038/srep25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawson MRL, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 66.Gibson EM, Purger D, Mount CW, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183): 1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholz J, Klein MC, Behrens TEJ, Johansen-berg H. Training induces changes in white-matter architecture. 2009; 12(11): 1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaddock-Heyman L, Erickson Kl, Holtrop JL, et al. Aerobic fitness is associated with greater white matter integrity in children. Front Hum Neurosci. 2014;8(August):1–7. doi: 10.3389/fnhum.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broman-Fulks JJ, Berman ME, Rabian B a., Webster MJ. Effects of aerobic exercise on anxiety sensitivity. Behav Res Ther. 2004;42(2): 125–136. doi: 10.1016/S0005-7967(03)00103-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.